CMS-10668 CLABSI Validation Template
Quality Measures and Administrative Procedures for the Hospital-Acquired Condition Reduction Program (CMS-10668)
FY27_CLABSI_ValTemp.xlsx
Hospital-Acquired Condition Reduction Program-NHSN HAI Measures Validation
OMB: 0938-1352
⚠️ Notice: This form may be outdated. More recent filings and information on OMB 0938-1352 can be found here:
Document [xlsx]
Download: xlsx | pdf
Template
NHSN ICU Location
FY2027 Submission Instructions
Overview
DefinitionsTemplate
NHSN ICU Location
FY2027 Submission Instructions
Sheet 1: Definitions
| Central line-associated bloodstream infection (CLABSI) Validation Template | ||
| In support of the Centers for Medicare & Medicaid Services (CMS) Hospital-Acquired Condition (HAC) Reduction Program inpatient data validation efforts | ||
| for the Fiscal Year (FY) 2027 program year: | ||
| • Each hospital selected for CLABSI validation is to produce a list of positive blood cultures for intensive care unit (ICU) patients, which is annotated to identify | ||
| patients with central lines placed during the stay. | ||
| • The line list should include all final results for positive blood cultures collected during an ICU stay. | ||
| • For each patient confirm: | ||
| ° The patient had an ICU admission during this hospital stay; and | ||
| ° The patient had a positive blood culture drawn during the ICU stay. | ||
| (The list should include all positive blood cultures for patients admitted in the ICU at the time the culture was drawn. | ||
| If the patient did not have an ICU admission when the culture was drawn, do not include these on the Validation Template.) | ||
| ° Whether a central line was in place at any time during the hospital stay. | ||
| FY 2027 - CLABSI Validation Template | ||
| (Use this template for 1Q 2024 through 4Q 2024 positive blood cultures - all quarters must be submitted on separate templates) | ||
| FIELD (* indicates required field) | DESCRIPTION | SECTION |
| NHSN Facility ID* | The National Healthcare Safety Network (NHSN)-assigned facility ID under which your hospital submits NHSN data. | |
| Provider ID/CCN* | Hospital's 6-digit CMS Certification Number (CCN). Do not include any hyphens. | Hospital Information Section |
| Hospital Name* | Hospital Name associated with CCN. | Complete the first row in the |
| State* | Enter the 2 character abbreviation for the state in which the hospital is located. | spreadsheet. The information |
| Calendar Quarter* | Select from the drop-down list the calendar quarter to which the CLABSI Validation Template pertains. | provided in the first row will be applied to all positive blood |
| Hospital Contact Name* | Hospital contact name for CMS to contact with questions. | cultures listed on the template. |
| Contact Phone* | Phone number for hospital contact listed. | |
| Contact Email* | Email address for hospital contact listed. | |
| Total discharges in quarter with ICU stay | The total number of patients discharged during the reporting quarter who had an ICU stay. Patients with positive blood cultures are a subset of this group. | |
| Positive Blood Cultures (Y/N)* | Select Yes or No from the drop-down list. Does the hospital have any final results for positive blood cultures for ICU patients in the calendar quarter referenced? | |
| Patient Identifier* | The patient identifier assigned by the hospital. Use the same patient identifier that would be submitted to NHSN if the episode of care (EOC) would be reported as a CLABSI event. | |
| Birthdate* | The patient date of birth using MM/DD/YYYY format. | |
| Sex* | Select Female, Male or unknown from the drop-down list to indicate the sex of the patient. | |
| Central line Y/N* | Select Yes or No from the drop-down list. Did the patient have a central line in place at any time during their hospital stay? Please include central lines already in place when the patient was admitted. | |
| Admit Date* | Enter date patient was admitted to hospital in MM/DD/YYYY format. | |
| Discharge Date* | Enter date patient was discharged from the hospital in MM/DD/YYYY format. If a patient has not been discharged from the hospital enter "Not Discharged" for the Discharge Date field. Discharge dates that fall within the reporting quarter will be eligible for validation. |
