NIST SRM1950 Customer Feedback Survey
Generic Clearance for Program Evaluation Data Collections
CollectionInstrument-NIST-SRM1950Survey-Version A-Existing Users.v2
NIST SRM1950 Customer Feedback Survey
OMB: 0693-0033
Title: NIST SRM1950 Customer Feedback Survey
Version: A. Existing Users (current or past)
OMB Control #0693-0033
Expiration Date: 9/30/2025
NIST Generic Clearance for Program Evaluation Data Collections
A Federal agency may not conduct or sponsor, and a person is not required to respond to, nor shall a person be subject to a penalty for failure to comply with an information collection subject to the requirements of the Paperwork Reduction Act of 1995 unless the information collection has a currently valid OMB Control Number. The approved OMB Control Number for this information collection is 0693-0033. Without this approval, we could not conduct this survey/information collection. Public reporting for this information collection is estimated to be approximately 10 minutes/hours per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the information collection. All responses to this information collection are voluntary. Send comments regarding this burden estimate or any other aspect of this information collection, including suggestions for reducing this burden to the National Institute of Standards and Technology at: 100 Bureau Drive, Gaithersburg, MD 20899, Attn: Yee-Yin Choong, yee-yin.choong@nist.gov.
Survey Landing page:

NOTE:
If respondents click the hyperlink “SRM1950”, the SRM1950 certificate of analysis will be displayed in a new window (https://tsapps.nist.gov/srmext/certificates/1950.pdf).
If respondents click the “Click for additional information about this survey”, the study’s information sheet (attached) will be display in a new window.
If respondents click the “survey link”, it will allow respondents to copy the link to forward to other participants.
Page 1:
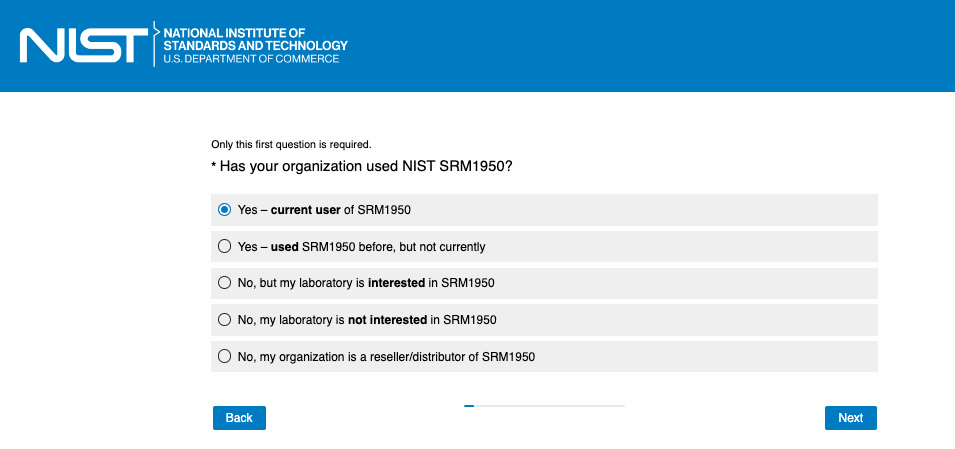
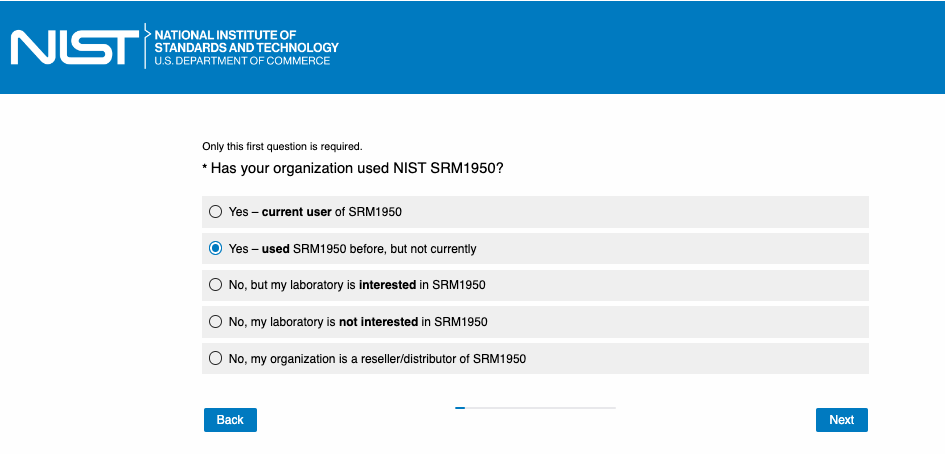
OR
NOTE:
Version A: If the answer is “Yes – current user of SRM1950” or “Yes – used SRM1950 before, but not currently.”
Version B: If the answer is “No, but my laboratory is interested in SRM1950.”
Version C: If the answer is “No, my laboratory is not interested in SRM1950.”
Version D: If the answer is “No, my organization is a reseller/distributor of SRM1950.”
Page 2:

Page 3:
Page 4:
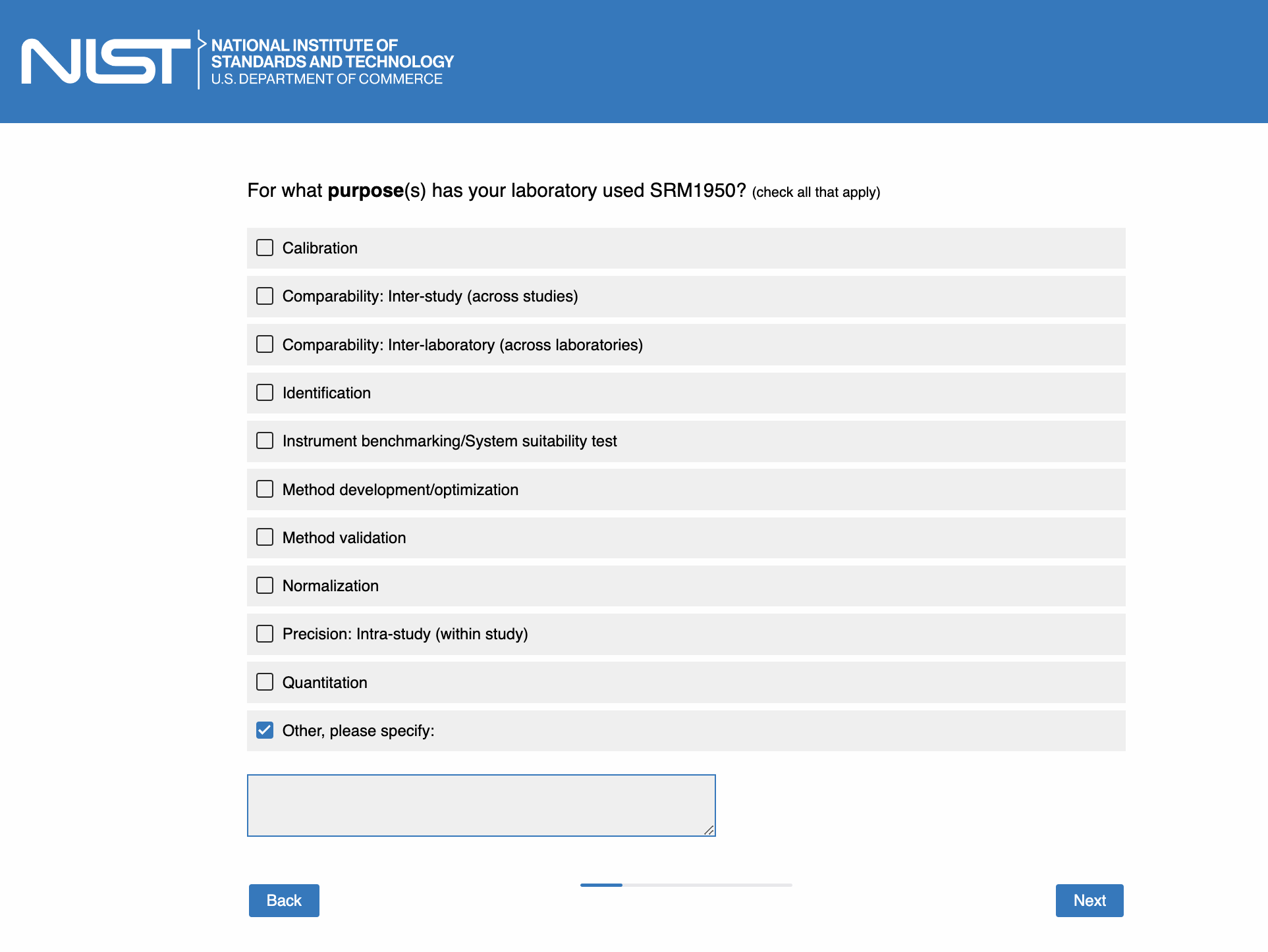
Page 5:

