Form Appendix A Appendix A 2023 QCDR and Registry Self-nomination User Guide
Quality Payment Program (QPP)/Merit-Based Incentive Payment System (MIPS) (CMS-10621)
Appendix A 2023MIPS QCDR and Registry Self-Nomination User Guide
CY 2024 Performance Period/2026 MIPS Payment Year Burden Summary
OMB: 0938-1314
2023 Merit-Based
Incentive Payment System (MIPS) Program: Self-Nomination User Guide
for Qualified Clinical Data Registries (QCDRs) and Qualified
Registries
June 2022
Table of Contents
Accessing the Quality Payment Program Website 5
Sign Up for a Quality Payment Program Account 5
Creating a Self-Nomination Form (prospective QCDRs/Qualified Registries) 16
Creating a Self-Nomination Form (existing QCDRs/Qualified Registries not in good standing) 19
Populating the 2023 Self-Nomination Form 21
Populating the 2023 Simplified Self-Nomination Form 22
MIPS Clinical Quality Measures 45
MIPS electronic Clinical Quality Measures 46
Benchmarking Capabilities(QCDR ONLY) 55
Pre-populated Existing QCDR Measures 58
Self-Nominate QCDR Measures 59
Co-Owned by two or more QCDRs 61
Measure Specification Fields 65
Supporting Documentation Section: 76
QCDR Measure Permission Checklist 80
Submission of the Self-Nomination Form 81
Modifying a Self-Nomination Form 82
Withdrawing a Self-Nomination Form 82
Help with QCDR Measure Development 85
Introduction
Purpose
The 2023 Self-Nomination User Guide provides prospective Qualified Clinical Data Registries (QCDRs) and Qualified Registries with guidance on how to self-nominate for the 2023 performance period of the Merit-Based Incentive Payment System (MIPS) program. The intent of the guide is to provide entities with step-by-step instructions on the information needed to populate and submit a completed Self-Nomination form for the Centers for Medicare & Medicaid Services (CMS) consideration.
Background
The Self-Nomination form is available through the CMS Quality Payment Program (QPP) website and should be accessed, completed, and submitted by entities seeking to participate in the 2023 MIPS performance period as a QCDR and/or Qualified Registry.
The QCDR and Qualified Registry Self-Nomination form contains the following tabs (please note, you are required to populate all required fields marked with an asterisk (*) and tabs prior to submitting your Self-Nomination form for CMS review):
Intermediary Contact Info tab – Intermediaries are required to enter their demographic and contact information.
Intermediary Details tab – Intermediaries are required to enter their participation details.
Qualified Posting Details tab – Intermediaries are required to enter their cost information, services included in cost, last date to accept new clients, reporting information/supported, data collection methods, reporting frameworks, MIPS Value Pathways (MVPs) supported, and performance categories. A qualified posting is developed for the approved QCDRs/Qualified Registries and include organization type, specialties, previous participation in MIPS (if applicable), program status (remedial action taken against the QCDR/Qualified Registry or terminated as a third-party intermediary, if applicable), contact information, last date to accept new clients, virtual groups specialty parameters (if applicable), the approved measures, reporting frameworks/performance categories/MVPs supported, services offered, and costs incurred by clients. All approved QCDRs/Qualified Registries are included in the Qualified Posting that is posted on the CMS QPP Resource Library.
Attestations tab – Intermediaries are required to attest that they understand and are able to meet the participation requirements for the 2023 MIPS performance period.
Improvement Activities tab – Intermediaries are required to select the improvement activities supported for the 2023 MIPS performance period. For more information, please see the 2022 MIPS Improvement Activities User Guide.
Promoting Interoperability tab – Intermediaries are required to select the Promoting Interoperability objectives and measures supported for the 2023 MIPS performance period. For the Promoting Interoperability performance category, if the MIPS eligible clinician, group, virtual group, or subgroup1 is using certified electronic health record technology (CEHRT); the third-party intermediary may be excepted from this requirement if ALL its MIPS eligible clinicians, groups, virtual groups, or subgroups fall under the reweighting policies. Health Information Technology (IT) vendors are required to submit data for at least one category. For more information, please see the 2022 MIPS Promoting Interoperability User Guide.
MIPS Clinical Quality Measure (CQM) tab – Intermediaries are required to select the individual MIPS CQMs supported for the 2023 MIPS performance period. QCDRs and Qualified Registries must support at least six quality measures, with at least one outcome measure. If an outcome measure is not available, at least one other high priority measure should be used. Please note, some MIPS CQMs are also available as MIPS electronic clinical quality measures (eCQMs) and should be used as specified. Changes to the measure specifications are not permitted.
MIPS electronic Clinical Quality Measure (eCQM) tab – Intermediaries may specify the eCQMs supported for the 2023 MIPS performance period. Please note, some MIPS eCQMs are also available as MIPS CQMs and should be used as specified. Changes to the measure specifications are not permitted.
MIPS Value Pathway (MVP) tab – Intermediaries may specify the MVPs supported for the 2023 MIPS performance period. Please note, the list of MVPs reflects what is finalized in the CY 2022 Physician Fee Schedule Final Rule for the QPP. MVP availability is subject to change, based upon what is finalized for inclusion and removal in the CY 2023 Physician Fee Schedule Final Rule for the QPP. In addition, voluntary, opt-ins, and virtual groups are currently excluded from MVP reporting.
Data Validation tab – Intermediaries are required to specify the methodology that they will use to validate the data submitted for the 2023 MIPS performance period.
Please refer to the 2023 QCDR Fact Sheet located in the QPP Resource Library for additional information on the QCDR participation requirements. The 2023 QCDR Fact Sheet is included in the 2023 Self-Nomination Toolkit.
Please refer to the 2023 Qualified Registry Fact Sheet located in the QPP Resource Library for additional information on the Qualified Registry participation requirements. The 2023 Qualified Registry Fact Sheet is included in the 2023 Self-Nomination Toolkit.
The QCDR Self-Nomination form contains the following additional tabs (please note, you are required to populate all required fields marked with an asterisk (*) and tabs prior to submitting your Self-Nomination form for CMS review):
Benchmarking Capabilities tab (optional) – Allows QCDRs to upload their benchmarking methodology.
QCDR Measures tab (optional) – Allows QCDRs to provide QCDR measures and/or supplemental QCDR measure documentation for consideration for the 2023 performance period. To utilize this QCDR measures tab your intermediary must choose ‘Yes’ under the “Do you plan to submit QCDR Measures?” question on the Intermediary Details tab to submit your QCDR measures. Please note, QCDRs who plan to submit QCDR measures for CMS consideration, must complete this tab.
To be considered for the 2023 MIPS performance period, prospective QCDRs and/or Qualified Registries will be required to submit their complete Self-Nomination form (inclusive of MIPS quality measures, QCDR measures (QCDRs only), and data validation) by 8 p.m. ET on September 1, 2022. Intermediaries who intend on participating in MIPS as a QCDR and Qualified Registry must complete and submit a Self-Nomination form for each intermediary type for the 2023 MIPS performance period. CMS will only approve the organization for the intermediary type identified within the Self-Nomination form. Applicants will not be able to update or submit their self-nomination after the deadline. CMS will not review late submissions.
Failure to meet participation requirements and/or the falsification of any information provided during self-nomination, may result in remedial action being taken against the QCDR and/or Qualified Registry, or termination as a QCDR and/or Qualified Registry in the current and future program years of MIPS.
CMS will post two qualified postings for the 2023 MIPS performance period, one for approved QCDRs and another for approved Qualified Registries. MIPS eligible clinicians, groups, virtual groups, subgroups, or Alternative Payment Model (APM) Entities, inclusive of voluntary and opt-in participants may utilize the qualified postings to select a CMS-approved QCDR or Qualified Registry as their method of data submission for MIPS reporting.
Additional resources regarding the 2023 MIPS performance period can be found on the QPP website.
Accessing the Quality Payment Program Website
Sign Up for a Quality Payment Program Account
If you do not have a user account, you must create a user account.
Review the QPP Access User Guide (zip) that contains these files:
QPP Access at a Glance - Gives an overview of what you need to do to sign in to http://www.qpp.cms.gov and how you will manage access to organizations you want to access to self-nominate.
Register for a Health Care Quality Information Systems (HCQIS) Access Roles and Profile (HARP) Account - Gives step-by-step instructions with screenshots for new users (those who have never signed into http://www.qpp.cms.gov) to create an account.
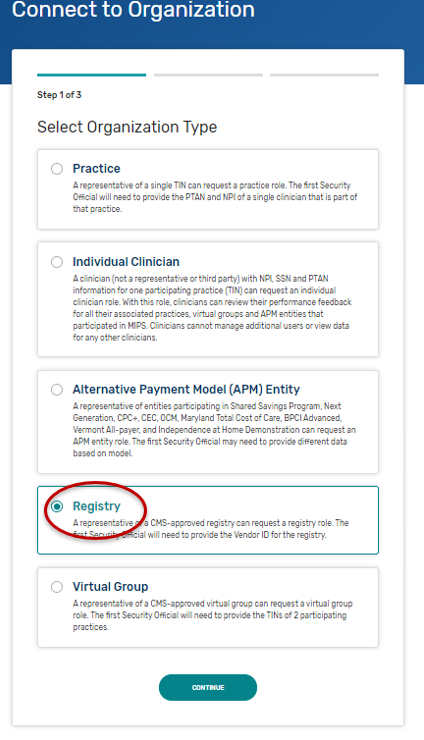
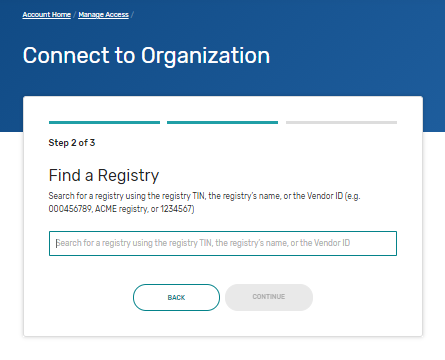
Connect to an Organization - Gives step-by-step instructions with screenshots for any user to request authorization for an organization. Follow these steps if you need to self-nominate for an organization that is currently submitting on the QPP website. This includes organizations that qualify for the simplified Self-Nomination form. You may need to connect to the organizations that you need to self-nominate.
Security Officials: Manage Access - Gives step-by-step instructions with screenshots for a small group of users (those with a Security Official role) to approve requests to access their organization. Follow these steps if you need to self-nominate for an organization that is currently submitting on the QPP website. This includes organizations that qualify for the simplified Self-Nomination form. You may need to request the Security Official role for the organizations that you need to self-nominate.
Navigate to the QPP website.
Click on Register.
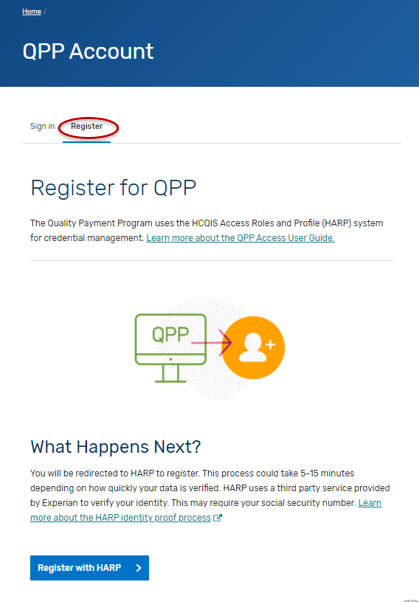
Click on “Register with HARP” to create your account. This process could take 5-15 minutes depending on how quickly your data is verified. HARP uses a third-party service provided by Experian to verify your identity. This may require your social security number. Please select the “Security Official” role.
If you are self-nominating for a new intermediary that is not currently active on the QPP website, then you are done.
If you are self-nominating for an existing intermediary that is active on the QPP website, then you will need to connect to that organization and request the “Security Official” role.
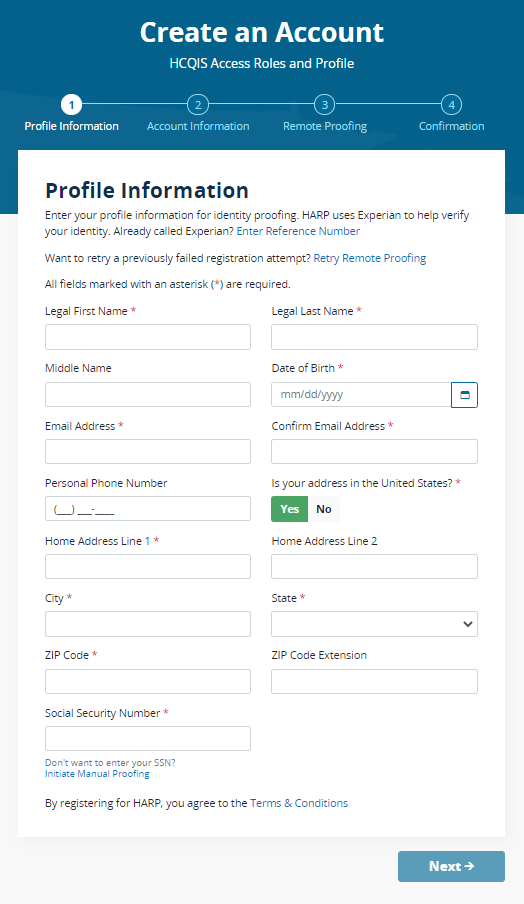
Once you have created an account and logged in, you will land on the Manage Access page in the QPP website and will see the “Registry/QCDR Self-Nomination” link to access the Self-Nomination form for QCDRs/Qualified Registries. You will see the organizations that you are connected to listed on the Manage Access page. If you are not seeing an existing intermediary that is active on the QPP website that you need to self-nominate, then you will need to click on “Connect to an Organization” and connect to that organization and request the “Security Official” role.

