Crosswalk of Changes
Att2_EIP2024_NonSub_Crosswalk_Sept2023.docx
[NCEZID] Emerging Infections Program
Crosswalk of Changes
OMB: 0920-0978
Cross walk - 2024 form changes
ABCs
ABCs Case Report Form - Attachment #3
-
2023 Form
2024 Form (Changes in yellow highlight)
a)
N/A
Added new question:
6a. Planning Region

-
2023 Form
2024 Form (Changes in yellow highlight)
a)
Updated overall design of the form to show available all data value sets.
b)
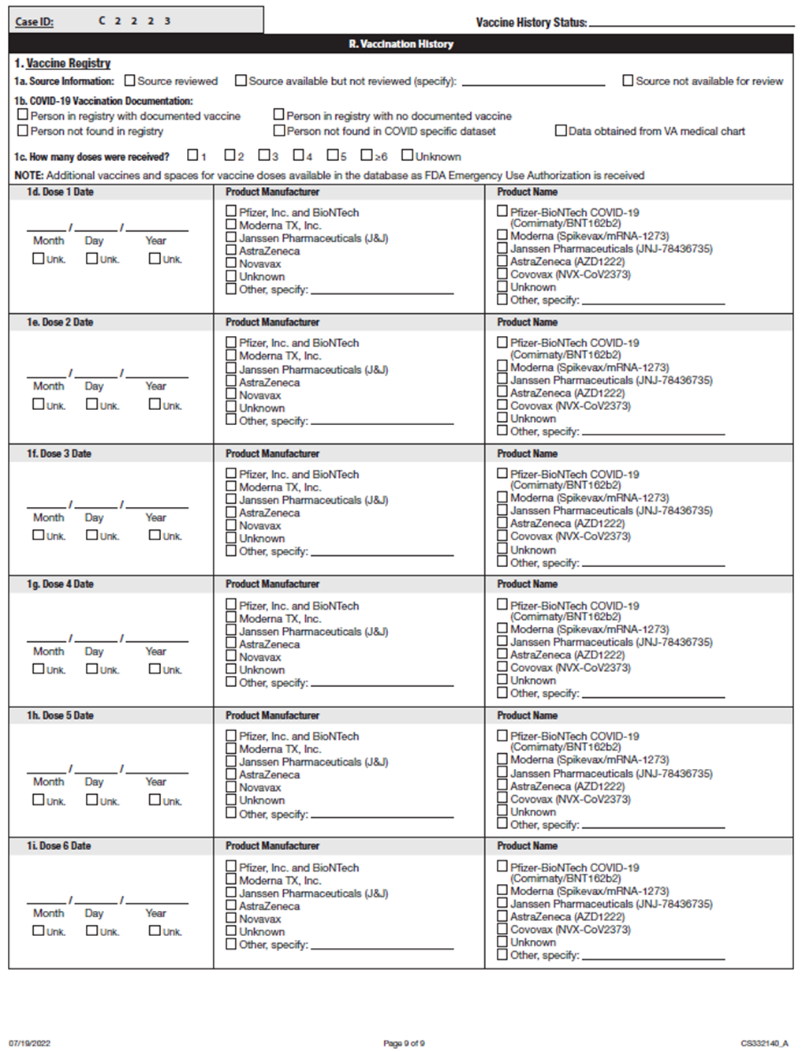
i) Added question on most recent influenza vaccine date.
ii) Added question on most recent COVID-19 vaccine date.

iii) Added question on RSV vaccine date
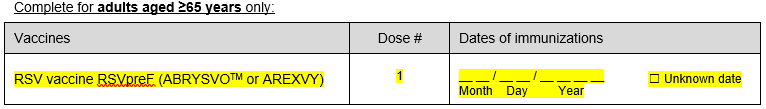
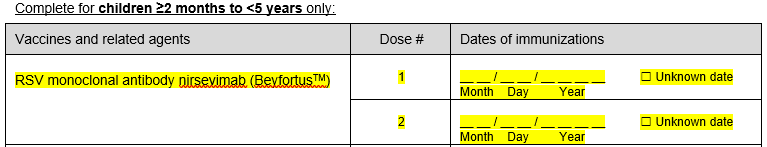
iv) Added question on RSV monoclonal antibody dates (complete for children <5 years only)
c)
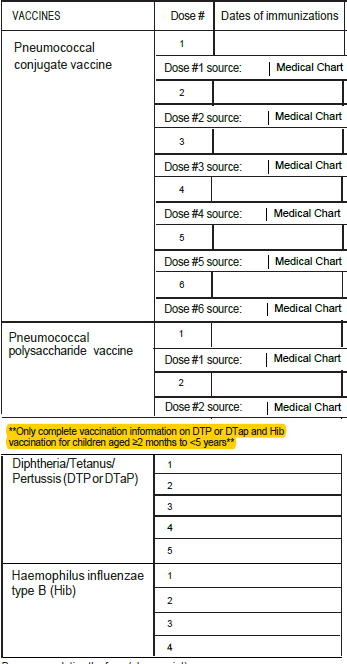
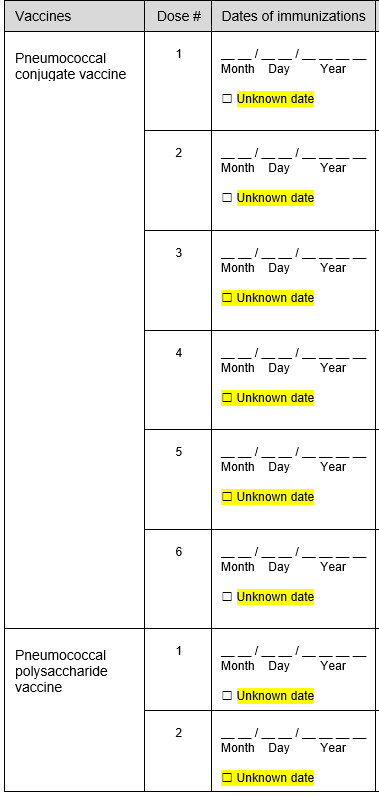
Addition of unknown checkboxes for all vaccination date variables.
