Form 0920-24CB Post-Clinic Visit Survey_Online_English
[NCCDPHP] Evaluation of a Prostate Cancer Decision Aid
Attachment 10h_Post-Clinic Visit Survey_Online_English
Post-clinic Survey
OMB: 0920-1438
Form approved
OMB # 0920-####
Exp. date MM/DD/YYYY
ATTACHMENT 10h
Post-Clinic Visit Survey:
Online, English
WEB SURVEY APPEARANCE |
Optimization for best viewing and ease of use on multiple devices (desktop, laptop, tablet, smartphone) |
Header Text: Prostate Cancer Screening Evaluation |
Footer Text: If you have any questions about the study, please email the study coordinator at danielle.nielsen@icf.com |
Logo: Planning to add the CDC logo |
Survey Termination: Thank you for your interest and time. You do not qualify for this study at this time |
Prompt for required questions: Please complete all the questions on this page before moving on. |
PROMPT FOR ALL OTHER QUESTIONS IF RESPONDENT TRIES TO SKIP You have not answered all the questions on this page. Please consider responding to all questions. If you are choosing not to answer a question(s), click next. |
Survey Complete text: Thank you for completing the survey. If you have any questions about the study, please email the study coordinator at danielle.nielsen@icf.com |
Imported Sample Variables
CLINIC
IMPORTED SAMPLE VARIABLE: Clinic Name
[TEXT BOX]
NAME
IMPORTED SAMPLE VARIABLE: Name
[TEXT BOX]
Informed Consent
[ASK ALL]
LANG.
In what language would you like to complete this survey?
¿En qué idioma le gustaría completar esta encuesta?
01 English
02 Español

[ASK ALL]
[REQUIRED]
C1CONSENT.
You are being asked to participate in this study because you receive care at [CLINIC] and are eligible for prostate cancer screening. The purpose of this survey is to gather information about your prostate cancer screening history and decision making. If you complete this survey, you will receive a $25 gift code.
Your completion of this survey is completely voluntary, and your responses are confidential.
Who is sending this survey? ICF is a consulting firm that is working with the Division of Cancer Prevention and Control (DCPC) at the Centers for Disease Control and Prevention (CDC) to evaluate different prostate cancer screening decision aids and their ability to help men aged 55-69 years make a decision whether to get a prostate-specific antigen (PSA) test. A PSA test is a blood test that measures the level of PSA in the blood. PSA is a substance made by the prostate.
How long will it take? This survey will take no longer than 20 minutes. Your participation in this study is 100% voluntary which means you can choose whether or not you want to take part in this study.
What are the risks and benefits of doing the survey? As a participant in our study, there is a minimal risk related to your privacy and/or confidentiality, but steps have been taken to remove your personal information so that you cannot be identified. Only members of the research team will have access to study information. Remember, you are free to choose not to participate in this study. You are also free to leave the study at any time. Leaving the study will not interfere with your care, payment for your health care, or your eligibility for health care benefits.
Is there a cost associated with the study? There are no costs to you for participating in this study.
What will happen next? This is the last activity for the study. After you complete this survey, you will receive an email with a $25 gift card. You will also receive a final thank you email with some additional materials with information about prostate cancer screening.
How will my information be shared outside of the study? Your personal responses will not be shared outside of the study. Summaries of survey results that are not linked to your name will be shared with CDC and/or may be published in a professional journal.
Who do I call about problems or questions? If you have questions about or concerns about your participation in this project, please contact the ICF project manager - Danielle Nielsen at Danielle.Nielsen@icf.com. For questions regarding your rights as a study participant, you can contact ICF’s Institutional Review Board (IRB) representative Christine Walrath at (646) 695-8154 or Christine.Walrath@icf.com.
If you agree to participate in this study, please click “Begin Survey.”
If you do not agree to participate, please click, “I decline.”
01 Begin survey
02 I decline
[Terminate if C1=02]

Section 1: Prostate Cancer Knowledge
[ASK ALL]
Q1.
For this first section, we’d like to get a sense of your prostate cancer knowledge now that you have reviewed the assigned material and had a visit with your health care provider. If you are unsure of the answer, please select the “Not sure” response.
The prostate gland is a reproductive organ located below the bladder.
01 True
02 False
03 Not
sure
[ASK ALL]
Q2. The prostate gland makes some of the fluid that’s part of semen.
01 True
02 False
03 Not sure

