SSA HIV Behavioral Surveillance BHBA_09302022
SSA HIV Behavioral Surveillance BHBA_09302022.docx
[NCHHSTP] National HIV Behavioral Surveillance: Brief HIV Bio-behavioral Assessment (NHBS-BHBA)
OMB: 0920-1398
National HIV Behavioral Surveillance:
Brief HIV Bio-behavioral Assessment (NHBS-BHBA)
OMB No. 0920-XXXX
Supporting Statement A
September 30, 2022
Project Officer:
Dr. Cyprian Wejnert
Team Lead, Behavioral Surveillance Team
National Center for HIV, Hepatitis, STD and TB Prevention
Centers for Disease Control and Prevention
1600 Clifton Rd, NE, MS US8-4
Atlanta, Georgia 30333
Phone: (404) 639-6055
Fax: (404) 639-8640
E-mail: dwy7@cdc.gov
National HIV Behavioral Surveillance: Brief HIV Bio-behavioral Assessment (NHBS-BHBA)
OMB No. 0920-XXXX
Table of Contents
SeCTION
1. Circumstances Making the Collection of Information Necessary
2. Purpose and Use of the Information Collection
3. Use of Improved Information Technology and Burden Reduction
4. Efforts to Identify Duplication and Use of Similar Information
5. Impact on Small Business or Other Small Entities
6. Consequences of Collecting the Information Less Frequently
7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
9. Explanation of Any Payment or Gift to Respondents
10. Protection of the Privacy and Confidentiality of Provided by Respondents
11. Institutional Review Baord (IRB) and Justification for Sensitive Questions
12. Estimates of Annualized Burden Hours and Costs
13. Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers
14. Annualized Cost to the Federal Government
15. Explanation for Program Changes or Adjustments
16. Plans for Tabulation and Publication and Project Time Schedule
17. Reason(s) Display of OMB Expiration Date is Inappropriate
18. Exceptions to Certification for Paperwork Reduction Act (PRA) Submissions
EXHIBITS
Exhibit 12.A Estimated Annualized Burden Hours
Exhibit 12.B Estimated Annualized Burden Costs
Exhibit 14.A Estimated Annualized Costs to the Government
LIST OF ATTACHMENTS
Attachment Number |
|
Document Description |
|
|
|
1 |
|
Section 301 of the Public Health Service Act |
2 |
|
60-Day FRN |
2a |
|
Public comments |
2b |
|
Memo in response to public comment |
3a |
|
Quantitative Base Eligibility Screener |
3b |
|
Quantitative Population Eligibility Screener |
3c |
|
Quantitative Core Survey |
3d |
|
Quantitative Population-Specific Questions |
3e |
|
Qualitative Eligibility Screener |
3f |
|
Model Qualitative Data Collection Guide |
4a |
|
Quantitative Base Eligibility Screener (Spanish) |
4b |
|
Quantitative Population Eligibility Screener (Spanish) |
4c |
|
Quantitative Core Survey (Spanish) |
4d |
|
Quantitative Population-Specific Questions (Spanish) |
4e |
|
Qualitative Eligibility Screener (Spanish) |
4f |
|
Model Qualitative Data Collection Guide (Spanish) |
5a |
|
Assurance of Confidentiality for HIV/AIDS Surveillance |
5b |
|
Privacy Impact Assessment |
6 |
|
Agreement to Abide by Restrictions on Release of Surveillance Data |
7 |
|
NHBS-BHBA Overview |
8a |
|
Model Quantitative Survey Consent Form |
8b |
|
Model Qualitative Professional Key Informant Interview Consent Form |
8c |
|
Model Qualitative Community Key Informant Interview Consent Form |
8d |
|
Model Qualitative Focus Group Consent Form |
9 |
|
Project Determination |
10 |
|
Sample Analysis Tables |
11 |
|
Summary of NHBS-BHBA Data Collection |
 Goal:
The
National HIV Behavioral Surveillance: Brief HIV Bio-behavioral
Assessment (NHBS-BHBA) is a supplemental surveillance project
designed to describe the HIV prevalence and behaviors related to HIV
acquisition and prevention among prioritized populations at high risk
for HIV in selected geographic areas across funded states in the
United States, such as, men who have sex with men, persons who inject
drugs, heterosexually active persons at increased risk for HIV
infection, as well as other locally
identified populations of
interest.
Goal:
The
National HIV Behavioral Surveillance: Brief HIV Bio-behavioral
Assessment (NHBS-BHBA) is a supplemental surveillance project
designed to describe the HIV prevalence and behaviors related to HIV
acquisition and prevention among prioritized populations at high risk
for HIV in selected geographic areas across funded states in the
United States, such as, men who have sex with men, persons who inject
drugs, heterosexually active persons at increased risk for HIV
infection, as well as other locally
identified populations of
interest.
Intended Use: To guide state and local prevention efforts and to monitor trends in HIV prevalence, receipt of HIV prevention services, and HIV-risk related behaviors.
Methods: Mixed methods quantitative and qualitative assessments and optional HIV testing of persons belonging to prioritized populations in selected geographic areas of interest recruited using methodologies appropriate for sampling hard-to-reach or hidden populations.
Subpopulation: Prioritized populations at risk for HIV infection in 2 U.S. states.
Analysis: Descriptive statistics and multivariable analyses to assess: 1) prevalence and awareness of HIV infection, 2) risk behaviors for HIV infection, 3) receipt of HIV prevention services.
A. Justification
Circumstances Making the Collection of Information Necessary
The Centers for Disease Control and Prevention (CDC), National Center for HIV, Viral Hepatitis, STD, and TB Prevention (NCHHSTP) requests a new 3-year approval for the National HIV Behavioral Surveillance: Brief HIV Bio-behavioral Assessment otherwise known as NHBS-BHBA, Notice of Funding Opportunity CDC-RFA-PS22-2201. NHBS-BHBA is a supplemental surveillance project designed to describe the HIV prevalence and behaviors related to HIV infection and prevention among priority populations at high risk for HIV in selected geographic areas of interest across states in the United States which lack bio-behavioral data.