Patient & Blood Culture Section Complete for every final positive blood culture. |
| First Name | First name of patient. | |
| Last Name | Last name of patient. | |
| NHSN ICU Location* | Select from the drop-down list, the NHSN ICU location to which the patient was assigned when the positive blood culture was collected. Include only cultures collected during an ICU stay. Only locations from the drop-down will be accepted; do not use a hospital-assigned location. |
|
| Lab ID* | Lab ID, accession number or specimen number corresponding to positive blood culture. | |
| Blood Culture Date* | Provide the date the blood culture was collected in MM/DD/YYYY format. | |
| Blood Culture Time | Provide the time the blood was drawn if easily available. | |
| Pathogen Name (A)* | Specify pathogen identified. Only pathogens from the drop-down will be accepted. The drop-down options are derived from the CDC's NHSN Organism List. It is possible your laboratory may identify an organism that cannot be found when referencing the drop-down. Organisms from the following genera are excluded from NHSN surveillance and cannot be used to meet any of the NHSN definitions: Blastomyces, Histoplasma, Coccidioides, Paracoccidioides, Cryptococcus, and Pneumocystis. Since they are excluded pathogens for all NHSN HAI reporting, they are not included on the NHSN organism list and do not need to be included on the HAI Validation Template. If you have an organism which is not found on the drop-down, other than those listed above, please contact nhsn@cdc.gov for guidance on appropriate reporting. |
|
| Pathogen Name (B) | Specify pathogen identified. Only pathogens from the drop-down will be accepted. | |
| Pathogen Name (C) | Specify pathogen identified. Only pathogens from the drop-down will be accepted. | |
| For additional information, view the appropriate CLABSI Abstraction Manual posted on the Inpatient Data Validation Resources | ||
| page of QualityNet (direct link): | https://qualitynet.cms.gov/inpatient/data-management/data-validation/resources | |
| For the purposes of CMS inpatient data validation, please note the differences between NHSN data submission | ||
| and validation template/medical record submission, as described below: | ||
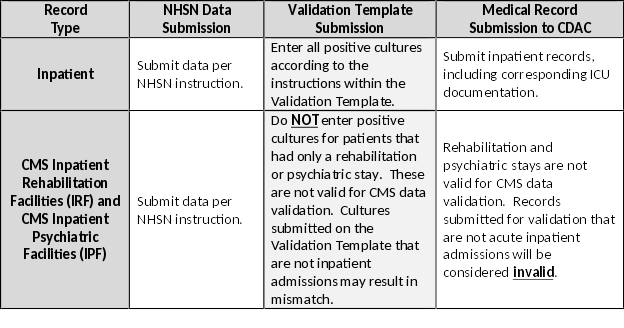
|
||
| PRA Disclosure Statement | ||
| According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this | ||
| information collection is 0938-1352 (Expires 01/31/2026). The time required to complete this information collection is estimated to average 10 hours per response, including the time to review instructions, | ||
| search existing data resources, gather the data needed, and complete and review the information collection. If you have comments concerning the accuracy of the time estimate(s) or suggestions for | ||
| improving this form, please write to CMS, 7500 Security Boulevard, Attn: PRA Reports Clearance Officer, Mail Stop C4-26-05, Baltimore, Maryland 21244-1850. ****CMS Disclosure**** Please do not send | ||
| applications, claims, payments, medical records or any documents containing sensitive information to the PRA Reports Clearance Office. Please note that any correspondence not pertaining to the | ||
| information collection burden approved under the associated OMB control number listed on this form will not be reviewed, forwarded, or retained. | ||
| If you have questions or concerns regarding where to submit your documents, please contact the Validation Support Contractor at validation@telligen.com. | ||
Sheet 2: Template
| NHSN Facility ID* | Provider ID/CCN* | Hospital Name* | State* | Calendar Quarter* | Hospital Contact Name* | Contact Phone* | Contact Email* | Total discharges in quarter with ICU stay | Positive Blood Cultures (Y/N)* | Patient Identifier* | Birthdate* | Sex* | Central line Y/N* | Admit Date* | Discharge Date* | First Name | Last Name | NHSN ICU Location* | Lab ID* | Blood Culture Date* | Blood Culture Time | Pathogen Name (A)* | Pathogen Name (B) | Pathogen Name (C) |
Sheet 3: NHSN ICU Location
| Below is a list of NHSN ICU Locations applicable for CMS inpatient data validation CLABSI reporting. | ||
| CDC LOCATION LABEL | CDC LOCATION CODE | LOCATION DESCRIPTION |