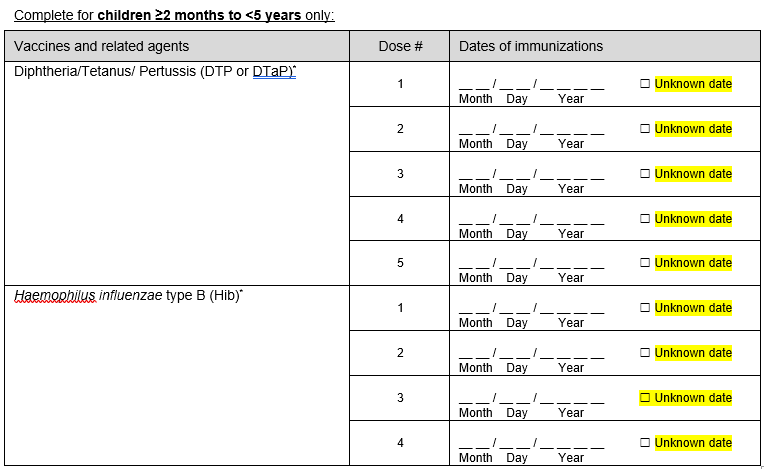
d)

Removed questions.
FoodNET
FoodNet Active Surveillance Data Elements List – Attachment #5
Refer to Attachment #5 - Changes are highlighted in Yellow
FluSurv-Net
FluSurv-NET Influenza Surveillance Project Case Report Form– Attachment #6
Question on 2022-23 Form |
Questions on 2023-24 Form |
Patient Data – This information is not sent to CDC
|
Patient Data – This information is not sent to CDC
|
Case Classification □ Prospective □ Surveillance Discharge Audit
|
Case Classification □ Surveillance Discharge Audit
|
C15. Where did the patient reside at the time of hospitalization (Indicate type of residence)
|
C15. Where did the patient reside at the time of hospitalization (Indicate type of residence)
|
N/A |
E5. Supplemental Oxygen?
|
F2. If patient discharged alive, please indicate to where:
|
F2. If patient discharged alive, please indicate to where:
|
G1. Reason for admission:
|
G1. Reason for admission:
|
G2. Acute signs/symptoms present at admission (began or worsened within 2 weeks prior to admission) (Select all that apply)
Non respiratory symptoms
|
G2. Acute signs/symptoms present at admission (began or worsened within 2 weeks prior to admission) (Select all that apply)
Non respiratory symptoms
|
G2. Acute signs/symptoms present at admission (began or worsened within 2 weeks prior to admission) (Select all that apply)
Respiratory symptoms
|
G2. Acute signs/symptoms present at admission (began or worsened within 2 weeks prior to admission) (Select all that apply)
Respiratory symptoms
|
G2. Acute signs/symptoms present at admission (began or worsened within 2 weeks prior to admission) (Select all that apply)
For cases <2 years
|
G2. Acute signs/symptoms present at admission (began or worsened within 2 weeks prior to admission) (Select all that apply)
For cases <12 years
|
N/A
|
G8. Environmental tobacco smoke exposure (for pediatric patients <12 years):
|
I1a. If yes, what is the specimen source?
|
I1a. If yes, what is the specimen source?
|
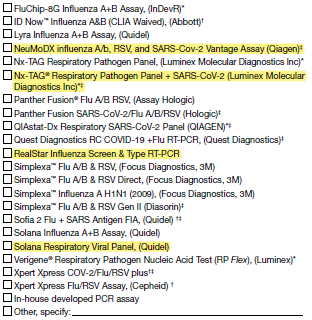
J1. Was patient tested for any of the following viral respiratory pathogens within 14 days prior to admission or ≤3 days after admission?
|
J1. Was patient tested for any of the following viral respiratory pathogens within 14 days prior to admission or ≤3 days after admission?
|
L. Chest Imaging – Based on radiology report only
2b. For the first abnormal chest x-ray, please check all that apply
|
L. Chest X-ray – Based on radiology report only
2b. For the first abnormal chest x-ray, please check all that apply
|
M1. Did the patient have any of the following new diagnoses at discharge? (Select all that apply)
|
M1. Did the patient have any of the following new diagnoses at discharge? (Select all that apply)
|
N/A |
O5. Pregnancy complications during current pregnancy? (Select all that apply)
|
O6a. If patient was pregnant on admission but no longer pregnant at discharge, indicate pregnancy outcome at discharge.
|
O6a. If patient was pregnant on admission but no longer pregnant at discharge, indicate pregnancy outcome at discharge. (If multiple fetuses, indicate outcome at discharge for each fetus in the database separately.)