Background
Historically, surveillance to describe the HIV/AIDS epidemic in the United States has primarily involved reporting of HIV and AIDS cases. CDC’S HIV surveillance system is the nation’s source for timely case surveillance information used to track the epidemic (OMB No. 0920-0573, National HIV Surveillance System). CDC funds and assists state and local health departments to collect the information. Health departments report their data to CDC so that information can be analyzed to determine who is being affected and why. Tragically, the rate of new HIV infections continues to be high: an estimated 34,800 Americans became infected with HIV in 2019 (CDC, 2021).
Because many years may pass between the time when a person is infected with HIV and the time that HIV infection is diagnosed, case surveillance for HIV infection does not reflect recent trends in the behaviors that fuel the epidemic. There remains a critical need to understand HIV related risk behaviors and the reach of prevention to groups at high risk (Gallagher et al., 2007). Supplemental surveillance systems and surveys have been used to provide additional information about behaviors related to HIV infection (e.g., OMB No. 0920-0770, National HIV Behavioral Surveillance System (NHBS); OMB No. 0920-1262, Barriers and Facilitators to Expanding the NHBS to Conduct HIV Behavioral Surveillance Among Transgender Women (NHBS-Trans)). However, existing behavioral surveillance systems are limited by geographic constraints and are not flexible to respond to emerging issues or explore other areas/populations of concern for HIV prevention.
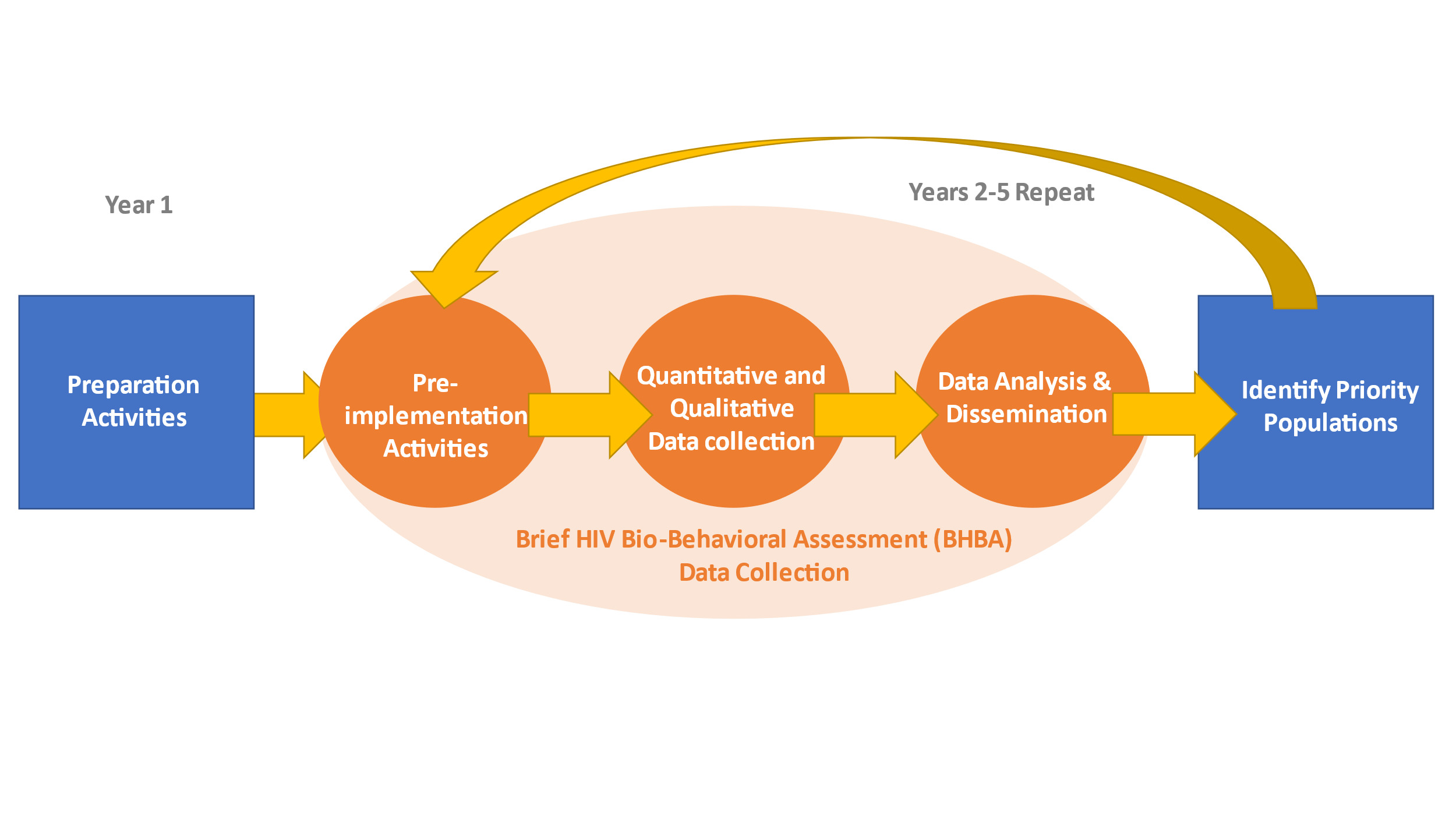
Systematic, targeted, flexible, and real-time collection and analysis of data are needed to understand HIV risk behaviors and prevention service utilization, and fill data gaps in geographic areas with limited information on populations at risk of HIV infection. In this information collection request, CDC requests OMB approval to conduct brief HIV bio-behavioral assessments (BHBAs). Through NHBS-BHBA, CDC will work with two funded states to conduct brief mixed-methods quantitative and qualitative assessments to describe the HIV prevalence and behaviors related to HIV infection and receipt of HIV prevention services among priority populations in selected geographic areas of interest across funded states where data are needed (Figure 1). Priority populations may include gay, bisexual and other men who have sex with men (MSM), persons who inject drugs (PWID), and heterosexually active persons at increased risk for HIV infection (HET), as well as other locally identified populations of interest (e.g., women who inject drugs and/or exchange sex). States will determine the priority population for each BHBA. CDC will provide technical assistance.