Page 6: [only show this page if “Cholesterol and Glycerides” is checked in Page 5]

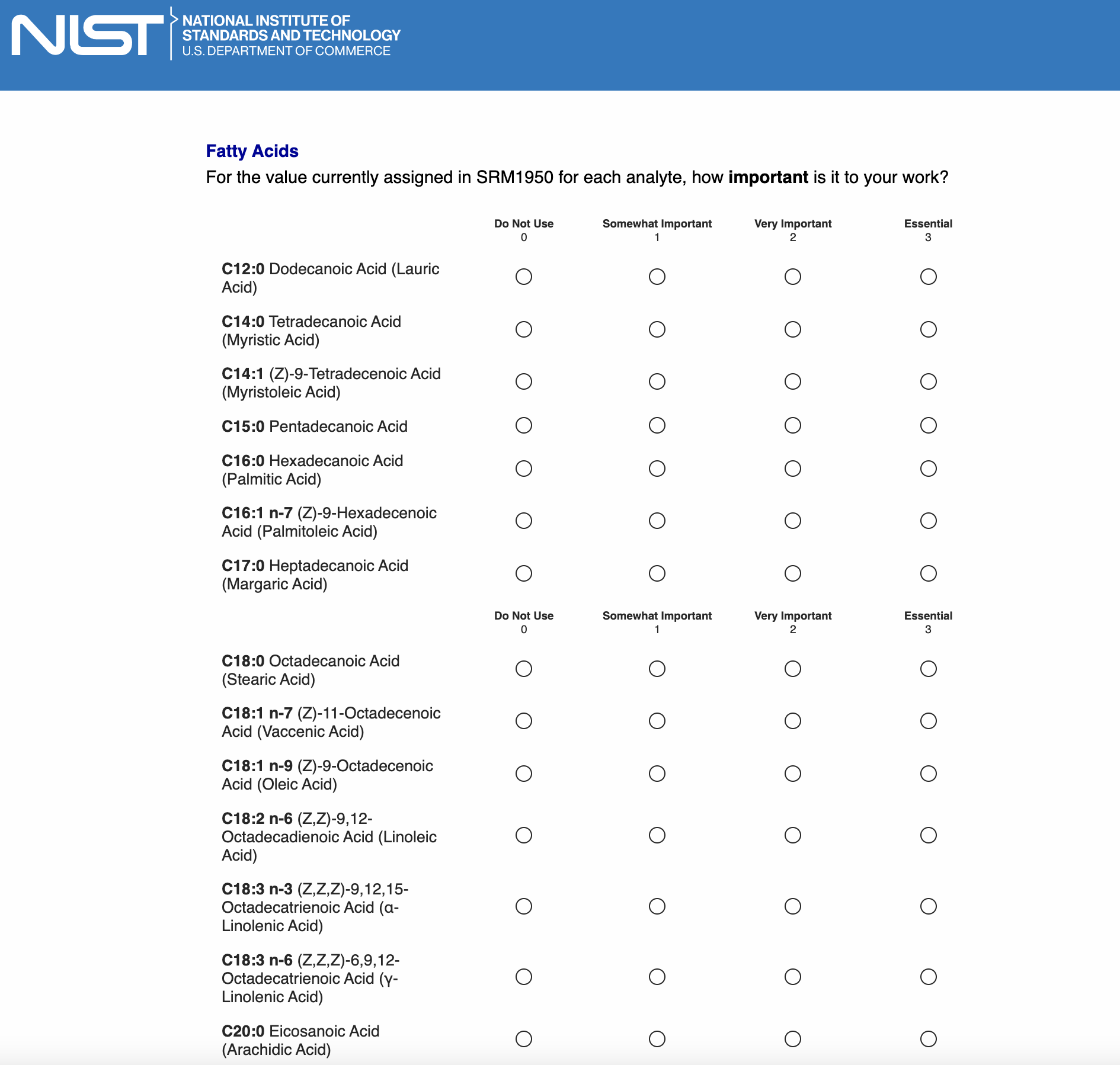
Page 7: [only show this page if “Fatty acids” is checked in Page 5]