|
FluSurv-NET/RSV Laboratory Survey– Attachment #7
Question on 2022-23 form |
Question on 2023-24 form |
N/A |
Title of person responding to questions for laboratory
|
N/A
|
3. Does the laboratory currently (or plan to in the next year) send out specimens to be tested with the Karius Test?
|
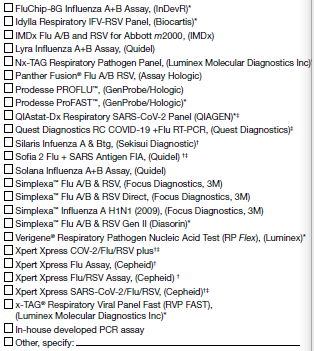
4A. Select the kit name(s) (manufacturer) for the rapid influenza antigen diagnostic test performed or planned to be used at the laboratory: (Check all that apply)
|
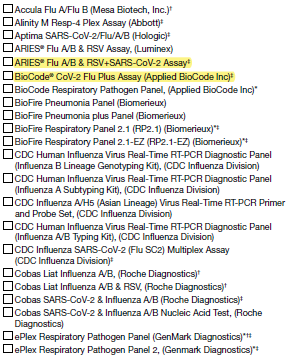
5A. Select the kit name(s) (manufacturer) for the rapid influenza antigen diagnostic test performed or planned to be used at the laboratory: (Check all that apply)
|
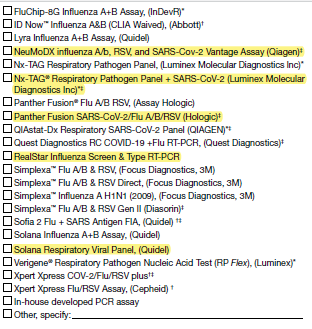
5a. Select the kit name(s) (manufacturer) for all molecular assays performed or planned to be used at the laboratory: (Check all that apply)
|
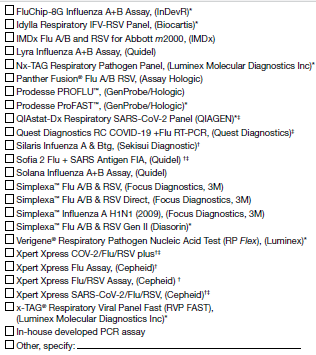
6a. Select the kit name(s) (manufacturer) for all molecular assays performed or planned to be used at the laboratory: (Check all that apply)
|
5b. If more than one kit is selected above, please select the one kit name that is (or will be) used most frequently for molecular assay at the laboratory during the current influenza season:
|
6b. If more than one kit is selected above, please select the one kit name that is (or will be) used most frequently for molecular assay at the laboratory during the current influenza season:
|
COVID19 Vaccination Status on FluSurv-NET Cases – Attachment #8
Questions on 2022-23 form |
Questions on 2023-24 form |
|
|
HAIC
Multi-site Gram-Negative Surveillance Initiative (MuGSI) Case Report Form (CRF) Attachment #9
Question on original 2023 form |
Question on 2024 form |
Description of change |
2023 Carbapenem Resistant Enterobacteriaceae (CRE)/ Carbapenem Resistant A. baumannii (CRAB) Multi-site Gram-Negative Surveillance Initiative (MuGSI) Healthcare-Associated Infections Community Interface (HAIC) Case Report |
2024 Multi-site Gram-Negative Surveillance Initiative (MuGSI) Healthcare-Associated Infections Community Interface (HAIC) Case Report |
I. Updated year to 2024 II. Removed the pathogens from the title since this one form covers all of MuGSI surveillance pathogens |
10. Organism: ð CRE ð CRAB
If CRE, select one of the following:
ð Escherichia coli ð Klebsiella aerogenes ð Klebsiella oxytoca ð Enterobacter cloacae ð Klebsiella pneumoniae |
10. Organism:
ð Carbapenem-Resistant Enterobacterales (CRE) ð Escherichia coli ð Klebsiella pneumoniae ð Klebsiella oxytoca ð Klebsiella aerogenes ð Enterobacter cloacae ð Extended-spectrum beta-lactamase-producing Enterobacterales (ESBL-E) ð Escherichia coli ð Klebsiella pneumoniae ð Klebsiella oxytoca
ð Carbapenem-Resistant A. baumannii (CRAB)
ð Invasive Escherichia coli (iEC) (not CRE or ESBL-E)
|
I. Updated all MuGSI pathogens and phenotypes under surveillance |
16. Patient Outcome:
On the day of or in the 6 calendar days before death, was the pathogen of interest isolated from a site that meets the case definition? ð Yes ð No ð Unknown
|
16. Patient Outcome
[Removed] |
I. Removed the specified question from “16. Patient Outcome:” on the 2024 form |
17a. Types of infection associated with culture(s): (Check all that apply) ð None ð Colonized ð Unknown
ð Abscess, not skin ð AV fistula/graft infection ð Bacteremia ð Bursitis ð Catheter site infection (CVC) ð Cellulitis ð Chronic Ulcer/wound (not decubitus) ð Decubitus/pressure ulcer ð Empyema ð Endocarditis ð Epidural abscess ð Meningitis ð Osteomyelitis ð Peritonitis ð Pneumonia (CRAB cases, complete Q23c) ð Pyelonephritis ð Septic arthritis ð Septic emboli ð Septic shock ð Skin abscess ð Surgical incision infection ð Surgical site infection (internal) ð Traumatic wound ð Urinary tract infection ð Other (specify):______________
|
17a. Types of infection associated with culture(s): (Check all that apply) ð None ð Colonized ð Unknown
ð Abscess, not skin ð AV fistula/graft infection ð Bacteremia ð Bursitis ð Catheter site infection (CVC) ð Cellulitis ð Chronic Ulcer/wound (not decubitus) ð Decubitus/pressure ulcer ð Empyema ð Endocarditis ð Epidural abscess ð Meningitis ð Osteomyelitis ð Peritonitis ð Pneumonia (CRAB cases, complete Q23c) ð Pyelonephritis ð Sepsis ð Urosepsis ð Septic arthritis ð Septic emboli ð Septic shock ð Skin abscess ð Surgical incision infection ð Surgical site infection (internal) ð Traumatic wound ð Urinary tract infection ð Other (specify):______________
|
I. Included “Sepsis” as an infection type, including a sub-choice for “Urosepsis” |
|
20. Risk factors: (Check all that apply) Invasive or diagnostic urologic procedure in the year before DISC: ð Yes ð No ð Unknown
If yes, check all that apply: ð Prostate procedure ð Cystoscopy ð Other
|
I. Added a new risk factor question. |
23b. Risk factors in the 7 days before the DISC: ð Non-invasive positive pressure ventilation (CPAP or BiPAP) at any time in the 7 calendar days before the DISC ð Nebulizer treatment at any time in the 7 calendar days before the DISC ð Mechanical ventilation at any time in the 7 calendar days before the DISC ð None |
23b. Risk factors prior to CRAB DISC: ð Non-invasive positive pressure ventilation (CPAP or BiPAP) at any time in the 7 calendar days before the DISC ð Nebulizer treatment at any time in the 7 calendar days before the DISC ð Mechanical ventilation at any time in the 7 calendar days before the DISC ð Visited a wound care clinic at any time in the year before the DISC ð None |
I. Revised the text for the question. II. Added an additional risk factor in the year before the DISC
|
|
24a. Is antimicrobial use (IV or Oral) in the 30 days before the DISC documented? ð Yes ð No ð Unknown |
I. Added question
Note: This question is not new to MuGSI surveillance nor the MuGSI database. It is being included in the consolidated 2024 form from the OMB-approved 2023 ESBL/iEC form |
|
24b.