[ASK ALL]
Q3.
Older men are more likely to get prostate cancer.
01 True
02 False
03 Not sure

[ASK ALL]
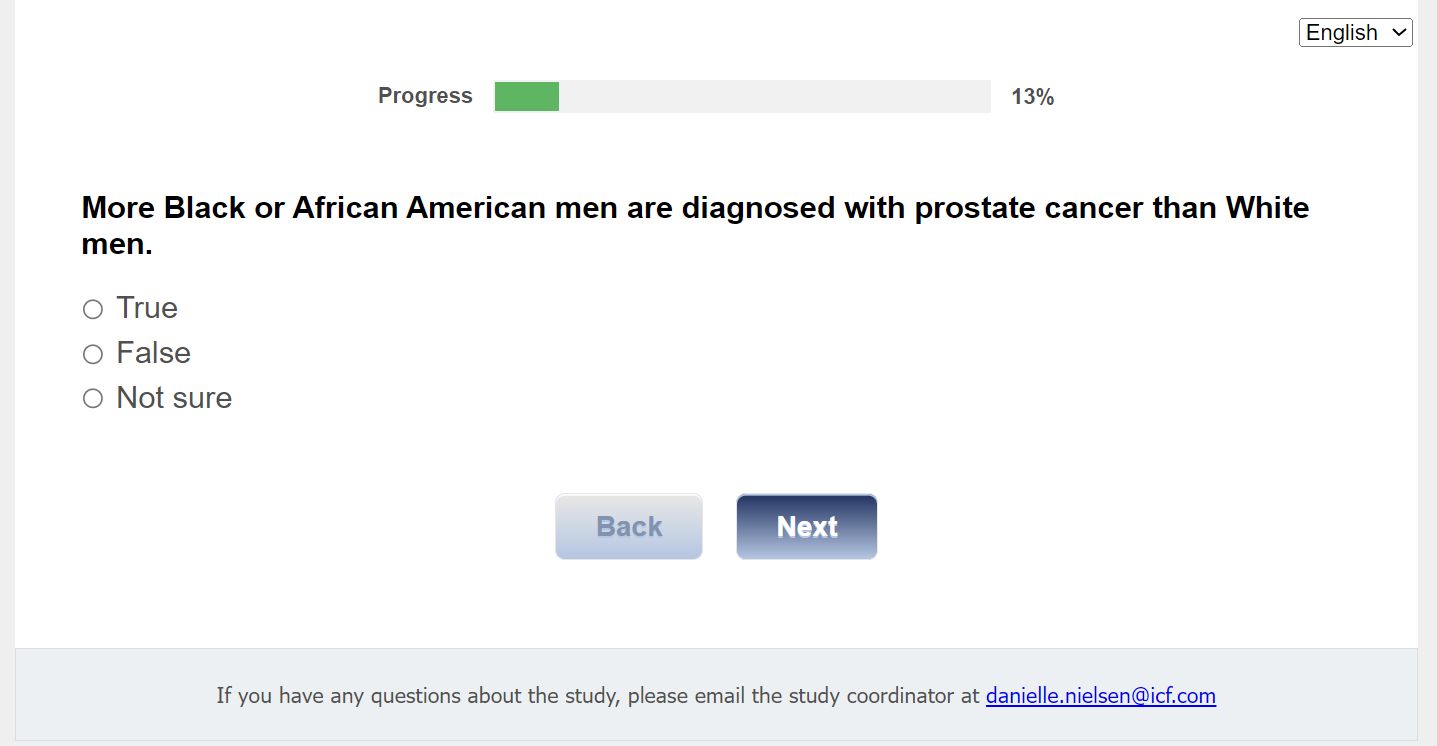
Q4. More Black or African American men are diagnosed with prostate cancer than White men.
01 True
02 False
03 Not sure
[ASK ALL]
Q5. Men who have fathers or brothers with prostate cancer are more likely to get prostate cancer than those who do not.
01 True
02 False
03 Not sure

[ASK ALL]
Q6. Who do you think is more likely to get prostate cancer?
01 White men
02 Black or African American men
03 Hispanic or Latino men
04 Asian men
05 Race or ethnicity is not a factor
06 Not sure