Page 8: [only show this page if “Amino acids” is checked in Page 5]

Page 9: [only show this page if “Fat-soluble vitamins and Carotenoids” is checked in Page 5]

Page 10: [only show this page if “Water-soluble vitamins” is checked in Page 5]

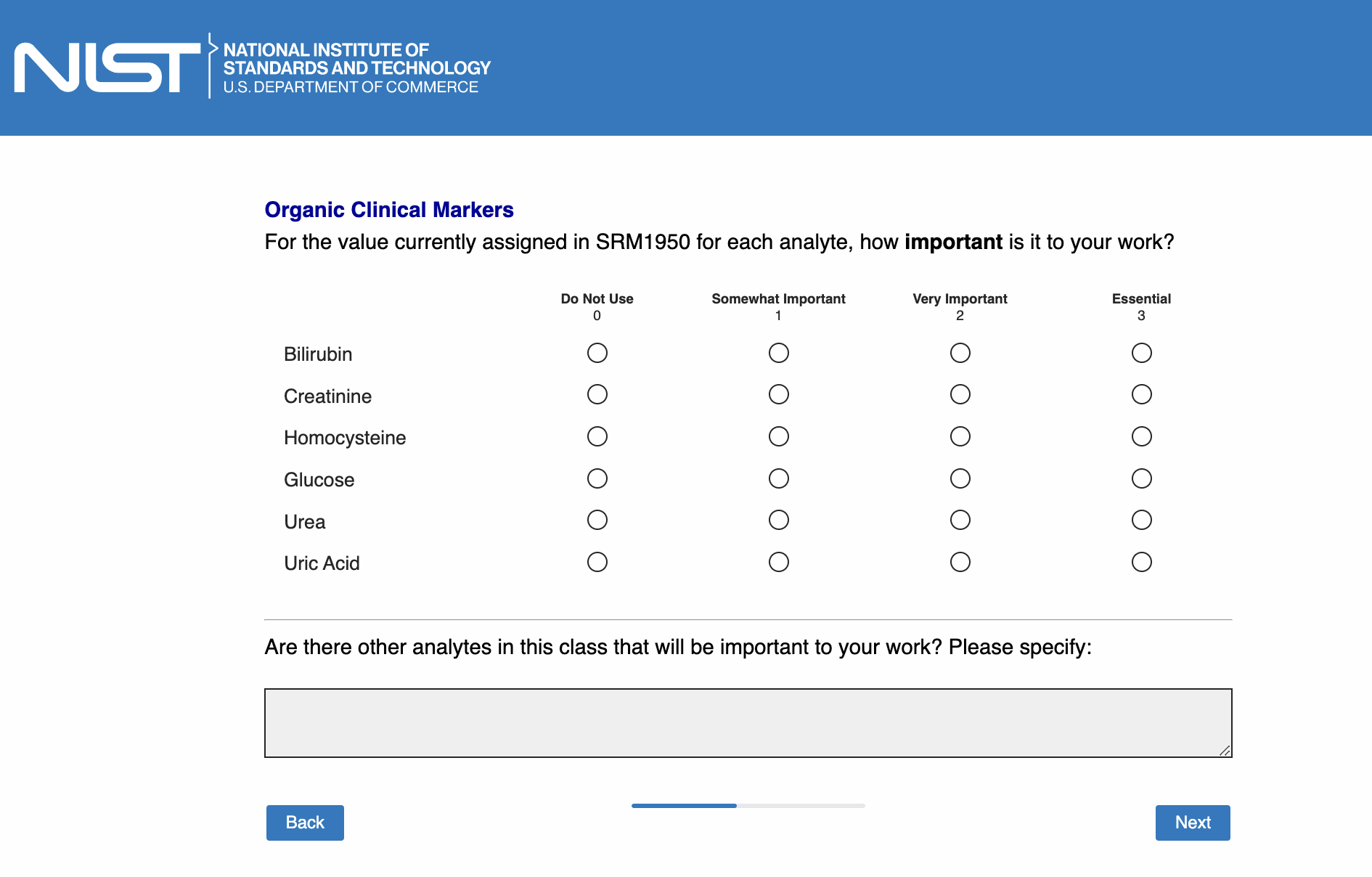