The ultimate surveillance goal is a system that combines information on cases, new HIV infections, and behaviors and characteristics of people at high risk for HIV infection. By meeting this goal, CDC can track the epidemic and direct prevention resources to where they are needed most. NHBS-BHBA provides a promising strategy for the collection of streamlined, relevant data that accelerate the timeframe for local public health action.
Figure 1. Overview of National HIV Behavioral Surveillance-Brief HIV Bio-behavioral Assessment (NHBS-BHBA)
Collection of HIV surveillance data is regulated by Title III – General Powers and Duties of Public Health Service, Section 301 (241.)a. Research and investigations generally (Attachment 1).
Purpose and Use of Information Collection
The primary objective of NHBS-BHBA is to conduct mixed-methods behavioral surveillance among populations at high risk for HIV in selected geographic areas of interest to assess: 1) risk behaviors and social determinants of health for HIV infection, 2) HIV testing behaviors and treatment, 3) HIV seroprevalence and incidence, and 4) exposure to, use of, and impact of HIV prevention services. Through this project, funded state health departments for NHBS-BHBA will work with local entities to collect surveillance data to understand HIV risk, especially in areas or populations with limited information.
National data from NHBS-BHBA will be useful for documenting the need for prevention resources and the reach of prevention programs targeting persons at risk of HIV infection. Data on changing patterns of utilization of prevention resources is critical to determining resource requirements for future funding cycles for prevention programs. Data from NHBS-BHBA may be used to answer questions about prevention service reach, gaps, and impact of allocated resources.
At the local level, NHBS-BHBA data will provide locally relevant data that may be used to respond to ongoing and emerging HIV hotspots, identify needs for HIV prevention and care, and evaluate, develop, or improve prevention programs directed to the populations of interest and their communities.
NHBS-BHBAs are brief mixed-methods bio-behavioral HIV assessments conducted in priority populations in selected geographic areas of interest (hereafter referred to as BHBA populations) which lack bio-behavioral data. The process will include 1) identification of geographic areas/populations at risk, 2) formative assessment for operations, 3) mixed methods data collection and HIV testing, and 4) data analysis and dissemination, and development of recommendations. At the end of each data collection year, funded states will identify two or more priority populations to conduct NHBS-BHBA data collection for the following year. This iterative process provides flexibility and is intended to ensure that BHBA data collection occurs in places and populations where data are needed.
Funded states will develop an identification process to prioritize at least two populations to conduct BHBAs for each year of data collection (i.e., years 2-5). Once a priority population is identified, funded states will work with CDC to formally define the population and develop specific criteria that will determine eligibility for participation in BHBA data collection, i.e., quantitative interviews and HIV testing, and qualitative data collection.
Project staff will conduct formative assessment for each BHBA to identify appropriate recruitment methods and develop operational procedures in the project area. Project staff will also garner the support of the local community for the project and identify questions of local interest for HIV prevention. Formative assessment will immediately precede BHBA data collection.
BHBAs will be conducted through mixed-methods quantitative and qualitative data collection. Project staff will implement recruitment strategies appropriate for reaching BHBA populations that may include venue-based time-space sampling (VBS), respondent-driven sampling (RDS), peer-to-peer recruitment, virtual recruitment, location-based methods, referrals from community partners, and other CDC-approved methods to recruit populations at high-risk for HIV infection. These methods are explained in more detail in Part B.
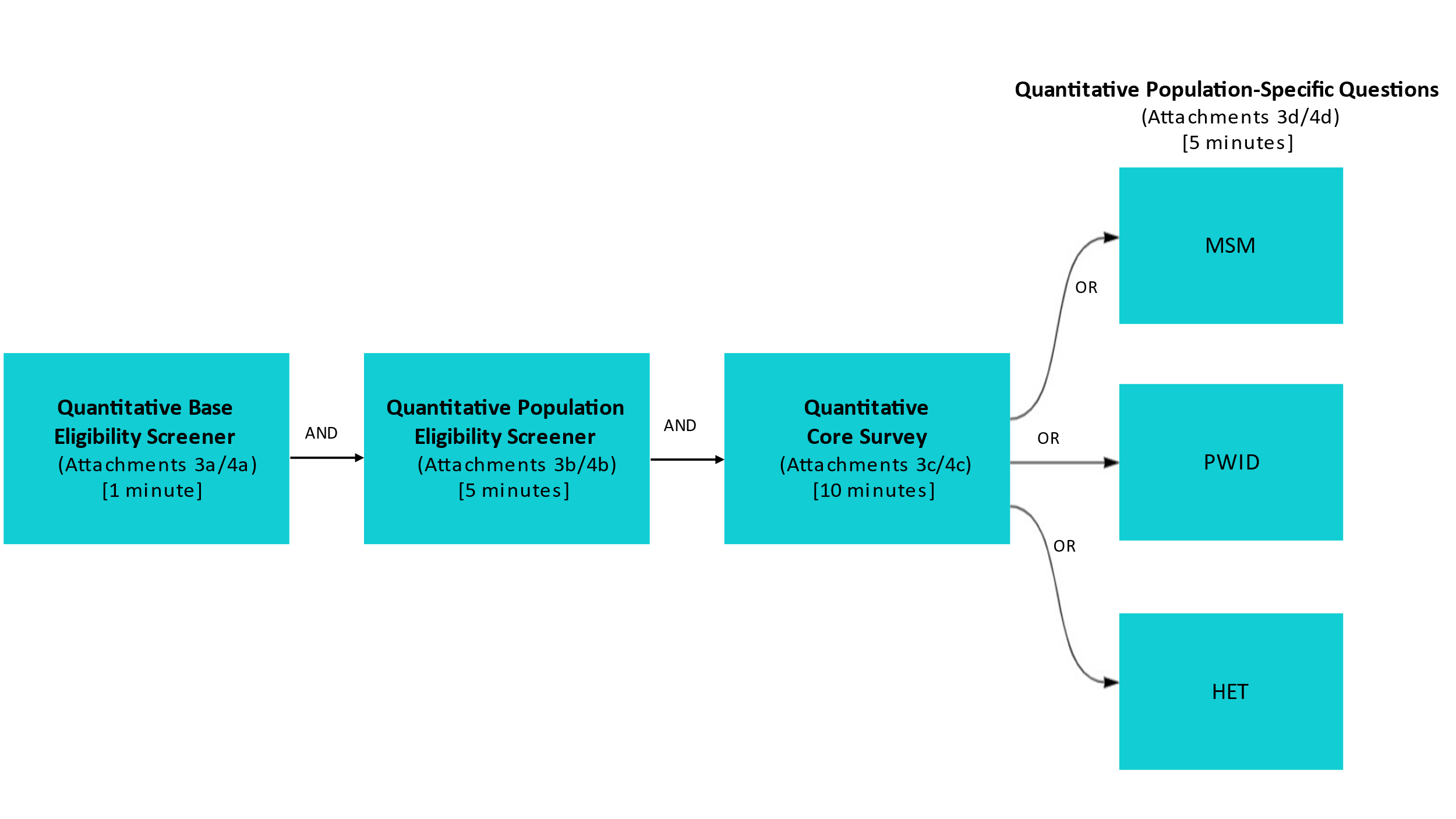
Quantitative data will be collected through brief, interviewer-administered, standardized quantitative interviews and HIV testing for 500 participants from at least two BHBAs per year of data collection (in years 2-5 of the funding). Anonymous blood-based rapid testing and supplemental testing for HIV infection will be offered to those who participate in quantitative interviews. A short screening to assess various eligibility criteria and limited demographics will be administered (Attachments 3a/3b and 4a/4b). The quantitative survey will include core questions on sexual risk behaviors, injection and non-injection drug use behaviors, testing for HIV and other sexually transmitted infections (STIs), and use of prevention and health care services (Attachments 3c/4c). Standard sets of questions tailored to specific populations will cover topics appropriate to the priority population (Attachments 3d/4d). These may include questions about sexual partners, drug treatment, and history of adverse health outcomes such as overdose, experiences with homelessness, experiences with law enforcement, and experiences with violence. To manage burden on respondents, the number of population-specific questions will be limited to 5 minutes per BHBA (Figure 2).