Log in to the QPP website.
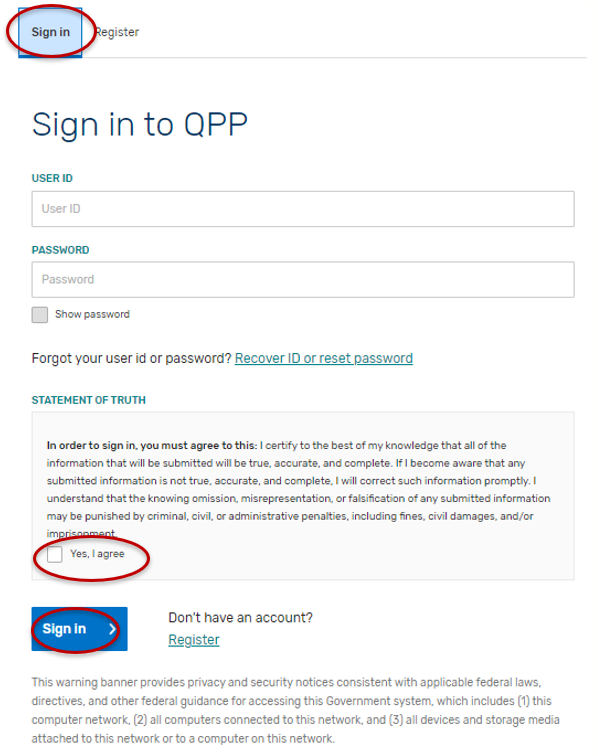
Select “Sign In.”
Enter your User Identifier (ID) and Password, check the “Yes, I agree” box, and click the “Sign In” button.
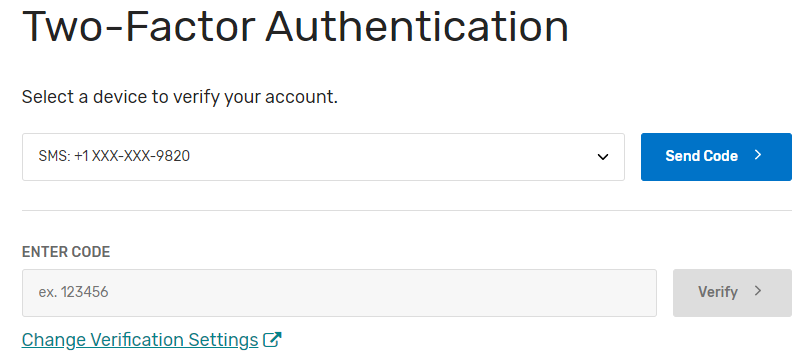
Please note, you must have the “Security Official” role assigned to access, complete, and submit the Self-Nomination form for your organization(s). If you do not have the “Security Official” role, please submit a request to your organization’s “Security Official” via the QPP website. If you are the “Security Official” for your organization, you may add this role to the appropriate staff via the QPP website. Users can request access as a “Security Official” on the Manage Access page by clicking the “Connect to another Organization” link. You will be able to identify the QCDRs/Qualified Registries where the “Security Official” role has been approved as they are listed on the Manage Access page. The list of organizations that you can self-nominate will match the list of organizations displayed on the Manage Access page under the Registry option (QCDR/Qualified Registry entities). If you know the organization that you need to self-nominate is active on the QPP website and it is missing from this list, then you need to follow the steps above to “Connect to an Organization” in the “Security Official” role.
Please note that sharing HARP account credentials with other users is not permitted and is considered a security violation. Each individual user must have their own HARP account to log in to the QPP website. Unauthorized or improper use of this system is prohibited and may result in disciplinary action and/or civil and criminal penalties.

Self-Nomination Form Tips
Please consider the following tips as you prepare to self-nominate:
QCDRs and Qualified Registries must enter and maintain a Health Insurance Portability and Accountable Act (HIPAA) compliant Business Associate Agreement (BAA) with its participating MIPS eligible clinicians, groups, virtual groups, subgroups, or APM Entities, inclusive of voluntary and opt-in participants that provides for receipt of patient-specific data. The BAA must account for the disclosure of quality measure results, numerator and denominator data, and/or patient-specific data on Medicare and non-Medicare patients on behalf of MIPS eligible clinicians, groups, virtual groups, subgroups, or APM Entities, inclusive of voluntary and opt-in participants.
All prospective QCDRs and Qualified Registries must complete and submit their Self-Nomination form via the QPP website. No other Self-Nomination form submission methods will be accepted.
Prepare the information needed to complete the Self-Nomination form, measure information and Data Validation in advance of the attempt to self-nominate via the QPP website. Please note, that the system will log you out after 30 minutes of inactivity.
A third party intermediary’s principal place of business and retention of associated CMS data must be within the U.S. Please note, CMS policy prohibits non-U.S. citizens from accessing CMS IT systems.
The time required to complete this information collection is estimated to average twelve hours per Self-Nomination form, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the Self-Nomination form.
All fields marked with a red asterisk (*) are required.
You will not be able to successfully submit a self-nomination unless all the required fields of all tabs have been filled out. However, once submitted, you may go back and edit your submission until 8 p.m. ET on September 1, 2022. Do not click “SUBMIT FOR REVIEW” until all the required fields of all tabs have been completed.
Comment functionality is available in the Self-Nomination tool. It may be used for specifying any updates or informing CMS about any changes that have been applied to the self-nomination or QCDR measures. Refer to “Comments” section of this User Guide for additional information.
Please contact the MIPS QCDR/Registry Support Team (PIMMS Team) at QCDRVendorSupport@gdit.com or RegistryVendorSupport@gdit.com, if you cannot find your simplified Self-Nomination form instead of creating and submitting a new Self-Nomination form for your organization.
Please ensure that a member of your organization has a QPP account with the appropriate associated role at all times and that appropriate coverage is in place if the lead self-nomination contact person is out of the office.
If an error message is generated while in the QPP self-nomination website, you should try refreshing the page and/or clearing your cache.
If you have questions about the 2023 Self-Nomination form, please contact the QPP
Service Center at 1-866-288-8292 or by email at: QPP@cms.hhs.gov (Monday through Friday from 8 a.m. to 8 p.m. ET). To receive assistance more quickly, please consider calling during non-peak hours, before 10 a.m. and after 2 p.m. ET. Customers who are hearing impaired can dial 711 to be connected to a TRS Communications Assistant.
Creating a Self-Nomination Form (prospective QCDRs/Qualified Registries)
Click “Registry/QCDR Self-Nomination” under Manage Access.
Click “Add New Intermediary.”
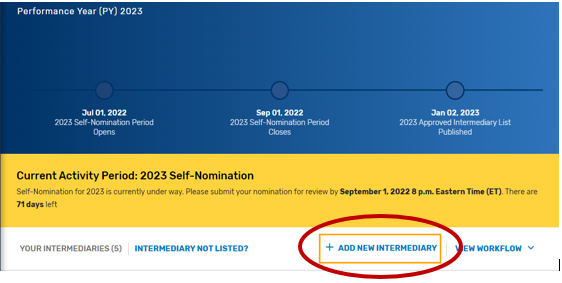
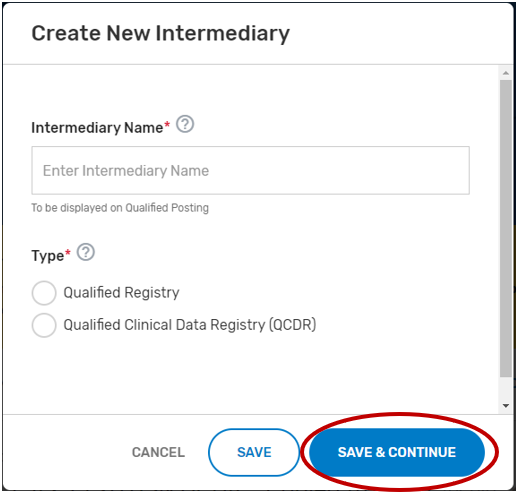
Enter your intermediary name (to be displayed on Qualified Posting) and indicate your prospective QCDR’s or Qualified Registry’s intermediary type. Please ensure you have selected the correct intermediary type, as this may affect your application. If you would like to self-nominate to become both a QCDR and Qualified Registry, you must complete an application for each intermediary type.
Click “Save & Continue.”
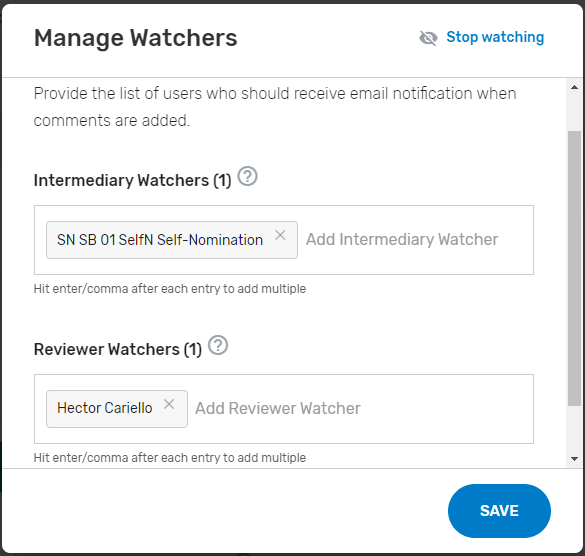
Add ‘Watchers’ to monitor the status of your Self-Nomination form. This feature allows entities to create a list of users who should receive email notification when comments and/or status changes are provided. Please note, make sure the ‘watcher(s)’ you provide are kept current to prevent delays with your self-nomination.
Creating a Self-Nomination Form (existing QCDRs/Qualified Registries not in good standing)
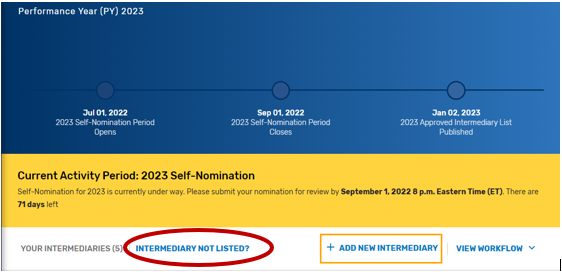
Find your intermediary name in the list on the Intermediary landing page with the Self-Nomination Form “Partially Renewed” status.
If you are not seeing an intermediary that you know is active on the QPP website, please refer to the Intermediary Not Listed link for instructions on how to connect to that organization via the Manage Access page.
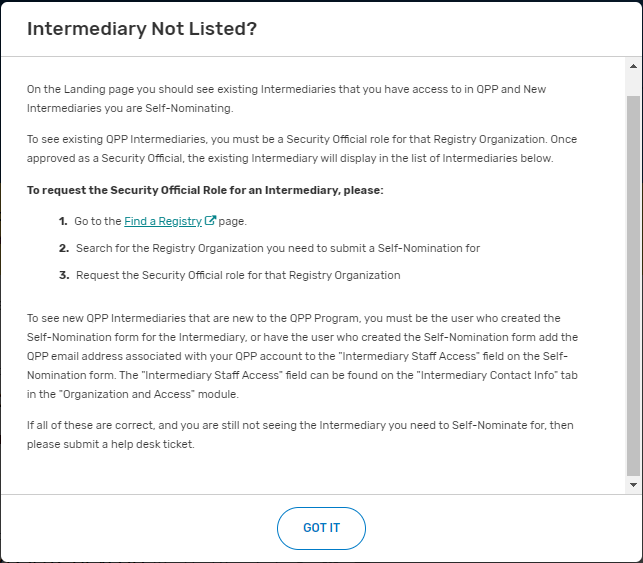
Click on “Intermediary Not Listed” button.
Follow the directions provided in the “Intermediary Not Listed?” pop-up window.
Populating the 2023 Self-Nomination Form
Disclaimer: A majority of the screenshots used in this section of the User Guide were taken from the QCDR Self-Nomination form. Corresponding fields of the Qualified Registry Self-Nomination form may slightly differ.
Please note, the “Populating the 2023 Self-Nomination Form” process should be completed by prospective QCDRs/Qualified Registries or existing QCDRs/Qualified Registries that currently are not in good standing (i.e., have remedial action taken against the intermediary). Existing QCDRs/Qualified Registries in good standing may refer to the “Populating the 2023 Simplified Self-Nomination Form” section of the User Guide for information on self-nominating for the 2023 MIPS performance period. Existing QCDRs/Qualified Registries in good standing should contact the MIPS QCDR/Registry Support Team (PIMMS Team) at QCDRVendorSupport@gdit.com or RegistryVendorSupport@gdit.com if they cannot find or access the simplified Self-Nomination form instead of submitting a new Self-Nomination form.
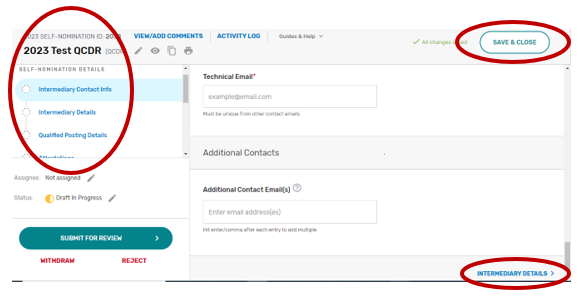
All input placed into the form is automatically saved when you move to the next field. If you cannot complete the Self-Nomination form in one session, you may click the “Save & Close” button on the Self-Nomination form and come back to it later.
You may navigate between the Self-Nomination form tabs by clicking the appropriate tab on the left-hand side of the screen or at the bottom of the screen.
Populating the 2023 Simplified Self-Nomination Form
A Simplified Self-Nomination form is available to reduce the burden of self-nomination for those existing QCDRs/Qualified Registries that have previously participated in MIPS and are in good standing (remedial action has not been taken against the intermediary). A Simplified Self-Nomination form is available only for existing QCDRs and Qualified Registries in good standing with minimal changes, and those with substantive changes as described below. Existing QCDRs/Qualified Registries in good standing should contact the MIPS QCDR/Registry Support Team (PIMMS Team) at QCDRVendorSupport@gdit.com and RegistryVendorSupport@gdit.com if they cannot find or access the Simplified Self-Nomination form instead of submitting a new Self-Nomination form.
QCDR/Qualified Registry with minimal changes – existing QCDRs/Qualified Registries in good standing that would like to submit minimal changes to their previously approved Self-Nomination form from the previous year and may submit these changes, and attest to no additional changes from their previously approved QCDR/Qualified Registry Self-Nomination form, for CMS review and consideration during the self-nomination period, from July 1 and September 1. Minimal changes include but are not limited to:
Limited changes to the supported performance categories.
Adding or removing MIPS quality measures.
Reporting frameworks.
MVPs supported.
Promoting Interoperability measures.
Improvement activities.
Adding or updating existing services offered.
Cost associated with using the QCDR/Qualified Registry.
QCDR/Qualified Registry with substantive changes – existing QCDRs/Qualified Registries in good standing, may also submit for CMS review and approval, updates to a QCDRs/Qualified Registries data validation, or a change in the organization structure that would impact any aspect of the QCDR/Qualified Registry. Substantive changes impacting the organizational structure should be communicated to CMS as soon as possible. Suggestions for new MIPS quality measures should go through the Annual Call for Measures process and updates to the existing MIPS quality measures should be submitted to the existing measure’s steward. QCDRs/Qualified Registries are not allowed to submit or create new MIPS quality measures during self-nomination.
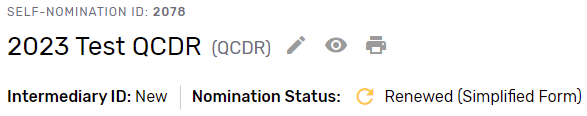
You may begin your 2023 Simplified Self-Nomination form by clicking “Get Started” on the self-nomination landing page. Please note, that existing QCDRs and Qualified Registries that are eligible for the Simplified Self-Nomination form will see a nomination status of “Renewed.”
Please note, the new fields in the 2023 Self-Nomination form, fields that are now required or include validation for a specific format (i.e., telephone number), will need to be populated or updated for the Self-Nomination form to pass validation and be successfully submitted.
2022 QPP performance period information will be available for public reporting on Care Compare starting in late 2023. If you have any questions about this, please contact the Care Compare support team at: PhysicianCompare1@westat.com.
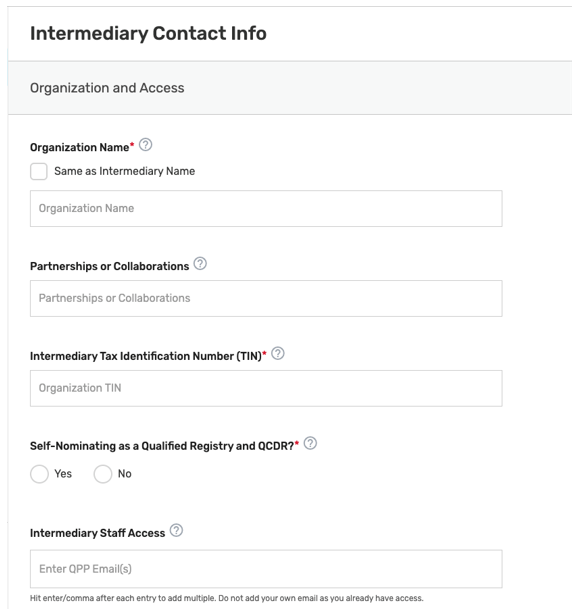
Intermediary Contact Info
Enter the name of your prospective QCDR or Qualified Registry (if different from the intermediary name) and your organization’s tax identification number (TIN). The intermediary name will be used as your QCDR or Qualified Registry name for CMS purposes. Please provide if your organization will be participating as both intermediary types (i.e., QCDR/Qualified Registry) for the 2023 MIPS performance period. The “Intermediary Staff Access” field will ONLY be seen if the Self-Nomination form is for a new intermediary. List QPP email addresses for any additional staff at your organization who need to have access to the Self-Nomination form. To access an existing intermediary’s Self-Nomination form, additional users must be connected to that organization as a “Security Official” role.
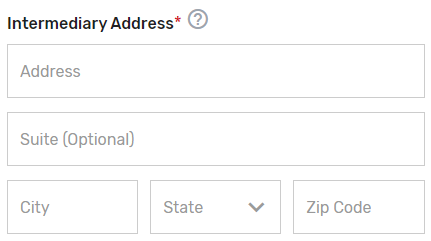
Enter the mailing address for your prospective QCDR or Qualified Registry.
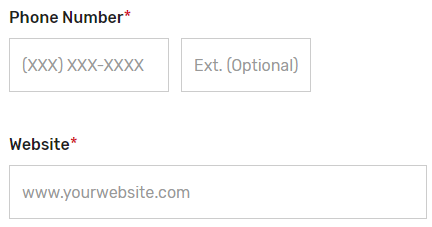
Enter the prospective QCDR’s or Qualified Registry’s contact information (i.e., Phone Number and Website). The “Phone Number” field should be populated as (XXX) XXX-XXXX.
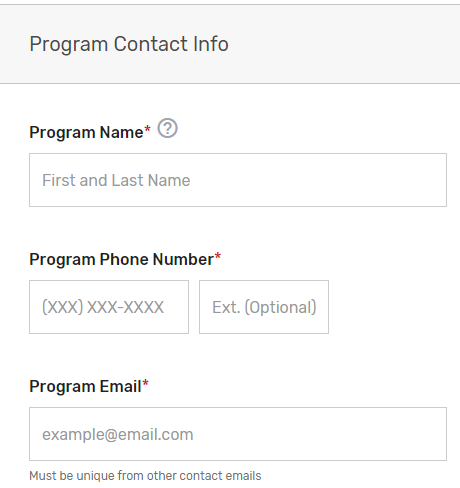
Enter information for a program, clinical, and technical contact. Please note, you are required to provide three unique points of contact (POCs) for these three fields. Entering only one POC or different email addresses for the same POC (i.e., the same name is entered for program and clinical contact with two different email addresses) is not acceptable. If you would like to add additional POCs to be included in the email distribution to receive program announcements and support call information (if approved), please add the email address(es) in the “Additional Contacts” field.
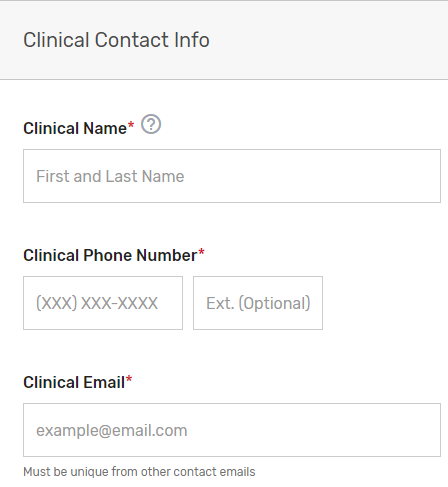
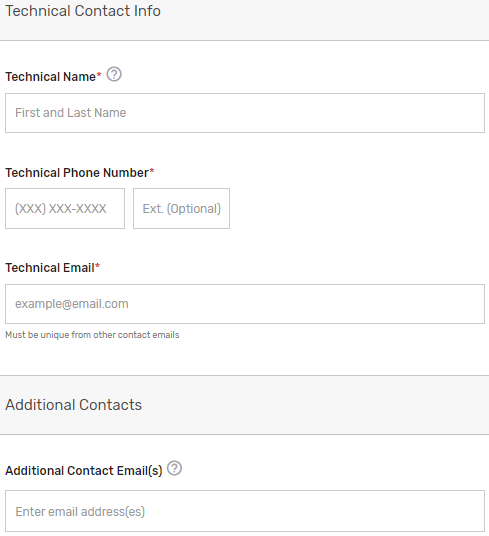
Please note, this contact information will only be used in relation to your potential participation in the program. To ensure notices are received, please have these contacts add the QPP to their safe/approved senders list.
Intermediary Details
Click the Intermediary Details tab.
Indicate your prospective QCDR’s or Qualified Registry’s intermediary type. If “Health IT Vendor” or “Other” is selected, please specify.
Indicate if this is a new or existing QCDR or Qualified Registry that participated under MIPS and Physician Quality Reporting System (PQRS). Select all applicable years your prospective QCDR or Qualified Registry has participated in MIPS and/or PQRS as a QCDR or Qualified Registry.
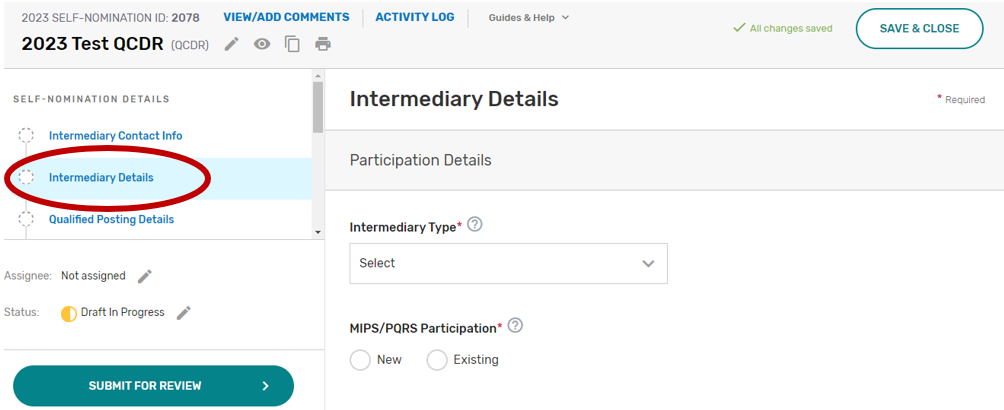
Has your intermediary participated as a QCDR or Qualified Registry during the last two performance periods and submitted any MIPS data on behalf of clinicians, groups, virtual groups, subgroups, or APM Entities, inclusive of voluntary and opt-in participants? Please select Yes or No. If your intermediary chooses “No”, your intermediary must populate the self-nomination participation plan section.