Page 11: [only show this page if “Organic clinical markers” is checked in Page 5]
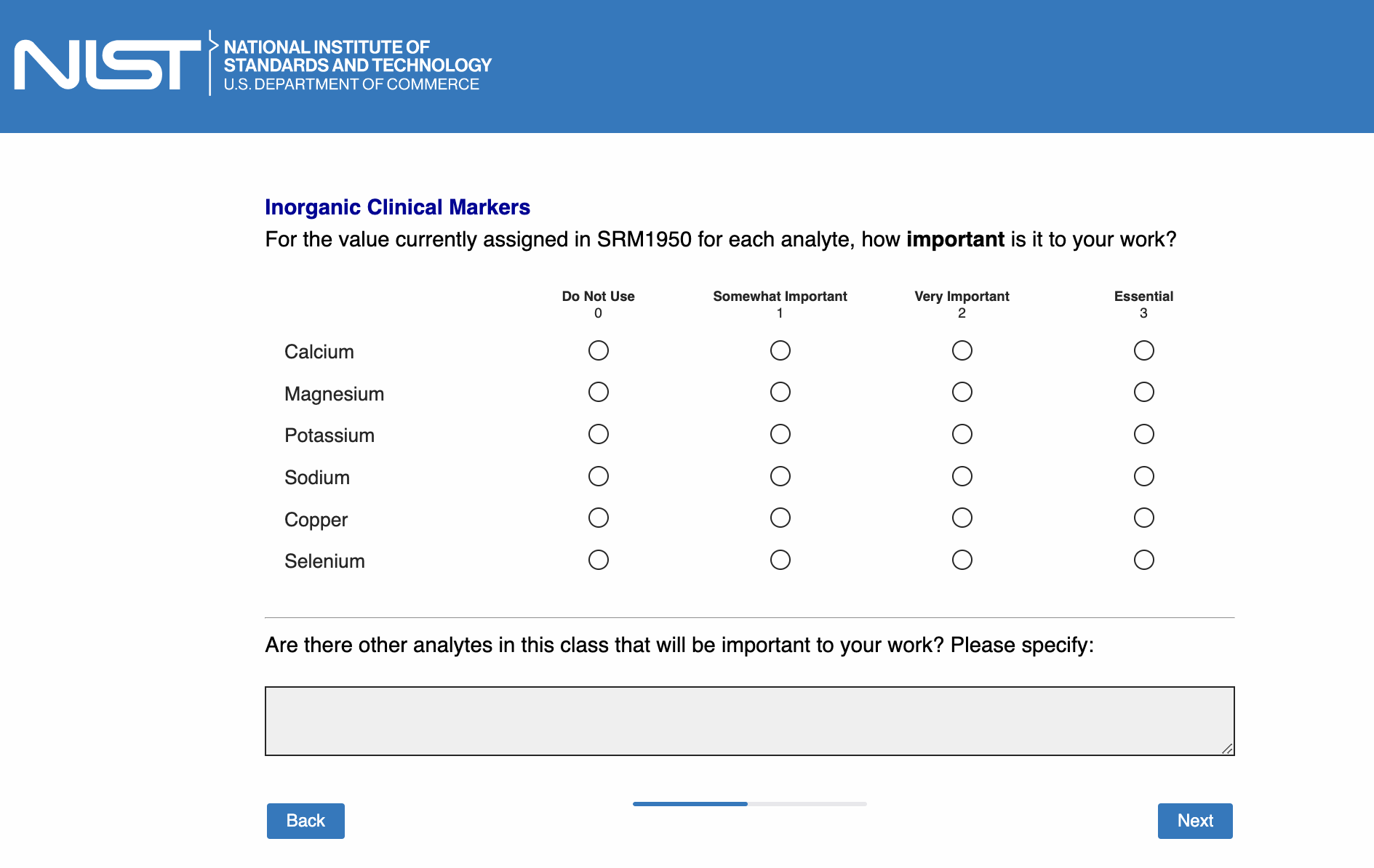
Page 12: [only show this page if “Inorganic clinical markers” is checked in Page 5]
Page 13: [only show this page if “Hormones (e.g., steroid hormones)” is checked in Page 5]