Figure 2. BHBA Quantitative Survey Overview
A sample schedule of NHBS-BHBA activities is provided (Figure 3). Upon request, CDC will submit annually a Change Request to OMB to reflect the priority populations for each year of NHBS-BHBA data collection. The Change Request will provide documentation of the priority populations and timeframes of data collection determined by the funded states to be most relevant to their HIV prevention and control efforts. With each Change Request, CDC will also update Attachment 11 (Summary of NHBS-BHBA Data Collection) to provide a quick-reference overview of all data collection conducted under NHBS-BHBA.
Figure 3. Sample NHBS-BHBA Data Collection for 2023-2025

Year |
BHBA |
State #1 |
State #2 |
2023 |
1 |
MSM |
MSM |
2 |
PWID |
HET |
|
2024 |
1 |
HET |
MSM |
2 |
MSM |
PWID |
|
2025 |
1 |
MSM |
HET |
2 |
PWID |
MSM |
Qualitative data will be collected through observations, key informant interviews with community members (CKI) and professionals (PKI) familiar with the BHBA population, focus groups and other activities to interpret standardized quantitative findings and inform development of recommendations for state and local public health partners. Qualitative data collection will be tailored to the population of interest and will evolve based on preceding and existing information. A short screening to assess population eligibility will be administered as needed, for key informant interviews and focus groups (Attachments 3e/4e). Qualitative interviews may include questions about healthcare access and services, sexual risk behaviors, HIV treatment and prevention, STIs, drug use, social determinants of health, stigma, discrimination, and social capital (Attachments 3f/4f). Recruitment for qualitative data collection will continue until thematic saturation is reached on the variables of interest – the point where no new information is emerging from the data. This point may occur with 20 people per homogenous group and will depend on several factors (e.g., sampling approach, intragroup or person-characteristics).
Funded states will analyze and triangulate quantitative and qualitative data to develop preliminary findings. They will identify emerging key themes and develop recommendations and share preliminary findings with advisory groups comprised of key partners from community organizations and institutions that serve populations at high-risk of HIV infection. Data will be used to develop a summary report for each BHBA population surveyed with integrated findings from quantitative and qualitative data and development of recommendations for local planning and evaluation. Funded states will collaborate with community partners to disseminate and promote use of NHBS-BHBA data.
The information collection described in this request is funded through cooperative agreements with state and local health departments (CDC surveillance activities are routinely funded through cooperative agreements with state and local health departments). The five-year funding announcement PS22-2201 was published August 2, 2021. From 2022 to 2026, 2 states are funded to collect data for NHBS-BHBA.
Use of Improved Information Technology and Burden Reduction
Data will be collected electronically to minimize burden to participants and interviewers. By entering data directly into the online REDCap computer system, the efficiency of data collection is improved as compared to using paper and then transferring that data into a computer database.
Computer-assisted personal interviews conducted by an interviewer reduce burden for the respondent because they may improve comprehension (compared with a self-administered questionnaire), and may improve response time. The computer “assists” by customizing the question wording for each respondent, allowing the interviewer to focus on explaining complex terms or definitions, giving instructions, ensuring that answers are relevant and entered accurately, and maintaining the respondent’s privacy.
CDC will conduct training and site visits to provide instructions and technical assistance on how to conduct the interviews, use the REDCap system, archive the collected data, and transfer the data. CDC will also provide training and detailed instructions on methods for conducting the interviews. CDC will require project staff providing supervision on the project to monitor interviewers regularly. CDC will convene lessons-learned meetings to identify and resolve the problems that can occur with the quantitative and qualitative instruments used for conducting interviews. Automated edit checks will be built into the computer software programs as a further quality control measure. Provision of electronic data collection software, training and technical assistance will help to reduce the burden on conducting NHBS-BHBA surveillance project.
The project data files will be transferred directly to the REDCap server and accessed by CDC. Qualitative data files will be transmitted to CDC using FileZilla, a secure file transfer protocol.