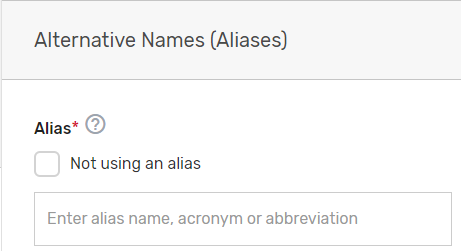
Enter any other aliases or acronyms your prospective QCDR or Qualified Registry currently uses or has used for participation as a QCDR or Qualified Registry in previous program periods.
Please indicate your prospective QCDR or Qualified Registry’s related clinical specialty(ies).
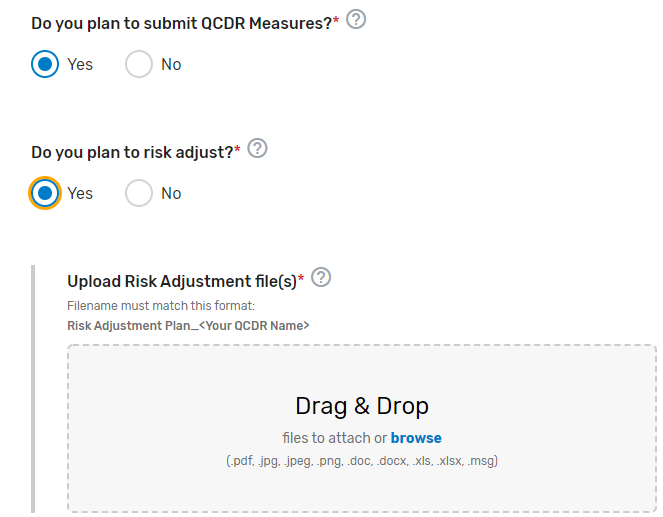
Does the prospective QCDR plan to submit QCDR measures? Please select Yes or No (QCDRs only). To populate the QCDR Measures tab your intermediary must choose ‘Yes’ under the “Do you plan to submit QCDR Measures?” question to submit your QCDR measures for CMS consideration.
Does the prospective QCDR plan to risk adjust the quality measures data that is integrated with the measure specifications? Please select Yes or No (QCDRs only). Upload your Risk Adjustment Plan in the “file uploads” section. Please note, do NOT upload the attachment in the comment section.
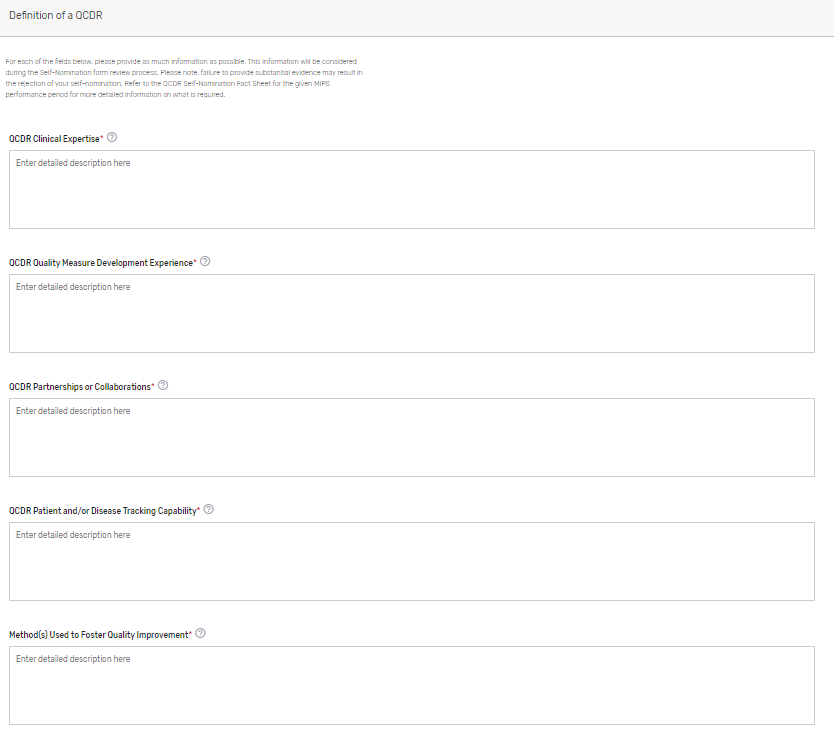
Describe how your prospective QCDR meets the definition of a QCDR. Please note, reiterating CMS’s definition of a QCDR does not suffice as justification as to how your prospective QCDR meets the definition. Please describe in detail your prospective QCDR’s clinical expertise and quality measure development experience, partnerships or collaborations, patient and/or disease tracking capability, method(s) used to foster quality improvement, and evidence of meeting the identified deficiencies to meet the definition of a QCDR (if applicable) (QCDRs Only).
Please refer to the 2023 QCDR Self-Nomination Fact Sheet for more detailed information on what is required.
Qualified Posting Details
Indicate the cost information as well as the type of services your prospective QCDR or Qualified Registry provides. If approved, this information will be included in the prospective QCDR’s or Qualified Registry’s qualified posting. Please include frequency (monthly, annual, per submission), and if the cost is per provider/practice. Make sure to provide in detail the differences in cost/services in each Self-Nomination form, if you are an intermediary that selected ‘Yes’ to self-nominate as both a QCDR and Qualified Registry in the Intermediary Contact Info tab.
Indicate the latest date your prospective QCDR or Qualified Registry can accept new clients. Please add the date as MM/DD/YYYY. The performance period starts on January 1, 2023, and ends December 31, 2023. As a reminder, data submissions begin January 2, 2024, and end on April 1, 2024.
Indicate the data collection method(s) your prospective QCDR or Qualified Registry supports, select the performance category(ies) you will be supporting, and which reporting options you will support. If “Other” is selected, please specify the data collection method in the corresponding field. The quality and improvement activities performance categories are grayed out as your prospective QCDR or Qualified Registry must support quality and improvement activities. Please note, QCDRs and Qualified Registries that submit data are REQUIRED to support the quality, Promoting Interoperability and improvement activities performance categories. A third party intermediary could be excepted from Promoting Interoperability if ALL its MIPS eligible clinicians, groups, virtual groups, and/or subgroups, fall under the reweighting policies. For more information, please see the 2022 MIPS Promoting Interoperability User Guide.
Indicate which reporting frameworks you will support for your MIPS eligible clinicians to participate as individual clinicians, groups, virtual groups, subgroups, or APM Entities, inclusive of voluntary and opt-in participants. QCDRs and Qualified Registries must support MVPs that are relevant to the specialties supported. QCDRs and Qualified Registries may also support the APM Performance Pathway (APP).
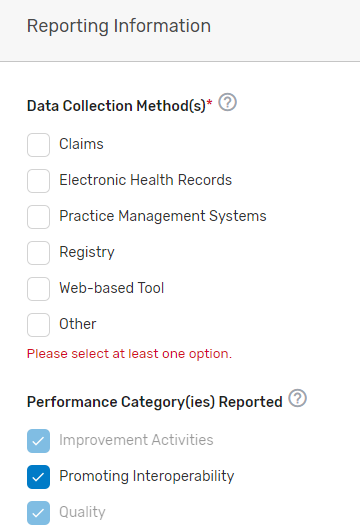
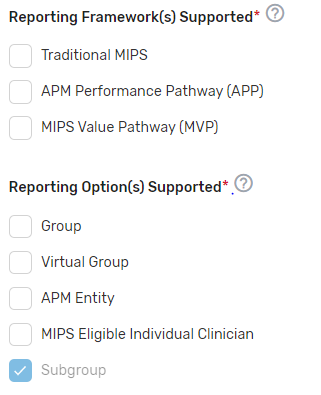
Attestations
Click the Attestations tab.
Review the “Participation” statement and enter your name to attest that your prospective QCDR or Qualified Registry meets the participation requirements. In addition, please review and answer the self-nomination attestation questions and indicate if your prospective QCDR or Qualified Registry is able to meet these program requirements. To be considered, a prospective QCDR or Qualified Registry must attest “Yes” to all the self-nomination attestations.