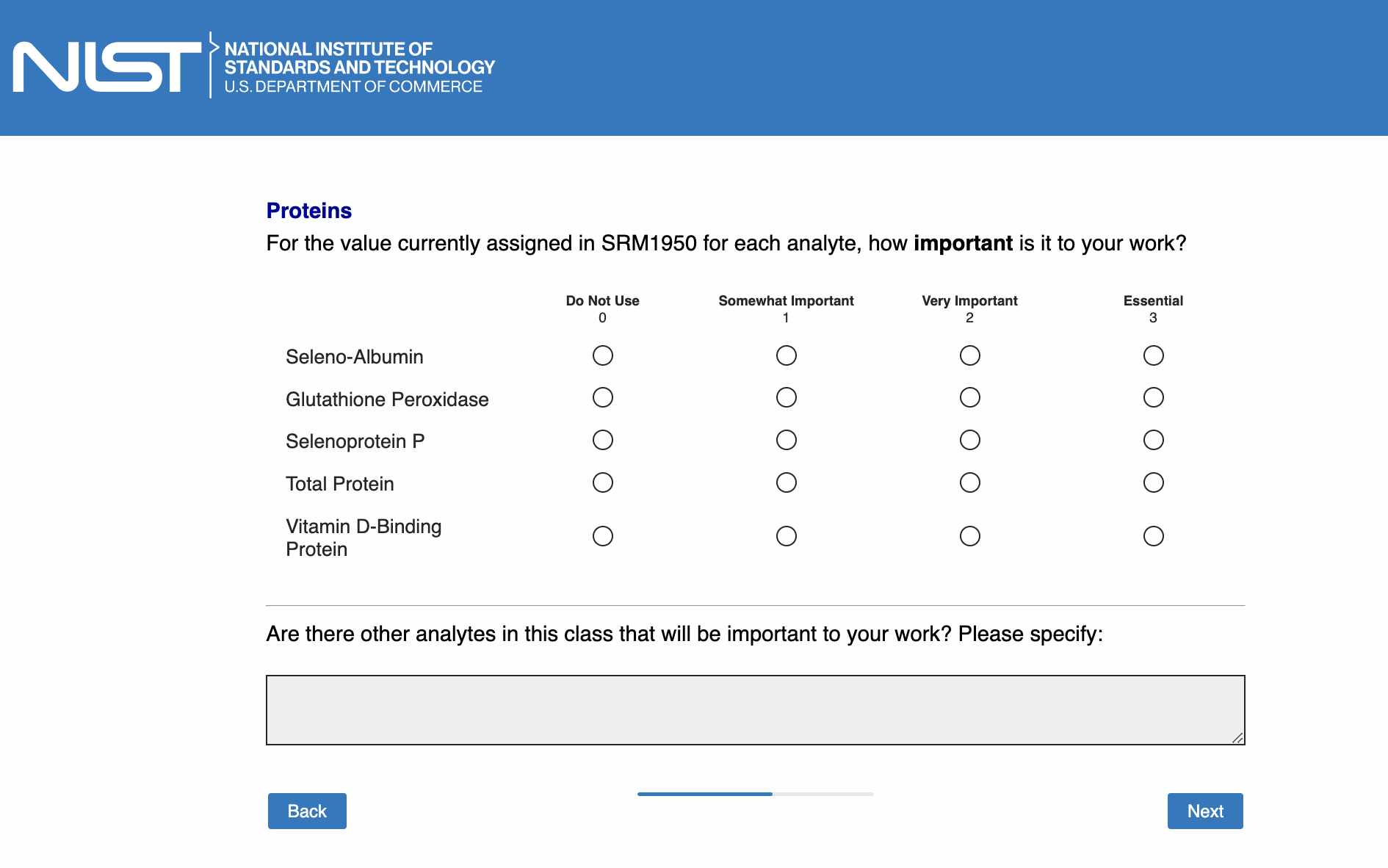
Page 14: [only show this page if “Proteins” is checked in Page 5]
Page 15: [only show this page if “Perfluorinated Compounds (PFCs)” is checked in Page 5]