| Inpatient Adult Critical Care Units | ||
| Burn Critical Care | IN:ACUTE:CC:B | Critical care area for the care of patients with significant/major burns. |
| Medical Cardiac Critical Care | IN:ACUTE:CC:C | Critical care area for the care of patients with serious heart problems that DO NOT require heart surgery. |
| Medical Critical Care | IN:ACUTE:CC:M | Critical care area for the care of patients who are being treated for nonsurgical conditions. |
| Medical-Surgical Critical Care | IN:ACUTE:CC:MS | Critical care area for the care of patients with medical and/or surgical conditions. |
| Neurologic Critical Care | IN:ACUTE:CC:N | Critical care area for the care of patients with life- threatening neurologic diseases. |
| Neurosurgical Critical Care | IN:ACUTE:CC:NS | Critical care area for the surgical management of patients with severe neurologic diseases or those at risk for neurologic injury as a result of surgery. |
| Oncology Medical Critical Care | IN:ACUTE:CC:ONC_M | Critical care area for the care of oncology patients who are being treated for nonsurgical conditions related to their malignancy. |
| Oncology Surgical Critical Care | IN:ACUTE:CC:ONC_S | Critical care area for the evaluation and management of oncology patients with serious illness before and/or after cancer-related surgery. |
| Oncology Medical-Surgical Critical Care | IN:ACUTE:CC:ONC_MS | Critical care area for the care of oncology patients with medical and/or surgical conditions related to their malignancy. |
| Onsite Overflow Critical Care | IN:ACUTE:CC:OF_ONSITE | Area previously used for non-patient care which has been repurposed to care for critically ill or injured patients. |
| Prenatal Critical Care | IN:ACUTE:CC:PNATL | Critical care area for the care of pregnant patients with complex medical or obstetric problems requiring a high level of care to prevent the loss of the fetus and to protect the life of the mother. |
| Respiratory Critical Care | IN:ACUTE:CC:R | Critical care area for the evaluation and treatment of patients with severe respiratory conditions. |
| Surgical Cardiothoracic Critical Care | IN:ACUTE:CC:CT | Critical care area for the care of patients following cardiac and/or thoracic surgery. |
| Surgical Critical Care | IN:ACUTE:CC:S | Critical care area for the evaluation and management of patients with serious illness before and/or after surgery. |
| Trauma Critical Care | IN:ACUTE:CC:T | Critical care area for the care of patients who require a high level of monitoring and/or intervention following trauma or during critical illness related to trauma. |
| Inpatient Pediatric Critical Care Units | ||
| ONC Pediatric Critical Care | IN:ACUTE:CC:ONC_PED | Critical care area for the care of oncology patients ≤18 years old who are being treated for surgical or nonsurgical conditions related to their malignancy. |
| Pediatric Burn Critical Care | IN:ACUTE:CC:B_PED | Critical care area for the care of patients ≤18 years old with significant/major burns. |
| Pediatric Surgical Cardiothoracic Critical Care | IN:ACUTE:CC:CT_PED | Critical care area for the care of patients ≤18 years old following cardiac and thoracic surgery. |
| Pediatric Medical Critical Care | IN:ACUTE:CC:M_PED | Critical care area for the care of patients ≤18 years old who are being treated for nonsurgical conditions. |
| Pediatric Medical-Surgical Critical Care | IN:ACUTE:CC:MS_PED | Critical care area for the care of patients ≤18 years old with medical and/or surgical conditions. |
| Pediatric Neurosurgical Critical Care | IN:ACUTE:CC:NS_PED | Critical care area for the surgical management of patients ≤18 years old with severe neurologic diseases or those at risk for neurologic injury as a result of surgery. |
| Pediatric Respiratory Critical Care | IN:ACUTE:CC:R_PED | Critical care area for the evaluation and treatment of patients ≤18 years old with severe respiratory conditions. |
| Pediatric Surgical Critical Care | IN:ACUTE:CC:S_PED | Critical care area for the evaluation and management of patients ≤18 years old with serious illness before and/or after surgery. |
| Pediatric Trauma Critical Care | IN:ACUTE:CC:T_PED | Critical care area for the care of patients ≤18 years old who require a high level of monitoring and/or intervention following trauma or during critical illness related to trauma. |