[ASK ALL]
Q7. Who do you think is more likely to get prostate cancer?
01 A man whose father has had prostate cancer
02 A man whose father has not had prostate cancer
03 It doesn’t make any difference
04 Not sure

[ASK ALL]
Q8.
A prostate-specific antigen (PSA) blood test can be done to check for prostate cancer.
01 True
02 False
03 Not sure

[ASK ALL]
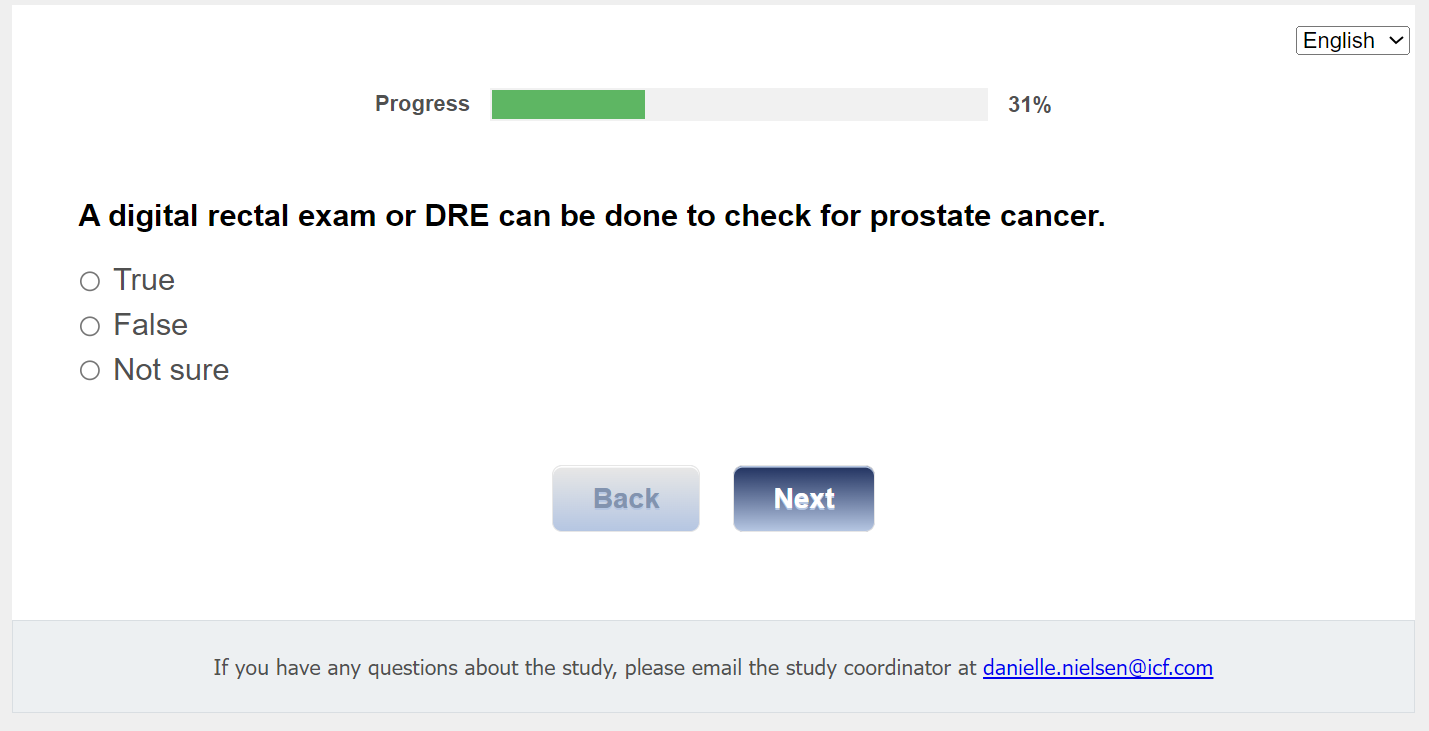