If yes, check all antimicrobials used in the 30 days before the
DISC:
ð Amikacin ð Amoxicillin ð Amoxicillin/clavulanic acid ð Ampicillin ð Ampicillin/sulbactam ð Azithromycin ð Aztreonam ð Cefadroxil ð Cefazolin ð Cefdinir ð Cefepime ð Cefiderocol ð Ceixime ð Cefotaxime ð Cefoxitin ð Cefpodoxime ð Ceftaroline ð Ceftazidime ð Ceftazidime/avibactam ð Ceftizoxime ð Ceftolozane/tazobactam ð Ceftriaxone ð Cefuroxime ð Cephalexin ð Ciprofloxacin ð Clarithromycin ð Clindamycin ð Dalbavancin ð Daptomycin ð Delafloxacin ð Doripenem ð Doxycycline ð Eravacycline ð Ertapenem ð Fidaxomicin ð Fosfomycin ð Gentamicin ð Imipenem/cilastatin ð Levofloxacin ð Linezolid ð Meropenem ð Meropenem/vaborbactam ð Metronidazole ð Moxifloxacin ð Nitrofurantoin ð Omadacycline ð Oritavancin ð Penicillin ð Piperacillin/tazobactam ð Polymyxin B ð Polymyxin E (colistin) ð Rifaximin ð Tedizolid ð Telavancin ð Tigecycline ð Tobramycin ð Trimethoprim ð Trimethoprim/sulfamethoxazole ð Vancomycin ð IV ð PO ð Other (specify):___________ ð Other (specify):___________
Reminder: Any prior antimicrobial use that is not noted above should be documented in the other (specify) field.
|
I. Added question
Note: This question is not new to MuGSI surveillance nor the MuGSI database. It is being included in the consolidated 2024 form from the OMB-approved 2023 ESBL/iEC form |
24c. COVID-Net Case ID:_________ |
25c. COVID-Net Case ID in the year before or day of DISC:_________
ð None or N/A
|
I. Updated the question number II. Added the specified timeframe III. Included a checkbox for “None or N/A”
|
Multi-site Gram-Negative Surveillance Initiative (MuGSI) Community-Associated Carbapenemase-Producing Carbapenem-Resistant Enterobacterales (CA CP-CRE) Health interview - Attachment #10
Original Instruction |
Proposed Change to Instruction |
[If answer to Q22 = 1, i.e., interviewee lives alone, skip to Section G] |
[If answer to Q22 = 1, i.e., interviewee lives alone, skip to Section 9] |
Multi-site Gram-Negative Surveillance Initiative (MuGSI) Supplemental Surveillance Officer Survey - Attachment #11
2023 Survey Question |
2024 Survey Question |
Description: Please answer the following questions for the year 2023. The purpose of the survey is to verify and document current surveillance procedures, including isolate collection and testing methods at clinical laboratories. Please enter your responses into the corresponding RedCap database. If you have any questions, please contact Julian Grass (hij3@cdc.gov) and Joshua Brandenburg (ode4@cdc.gov).
|
Description: Please answer the following questions for the year 2024, unless otherwise specified. The purpose of the survey is to verify and document current surveillance procedures, including isolate collection and testing methods at clinical laboratories. Please enter your responses into the corresponding REDCap database. If you have questions, please contact Julian Grass (hij3@cdc.gov) and Joshua Brandenburg (ode4@cdc.gov). |
Surveillance area characteristics:
|
Surveillance area characteristics:
|
Surveillance area characteristics:
_______ Statewide _______ Defined catchment area, please specify__________
_______ yes _______ no
_______ Agent of the state _______ State Health Department Regulation _______ Other, please explain: __________________________________
|
Surveillance area characteristics: 2. Is CRE reportable at your state/site? ___ yes ___ no
_______ Statewide _______ Defined area, such as a county(ies). Please specify
_______ yes _______ no specify ___________
_______ Agent of the state _______ State Health Department Regulation _______ Other, please explain: _____________
|
Surveillance area characteristics: 3. Is CRAB state reportable at your site? ___ yes___ no
_______ Statewide _______ Defined catchment area, please specify__________
_______ yes _______ no
_______ Agent of the state _______ State Health Department Regulation _______ Other, please explain: __________________________________
|
Surveillance area characteristics:
_______ Statewide _______ Defined area, such as a county(ies). Please specify
_______ yes _______ no specify ___________
_______ Agent of the state _______ State Health Department Regulation _______ Other, please explain: _____________
|
Surveillance area characteristics:
_______ Statewide _______ Defined catchment area, please specify__________
_______ yes _______ no
_______ Agent of the state _______ State Health Department Regulation _______ Other, please explain: __________________________________
|
Surveillance area characteristics: 4. Is ESBL-E reportable at your state/site? ___ yes ___ no
_______ Statewide _______ Defined area, such as a county(ies). Please specify
_______ yes _______ no specify ___________
_______ Agent of the state _______ State Health Department Regulation _______ Other, please explain: _____________
|
|
Surveillance area characteristics:
_______ Statewide _______ Defined area, such as a county(ies). Please specify
_______ yes _______ no specify ___________
_______ Agent of the state _______ State Health Department Regulation _______ Other, please explain: _____________
|
Laboratory Participation and Isolate Testing
|
Laboratory Participation and Isolate Testing – Part 1
|
|
Laboratory Participation and Isolate Testing – Part 1
________________________________________________________________________ ________________________________________________________________________
|
|
Laboratory Participation and Isolate Testing – Part 1
_______ yes _______ no
__________________________________________________________________
_____________________________________________________________________
|
Laboratory Participation and Isolate Testing 2. Did your site send MuGSI isolates to CDC for characterization in 2023? ____yes ____no
|
Laboratory Participation and Isolate Testing – Part 1
|
Laboratory Participation and Isolate Testing
_______ CRE; _______ CRAB; _______ ESBL |
Laboratory Participation and Isolate Testing – Part 1
_______ CRE; _______ CRAB; _______ ESBL; ________iEC |
Laboratory Participation and Isolate Testing d.If yes, what was the total number of isolates collected from the clinical laboratories in 2023? _______ CRE; _______ CRAB; _______ ESBL
|
Laboratory Participation and Isolate Testing – Part 1
_______ CRE; _______ CRAB; _______ ESBL; _______iEC
|
Laboratory Participation and Isolate Testing
Type of Laboratory |
Laboratory Participation and Isolate Testing – Part 2
_____clinical laboratory _____public health laboratory _____research laboratory _____reference laboratory |
Laboratory Participation and Isolate Testing