| Inpatient Critical Care Neonatal Units | ||
| Neonatal Critical Care (Level II/III) | IN:ACUTE:CC_STEP:NURS | Combined nursery housing both Level II and III newborns and infants, as per the NHSN level definitions above and below. This is analogous to a mixed acuity unit specifically for Neonatal Critical Care patients. |
| Neonatal Critical Care (Level III) | IN:ACUTE:CC:NURS | A hospital neonatal intensive care unit (NICU) organized with personnel and equipment to provide continuous life support and comprehensive care for extremely high- risk newborn infants and those with complex and critical illness. The capabilities of Level III, listed below, are from the American Academy of Pediatrics definitions of levels of neonatal care.1 Level III NICU Level II capabilities plus: -Provide sustained life support -Provide comprehensive care for infants born < 32 wks. gestation and weighing <1500 g and infants born at all gestational ages and birth weights with critical illness -Provide prompt and readily available access to a full range of pediatric medical subspecialists, pediatric surgical specialists, pediatric anesthesiologists, and pediatric ophthalmologists -Provide a full range of respiratory support that may include conventional and/or high-frequency ventilation and inhaled nitric oxide -Perform advanced imaging, with interpretation on an urgent basis, including computed tomography, MRI, and echocardiography |
| Neonatal Critical Care (Level IV) | IN:ACUTE:CC:NURS_IV | Critical care area for the care of newborns and infants with serious illness requiring Level IV care; area is supervised by a neonatologist Level IV Level III capabilities plus: -Located within an institution with the capability to provide surgical repair of complex congenital or acquired conditions -Maintain a full range of pediatric medical subspecialists, pediatric surgical subspecialists, and pediatric subspecialists at the site -Facilitate transport and provide outreach education |
Sheet 4: FY2027 Submission Instructions
| USER GUIDE AND SUBMISSION INSTRUCTIONS | ||||||||||||||||
| ---> | The FY 2027 Validation Template User Guide and Submission Instructions, along with supporting documentation, can be found on the CMS QualityNet website. | |||||||||||||||
| To access, select [Hospitals–Inpatient], and then [Data Management], followed by [Data Validation], and lastly [Resources]: | ||||||||||||||||
| https://qualitynet.cms.gov/inpatient/data-management/data-validation/resources | ||||||||||||||||
| The only acceptable method of sending HAI Validation Templates is through the CMS Managed File Transfer (MFT) application: | ||||||||||||||||
| https://qnetmft.cms.gov | ||||||||||||||||
| HAI Validation Templates contain Protected Health Information (PHI) and cannot be sent via email -- even if a template were sent encrypted from a secure | ||||||||||||||||
| workplace email, it would still be considered a security violation. | ||||||||||||||||
| It is recommended to submit HAI Validation Templates at least a week prior to the submission deadline in case there are difficulties with | ||||||||||||||||
| transmitting files, and to allow time for revisions/corrections when necessary. | ||||||||||||||||
| If you are unable to log in to the CMS MFT application, the first person to contact is your hospital's Security Official (SO). | ||||||||||||||||
| If your SO is unable to establish your access, you will need to contact the Center for Clinical Standards & Quality (CCSQ) Service Center by phone at 866-288-8912. | ||||||||||||||||
| It is recommended hospitals have two SOs at all times to ensure the ability to upload Validation Templates by the established submission deadlines. | ||||||||||||||||
| We suggest hospitals ask their IT department to add validation@telligen.com to their ‘Safe Senders List’ to ensure validation-related email notifications are received. | ||||||||||||||||
| HAI VALIDATION TEMPLATE COMPLETION & SUBMISSION TIPS | ||||||||||||||||
| Prior to submitting HAI Validation Templates to CMS, it is recommended that quality assurance is performed on the data within the template. | ||||||||||||||||
| Review the [Definitions] tab to ensure correct information is entered in each field. | ||||||||||||||||