Q9. A digital rectal exam or DRE can be done to check for prostate cancer.
01 True
02 False
03 Not sure
[ASK ALL]
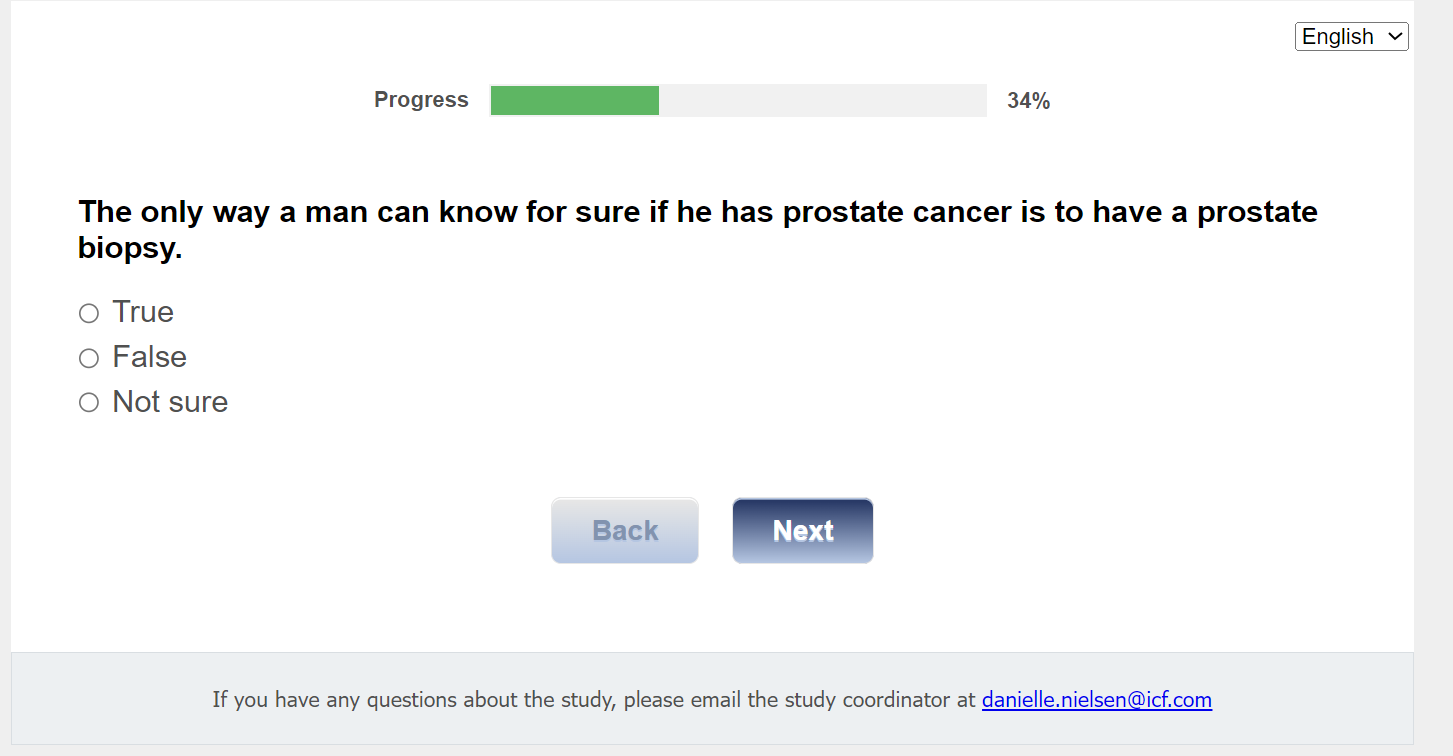
Q10. The only way a man can know for sure if he has prostate cancer is to have a prostate biopsy.
01 True
02 False
03 Not sure
[ASK ALL]
Q11. A prostate biopsy is when a blood test is used to check for proteins in the blood.
01 True
02 False
03 Not sure