MuGSI pathogens under surveillance |
Laboratory Participation and Isolate Testing – Part 2
_____CRE _____CRAB _____ESBL _____iEC |
Laboratory Participation and Isolate Testing
|
Laboratory Participation and Isolate Testing – Part 2
_____electronic messaging, such as HL7 _____e-mail _____fax _____EIP staff manually generate reports on-site _____other, please specify_____________________ _____unknown |
Laboratory Participation and Isolate Testing
Method for case identification |
Laboratory Participation and Isolate Testing – Part 2
_____automated testing instrument _____laboratory information system _____medical record _____other, please specify_____________________ _____unknown |
Laboratory Participation and Isolate Testing
Carbapenem confirmatory testing and method |
Laboratory Participation and Isolate Testing – Part 2 7.Carbapenem confirmatory testing method
kirby bauer:_____CRE _____CRAB _____ESBL _____iEC other, please specify: _____CRE _____CRAB _____ESBL _____iEC laboratory not testing _____CRE _____CRAB _____ESBL _____iEC unknown _____CRE _____CRAB _____ESBL _____iEC
|
Laboratory Participation and Isolate Testing
Carbapenemase testing method |
Laboratory Participation and Isolate Testing – Part 2 8.Carbapenemase testing method
Non-molecular test methods carbaNP: _____CRE _____CRAB _____ESBL _____iEC carbapenemase inactivation method: _____CRE _____CRAB _____ESBL _____iEC CPO detect: _____CRE _____CRAB _____ESBL _____iEC disk diffusion/ROSCO disk e-test: _____CRE _____CRAB _____ESBL _____iEC modified carbapenemase inactivation method: _____CRE _____CRAB _____ESBL _____iEC modified hodge test: _____CRE _____CRAB _____ESBL _____iEC RAPIDEC: _____CRE _____CRAB _____ESBL _____iEC Other, please specify: _____CRE _____CRAB _____ESBL _____iEC laboratory not testing: _____CRE _____CRAB _____ESBL _____iEC unknown: _____CRE _____CRAB _____ESBL _____iEC Molecular test methods automated molecular assay: _____CRE _____CRAB _____ESBL _____iEC carba-R: _____CRE _____CRAB _____ESBL _____iEC check points: _____CRE _____CRAB _____ESBL _____iEC MALDI-TOF MS: _____CRE _____CRAB _____ESBL _____iEC next generation nucleic acid sequencing: _____CRE _____CRAB _____ESBL _____iEC polymerase chain reaction: _____CRE _____CRAB _____ESBL _____iEC streck ARM-D: _____CRE _____CRAB _____ESBL _____iEC other, please specify:____________________ _____CRE _____CRAB _____ESBL _____iEC laboratory not testing: _____CRE _____CRAB _____ESBL _____iEC unknown: _____CRE _____CRAB _____ESBL _____iEC |
Laboratory Participation and Isolate Testing
ESBL production testing and method |
Laboratory Participation and Isolate Testing – Part 2
broth microdilution – ESBL well:_____CRE _____CRAB _____ESBL _____iEC broth microdilution – ATI flag: _____CRE _____CRAB _____ESBL _____iEC broth microdilution – manual: _____CRE _____CRAB _____ESBL _____iEC disk diffusion: _____CRE _____CRAB _____ESBL _____iEC e-test: _____CRE _____CRAB _____ESBL _____iEC molecular test, please specify_____CRE _____CRAB _____ESBL _____iEC other non-molecular test, please specify:_____CRE _____CRAB _____ESBL _____iEC laboratory not testing: _____CRE _____CRAB _____ESBL _____iEC unknown: _____CRE _____CRAB _____ESBL _____iEC
|
Laboratory Participation and Isolate Testing
Organism identification method |
Laboratory Participation and Isolate Testing – Part 2
MALDI-TOF: _____CRE _____CRAB _____ESBL _____iEC polymerase chain reaction: _____CRE _____CRAB _____ESBL _____iEC whole genome sequencing: _____CRE _____CRAB _____ESBL _____iEC DNA sequencing, please specify:_____CRE _____CRAB _____ESBL _____iEC rRNA gene sequencing, please specify:_____CRE _____CRAB _____ESBL _____iEC biochemical tests, please specify:_____CRE _____CRAB _____ESBL _____iEC immunological techniques, please specify:_____CRE _____CRAB _____ESBL _____iEC other, please specify:_____CRE _____CRAB _____ESBL _____iEC laboratory not testing:_____CRE _____CRAB _____ESBL _____iEC unknown: _____CRE _____CRAB _____ESBL _____iEC
|
Laboratory Participation and Isolate Testing
Culture-independent diagnostic test |
Laboratory Participation and Isolate Testing – Part 2
_____yes, please specify the type of test________________ If yes, is a positive test result always followed up by a culture? _______ yes _______ no _______ unknown _____no _____unknown |
Laboratory Participation and Isolate Testing
Isolate submission to state public health laboratory |
Laboratory Participation and Isolate Testing – Part 2
_____yes _____no _____unknown |
|
Laboratory Participation and Isolate Testing – Part 2
|
|
Laboratory Participation and Isolate Testing – Part 2
|
|
Additional information on MuGSI surveillance activities
_______________________________________________________________________________________________________________ _______________________________________________________________________________________________________________
|
|
Additional information on MuGSI surveillance activities
_______ Monthly _______ Quarterly _______ Other time frame, specify: ______________________________________ _______ Never b. If yes, what type of edits are you running? Do you think they would be helpful to add to edits generated by CDC? ________________________ |
|
Additional information on MuGSI surveillance activities
a. If yes, what is the most recent year of surveillance data that was geocoded? ___________________ b. If no, why not? ________________________________________________________________________________________________________________________________________________________________________________________________________________________ |
|
Additional information on MuGSI surveillance activities
|
|
Additional information on MuGSI surveillance activities
a. If yes, what was the most recent year of surveillance data with CRFs re-abstracted? ___________________ b. If no, why not? ________________________________ |
Additional information on MuGSI surveillance activities
|
Additional information on MuGSI surveillance activities
|
|
Additional information on MuGSI surveillance activities
|
2023 CRF Question |
Changes to the 2023 CRF Question |
|||||||||||||||||||||||||
|
15a. Is the isolate MRSA or MSSA? □ MRSA □ MSSA □ Unknown [new question] |
|||||||||||||||||||||||||
22. SUSCEPTIBILITY RESUTLS (S=Sensitive (1), I=Intermediate (2), R=Resistant (3), U=Unknown/Not Reported (9)
|
22. SUSCEPTIBILITY RESULTS (S=Sensitive (1), I=Intermediate (2), R=Resistant (3), NS=Non-susceptible (4), SDD=Susceptible dose-dependent (5), U=Unknown/Not Reported (9)
|
|||||||||||||||||||||||||
|
28a.