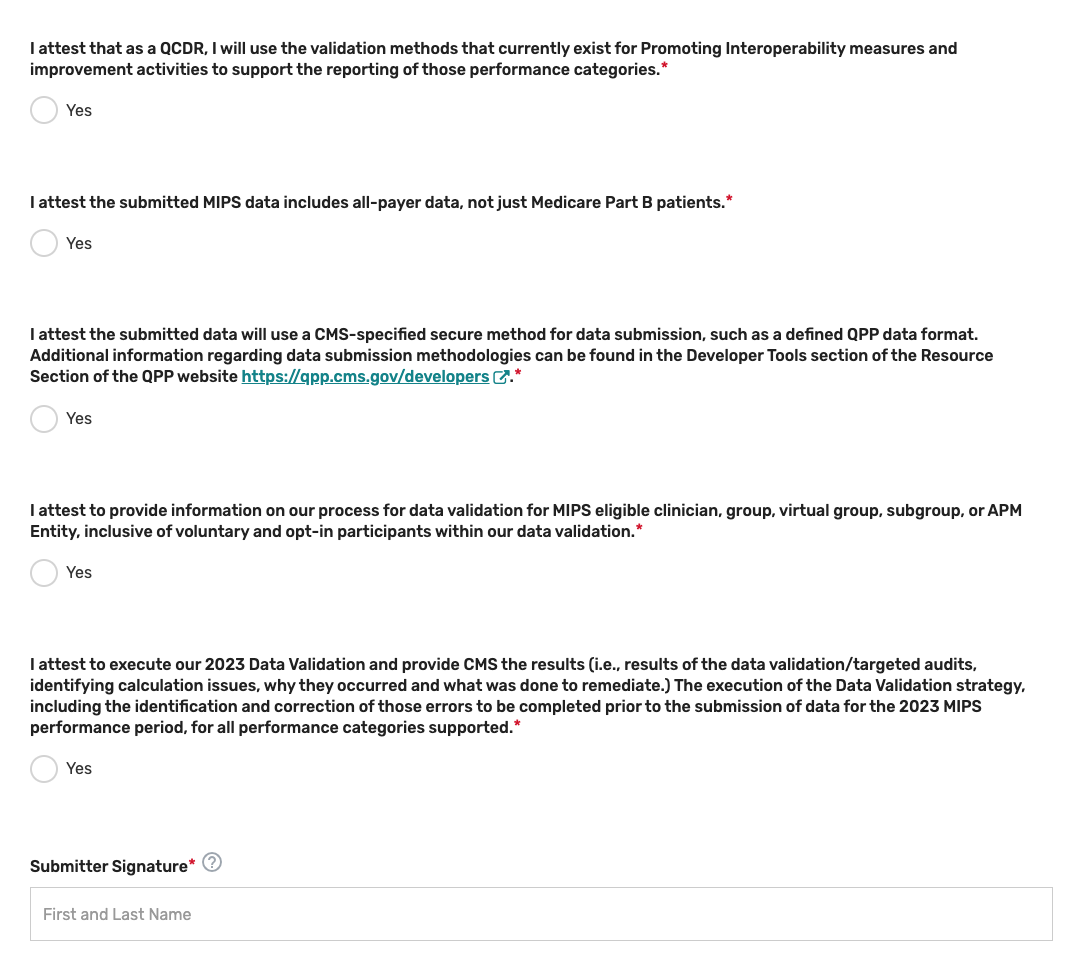
Please note, these attestations include the acceptance of data exports directly from an EHR or other data sources. If you become aware that any submitted information is not true, accurate, and complete, you will correct such issues promptly prior to submission, or refrain from submitting it.
Improvement Activities
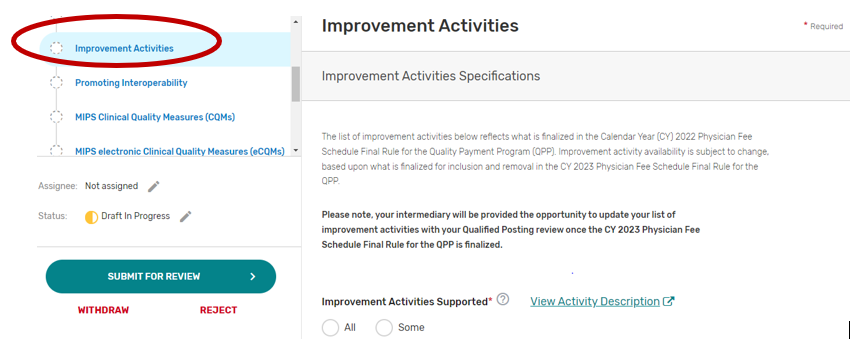
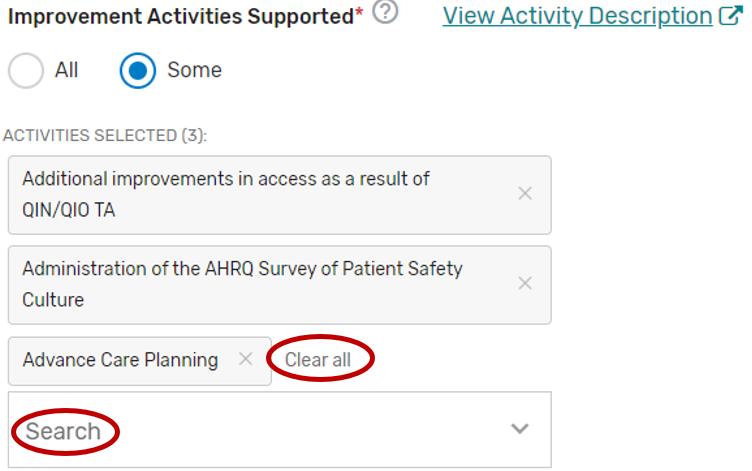
You may select “All” or “Some.” The list of supported improvement activities will appear if “Some” is selected. For more information, please see the 2022 Improvement Activities User Guide.
You may select each improvement activity you will support from the list of all the improvement activities. A list will display and update after each individual improvement activity is selected. Select “All”, instead of selecting each improvement activity from the drop down if you will support all the improvement activities.
You may remove a selected individual improvement activity by clicking the “X” next to each selected improvement activity or click “Clear all” to remove all the selected improvement activities. In addition, you may use the “Search” function to look-up specific improvement activities.
To edit your improvement activities, refer to the “Modifying a Self-Nomination” section of this User Guide.
Promoting Interoperability
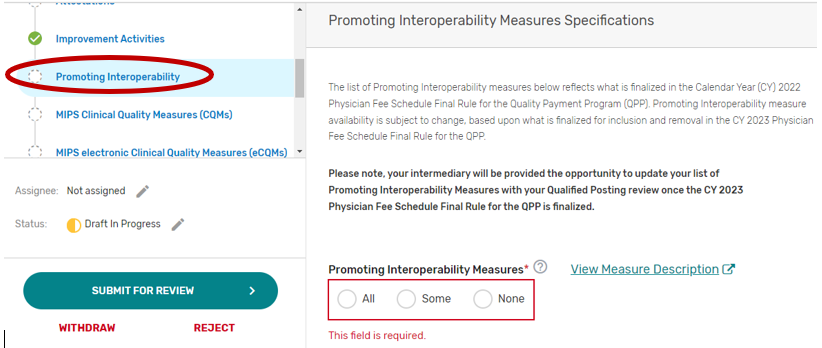
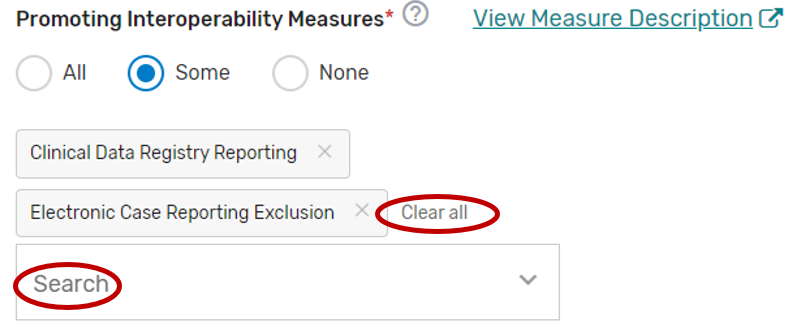
2. You may select “All”, “Some”, or “None.” The list of supported Promoting Interoperability objectives and measures will appear if “Some” is selected. Please note, a third party intermediary could be excepted from Promoting Interoperability if ALL its MIPS eligible clinicians, groups, virtual groups, or subgroups fall under the reweighting policies. If you select “None”, please make sure you uncheck “Promoting Interoperability” under the Reporting Options section on the Qualified Posting Details tab. A field will display for your intermediary to provide an attestation of the exception criteria. For more information, please see the 2022 MIPS Promoting Interoperability User Guide.
You may select each Promoting Interoperability you will support from the list of all the Promoting Interoperability objectives and measures. Each individual objective or measure selected will show after each objective or measure is selected. Select “All”, instead of selecting each Promoting Interoperability objective and measure from the drop down if you will support all the Promoting Interoperability objectives and measures.
You may remove a selected objective or measure by clicking the “X” next to each selected objective or measure or click “Clear all” to remove all the selected objectives or measures. In addition, you may use the “Search” function to look-up specific objectives or measures.
To edit your objectives or measures, refer to the “Modifying a Self-Nomination” section of this User Guide.
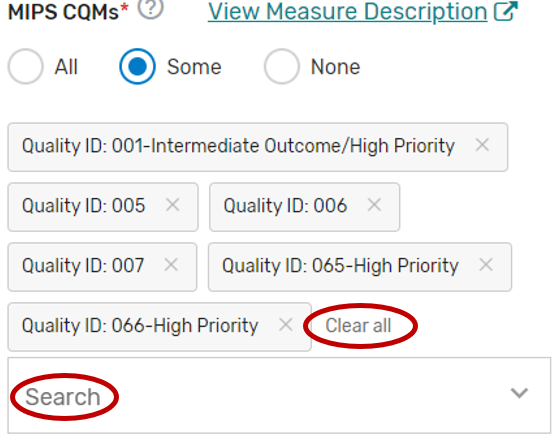
MIPS Clinical Quality Measures
Click the MIPS Clinical Quality Measures (CQMs) tab.
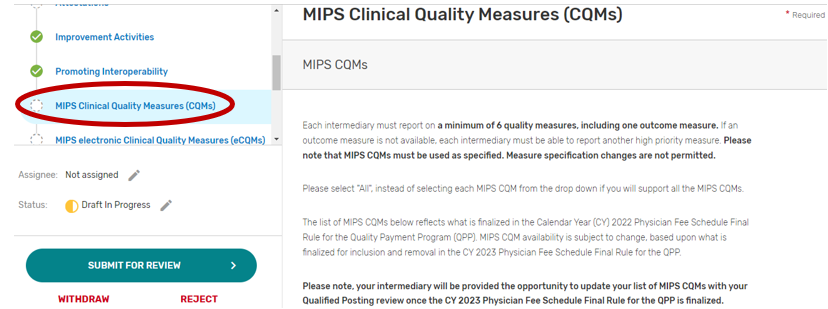
You may select “All”, “Some”, or “None.” The list of individual MIPS CQMs will appear if “Some” is selected. Please note that the MIPS CQM must be used as specified. Measure specification changes are not permitted.
You may select each MIPS CQM you will support from the list of all the MIPS CQMs. Each MIPS CQM selected will show after each measure is selected. Select “All”, instead of selecting each MIPS CQM from the drop down if you will support all the MIPS CQMs.
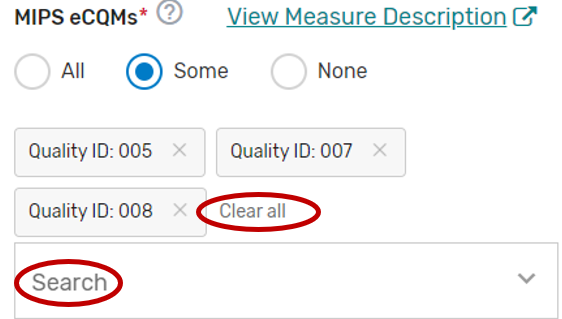
You may remove a selected MIPS CQM by clicking the “X” next to each selected measure or click “Clear all” to remove all the selected measures. In addition, you may use the “Search” function to look-up specific measures.
To edit your individual MIPS CQMs, refer to the “Modifying a Self-Nomination” section of this User Guide.
MIPS electronic Clinical Quality Measures
Click the MIPS electronic Clinical Quality Measures (eCQMs) tab.
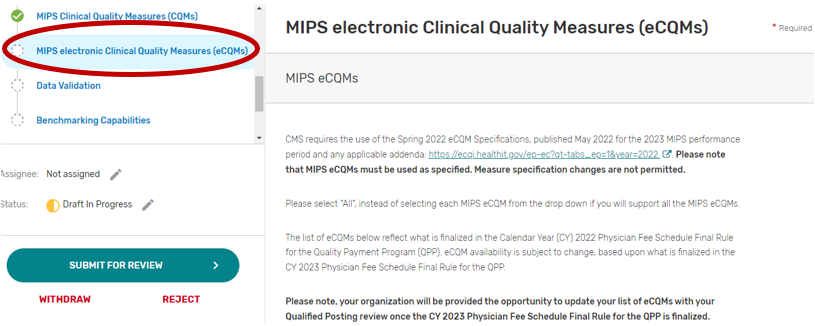
You may select “All”, “Some”, or “None.” The list of individual MIPS eCQMs will appear if “Some” is selected. Please note, the MIPS eCQM must be used as specified. Measure specification changes are not permitted.
You may select each eCQM you will support from the list of all the eCQMs. Each MIPS eCQM selected will show after each measure is selected. Select “All”, instead of selecting each MIPS eCQM from the drop down if you will support all the MIPS eCQMs.
You may remove a selected MIPS eCQM by clicking the “X” next to each selected measure or click “Clear all” to remove all of the selected measures. In addition, you may use the “Search” function to look-up specific measures.
To edit your individual MIPS CQMs, refer to the “Modifying a Self-Nomination” section of this User Guide.
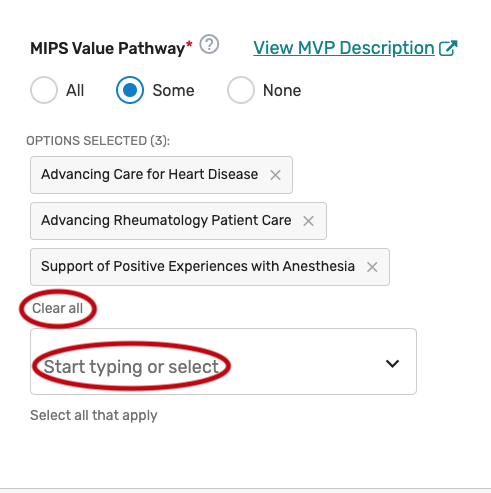
MIPS Value Pathway (MVP)
Click the MIPS Value Pathway (MVP) tab.