| ü | Do not add, delete, rename, or change the order of the tabs. | |||||||||||||||
| ü | Do not add, delete, or rename column headings. | |||||||||||||||
| ü | Do not leave the first row blank or skip rows between patient data. | |||||||||||||||
| ü | Make sure the Provider ID/CCN field is exactly 6 numeric characters (do not add a hyphen). | |||||||||||||||
| ü | Make sure the State field contains the 2 character abbreviation for your state, not the full state name. | |||||||||||||||
| ü | Verify the Calendar Quarter listed on each Validation Template is correct. | |||||||||||||||
| ü | Review all dates for accuracy and correct format as specified on the [Definitions] tab. | |||||||||||||||
| ü | Make sure pathogens entered on each row of the template are found within the drop-down provided. | |||||||||||||||
| ü | If a patient has not been discharged from the hospital, enter ‘Not Discharged’ for the Discharge Date field. | |||||||||||||||
| ü | The 'Positive Blood Cultures' column cannot include rows listing both "Yes" and "No"; entering "No" indicates no positive cultures for the quarter. | |||||||||||||||
| ü | Ensure all NHSN ICU locations are within the approved NHSN drop down on the template. Hospital-assigned locations will not be accepted. | |||||||||||||||
| ü | Be sure to populate all required fields on each consecutive row if there were multiple final positive cultures collected for the same patient. | |||||||||||||||
| ü | Perform quality check of data entered into this template against what was entered into NHSN; stay mindful of differing CMS and NHSN deadlines. | |||||||||||||||
| ü | Check to ensure any cases with a separate Inpatient Rehabilitation Facility (IRF) or Inpatient Psychiatric Facility (IPF) CCN are not included on the template. | |||||||||||||||
| ü | Append the file name with the 6-digit CMS Certification Number (CCN)/Provider ID, followed by an underscore and the quarter. | |||||||||||||||
| For example: 012345_1QYY_FYXX_CLABSI_ValTemp.xlsx | ||||||||||||||||
| • When submitting templates via the [Compose] button within the Mail area of the CMS MFT dashboard, input the subject of the message | ||||||||||||||||
| with the 6-digit CCN/Provider ID, Submission Quarter, and Template type(s) attached. | ||||||||||||||||
| For example: 012345 1QYY FYXX CLABSI & CAUTI Validation Templates | ||||||||||||||||
| • When choosing a recipient, select the ellipsis button to the right of the To field and then select the [Groups] tab to locate the "Validation Support Contractor" group. | ||||||||||||||||
| Do NOT select any individual person(s) from the recipient list; only select the "Validation Support Contract" Group. | ||||||||||||||||
| Some individual accounts are not regularly monitored—sending to any one individual risks delay in processing. | ||||||||||||||||
| • Leave the 'Require Registered Users' box checked under Options. Un-checking this box puts the message at risk of not being processed. | ||||||||||||||||
| • We strongly encourage hospitals to add a check on the 'Read Receipt' box under Options. | ||||||||||||||||
| After a file has been downloaded by someone on the Validation Support Contractor team, it will be in the queue for processing. | ||||||||||||||||
| • It is suggested that users verify a message has been sent by clicking on the [Sent Items] button from the left-side navigation panel of the MFT dashboard. | ||||||||||||||||
| NOTE: It can take a couple minutes for messages to appear in the Sent Items folder. Please, do NOT re-send messages multiple times, | ||||||||||||||||
| as this significantly delays processing and requires version confirmation. | ||||||||||||||||
| • You will receive email confirmation (usually within 2 business days of being downloaded) from the Validation Support Contractor letting you know the Validation | ||||||||||||||||
| Templates were processed. If you do not receive a processing confirmation, please include your hospital's 6-digit CCN/Provider ID in an | ||||||||||||||||
| email to | validation@telligen.com | |||||||||||||||
| File Type | application/vnd.openxmlformats-officedocument.spreadsheetml.sheet |
| File Modified | 0000-00-00 |
| File Created | 0000-00-00 |
© 2026 OMB.report | Privacy Policy