|
|||||||||||||||||||||||||
|
28b. Does the patient have another type of indwelling prosthetic device associated with the infection? □ Yes, specify:_____________ □ No □ Unknown |
|||||||||||||||||||||||||
34a. COVID-NET CASE ID: ________________ |
34a. COVID-NET CASE ID in the year before or day of the DISC: ________________ □ None or N/A [updated language, added checkbox] |
|||||||||||||||||||||||||
Invasive Staphylococcus aureus Supplemental Surveillance Officer Survey - Attachment #13
2022 Survey Question |
Changes to the 2022 Survey Question |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Surveillance area characteristics 5a. If yes:
_______ Hospital MRSA LabID event _______ Hospital central line-associated bloodstream infection (CLABSI) data _______ Dialysis event
|
Surveillance area characteristics If yes:
_______ Hospital MRSA LabID event _______ Hospital central line-associated bloodstream infection (CLABSI) data _______ Hospital Antimicrobial Use and Resistance (AUR) Option _______ Dialysis event [Added a checkbox] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Surveillance area characteristics 5b. If no:
_______ Hospital MRSA LabID event _______ Hospital CLABSI data _______ Dialysis event |
Surveillance area characteristics 5b. If no:
_______ Hospital MRSA LabID event _______ Hospital CLABSI data _______ Hospital AUR Option _______ Dialysis event [Added a checkbox] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Lab participation and case finding
|
Lab participation and case finding
[Updated question wording] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Lab participation and case finding
|
Lab participation and case finding
[Updated question wording] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Lab participation and case finding 4. Indicate the percentage contribution of each case finding method to your site’s total SA case counts (100%) in 2022.
|
Lab participation and case finding 4. Indicate the percentage contribution of each case finding method to your site’s total SA case counts (100%) in 2023.
[updated wording to second method listed]
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Lab participation and case finding 5. For labs reporting invasive SA, how many of the participating labs are providing case reports through direct electronic messaging, such as HL7 messaging? ________
a. If less <100%, how else are you receiving reports? _____________________________
|
Lab participation and case finding 5. For labs reporting invasive SA, how many of the participating labs are providing case reports through direct electronic messaging, such as HL7 messaging? ________
a. If less <100%, how else are you receiving reports (check all that apply)? □ Secure email □ Fax □ Manual surveillance on-site □ Mailed hard copies □ State electronic reporting system □ Other, specify: _____________________________ [Added checkboxes in place of free text] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Lab participation and case finding
_______ yes _______ no
|
Lab participation and case finding
_______ yes _______ no
[Added 6c] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ascertainment of surveillance area and case audits
|
Ascertainment of surveillance area and case audits 2. Indicate the percentage contribution of each finding method to your site’s audit counts (100%)
[updated wording to second method listed] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ascertainment of surveillance area and case audits
_______ yes _______ no a. If yes, please describe the check(s) that you use____________
a.If yes, do you plan to use these for MSSA once more surveillance data are available? ___yes ___ no |
Ascertainment of surveillance area and case audits
_______ yes _______ no a. If yes, please describe the check(s) that you use ___________
b. If yes, how often are the check(s) used?
[deleted 7ba] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Ascertainment of surveillance area and case audits
_______ yes _______ no a. If yes, please describe the check(s) that you use
b. If yes, how often are the check(s) used? [Added] |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
COVID-19 impact section 1. Did COVID-19 response activities affect or delay 2022 iSA surveillance work (e.g., unable to meet iSA deadlines during 2022)? ___ yes __ no a. If no, how were you able to meet iSA deadlines? b. If yes, how did COVID-19 response activities delay your iSA work?
[deleted] |
|
Invasive Staphylococcus aureus Laboratory Survey: Use of Nucleic Acid Amplification Testing (NAAT) - Attachment #14
2023 Survey Question |
2024 Survey Question |
||||||||||||||||||||||||||||
|
Date Last Survey Completed: _____________ [Added question to header section] |
||||||||||||||||||||||||||||
2. During the past year, has your lab changed testing methods used to detect any of the following pathogens:
|
2. During the past year (i.e., in the past 12 months or since the completion of the last lab survey), has your lab changed testing methods used to detect any of the following pathogens:
|
||||||||||||||||||||||||||||
5b. Which tests do you use to detect S. aureus directly from a sterile site source without culture (sterile site sources only, i.e., blood, CSF, pleural fluid, bone, etc.)? Please check all that apply. □ T2Bacteria® Panel…Date started ______ □ Karius TestTM… Date started______ □ Other, Lab developed test (detects MRSA or SA)… Date started _____ □ Other commercial test, specify___ … Date started _____
|
5b. Which tests do you use to detect S. aureus directly from a sterile site source without culture (sterile site sources only, i.e., blood, CSF, pleural fluid, bone, etc.)? Please check all that apply. □ T2Bacteria® Panel…Date started ______ □ Other FDA-approved test, specify___ Date started __ Method: □ PCR □ Next generation sequencing (NGS) □ Other, specify __________ □ Karius TestTM… Date started______ □ Other, Lab developed test (detects MRSA or SA)… Date started _____ Method: □ PCR □ Next generation sequencing (NGS) □ Other, specify __________ [changed wording and option order for other commercial test option; added a sub question ‘Method’ for two of the options] |
||||||||||||||||||||||||||||
5g. Where do you plan to have these tests performed? □ On-site □ Send out, please specify lab _______
|
5g. Where do you plan to have these tests performed? □ On-site □ Send out, please specify lab _______ - GO TO Q5i [Added skip pattern] |
||||||||||||||||||||||||||||
|
5h. Which tests do you plan to use to detect S. aureus directly from a sterile site source without culture? (sterile site sources only, i.e., blood, CSF, pleural fluid, bone, etc.)? Please check all the apply. □ T2Bacteria® Panel…Date started ______ □ Other FDA-approved test, specify___ Date started __ □ Karius TestTM… Date started______ □ Other, Lab developed test (detects MRSA or SA)… Date started _____ [new question] |
||||||||||||||||||||||||||||
|
5i. Will all positive tests directly from sterile sources (without positive culture) appear in the S. aureus surveillance laboratory line lists? □ Yes □ No □ Unknown [new question] |
||||||||||||||||||||||||||||
|
5j. Will you still obtain an isolate for S. aureus or MRSA if these tests are used? □ Yes-END SURVEY □ No-END SURVEY □ Unknown – END SURVEY [new question] |
||||||||||||||||||||||||||||
Clostridiodies difficile Infection (CDI) Case Report and Treatment Form - Attachment #15
2023 CRF |
2024 CRF |
Changes |
9a. EIA
|
9a. EIA
|
Added option for “unknown” |
9b. GDH
|
9b. GDH
|
Added option for “unknown” |
9c. Cytotoxin
|
9c. Cytotoxin
|
Added option for “unknown” |
9d. NAAT (C. diff only)
|
9d. NAAT (C. diff only)
|
Added option for “unknown” |
9e. NAAT (GI panel)
|
9e. NAAT (GI panel)
|
Added option for “unknown” |