You may select “All”, “Some”, or “None.” The list of individual MVPs will appear if “Some” is selected. Please note, the list of MVPs reflects what is finalized in the CY 2022 Physician Fee Schedule Final Rule for the QPP. MVP availability is subject to change, based upon what is finalized for inclusion and removal in the CY 2023 Physician Fee Schedule Final Rule for the QPP.
You may select each MVP you will support from the list of all currently available MVPs. Each MVP selected will show after one is selected. Select “All”, instead of selecting each individual MVP from the drop down if you will support all the MVPs. Please note, QCDRs are expected to support all QCDR measures within an MVP and should obtain permission to borrow the QCDR measure(s) from the applicable QCDR measure owner(s).
You may remove a selected MVP by clicking the “X” next to each selected MVP or click “Clear all” to remove all the selected MVPs. In addition, you may use the “Search” function to look-up specific MVPs.
To edit your individual MVPs, refer to the “Modifying a Self-Nomination” section of this User Guide.
Data Validation
On this tab, you will be asked to specify the methodology your prospective QCDR, or Qualified Registry will use for validating the data being submitted to CMS for traditional MIPS and MVP reporting. All fields are required to be populated. The data validation strategy must be populated into the pre-formulated fields in the Self-Nomination form. CMS will only review the information populated in the pre-formulated fields for purposes of satisfying the data validation strategy. Please note, execution of your data validation strategy must be completed prior to the 2023 data submission period for all performance categories supported, so errors can be corrected prior to data submission for the 2023 MIPS performance period. All data that is eligible to be submitted for purposes of the MIPS program (traditional MIPS, MVP reporting) should be subject to validation, regardless of whether they are MIPS eligible clinicians, groups, virtual groups, subgroup, or APM Entities, inclusive of voluntary and opt-in participants. Please note, voluntary, opt-ins, and virtual groups are currently excluded from MVP reporting.

Populating Data Validation
Describe how your prospective QCDR or Qualified Registry will verify MIPS eligibility for each clinician, group, virtual group, subgroup, or APM Entity, inclusive of voluntary and/or opt-in participant (i.e., verify all participation categories meet the eligibility thresholds). Please note, MIPS eligible clinician, group, virtual group, subgroup, or APM Entity, inclusive of voluntary and opt-in participants self-attestation does not suffice.

Indicate the method your prospective QCDR or Qualified Registry will use to verify the accuracy of TINs and/or National Provider Identifiers (NPIs) intended for submission (i.e., CMS National Plan and Provider Enumeration System (NPPES), CMS claims, and/or tax documentation). Please note, each MIPS eligible clinician, group, virtual group, subgroup, or APM Entity, inclusive of voluntary and opt-in participants self-attestation does not suffice.

Describe the method your prospective QCDR or Qualified Registry will use to accurately calculate reporting and performance rates (i.e., formulas included in the quality measure specifications).
Describe the system your prospective QCDR or Qualified Registry will use to verify that only 2023 versions of MIPS quality measures, QCDR measures, MVPs, improvement activities, and Promoting Interoperability measures are reported for MIPS submission.

Describe the process used for completion of the data validation audit of a subset of data prior to the submission to CMS. Periodic examinations may be completed to compare patient record data with submitted data and/or ensure the MIPS measures were accurately reported based on the appropriate measure specifications (that is, accuracy of numerator, denominator, and exclusion criteria). The QCDR/Qualified Registry must provide their sampling methodology that would be used to conduct the audits. The QCDR or Qualified Registry, at a minimum must meet the following sampling methodology to meet participation requirements: Sample 3% of the TIN-NPIs submitted to CMS, with a minimum of 10 TIN-NPIs or a maximum sample of 50 TIN-NPIs. At least 25% of the TIN-NPI’s patients (with a minimum sample of 5 patients or a maximum sample of 50 patients) should be reviewed for all measures applicable to the patient. Please note, the data validation audit and targeted audit are not the same audit. See further details in targeted audit section.
Describe the process used for completion of a targeted audit if data validation inaccuracies are identified during the data validation audit. Please note, targeted audit and data validation audit are not the same type of audit. The targeted audit should include a description of the root cause analysis, how the error was corrected, and the percentage of your total MIPS eligible clinicians, groups, virtual groups, subgroups, or APM Entities, inclusive of voluntary and opt-in participants impacted by the data error. Please note, the sample used for auditing in the targeted audit must not include data used for the data validation audit in which the deficiency or data error was identified (i.e., select an additional 3% sample in addition to the MIPS eligible clinicians, groups, virtual groups, subgroups, or APM Entities, inclusive of voluntary and opt-in participants impacted by the identified error). The aspect of the audit that is considered “the detail” is the specific error you are auditing for.
Benchmarking Capabilities(QCDR ONLY)
This tab is optional and should ONLY be used by QCDRs if they are submitting QCDR Benchmarking Capabilities for CMS consideration.
Indicate whether you have benchmarking capability (QCDR only). Upload your benchmarking plan in the “file uploads” section if ‘Yes’ is selected. Please note, do NOT upload the attachment in the comment section.
QCDR Measures (QCDR ONLY)
This tab is optional and should ONLY be used by QCDRs if they are submitting QCDR measures for CMS consideration.
QCDR measures are required to be submitted using the QCDR Measures tab. This tab should ONLY be filled out by QCDRs who meet the definition of a QCDR, are self-nominating as a QCDR for 2023, and wish to self-nominate QCDR measures for CMS consideration.
Each QCDR may self-nominate a maximum of 30 QCDR measures for CMS consideration.
QCDR measures may be approved for 2 years, at CMS discretion, by attaining approval status and meeting QCDR measure considerations and requirements. Upon annual review, CMS may revoke the QCDR measure second year approval, if the QCDR measure is found to be:
Topped out.
Duplicative of a more robust measure.
Reflects outdated clinical guideline.
The QCDR measure steward is no longer in good standing or participating as a QCDR.
Existing QCDRs (in good standing) will be provided a pre-populated QCDR Measures tab which includes their 2022 MIPS performance period approved QCDR measure specifications. The pre-populated QCDR measures (for existing QCDRs in good standing only) will be added to each existing QCDR’s respective 2023 Simplified Self-Nomination form in the QPP website. Please note, pre-populated QCDR measures will need to be updated to include information for the new required fields.
In addition, any updates to previously approved QCDR measure specifications from the 2022 MIPS performance period will need to be included in the QCDR measure submission for CMS consideration. Furthermore, QCDRs in good standing may also submit new QCDR measures for CMS consideration for the 2023 MIPS performance period.
In instances where a QCDR believes a low-reported QCDR measure that did not meet benchmarking thresholds is still important and relevant to a specialist’s practice, the QCDR may develop and submit a QCDR measure participation plan for CMS consideration. This QCDR measure participation plan must include the QCDR’s detailed plans and changes to encourage MIPS eligible clinicians, groups, virtual groups, subgroups, or APM Entities, inclusive of voluntary and opt-in participants to submit data on the low-reported QCDR measure for purposes of the MIPS program.
As examples, a QCDR measure participation plan could include one or more of the following:
Development of an education and communication plan.
Update the QCDR measure’s specification with changes to encourage broader participation.
Require reporting on the QCDR measure as a condition of reporting through the QCDR.
Populating QCDR Measures
All fields denoted with an asterisk (*) are required. The QCDR Measures tab has built in validation to ensure that all required fields are completed. Please ensure that all fields are checked for grammar and typographical errors. A complete list of fields with instructions can be found at the end of this section.
Select the QCDR Measures tab under QCDR Measure Submission to begin the QCDR measure submission process:

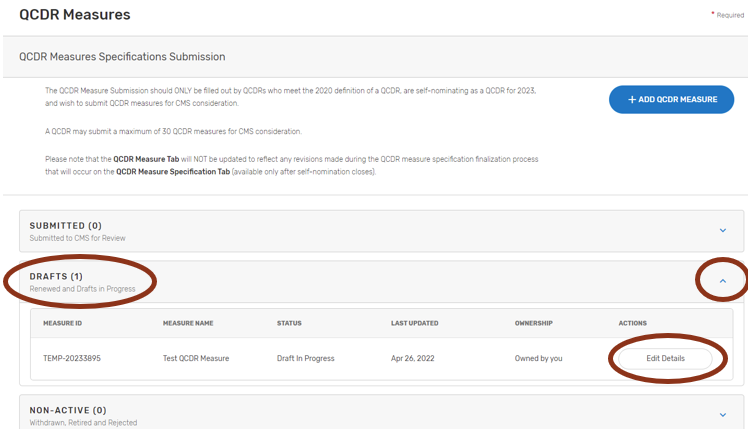
Pre-populated Existing QCDR Measures
The QCDR measures are pre-populated in the “DRAFTS” section under the QCDR Measures tab as part of the Simplified Self-Nomination form process for QCDRs in good standing from the previous MIPS performance period.
To view your pre-populated existing QCDR measures:
Click on “DRAFTS”.
Click the down arrow in the “Drafts” box, click on the “Edit Details” button of the QCDR measure to be completed.
Complete all required fields. Please note that required fields will vary depending on the QCDR measure owner status, measure adoption, and new versus existing QCDR measure status.
When all required fields are completed and ready for CMS submission, click on the “Submit to CMS” button located at the bottom of your screen. If the button is greyed out, please review and complete the missing required fields.
Self-Nominate QCDR Measures
These QCDR measures can be new or existing QCDR measures that the QCDR would like to submit for CMS consideration. This would include existing QCDR measures that did not meet the criteria to be in the pre-populated form (i.e., a QCDR measure borrowed from another QCDR, a QCDR measure that was not approved for the prior year but had previously been in the program).
To submit a QCDR measure for CMS consideration:
Click on “+Add QCDR Measure”.