4. Efforts to Identify Duplication and Use of Similar Information
We reviewed currently funded programs on Reginfo.gov and did not identify potential areas of duplication. We are not aware of any department or agency that systematically collects or maintains mixed-methods surveillance data on HIV risk behavior among these populations at risk for HIV infection in the U.S. Although NHBS (OMB# 0920-0770, exp. 01/31/2023) collects similar quantitative data elements as are being proposed for NHBS-BHBA, NHBS currently monitors adult men who have sex with men (MSM cycle), persons who inject drugs (PWID cycle) and heterosexually active persons at increased risk for HIV (HET cycle) in urban areas and does not consistently include additional priority populations, or extend reach to rural areas and other geographically underserved communities. Similarly, the Medical Monitoring Project (OMB# 0920-0740, exp. 05/31/2024) collects similar data elements, but data collection is limited to individuals with HIV. Additionally, no other funded programs systematically combine findings from rigorous quantitative methods with qualitative data. Previous cycles of NHBS have demonstrated that the NHBS framework is well suited for behavioral surveillance among people at increased risk or overburdened by HIV infection.
5. Impact on Small Businesses or Other Small Entities
This data collection will not involve small businesses.
Consequences of Collecting the Information Less Frequently
NHBS-BHBA data collection activities are planned for 2023-2026. The overall strategy for NHBS-BHBA involves conducting at least two BHBAs per year of data collection (Attachment 7, NHBS-BHBA Overview). Because it is a surveillance system from which data are needed to monitor progress, it is expected that if successful, NHBS-BHBA will continue beyond 2026. CDC will maintain OMB approval by submitting the necessary Extension ICR or Revision ICR, as needed.
Respondents are eligible to participate once in a quantitative interview and qualitative interview for a specific BHBA in a funded state. Each person is asked if they have been interviewed for the same BHBA quantitative or qualitative interview; those who indicate that they have participated in the same interview will not be interviewed again. It is possible that a person could be recruited for participation in multiple BHBAs, as some may engage in multiple risk behaviors.
If NHBS-BHBA was not implemented, we would continue to lack a systematic way to examine HIV prevention and risk behaviors and HIV-associated health outcomes rapidly in key populations and geographic areas with limited bio-behavioral data.
There are no legal obstacles to reduce the burden.
Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
None of the special circumstances in the guidelines of 5 CFR 1320.5 applies.
Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A 60-day notice to solicit public comments was published in the Federal Register on May 13, 2022, Vol. 87, Number 93, Pages 29316-29318 (Attachment 2). One commenter submitted one comment but was overall supportive (Attachment 2a). They asked for the release of information about which locations and populations will be studied for NHBS-BHBA. However, these decisions will be determined by the funded states’ needs assessments and input from their advisory groups to identify priority populations (such as, men who have sex with men, persons who inject drugs, heterosexually active persons at increased risk for HIV infection), in specific geographic areas for each year of data collection throughout the cooperative agreement (Attachment 2b).
Explanation of any Payment or Gift to Respondents
Incentives are used in NHBS-BHBA, as the project seeks to conduct interviews with hard-to-reach and highly selective populations and to ask them highly sensitive questions about issues such as sexual behavior and substance use (Kulka, 1995). Because the quantitative interview takes up to 20 minutes to complete and qualitative interview takes 1-2 hours to complete, to increase response rates, eligible persons will be offered an incentive following participation. We anticipate that increased response rates will lead to improved representativeness of the population of interest.
Participants are given $20-$50 for completing an interview, amount and form (e.g., cash, gift cards, cash cards, bus or subway tokens) are determined locally based on local regulations, city characteristics (e.g., cost of living), and previous research experience. Participants may receive incentive payments in-person (cash, physical gift card, etc.) or electronically (Venmo, PayPal, email, text, etc.). Participants who agree to HIV testing are offered an additional incentive. Participants who give a specimen for HIV testing are given $10-$50 for participation, amount and form (e.g., cash, gift cards, cash cards, bus or subway tokens) are determined locally based on local regulations, city characteristics (e.g., cost of living), and previous research experience.
In the RDS methodology, participants also receive an incentive for successfully recruiting one or more of their peers. If RDS is used for recruiting the BHBA population, providing the incentive (the “recruiter reward”) for recruiting a peer to the assessment increases peer recruitment. Recruiter rewards are $10-$25 for each of up to five peer referrals, which is standard for RDS studies (Heckathorn, Semaan, et al., 2002; Ramirez-Valles, 2005; Wang, 2005). As for the interview and testing, amount and form (e.g., cash, gift cards, cash cards, bus or subway tokens) are determined locally based on local regulations, city characteristics (e.g., cost of living), and previous research experience.
The need for and amount of the incentive is based, in part, on the fact that other, similar research projects that ask HIV risk behavior questions in the participating areas offer similar incentives. Thus, NHBS-BHBA would be competing with local researchers who do offer incentives; without incentives, it is likely that participation in NHBS-BHBA would be reduced (McKnight, 2006; Stueve, 2001; Valleroy, 2000). Incentives have been used in other complementary CDC data collection efforts such as for the Medical Monitoring Project (OMB 0920-0740, exp. 5/31/2024), described in section 4 above. These incentives were used to help increase participation rates; participants were offered approximately $50. Incentives have been shown to increase response rates, which in turn improves the validity and reliability of the data (Abreu and Winters, 1999; Shettle and Mooney, 1999; Whiteman et al., 2003). A meta-analysis (Church, 1993) of survey methodologies found that studies using monetary incentives yielded an average increase in response rates of 19.1 percentage points, representing a 65% average increase in response. Incentives – particularly, the dual-incentive structure in which participants who agree to recruit others are given a small incentive for recruiting their peers to participate - are an important aspect of respondent-driven sampling (Heckathorn, 1997). The incentive increases the likelihood that a participant will identify a member of his or her network that would be eligible for the study, thereby improving response rates and increasing the overall proportion of eligible participants.
Protection of the Privacy and Confidentiality of Information Provided by Respondents
The CDC Privacy Officer has assessed this package for applicability of 5 U.S.C. § 552a and determined that the Privacy Act does apply to the overall information collection. This activity is covered under the Privacy Act System of Records Notice (SORN) #09-20-0136, “Epidemiologic Studies and Surveillance of Disease Problems. HHS/CDC”, which enables the Centers for Disease Control and Prevention (CDC) officials to collect information to better understand disease patterns in the United States, develop programs for prevention and control of health problems, and communicate new knowledge to the health community.