9f. Other (specify)
|
9f. Other (specify)
|
Added option for “unknown” |
21. Underlying conditions
|
21. Underlying conditions
____________________ |
Added a field to specify organ transplanted |
34f.1 If YES, which medication was taken |
34f.1 If YES, which treatment was taken? |
Changed “medication” to “treatment” |
37. COVID-NET Case IDs: ________ |
37. COVID-NET Case IDs in the year before or day of DISC: _________
|
Clarified the time period of the question Added a checkbox for “none or N/A” |
Clostridiodies difficile Infection (CDI) Annual Surveillance Officers Survey - Attachment #16
Existing question |
Modified question |
2. In 2022, did any laboratories drop out of participation? |
2. In 2023, did any laboratories drop out of participation? (changed year to 2023 to reflect change in survey year) |
3. In 2022, did you identify any additional laboratories inside or outside of your catchment area which identify C.diff assays from persons who are residents of your catchment area? |
3. In 2023, did you identify any additional laboratories inside or outside of your catchment area which identify C.diff assays from persons who are residents of your catchment area? (changed year to 2023 to reflect change in survey year) |
10. Did your site complete a physician/outpatient provider survey in 2022? |
10. Did your site complete a physician/outpatient provider survey in 2023? (changed year to 2023 to reflect change in survey year) |
13. For each facility that treated a case in 2022, please provide the following |
13. For each facility that treated a case in 2023, please provide the following (changed year to 2023 to reflect change in survey year) |
Annual Survey of Laboratory Testing Practices for C. difficile Infections - Attachment #17
Existing question |
Modified question |
Was this a new laboratory in 2022? |
Was this a new laboratory in 2023? |
How often did you receive line lists from this lab in 2022? |
How often did you receive line lists from this lab in 2023? |
How did you receive line lists from this lab in 2022? |
How did you receive line lists from this lab in 2023? |
Did you receive specimens from this lab in 2022? |
Did you receive specimens from this lab in 2023? |
Was this lab audited in 2022? |
Was this lab audited in 2023? |
Types of facilities in your catchment area served by this lab in 2022 |
Types of facilities in your catchment area served by this lab in 2023 |
Did your laboratory ever send specimens off-site for Clostridioides difficile testing in 2022? |
Did your laboratory ever send specimens off-site for Clostridioides difficile testing in 2023? |
2a. Which testing method(s) for Clostridioides difficile (C. difficile) did your laboratory perform in 2022? |
2a. Which testing method(s) for Clostridioides difficile (C. difficile) did your laboratory perform in 2023? |
Did your laboratory use this testing method for Clostridioides difficile (C. difficile) in 2022? |
Did your laboratory use this testing method for Clostridioides difficile (C. difficile) in 2023? |
Did you use this testing method in this way for all of 2022? |
Did you use this testing method in this way for all of 2023? |
3a. Which EIA test kit was used by your laboratory in 2022? |
3a. Which EIA test kit was used by your laboratory in 2023? |
3b. Which Nucleic Acid Amplification test was used by your laboratory in 2022? |
3b. Which Nucleic Acid Amplification test was used by your laboratory in 2023? |
4a. If your laboratory used a multiplexed molecular diagnostic (e.g., Biofire Filmarray GI Panel, Luminex xTAG GPP) to test for several GI pathogens in 2022, did your laboratory suppress the C. difficile result so that clinicians could not see it? |
4a. If your laboratory used a multiplexed molecular diagnostic (e.g., Biofire Filmarray GI Panel, Luminex xTAG GPP) to test for several GI pathogens in 2023, did your laboratory suppress the C. difficile result so that clinicians could not see it? |
4b. If your laboratory used a multiplexed diagnostic in 2022 and the result was suppressed, where does the suppression occur? |
4b. If your laboratory used a multiplexed diagnostic in 2023 and the result was suppressed, where does the suppression occur? |
5a. If your laboratory used a nucleic acid amplification test (NAAT) (e.g., Cepheid Xpert C. difficile) as first line testing followed by a toxin EIA test (whenever NAAT result is positive) in 2022, did your laboratory suppress the positive NAAT result so that clinicians could not see it? |
5a. If your laboratory used a nucleic acid amplification test (NAAT) (e.g., Cepheid Xpert C. difficile) as first line testing followed by a toxin EIA test (whenever NAAT result is positive) in 2023, did your laboratory suppress the positive NAAT result so that clinicians could not see it? |
5b. If your laboratory used NAAT as first line testing followed by confirmatory toxin EIA testing in 2022, and both the NAAT and toxin EIA results were released to the clinician, did your laboratory provide any comments to help the clinician interpret the test results (e.g., NAAT-positive only result might represent colonization, etc.)? |
5b. If your laboratory used NAAT as first line testing followed by confirmatory toxin EIA testing in 2023, and both the NAAT and toxin EIA results were released to the clinician, did your laboratory provide any comments to help the clinician interpret the test results (e.g., NAAT-positive only result might represent colonization, etc.)? |
6. What are the LOINC or internal testing codes associated with the tests your lab used in 2022 (e.g. LOINC codes 13957-6, 34713-8, or 54067-4)? |
6. What are the LOINC or internal testing codes associated with the tests your lab used in 2023 (e.g. LOINC codes 13957-6, 34713-8, or 54067-4)? |
7. Did your lab have a policy to reject stool specimens for C. difficile testing in 2022? |
7. Did your lab have a policy to reject stool specimens for C. difficile testing in 2023? |
7a. Did your rejection policy for stool specimens change between January 1, 2022 and December 31, 2022? |
7a. Did your rejection policy for stool specimens change between January 1, 2023 and December 31, 2023? |
8. How many stool samples did you test for C. difficile each month in 2022? |
8. How many stool samples did you test for C. difficile each month in 2023? |
2023 CRF Question |
2024 CRF Question |
CANDIDEMIA 2023 CASE REPORT FORM (header)
|
CANDIDEMIA 2024 CASE REPORT FORM (header) (changed year) |
Version: Short Form 2023, Last Updated: 07/29/2022 (footnotes)
|
Version: Short Form 2024, Last Updated: 07/29/2023 (footnotes) (changed year and date) |
23. Candida species from initial positive blood culture (check all that apply): Candida albicans (CA) Candida glabrata (CG) Candida parapsilosis (CP) Candida tropicalis (CT) Candida dubliniensis (CD) Candida lusitaniae (CL) Candida krusei (CK) Candida guilliermondii (CGM) Candida, other (CO) specify: ______________ Candida, germ tube negative/non albicans (CGN) Candida species (CS) Pending
|
23. Candida species from initial positive blood culture (check all that apply): Candida albicans (CA) Candida auris (CAU) Candida glabrata (CG) Candida parapsilosis (CP) Candida tropicalis (CT) Candida dubliniensis (CD) Candida lusitaniae (CL) Candida krusei (CK) Candida guilliermondii (CGM) Candida, other (CO) specify: ______________ Candida, germ tube negative/non albicans (CGN) Candida species (CS) Pending (added new response option) |
24. Antifungal susceptibility testing Species CA CG CP CT CD CL CK CGM CO CGN CS Pending
|
24. Antifungal susceptibility testing Species CA CAU CG CP CT CD CL CK CGM CO CGN CS Pending (added new response option) |
25. Did the patient have a culture-independent diagnostic test (CIDT) for Candida, (e.g., T2), on the day of or in the 6 days before the DISC?