Indicate the measure owner status. Select "Yes", "No", or "Co-owned by two or more QCDRs" for this field.
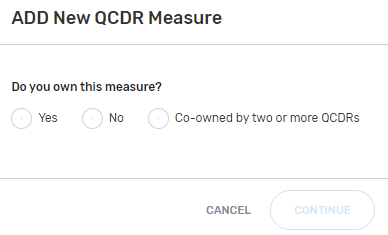
By selecting “Yes”, you are attesting that you own or co-own the QCDR measure.
By selecting "No", you are attesting that you do not own or co-own the QCDR measure and currently have written permission from the QCDR measure owner to use the QCDR measure. Please note that the QCDR who owns the QCDR measure must be an active and approved QCDR for the given self-nomination period. CMS/the MIPS QCDR/Registry Support Team (PIMMS Team) will confirm that the appropriate permission has been given by the QCDR measure owner as part of the QCDR measure review process. (During the measure specification process the QCDR measure owner will verify that all QCDRs attesting to borrowing their QCDR measure do in fact have permission. Each QCDR measure owner will be provided with names of other QCDRs that have attested to borrowing their QCDR measure.)
By selecting “Co-owned by two or more QCDRs”, you are attesting that you are a co-owner of the QCDR measure.
“Are you the primary steward?” This field needs to be answered “Yes” by the co-owner taking primary responsibility for the QCDR measure. The purpose of assigning a primary steward is to aid in determining appropriate measure specifications if there is conflicting information between QCDR measure owners.
Owned QCDR Measures
“Do you own this measure?” Select “Yes” and click continue.
Complete all required fields. Please note that required fields will vary depending on the QCDR measure owner status.
When all required fields are completed and ready for CMS submission, click on the “Submit to CMS” button located at the bottom of your screen. If the button is greyed out, please review and complete the missing required fields.
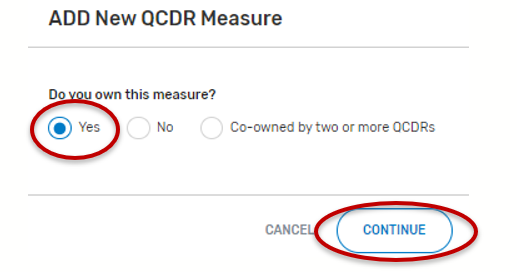
Co-Owned by two or more QCDRs
For the field, “Do you own this measure?” Select “Co-owned by two or more QCDRs”.
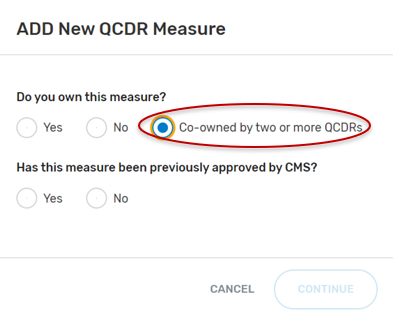
“Has this measure been previously approved by CMS?”, select “Yes” or “No” and then the “Continue” button located at the bottom of your screen.
If “No” was selected:
Populate the required fields that are present.
If “Yes” was selected:
Type in the “Assigned Measure ID”. Any QCDR measure that was approved for the previous MIPS performance period can be entered.
Click on “Find Measure”. If the correct QCDR measure information appears, select the “Continue” button located at the bottom of your screen.
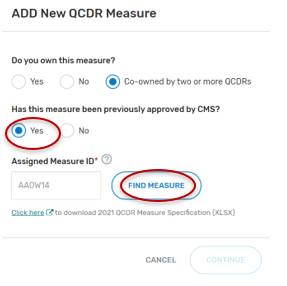
Complete all required fields. Please note that required fields will vary depending on the QCDR measure owner status.
When all required fields are completed and ready for CMS submission, click on the “Submit to CMS” button located at the bottom of your screen. If the button is greyed out, please review and complete the missing required fields.
Borrowed QCDR Measures
Please note that the QCDR who owns the QCDR measure must be an active and approved QCDR for the given self-nomination period. CMS/the MIPS QCDR/Registry Support Team (PIMMS Team) will confirm that the appropriate permission has been given by the QCDR measure owner as part of the QCDR measure review process.
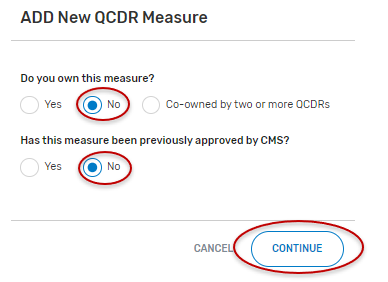
Select “No” for “Do you own this measure?”.
“Has this measure been previously approved by CMS?”, select “Yes” or “No” and the “Continue” button located at the bottom of your screen.
If “No” is selected:
Populate the required fields.
When uploading the written permission documentation from the QCDR measure owner to use the QCDR measure, please include the date of the upload in the file name.
Note: The required fields are limited to fields that a borrowing QCDR needs to complete and differ from the QCDR measure owner. The remaining QCDR measure specifications will be collected from the QCDR measure owner.
When all required fields are completed and ready for CMS submission, click on the “Submit to CMS” button located at the bottom of your screen. If the button is greyed out, please review the submission for missing required fields.
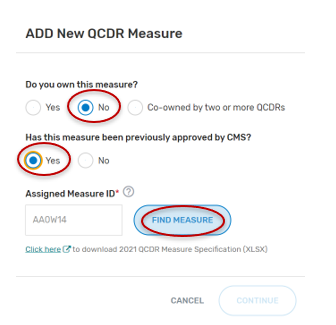
If “Yes” is selected:
Type in the “Assigned Measure ID” for the given QCDR measure. Any QCDR measure that was approved for the prior MIPS performance period can be entered.
Click on “Find Measure”. If the correct QCDR measure information appears, select “Continue” located at the bottom of your screen.
If an incorrect Assigned Measure ID is entered, you will not be able to continue until an existing Measure ID is entered (no spaces, see example below). Please ensure the QCDR measure you are attempting to locate was approved for the previous MIPS performance period.
Populate the required fields.
Note: The required fields are limited to fields that a borrowing QCDR needs to complete and differ from the QCDR measure owner. The remaining QCDR measure specifications will be collected from the QCDR measure owner.
When all required fields are completed and ready for CMS submission, click on the “Submit to CMS” button located at the bottom of your screen. If the button is greyed out, please review the submission for missing required fields.
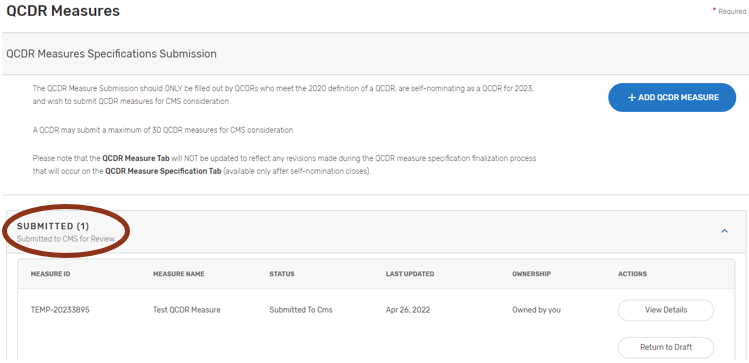
Submitting QCDR Measures for CMS Consideration
Please ensure that all QCDR measures are in the “Submitted to CMS” status before the close of the self-nomination period (8 p.m. ET on September 1). QCDR measures that are in a “Draft”, or “Non-active” status will not go through the CMS consideration process for use by the self-nominating QCDR.
Measure Specification Fields
The following section provides detailed information on each QCDR measure specification field. Please note that required fields will vary depending on the QCDR measure owner status, measure adoption, and new versus existing QCDR measure’s status.
Measure Info Section:
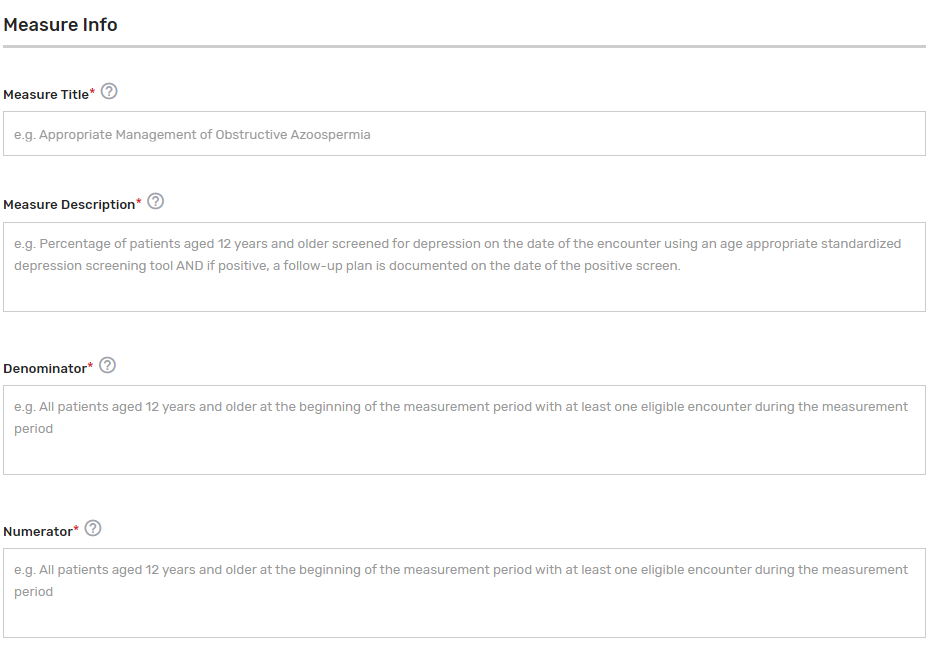
Measure Title* – Provide the measure title, which should begin with a clinical condition of focus, followed by a brief description of action. Example: Preventive Care and Screening: Screening for Depression and Follow-Up Plan.
Measure Description* – Describe the measure in full detail. Example: Percentage of patients aged 12 years and older screened for depression on the date of the encounter using an age-appropriate standardized depression screening tool and if positive, a follow-up plan is documented on the date of the positive screen.
Denominator* – Describe the eligible patient population to be counted to meet the measures’ inclusion requirements. Example: All patients aged 12 years and older at the beginning of the measurement period with at least one eligible encounter during the measurement period.
Numerator* – Provide the clinical action that meets the requirements of the measure. Example: Patients screened for depression on the date of the encounter using an age-appropriate standardized tool and, if positive, a follow-up plan is documented on the date of the positive screen.
Denominator Exclusions* – Provide a denominator exclusion that would remove the patient, procedure, or unit of measurement from the denominator. Enter “N/A” if not applicable. Example: Women who had a bilateral mastectomy or who have a history of a bilateral mastectomy or for whom there is evidence of a right and a left unilateral mastectomy.
Denominator Exceptions* – Provide the denominator exception that would allow for the exercise of clinical judgement. Applied after the numerator calculation and only if the numerator conditions are not met. Enter “N/A” if not applicable. Examples: Medical Reason(s): Patient is in an urgent or emergent situation where time is of the essence and to delay treatment would jeopardize the patient’s health status. Situations where the patient’s functional capacity or motivation to improve may impact the accuracy of results of standardized depression assessment tools. Example: Certain court appointed cases or cases of delirium.
Numerator Exclusions* – Provide a numerator exclusion that would remove the patient, procedure, or unit of measurement from the numerator, used only in ratio measures. Applied before the numerator calculation. Enter “N/A” if not applicable. Example: If the number of central line blood stream infections per 1,000 catheter days were to exclude infections with a specific bacterium, that bacterium would be listed as a numerator exclusion.