NHBS-BHBA data will be anonymous. Data collected through NHBS-BHBA, both locally and at CDC, are stored and accessed by a survey identification number. Other data collected, while sensitive, are not personally identifying; these assessment questions are described in Section 11.
In addition to limiting the amount of personally identifying information (PII) collected, this submission is covered by an Assurance of Confidentiality for HIV/AIDS surveillance data (Attachment 5a). The Assurance provides the highest level of legal confidentiality protections to the individual persons who are the subject of this data collection, and to the individuals and organizations responsible for data collection. The terms of the Assurance of Confidentiality reflect the collective experience of CDC, health departments, and the Council of State and Territorial Epidemiologists with respect to the collection, electronic transmission, and dissemination of HIV/AIDS surveillance data. The Assurance includes established policies and procedures governing all aspects of data collection and de-identification, physical security for paper forms and records, electronic data storage and transmission, and the release of aggregate data in forms that cannot be linked back to individual participants. The protections afforded by the Assurance of Confidentiality last forever and endure even after the respondent’s death.
For participants’ convenience or benefit, participants may have the option to provide contact information to BHBA project staff on a voluntary basis. Examples of participants providing contact information for convenience include but are not limited to: providing a phone number for phone text reminders of interview appointments; providing payment information (e.g., Venmo, PayPal, etc. name) so incentives can be provided electronically; providing an email address to facilitate videoconference interviews; or providing an address to receive self-collection or self-testing kits via mail. Examples of participants providing contact information for participant benefit include but are not limited to: providing telephone contact information so that project staff can call participants when their HIV (or additional testing offered) test results are ready; providing contact information to help participants with linkage to HIV care or other services (e.g., PrEP, housing, legal, substance use disorder treatment) they may need. Provision of contact information will be optional. In all cases, participants also will be provided information and instructions for how to participate fully without providing contact information (e.g., participants can participate in-person or call the project (rather than be called by the project) for interview, linkage to services, or test results. In all cases, participant contact information will not be linked or linkable to the participant’s responses to assessment questions in the quantitative or qualitative interviews. Contact information will be stored and secured locally and never shared with CDC. Contact information will be destroyed by the end of the data collection.
Privacy Impact Assessment
The data collection was assessed for privacy impact (Attachment 5b).
The Assurance of Confidentiality is enforced with appropriate training and contractual agreements, which clarify the responsibilities of all participants in HIV/AIDS surveillance activities who have access to directly identifiable data or to data that are potentially identifiable through indirect means. State and local health department personnel who conduct HIV/AIDS surveillance are subject to the confidentiality obligations described in the Data Security and Confidentiality Guidelines for HIV, Viral Hepatitis, Sexually Transmitted Disease, and Tuberculosis Programs: Standards to Facilitate Sharing and Use of Surveillance Data for Public Health Action. Centers for Disease Control and Prevention; 2011 available at: http://www.cdc.gov/nchhstp/programintegration/docs/PCSIDataSecurityGuidelines.pdf and are required to undergo security and confidentiality training.
NHBS-BHBA interviewers and data managers will undergo the same security and confidentiality training as required for health department staff. CDC’s Procurement and Grants Office will require the inclusion of 308(d) clauses in any HIV/AIDS support services work done by contractors (e.g., data analysis, computer programming, LAN support). All CDC permanent employees and their contractors will be required to attend annual confidentiality training to sign a Nondisclosure Agreement (Attachment 6), to attach this and to update their confidentiality agreements on an annual basis. Contractors must sign a “Contractor’s Pledge of Confidentiality.” Access to HIV/AIDS surveillance data maintained at CDC is restricted to authorized personnel who have signed the “Agreement to Abide by Restrictions on Release of Data.” CDC-funded cooperative agreements with state and local health departments reference the Assurance of Confidentiality as a condition of award. Any NHBS data maintained at CDC that are released to persons other than project staff will not include full date of birth or other PII.
The informed consent process for respondents may be fulfilled by obtaining oral consent. All funded states must obtain oral consent from respondents and document it in data collection forms on the portable computer. Example model consent documents for quantitative and qualitative interviews are included as Attachment 8a-d. Consent must be obtained for the quantitative interview and HIV testing separately. Participants may elect to complete the quantitative interview and not be tested; however, they may not be tested without completing the quantitative interview (those persons who only want an HIV test may be given information on where to seek an HIV test elsewhere). Respondents will be informed that data collected from them for NHBS-BHBA will be kept private and secure and that the data will be reported in aggregate format.
Institutional Review Board (IRB) and Justification for Sensitive Questions
The approved Project Determination Form (Attachment 9) indicates that because the project is a routine disease surveillance activity, the protocol will not be reviewed by CDC’s IRB. Each participating health department will be required to obtain approval for this project from their IRB as required by their local review and approval processes and federal regulations before data collection.
The collection of HIV status itself is sensitive because of stigma associated with HIV infection. In addition, the modes of transmission of HIV (through sexual contact and the sharing of HIV-contaminated needles and syringes) necessitate the collection of sensitive data regarding sexual practices and drug use. In keeping with the purpose of this project, other sensitive data are collected about specific behaviors, experiences or conditions that have been shown to be associated with HIV infection. For NHBS-BHBA, this includes the collection of STD and HIV diagnosis and testing, hepatitis diagnosis, history of homelessness and incarceration in the past 12 months, overdose, violence, and income. Questions about race and ethnicity will be asked using OMB’s two question format. These questions will be used to report on racial and ethnic disparities that have been well documented in other research on HIV risk and risk behaviors.
Although the information requested from participants is highly sensitive, the purposes of NHBS-BHBA cannot be accomplished without their collection. Collection of the data is used to understand barriers to engaging in protective behaviors and to using HIV prevention services, and to other services that improve the health of people at risk for HIV. These data are also used to enhance HIV prevention programs designed to reduce high-risk behaviors in persons most likely to acquire or transmit HIV.
The context in which questions are asked helps to overcome their potential sensitivity. There are several steps taken in NHBS-BHBA to minimize sensitivity and reiterate to the respondent the legitimate need for the information:
Nearly all questions allow for responses of “don’t know” or “refuse to answer.”