1 Yes 0 No 9 Unknown
|
25. Did the patient have a PCR molecular test for Candida (e.g., T2) in the 6 days before or two days after the DISC?
1 Yes 0 No 9 Unknown
(changed question wording) |
26a. If yes, provide dates of all subsequent positive Candida blood cultures and select the species:
Date Drawn (mm-dd-yyyy) ___ ___ - ___ ___ - ___ ___ ___ ___ Species identified* CA CG CP CT CD CL CK CGM CO:_________ CGN CS Pending
|
26a. If yes, provide dates of all subsequent positive Candida blood cultures and select the species:
Date Drawn (mm-dd-yyyy) ___ ___ - ___ ___ - ___ ___ ___ ___ Species identified* CA CAU CG CP CT CD CL CK CGM CO:_________ CGN CS Pending (added new response option) |
40. Underlying conditions (Check all that apply): Chronic Lung Disease Cystic Fibrosis Chronic Pulmonary disease Chronic Metabolic Disease Diabetes Mellitus With Chronic Complications Cardiovascular Disease CVA/Stroke/TIA Congenital Heart disease Congestive Heart Failure Myocardial infarction Peripheral Vascular Disease (PVD) Gastrointestinal Disease Diverticular disease Inflammatory Bowel Disease Peptic Ulcer Disease Short gut syndrome Immunocompromised Condition HIV infection AIDS/CD4 count <200 Primary Immunodeficiency Transplant, Hematopoietic Stem Cell Transplant, Solid Organ |
40. Underlying conditions (Check all that apply): Chronic Lung Disease Cystic Fibrosis Chronic Pulmonary disease Chronic Metabolic Disease Diabetes Mellitus With Chronic Complications Cardiovascular Disease CVA/Stroke/TIA Congenital Heart disease Congestive Heart Failure Myocardial infarction Peripheral Vascular Disease (PVD) Gastrointestinal Disease Diverticular disease Inflammatory Bowel Disease Peptic Ulcer Disease Short gut syndrome Immunocompromised Condition HIV infection AIDS/CD4 count <200 Primary Immunodeficiency Transplant, Hematopoietic Stem Cell Transplant, Solid Organ (specify): ________ (added new response option) |
52. Did the patient have a CVC in the 2 calendar days before, not including the DISC?
1 Yes 2 No 3 Had CVC but can’t find dates 9 Unknown If yes, check here if central line in place for > 2 calendar days: |
52. Did the patient have a CVC in the 2 calendar days before, not including the DISC?
1 Yes 2 No 3 Had CVC but can’t find dates 9 Unknown If yes, was the central line in place for > 2 calendar days: 1 Yes 0 No 9 Unknown (changed question wording, added additional response options) |
55b. If yes, EIP COVID-NET Case ID: ____________ 9 Unknown Out of EIP COVID-NET catchment area |
55b. If yes, EIP COVID-NET Case ID: ____________ None or N/A
(added new response option) |
AFST results for additional Candida isolates Species CA CG CP CT CD CL CK CGM CO CGN CS Pending
|
AFST results for additional Candida isolates Species CA CAU CG CP CT CD CL CK CGM CO CGN CS Pending (added new response option) |
Laboratory Testing Practices for Candidemia Questionnaire - Attachment #19
2023 Lab Survey Question |
2024 Lab Survey Question |
2023 LABORATORY TESTING PRACTICES FOR CANDIDEMIA QUESTIONNAIRE (header)
|
2024 LABORATORY TESTING PRACTICES FOR CANDIDEMIA QUESTIONNAIRE (header)
(changed year) |
2023 Page # of # (footnotes)
|
2024 Page # of # (footnotes)
(changed year) |
Yes (got to Q14) No (got to Q17) Unknown
|
Yes (go to Q14) No (go to Q17) Unknown
(changed question wording) |
Yes (specify) _____________ No Unknown Not applicable
|
Yes (specify) _____________ No Unknown Not applicable
(changed question wording) |
17) If No for Question 13, does this laboratory have plans to employ culture independent diagnostics for Candida identification in the near future (e.g., T2Candida Panel, BioFire)?
Yes No Unknown Not applicable
|
17) If No for Question 13, does this laboratory have plans to employ PCR molecular tests for Candida identification in the near future (e.g., T2Candida Panel, BioFire)?
Yes No Unknown Not applicable
(changed question wording) |
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Nti-Berko, Sonja Mali (CDC/DDID/NCEZID/DPEI) |
| File Modified | 0000-00-00 |
| File Created | 2023-09-13 |
© 2026 OMB.report | Privacy Policy