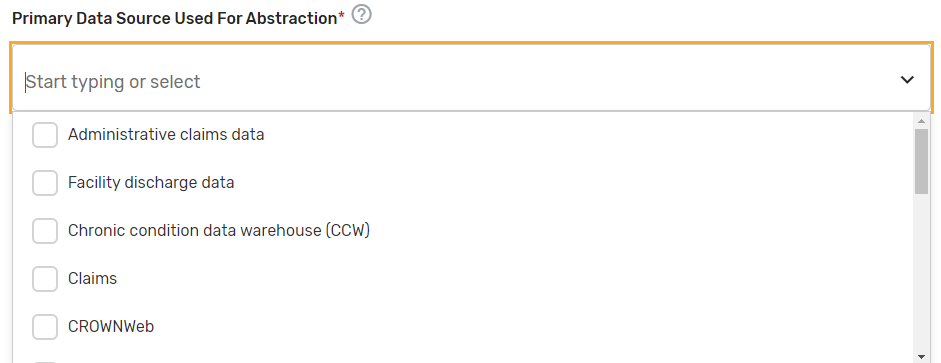
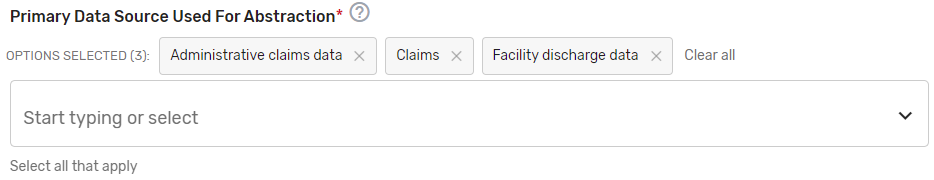
Primary Data Source Used for Abstraction* – Select all applicable primary data sources used for the QCDR measure. This may include, but is not limited to, administrative claims data, facility discharge data, chronic condition data warehouse (CCW), claims, CROWNWeb, EHR (enter relevant parts), Hybrid, Inpatient Rehabilitation Facility – Patient Assessment Instrument (IRF-PAI), Long-term Care Hospital (LTCH) CARE data set, National Healthcare Safety Network (NHSN), Outcomes and Assessment Information Set (OASIS-C1), paper medical record, Prescription Drug Event Data Elements, Patient-Reported Outcomes Measurement Information System (PROMIS), record review, Registry (enter which Registry), Survey, and/or Other (describe source).
If applicable, please enter additional information regarding the data source used – Provide additional information when “EHR”, "Registry" and/or "Other" is selected in the text box that appears as needed. You may list additional data sources used in addition to the primary data source. Example: ABC Registry.
National Quality Forum (NQF) ID (Identifier) Number (if applicable) –
Provide the assigned NQF ID number, if the submitted QCDR measure fully aligns with the NQF endorsed version of the measure. If no NQF ID number, enter “0000”. Examples: 0418, 0000.

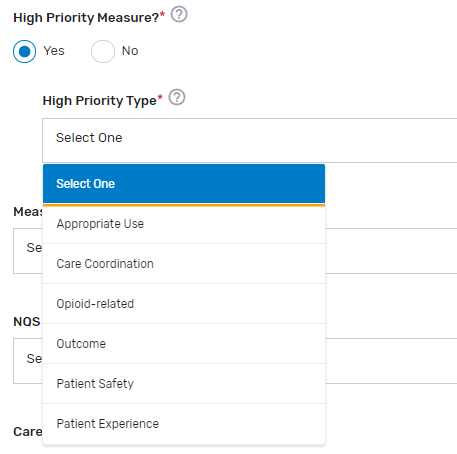
High Priority Measure?* – Enter "Yes" or "No" to indicate if the measure is a high priority measure.
High Priority Type* – If “Yes” is selected for “High Priority Measure?”, select the 1 appropriate high priority measure type from the drop-down list.
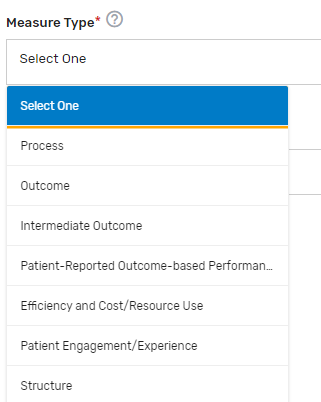
Measure Type* – Select which 1 measure type applies to the QCDR measure from the drop-down list.
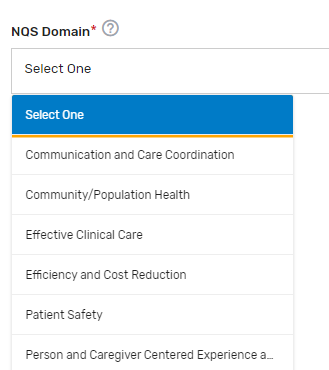
National Quality Strategy (NQS) Domain* – Select which 1 NQS domain applies to the QCDR measure from the drop-down list.
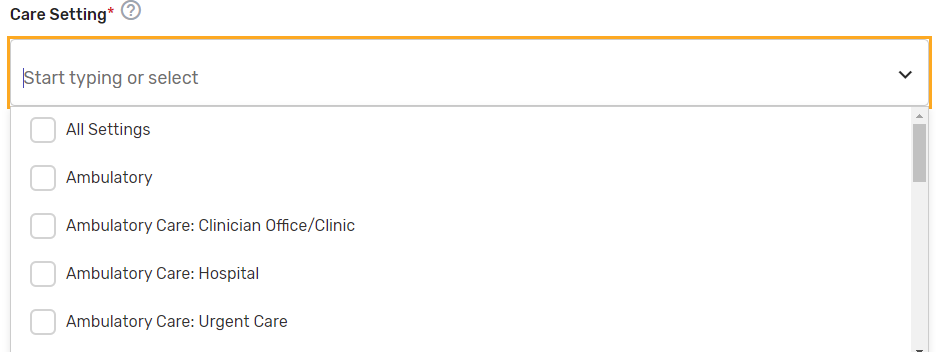
Care Setting* – Select all applicable care settings that are included within the QCDR measure. If “Other” is selected, a text box will appear for free text to be entered.
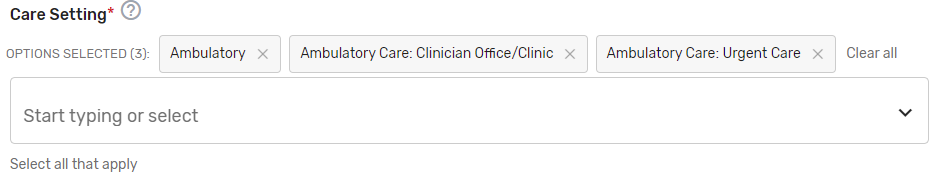
Includes Telehealth?* – Please answer “Yes” or “No” if the QCDR measure’s denominator includes services that are able to be provided via telehealth. (Please review the quality action to ensure that it is appropriate via telehealth).
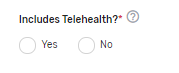
Which Meaningful Measure Area applies to this measure?* – Select 1 meaningful measure area that best applies to the measure from the drop-down list.
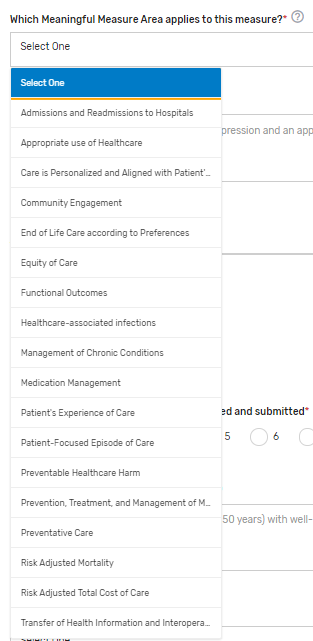
Meaningful Measure Area Rationale* – Provide a rationale for the selected “Meaningful Measure Area” for the QCDR measure. Example: This measure identifies patients with depression and an appropriate follow-up treatment plan.
Analytics Section:
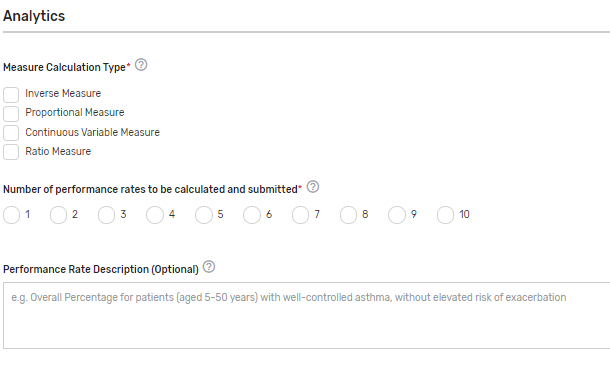
Measure Calculation Type* – Indicate the calculation type of QCDR measure. If “Continuous Variable” or Ratio” is chosen, provide a defined range of scores and the topics it describes in the text box that appears. Example: 0-250 minutes
Inverse Measure – This is a measure where a lower calculated performance rate for this type of measure would indicate better clinical care or control. The “Performance Not Met” numerator option for an inverse measure is the representation of the better clinical quality or control. Submitting that numerator option will produce a performance rate that trends closer to 0%, as quality increases.
Proportional Measure – This is a measure where the score is derived by dividing the number of cases that meet a criterion for quality (the numerator) by the number of eligible cases within a given time frame (the denominator). The numerator cases are a subset of the denominator cases. Example: Percentage of eligible women with a mammogram performed in the last year.
Continuous Variable Measure – This is a measure where a measure score in which each individual value for the measure can fall anywhere along a continuous scale and can be aggregated using a variety of methods such as the calculation of a mean or median. Example: Mean time to thrombolytics which aggregates the time in minutes from a case presenting with chest pain to the time of administration of thrombolytics.
CMS encourages QCDRs to construct the numerators to be proportional by establishing an expected benchmark based on guidelines or national performance data. Applying MIPS scoring methodology has proven to be challenging for non-proportional measures because variability in the data points makes decile creation based on a mathematical analysis very unpredictable.
Ratio Measure – Indicate if the measure is a ratio measure. This is a measure where a score that may have a value of zero or greater that is derived by dividing a count of one type of data by a count of another type of data. The key to the definition of a ratio is that the numerator is not in the denominator. Example: The number of patients with central lines who develop infection divided by the number of central line days). Rates closer to 1 represent the expected outcome.
Number of performance rates to be calculated and submitted* – Indicate the number of performance rates submitted for the measure.
Performance Rate Description(s) – Provide a brief description for each performance rate to be calculated and submitted. This is required for a QCDR measure that has more than 1 performance rate. Example: This measure will be calculated with 7 performance rates:
Rate 1: Overall Percentage for patients (aged 5-50 years) with well-controlled asthma, without elevated risk of exacerbation
Rate 2: Percentage of pediatric patients (aged 5-17 years) with well-controlled asthma, without elevated risk of exacerbation
Rate 3: Percentage of adult patients (aged 18-50 years) with well-controlled asthma, without elevated risk of exacerbation
Rate 4: Asthma well-controlled (submit the most recent specified asthma control tool result) for patients (ages 5 to 17 years) with Asthma.
Rate 5: Asthma well-controlled (submit the most recent specified asthma control tool result) for patients (ages 18 to 50 years) with Asthma.
Rate 6: Patient not at elevated risk of exacerbation for patients (ages 5 to 17 years) with Asthma
Rate 7: Patient not at elevated risk of exacerbation for patients (ages 18 to 50 years) with Asthma
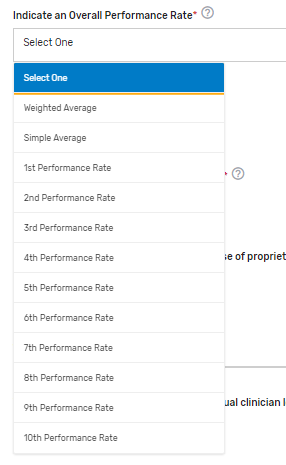
Indicate an Overall Performance Rate* – Select from the drop-down list which of the submitted rates will represent an overall performance rate for the measure or how an overall performance rate could be calculated based on the data submitted (for example, simple average of the performance rates submitted) or weighted average (sum of the numerators divided by the sum of the denominators), etc. If only 1 performance rate is being submitted, enter “1st Performance Rate”.
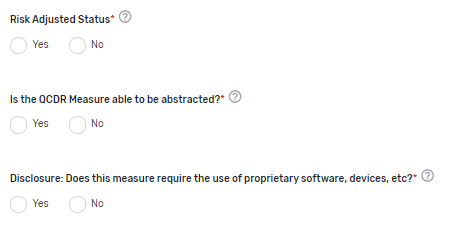
Risk-Adjusted Status* – Indicate if the measure is risk-adjusted.
If risk-adjusted, indicate which score is risk-adjusted* – Indicate the score that is risk-adjusted for the measure. If social determinants of health are built into the risk adjustment model, please specify.
Is the QCDR Measure able to be abstracted?* – Please attest the measure elements can be abstracted and are feasible. If borrowing the QCDR measure, it is expected that the ability to abstract the data according to the QCDR measure owner’s specifications is confirmed prior to self-nominating the QCDR measure. Withdrawing of the QCDR measure during an active performance period is not acceptable.
Disclosure: Does this measure require the use of proprietary software, devices, etc.?* – Indicate if the QCDR measure requires use of a proprietary software, device, survey, etc. that a clinician, group, virtual group, or subgroup would have to purchase or pay to utilize.
Testing Section:

Was the QCDR measure tested at the individual clinician level? – Select “Yes” or “No” to indicate if the QCDR measure was tested at the MIPS eligible clinician level.
Validity Testing Summary* – Provide validity testing summary, at a minimum, face validity is required by the QCDR measure owner. For face validity, list the total number of voting members in addition to the percentage that voted in favor of the measure’s face validity. If further validity testing was completed, please include the type of validity testing analysis (Correlation, Construct Validity, Gold Standard Comparison, Internal Consistency, Predictive Validity, Structural Validity, etc.), the number included within the testing sample, and any summary/interpretation of your results.
Feasibility Testing Summary (optional) – Provide feasibility testing summary, if available. For a patient reported outcome in performance measurement (PRO-PM) measure, select the data collection format(s). Please include a summary of the extent that the specified data elements are available in electronically defined fields. Examples of extent: ALL data elements are in defined fields in administrative claims, ALL data elements are in defined fields in EHRs, ALL data elements are in defined fields in electronic clinical data ([e.g., clinical registry, nursing home minimum data set, or MDS, home health Outcome and Assessment Information Set (OASIS)]), ALL data elements are in defined fields in a combination of electronic sources, SOME data elements are in defined fields in electronic sources, NO data elements are in defined fields in electronic sources, Patient/family-reported information: electronic, Patient/family-reported information: paper.
Reliability Testing Summary (optional) – Provide reliability testing summary, if available. Please include type of reliability testing such as Measure Score Reliability, Data Element Reliability, or please note if not available. Include type of reliability testing analysis (Signal to Noise, Random Split Half Correlation, IRR [Inter-rater reliability], ICC (Intraclass correlation coefficient), Test-Retest, Internal Consistency, or Other), the number included within the testing sample, and any summary/interpretation of your results.
Supporting Documentation Section:

Describe Link to Cost Measure/Improvement Activity* – Describe the link between the QCDR measure, cost measure, and an improvement activity. Please document “No Link Identified” if there is no link to a cost measure or an improvement activity. In cases where a QCDR measure does not have a clear link to a cost measure and an improvement activity, we would consider exceptions if the potential QCDR measure otherwise meets the QCDR measure requirements and considerations.
Clinical Recommendation Statement* – Provide a concise statement regarding the clinical recommendation for this QCDR measure including the current clinical guideline the measure is derived. Please list the type of evidence that was used to support the measure such as clinical guidelines, USPSTF (U.S. Preventive Services Task Force) guidelines, systematic review, empirical data, or define any other type of evidence. Specify the grade of the guidelines/systematic review, if available. Example: Adolescent Recommendation (12-18 years) “The USPSTF recommends screening for MDD in adolescents aged 12 to 18 years. Screening should be implemented with adequate systems in place to ensure accurate diagnosis, effective treatment, and appropriate follow-up (B recommendation)” (Sui, A. and USPSTF, 2016, p. 360).
Rationale for the QCDR measure* – Provide a concise statement regarding the rationale for the QCDR measure. Example: Depression is a serious medical illness associated with higher rates of chronic disease increased health care utilization, and impaired functioning (Pratt, Brody 2014). 2014 U.S. survey data indicate that 2.8 million (11.4%) adolescents aged 12 to 17 had a major depressive episode (MDE) in the past year and that 15.7 million (6.6%) adults aged 18 or older had at least one MDE in the past year, with 10.2 million adults (4.3%) having one MDE with severe impairment in the past year (Center for Behavioral Health Statistics and Quality, 2015).
Provide measure performance data (# months data collected, average performance rate, performance range, and number of clinicians or groups)* – Please provide the number of months the data was collected, average performance rate, standard deviation, performance range, and the number of eligible clinicians and/or TINs submitting the measure.
Can the measure be benchmarked against the previous performance period data? Enter "Yes" or "No" to indicate if the benchmark from prior years is able to be used for comparison. If this is an existing measure, an answer is required.
If applicable, please provide details why the previous benchmark can or cannot be used. Provide details regarding why the previous benchmark can or cannot be used in response to the changes to the existing measure. Example: The improvement addition to the numerator will make this measure an Outcome measure and therefore cannot be compared to the measure from last year.
If applicable, provide the study citation to support performance gap for the measure – Provide the study citation for the measure to support the performance gap. Citations should be the most current available or within 5 years. Example: Negative outcomes associated with depression make it crucial to screen to identify and treat depression in its early stages. While Primary Care Providers (PCPs) serve as the first line of defense in the detection of depression, studies show that PCPs fail to recognize up to 50% of depressed patients (Borner, 2010, p. 948).
If applicable, provide a Participation Plan if QCDR measure has low adoption by clinicians – If a QCDR measure fails to meet benchmarking thresholds for 2 consecutive performance periods (i.e., the data submitted is insufficient in meeting the case minimum and volume thresholds required for benchmarking), the QCDR may submit a participation plan for CMS consideration if is believed that the measure is important and relevant to a specialist’s practice.
Participation Plan requirements:
Detailed plan and methods to encourage eligible clinicians and groups to increase QCDR measure adoption.
Example: A QCDR measure participation plan could include 1 or more of the following:
Development of an education and communication plan
Update the QCDR measure’s specification with changes to encourage broader participation
Require reporting on the QCDR measure as a condition of reporting through the QCDR
Please indicate applicable specialty/specialties* – Select 1 or more applicable specialties. Multiple specialties can be selected. If “Other” is selected, please enter free text in the “Other Specialties” text box that appears.
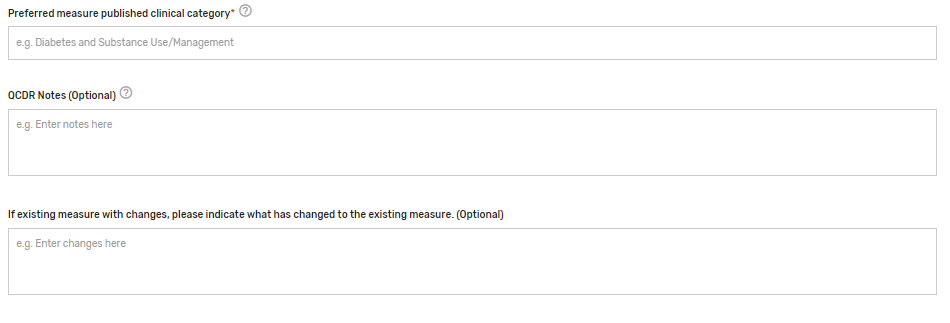
Preferred measure published clinical category* – Please provide a preferred clinical or specialty category. Please note that if a preferred measure published clinical category is not provided, one will be assigned to the measure by CMS. Example: Diabetes and Substance Use/Management.
QCDR Notes – Provide any additional notes that would assist in the review or clarification of the QCDR measure.
If existing measure with changes, please indicate what has changed to the existing measure. (Optional) – Please provide information on what changes were made to the existing measure(s).
QCDR Measure Permission Checklist
If a QCDR has been granted permission to use a QCDR measure owned by another active and approved QCDR, the permission must be obtained prior to self-nominating the borrowed QCDR measure for each MIPS performance period. It is expected that the ability to abstract the data according to the QCDR measure owner’s specifications is a condition of self-nominating the QCDR measure. Withdrawal of a QCDR measure mid-performance period is not acceptable and may lead to remedial action, up to and including termination. The following is a QCDR measure permission checklist:
Identify the QCDR measure(s) your QCDR will request for permission to use.
Contact the QCDR measure owner to request permission to use their QCDR measure(s).
The QCDR Qualified Posting and QCDR Measure Specifications located on the CMS QPP Resource Library page may be used to identify the appropriate point of contact.
Obtain written permission from the QCDR measure owner to use their QCDR measure(s).
Upload the documentation at the time of self-nomination for CMS reference. Please use the “Documentation of Approval from other QCDR” section to upload your supporting documentation.
Note: CMS does not provide agreement templates and defers to the QCDRs to the appropriate content included in such an agreement.
Submission of the Self-Nomination Form
Once the required fields of all tabs are completed, the self-nomination status will update to “Draft Complete”. Please note, you will not be able to successfully submit a Self-Nomination form unless all the required fields, marked with a red asterisk (*), of all tabs have been populated.
Once the form is filled out in its entirety and all edits are finalized, you will need to click on the “Submit For Review” button to save the entire Self-Nomination form and complete the submission process. The “Submit For Review” button is located at the bottom of the left-side of the page under the Self-Nomination form tabs, or can also be found on the self-nomination landing page.

You will receive a confirmation email with your self-nomination number. Please save the email for future reference.
It is suggested that you export a copy of your approved Self-Nomination form for your records.
Add ‘Watchers’ to monitor the status of your Self-Nomination form. This feature allows intermediaries to create a list of users who should receive an email notification when comments are added.
Modifying a Self-Nomination Form
To review or modify your Self-Nomination form, click “EDIT” on the self-nomination landing page. As a reminder, you will not be able to modify your submission after 8 p.m. ET on September 1, 2022.

If you have any questions about the 2023 QPP Self-Nomination form, please contact the QPP Service Center at 1-866-288-8292 or by email at: QPP@cms.hhs.gov (Monday through Friday from 8 a.m. to 8 p.m. ET). To receive assistance more quickly, please consider calling during non-peak hours, before 10 a.m. and after 2 p.m. ET. Customers who are hearing impaired can dial 711 to be connected to a TRS Communications Assistant.
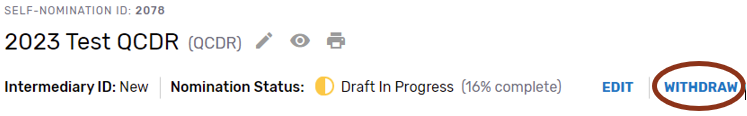
Withdrawing a Self-Nomination Form
If you wish to withdraw a Self-Nomination form that has already been submitted, click on the “Withdraw” button - withdrawing the entire Self-Nomination form from consideration to participate in MIPS as a prospective QCDR or Qualified Registry. The “Withdraw” option can be found on the Self-Nomination landing page or within the Self-Nomination form.

Comments
How to View/Add Comment
Please periodically monitor for comments posted, as there are instances where email notifications are delayed or blocked by your email.
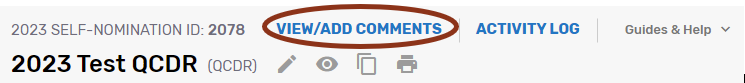
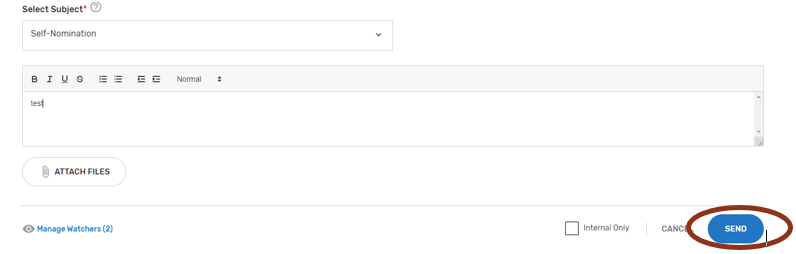
Click on ‘View/Add Comments’ to view, respond, or add a comment.
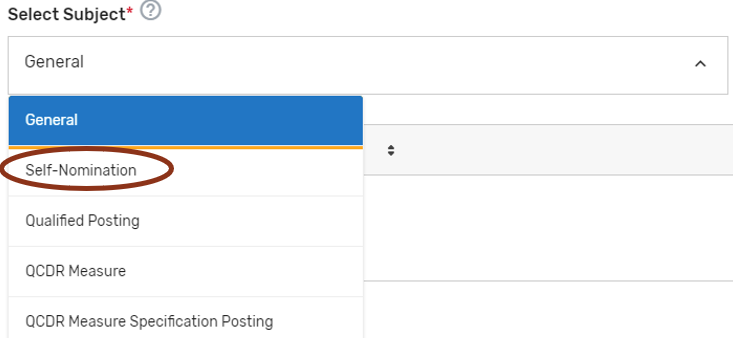
Click ‘Add Comment.’

Please make sure to indicate the correct subject in the ‘Select Subject’ dropdown that coordinates with the topic. For example, if you are submitting/responding to a comment on your Self-Nomination form choose the ‘Self-Nomination’ category from the ‘Select Subject’ dropdown.
When ready to submit your comment, click ‘Send.’
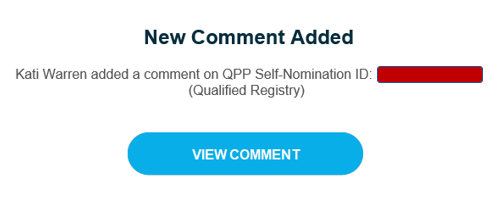
Once a comment has been submitted, all ‘Watchers’ will receive an email notification.
Resources
Help with Self-Nomination
Refer to the 2023 QCDR Fact Sheet and 2023 Qualified Registry Fact Sheet located in the QPP Resource Library page. In addition, lists with the criteria used to audit and validate data submitted in each of the MIPS performance categories will be available on the website.
For assistance with completing the Self-Nomination form, the comment box may be used to ask questions about populating form fields or submitting additional information. Refer to “Comments” section of the User Guide for additional information.
For additional assistance regarding self-nomination criteria, contact the QPP at 1-866-288-8292 or by email at: QPP@cms.hhs.gov (Monday through Friday from 8 a.m. to 8 p.m. ET). To receive assistance more quickly, please consider calling during non-peak house, before 10 a.m. and after 2 p.m. ET. Customers who are hearing impaired can dial 711 to be connected to a TRS Communications Assistant. To avoid security violations, do not include personal identifying information (PII) or protected health information (PHI), such as Social Security Number or TIN, in email inquiries to the QPP.
Help with QCDR Measure Development
Blueprint for the CMS Measures Management System - Provides a standardized system for developing and maintaining the quality measures used in CMS’s various quality initiatives and programs. The primary goal is to provide guidance to measure developers to help them produce high-caliber healthcare quality measures and documents the core set of business processes and decisions criteria when developing, implementing, and maintaining measures.
Measure Development Plan - Is a focused framework to help CMS build and improve quality measures that MIPS eligible clinicians, groups, virtual groups, APM Entities, voluntary participants, and/or opt-in participants could report under the MIPS and as participants in APM Entities (collectively known as the QPP).
QCDR Measure Development Handbook - Provides guidance and suggestions to QCDR measure developers on QCDR measure structure, analytics, and types. As well as a QCDR measure development checklist, resources for QCDR measure development and definitions used by CMS to communicate QCDR measure review decisions. The QCDR measure development handbook may be found in the Self-Nomination “Resources.”
According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is 0938-1314 (Expiration date: 01/31/2025). The time required to complete this information collection varies per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection. If you have comments concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to: CMS, 7500 Security Boulevard, Attn: PRA Reports Clearance Officer, Mail Stop C4-26-05, Baltimore, Maryland 21244-1850. ****CMS Disclosure**** Please do not send applications, claims, payments, medical records or any documents containing sensitive information to the PRA Reports Clearance Office. Please note that any correspondence not pertaining to the information collection burden approved under the associated OMB control number listed on this form will not be reviewed, forwarded, or retained. If you have questions or concerns regarding where to submit your documents, please contact QPP at qpp@cms.hhs.gov
1 Subgroup reporting is not available through traditional MIPS
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | 2022 MIPS QCDR/Qualified Registry Self-Nomination User Guide |
| Subject | Self-Nomination User Guide |
| Author | Stephenie Rudig |
| File Modified | 0000-00-00 |
| File Created | 2023-08-25 |
© 2026 OMB.report | Privacy Policy