Page 16:

Page 17:

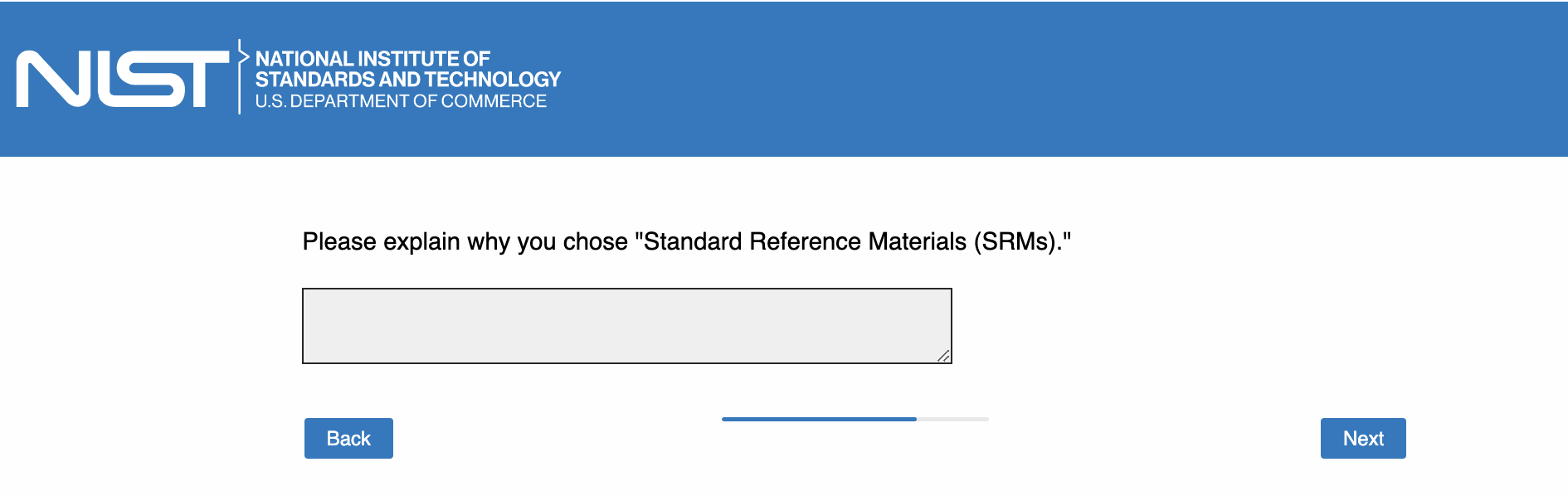
Page 18: [only show this page if “Reference Materials (RMs)” OR “Standard Reference Materials (SRMs)” OR “Both RMs and SRMs” is checked in Page 17; the text in quotation marks will be dynamically determined based on the selection]
Page 19:

Page 20:

Page 21:

| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Argent, Nina E. (Fed) |
| File Modified | 0000-00-00 |
| File Created | 2024-07-20 |
© 2026 OMB.report | Privacy Policy