[ASK ALL]
Q12. Neither the PSA nor DRE are 100% accurate.
01 True
02 False
03 Not sure

[ASK ALL]
Q13.
A man can have prostate cancer and have no symptoms.
01 True
02 False
03 Not sure

Section 2: Decisional Conflict
[ASK ALL]
Q21. As it relates to prostate cancer screening, after meeting with your health care provider, please indicate your agreement with each statement, using either strongly agree, agree, neither, disagree, or strongly disagree.
|
01 Strongly Agree |
02 Agree |
03 Neither |
04 Disagree |
05 Strongly Disagree |
Q21A. I know which prostate cancer screening options are available to me. |
|
|
|
|
|
Q21B. I know the benefits of each option. |
|
|
|
|
|
Q21C. I know the risks and side effects of each option. |
|
|
|
|
|
Q21D. I am clear about which benefits matter most to me. |
|
|
|
|
|
Q21E. I am clear about which risks and side effects matter most. |
|
|
|
|
|
Q21F. I am clear about which is more important to me (the benefits or the risks and side effects). |
|
|
|
|
|
Q21G. I have enough support from others to make a choice about prostate cancer screening. |
|
|
|
|
|
Q21H. I am choosing without pressure from others. |
|
|
|
|
|
Q21I. I have enough advice to make a choice. |
|
|
|
|
|
Q21J. I am clear about the best choice for me. |
|
|
|
|
|
Q21K. I feel sure about what to choose. |
|
|
|
|
|
Q21L. This decision is easy for me to make. |
|
|
|
|
|
Q21M. I feel I have made an informed choice. |
|
|
|
|
|
Q21N. My decision shows what is important to me. |
|
|
|
|
|
Q21O. I expect to stick with my decision. |
|
|
|
|
|
Q21P. I am satisfied with my decision. |
|
|
|
|
|
Section 3: Autonomous Decision Making
[ASK ALL]
Q22. Please check the response that best describes how you were involved in making decisions about screening for prostate cancer.
01 I was less involved than I wanted to be.
02 I was as involved as I wanted to be.
03 I was more involved than I wanted to be.
Section 4: Shared Decision Making
[ASK ALL]
Q23. Please answer the following questions about what happened when you talked with your health care provider about screening for prostate cancer.
Did you discuss prostate cancer screening with your provider?
01 Yes
02 No

[ASK IF Q23=01]
Q24. How much did you and your health care provider talk about the reasons you might want to have prostate cancer screening?
01 A lot
02 Some
03 A little
04 Not at all

[ASK IF Q23=01]
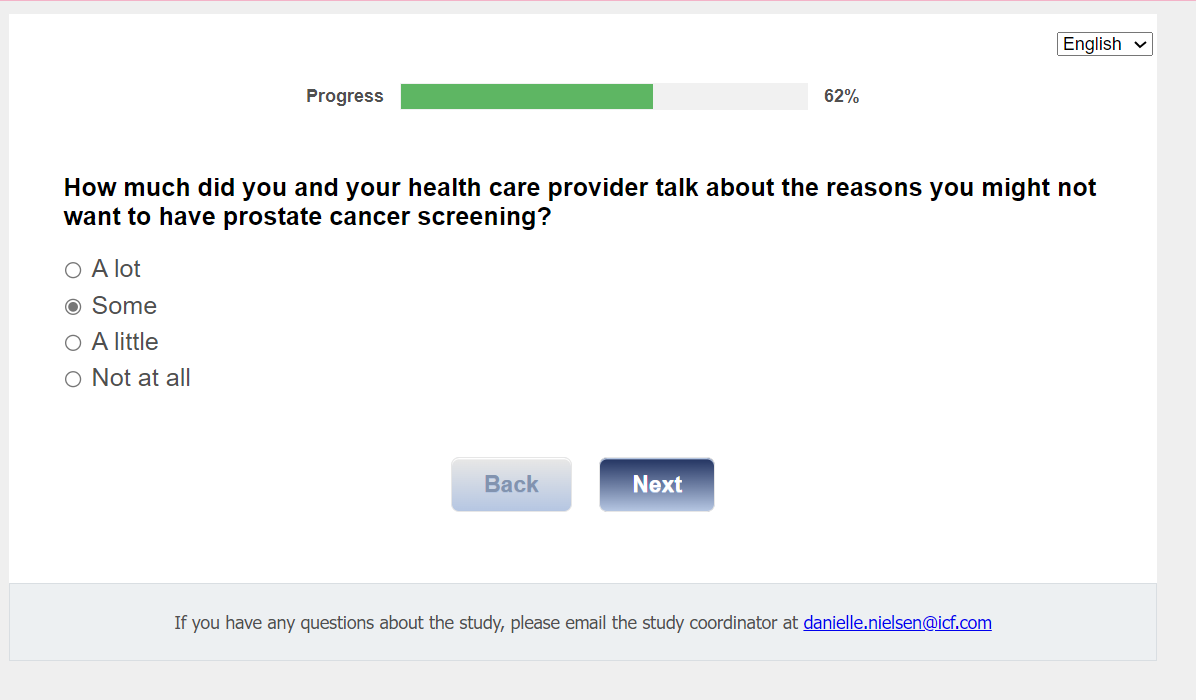
Q25. How much did you and your health care provider talk about the reasons you might not want to have prostate cancer screening?
01 A lot
02 Some
03 A little
04 Not at all
[ASK IF Q23=01]
Q26. Did your health care provider talk about a PSA blood test as something that you should seriously consider?
01 Yes
02 No