Consent scripts make it clear that the project is sponsored by CDC and the local health department, and that the information will be put to important uses.
Local phone numbers are provided if the respondent has questions about the survey.
The questionnaire is carefully organized to lead smoothly from one topic to another. Transitions are made clear to respondents and the need for the information explained. Assurances about the privacy and confidentiality of the data are reiterated.
If at any point during a quantitative interview or qualitative interview respondents feel uncomfortable, they may skip any questions or stop altogether.
All interviews will be conducted by trained project staff in a private location during established operating hours at local field site locations or remotely. Remote interviews will not proceed if the participant’s privacy cannot be ensured. Interviewers will be trained to administer the consent script and all interview questions by reading each item verbatim, thus ensuring that all respondents receive the same information for the consent and each question. No interviews will be conducted without the verbal consent of the respondent.
Social security numbers will not be collected from respondents.
No data will be collected from agencies regarding their policies, performance data or other practices.
Estimates of Annualized Burden Hours and Costs
NHBS-BHBA will involve conducting at least two BHBAs per year of data collection. The number of participants is expected to vary per BHBA. The annualized estimates of respondent burden for each data collection form provided below represent averages across the three years.
For quantitative data collection, base and population eligibility will be determined by assessing the respondent’s age, previous participation, county of residence, sex at birth, gender, race/ethnicity, history of homelessness, income, and history of sexual behavior or drug injection (Attachments 3a/3b and 4a/4b). Approximately 1,338 individuals will complete the base eligibility screener annually, and 1,204 individuals will complete the population eligibility screener annually. We estimate that it will take one minute to complete the base eligibility screener, and five minutes to complete the population eligibility screener. We anticipate that, on average, 338 of the respondents (25.3%) will be either not interested in a quantitative interview or will be ineligible after completing the base and population eligibility screeners, yielding a total of 1,000 eligible respondents over a 12-month period (Attachments 3c/3d) and 4c/4d). We estimate that it will take 10 minutes for a respondent to complete the quantitative core survey, and 5 minutes for completing the population-specific survey (averaged across the population-specific questions). The time for completion of population-specific questions will vary because the different assessment forms focus on different risk behaviors. Because HIV testing is a clinical procedure, it is not included in the burden estimates.
For qualitative data collection, a short screening to assess population eligibility will be administered as needed for key informant interviews and focus groups (Attachments 3e/4e). Approximately 96 individuals will complete the eligibility screener annually. We estimate that it will take 1 minute to complete the qualitative eligibility screener. We anticipate that, on average 16 of the respondents (16.7%) will be either not interested in completing a qualitative interview or will be ineligible after completing the qualitative eligibility screener, yielding a total of 80 eligible respondents over a 12-month period (Attachments 3f/4f). We estimate that it will take 1.5 hours for a respondent to complete the qualitative interview. The time for completion of qualitative interviews will vary depending on modality (focus group, individual interview), target audience (PKI, CKI), and topics of local interest.
The estimates in Exhibit 12.A cover the time that each respondent will spend communicating with the project staff and answering interview questions.
Exhibit 12.A: Estimate of Annualized Burden Hours
Respondent |
Form |
No. of Respondents |
No. of Responses per Respondent |
Average Burden per Response (hours) |
Total Burden(in hours) |
Persons Screened |
Quantitative Base Eligibility Screener (att 3a/4a)
|
1,338
|
1 |
1/60 |
22
|
Persons Screened |
Quantitative Population Eligibility Screener (att 3b/4b)
|
1,204 |
1 |
5/60 |
100 |
Eligible Participants
|
Quantitative Core Survey (att 3c/4c) |
1,000
|
1
|
10/60
|
167
|
Eligible Participants
|
Quantitative Population-specific Questions (att 3d/4d)
|
1,000
|
1 |
5/60 |
83 |
Persons Screened
|
Qualitative Eligibility Screener (att 3e/4e)
|
96 |
1 |
1/60 |
2 |
Eligible Participant |
Qualitative interviews (att 3f/4f)
|
80
|
1 |
1.5 |
120 |
Total Annualized Burden |
|
|
|
|
494 |
B. Estimated Annualized Cost to Respondents
Note: The hourly rate was determined by using information obtained from the US Department of Labor, Bureau of Labor Statistics:
http://www.bls.gov/cps/cpsaat39.htm
Exhibit 12.B: Annualized Cost to Respondents
Type of Respondent |
No. of Respondents |
No. of Responses per Respondent |
Total Burden Hours |
Hourly wage rate |
Total Respondent Cost |
Persons Screened, Quantitative Base Eligibility (Att 3a/4a) |
1,338 |
1 |
22 |
$24.60 |
$541.20 |
Persons Screened, Quantitative Population Eligibility (Att 3b/4b) |
1,204 |
1 |
100 |
$24.60 |
$2,460 |
Eligible Participants, Quantitative Core Survey (Att 3c/4c) |
1,000 |
1 |
167 |
$24.60 |
$4,108.20 |
Eligible Participants, Quantitative Population-specific Questions (Att 3d/4d) |
1,000 |
1 |
83 |
$24.60 |
$2,041.80
|
Persons Screened, Qualitative Eligibility (Att 3e/4e) |
96 |
1 |
2 |
$24.60 |
$49.20 |
Eligible Participants, Qualitative interviews (Att 3f/4f) |
80 |
1 |
120 |
$24.60 |
$2,952.00 |
Total Annualized Cost |
|
|
|
|
$12,152.40 |
Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
There are no other costs to respondents associated with this proposed collection of information.
Annualized Cost to the Federal Government
The annualized cost to the government is $1,239,795. The cost of this project for the three years is estimated to be $3,719,385. The annualized cost is summarized in Exhibit 14.A.