[ASK IF Q23=01]
Q27. Did your health care provider ask if you wanted to have prostate cancer screening?
01 Yes
02 No
Section 5: Time Spent with Provider
[ASK IF Q23=01]
Q28. Please answer the following questions about the amount of time spent talking with your health care provider about the PSA test.
Did you talk with your health care provider about the PSA test?
01 Yes
02 No
[ASK IF Q28=01]
Q29. About how many minutes did you spend with your health care provider discussing the PSA test?
RANGE 0-90 [TEXT BOX] Minutes

Section 6: Screening Behavior
[ASK ALL]
Q31. Please answer the following question about getting a PSA test.
Did you get a blood test to screen for prostate cancer?
01 Yes
02 No, but I intend to
03 No, and I do not intend to

Section 7: Informational Materials Used in Making Screening Decision
[ASK ALL]
Q32.
Please describe any informational tools or materials you have read or used to help you make a decision about prostate cancer screening.
[TEXT BOX]

Section 8: Gift Code
[ASK ALL]
[REQUIRED]
J1int. In appreciation for the time you have spent answering our questions, we would like to give you a $25 Amazon gift code. Would you like the gift code?
01 Yes
02 No
[ASK IF J1int=01]
J1.
Thank you for completing this survey. Your $25 Amazon gift code is displayed below. You will also receive an email with this $25 gift code for your records.
<insert GIFT_CODE>
The code is a unique number you can use to purchase items online at Amazon.com. You may enter the code online when you are ready to make a purchase at Amazon.com. There is no expiration date.
If you have any questions, please contact the project manager, Danielle Nielsen at Danielle.Nielsen@icf.com.

[ASK IF J1INT=01]
gcode email
To receive your code via email, please enter your email address here:
[TEXTBOX]

Send <gcode email> to <Email1> from CDCsurvey@icfsurvey.com
[ASK IF J1int=01]
Hello!
Thank you for completing this survey for the CDC Prostate Cancer Evaluation Study!
Here is your Amazon gift code for $25:
<GIFT_CODE>
HOW TO USE YOUR AMAZON GIFT CODE
The code is a unique number you can use to purchase items online at amazon.com. You may enter the code online when you are ready to make a purchase at amazon.com. There is no expiration date.
Save this code in a safe space until you are ready to use it. Some ideas to keep it safe are:
Write it down on a sheet of paper and keep it in a safe and hidden location.
Take a photo of the code with your phone.
Save the code in your Amazon.com account. If you have an amazon.com account, you can save your code in your account until you are ready to spend it.
Type in this link: https://www.amazon.com/gc/redeem/
Or, follow these instructions:
Go to amazon.com
In the blue banner, click on "Gift Cards & Registry"
On the gift cards page, choose "redeem a gift card"
Type or copy/paste the gift code into the "Enter claim code" field
Thank you once again for your time and feedback!
regards,
Debbie Krugipudi
CDC Prostate Evaluation Study Support Staff
[ASK ALL]
CLOSE.
Thank you for your responses to this survey.
We greatly appreciate your participation in the study on prostate cancer screening. Your participation in the various surveys and discussions have helped us gather information that will provide a deeper understanding of how well interactive tools and educational materials will help men make decisions about prostate cancer screening.
We assure you that your responses will be kept confidential and will not be shared outside of the study. Summaries of survey results and discussions will not be linked to your name or clinic when shared with CDC and/or when published in a professional journal.
If you have any questions, please contact the project manager, Danielle Nielsen at Danielle.Nielsen@icf.com.
Public reporting burden of this collection of information is estimated to average 20 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Reports Clearance Officer; 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA (0920-####).
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Thomas, Cheryll C. (CDC/DDNID/NCCDPHP/DCPC) |
| File Modified | 0000-00-00 |
| File Created | 2024-09-05 |
© 2026 OMB.report | Privacy Policy