Exhibit 14.A. NHBS-BHBA Annualized Cost to the Federal Government
Expense Type |
Expense Explanation |
Annual Costs (dollars) |
Direct Costs to the Federal Government |
NHBS – Personnel |
$206,795 |
Epidemiologist-14 1 @ 20% $25,108 |
||
|
||
Epidemiologist-13 2 @ 50% $53,117
|
||
Epidemiologist-13 2 @ 25% $26,559 |
||
|
||
|
||
Epidemiologist-12 1 @ 25% $22,335 |
||
|
||
|
Cooperative agreement funds to project areas |
$900,000 |
Contractor and Other Expenses |
|
|
|
Contracted Data Analyst (1 @ 50%) |
$40,000 |
|
ORISE Fellow |
$80,000 |
|
Travel (4 trips @ $1,500) |
$6,000 |
|
Meetings and Trainings |
$1,000 |
|
Spanish language translation |
$3,000 |
|
Analytic software |
$2,000 |
|
Printing |
$1,000 |
|
TOTAL COST TO THE GOVERNMENT |
$1,239,795 |
*Salary estimates were obtained from the US Office of Personnel Management salary scale at https://www.opm.gov/policy-data-oversight/pay-leave/salaries-wages/salary-tables/pdf/2022/ATL.pdf
The personnel related to the NHBS-BHBA data collection include project officers (epidemiologists) at the GS-12, 13, and 14 levels, a data analyst, and a fellow. Travel is related to providing technical assistance and conducting site visits. Examples of meetings that will be held include field operations training and the local principal investigators’ meeting that will be held in government space at no cost.
The information collection described in this request will be funded through cooperative agreements with state and local health departments starting in fiscal year 2022. CDC surveillance activities are routinely funded through cooperative agreements with state and local health departments.
Explanation for Program Changes or Adjustments
This is a new data/information collection.
Plans for Tabulation and Publication and Project Time Schedule
Data will be collected from at least two priority populations per funded state during each year of data collection (Attachment 7); clearance is requested for 3 years. The following is a brief overview of the estimated NHBS-BHBA timeline for the first year of data collection in a funded state; subsequent data collection years are expected to follow a similar time schedule.
Activities |
Estimated Time Schedule (Based on Expected OMB Approval: December 2022) |
Interviewer Training |
4 months after OMB approval |
Begin Data Collection |
5 months after OMB approval |
End Data Collection |
11 months after OMB approval |
Local Data Analysis and Dissemination |
12 months after OMB approval |
Analysis of NHBA-BHBA data |
18 months after OMB approval |
Publication of NHBS-BHBA data |
24 months after OMB approval |
Data from NHBS-BHBA will inform prevention programs services and increase existing knowledge in the behaviors that lead to acquisition of HIV infection. See Attachment 10 for sample analysis tables.
Each participating health department has responsibility for the release of local data. CDC has primary responsibility for the release of data aggregated from all funded states. These data are distributed to the participating agencies, researchers, policy makers and other interested parties through presentations at local, national and international conferences, publications in peer reviewed journals, and presentations at different forums such as continuing medical education courses and seminars. Funded states will create a dissemination plan for each BHBA, including at least one data product or report and at least one presentation to community partners. Funded states will have principal responsibility for analyzing their local quantitative and qualitative data and developing tailored recommendations. NHBS-BHBA project areas and CDC may collaborate on articles and reports when appropriate. Funded states will contribute to national reporting of data collection outcomes such as surveillance reports and other publications by reviewing analysis notifications, concept proposals, table shells and manuscript drafts within specified timelines and participating in discussions during monthly conference calls and annual meetings. CDC may disseminate reports, e.g., CDC HIV surveillance Special reports and other CDC reports, the Morbidity and Mortality Weekly report (MMWR), and peer-reviewed journals. CDC may also present results at national conferences and meetings.
Reason(s) Display of OMB Expiration Date is Inappropriate
The display of the OMB expiration date is not inappropriate.
Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification.
References
Abreu, D. A., & Winters, F. (1999). Using monetary incentives to reduce attrition in the survey of income and program participation. Proceedings of the Survey Research Methods Section of the American Statistical Association.
Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2015–2019. HIV Surveillance Supplemental Report 2021;26(No. 1). https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published May 2021. Accessed January 5, 2022.
Church, A. H. Estimating the Effect of Incentives on Mail Survey Response Rates: A Meta-Analysis. Public Opinion Quarterly 1993; 57(1): 62-79.
Heckathorn D. Respondent-driven sampling: a new approach to the study of hidden populations. Social Problems 1997; 44(2):174-199.
Heckathorn D, Semaan S, Broadhead R, James Hughes. Extensions of respondent-driven sampling: a new approach to the study of injeciton drug users aged 18-25. AIDS and Behavior 2002; 6(1):55-67.
Kulka R. The use of incentives to survey "hard to reach" respondents:a brief review of empirical research and current research practice. Seminar on New Directions in Statistical Methodology, 1995 #23, 256-289. 1995. FCSM Statistical Policy Working Papers. Ref Type: Report
McKnight C, Des Jarlais D, Bramson H et al. Respondent-driven sampling in a study of drug users in New York City: Notes from the field. Journal of Urban Health 2006; 83(7):i54-i59.
Ramirez-Valles J, Heckathorn D, Vazquez R, Diaz RM, Carlson R. From networks to populations: The development and application of respondent-driven sampling among IDUs and Latino gay men. AIDS and Behavior 2005; 9(4):387-402.
Shettle, C., & Mooney, G. (1999). Monetary incentives in U.S. government surveys. Journal of Official Statistics, 15, 231–250.
Stueve A, O'Donnell L, Duran R, San Doval A, Blome J. Time-space sampling in minority communities: Results with young Latino men who have sex with men. American Journal of Public Health 2001;91(6):922-926.
Valleroy L, Mackellar D, Karon J et al. HIV prevalence and associated risks in young men who have sex with men. JAMA 2000; 284(2):198-204.
Wang J, Carlson R, Falck R, Siegal H, Rahman A, Li L. Respondent-driven sampling to recruit MDMA users: a methodological assessment. Drug and Alcohol Dependence 2005; 78(5):147-157.
Whiteman, MK, P Langenberg, K Kjerulff, et al. A randomized trial or incentives to improve response rates to a mailed women’s health questionnaire. Journal of Womens Health 2003; 12: 821-828.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | ziy6 |
| File Modified | 0000-00-00 |
| File Created | 2023-08-28 |
© 2026 OMB.report | Privacy Policy