Regulatory Analysis for Part 26 Fitness for Duty Drug Testing Requirements Final Rule
Reg Analysis Part 26 Fitness for Duty Drug Testing Requirements Final Rule.docx
Information collections contained in 10 CFR Part 26, Fitness for Duty Drug Testing Requirements Final Rule
Regulatory Analysis for Part 26 Fitness for Duty Drug Testing Requirements Final Rule
OMB: 3150-0252
Regulatory Analysis for the
10 CFR Part 26 Fitness for Duty Drug Testing Requirements Final Rule
[Docket ID NRC-2009-0225]

U.S. Nuclear Regulatory Commission
Office of Nuclear Material Safety and Safeguards
Division of Rulemaking, Environmental, and Financial Support
[ENTER DATE WHEN READY TO ISSUE]

[Page Intentionally Blank]
Abbreviations and Acronyms iii
1.2 Statement of the Problem and U.S. Nuclear Regulatory Commission Objectives for the Rulemaking 3
2. Identification and Preliminary Analysis of Alternative Approaches 6
2.1 Alternative 1: Take No Action 6
2.2 Alternative 2: Amend 10 CFR Part 26 7
2.3 Alternative 3: Address Issues Without Rulemaking 8
4. Evaluation of Benefits and Costs 13
4.1 Identification of Affected Attributes 13
4.2.1 Baseline for Analysis 19
4.2.2 Affected Entities (Sites and Fitness for Duty Programs) 19
4.2.3 Cost and Benefit Calculations 20
4.2.4 Incremental Requirements in the Final Rule 24
5.1 Benefits and Costs of the Final Rule 34
5.1.1 One-Time Policy, Procedure, and Training Costs 38
5.1.4 Expanded Initial and Confirmatory Drug Testing Panels to Include Ecstasy 41
5.1.5 Expand the Initial and Confirmatory Drug Testing Panels to Include Four Opioids 42
5.1.8 Alternative Specimen (Oral Fluid) Drug Testing 45
5.1.9 Workplace Free of Drugs and the Effects of Such Substances 45
5.1.9 Security Vulnerability 47
5.1.10 Improve Subversion Detection 48
5.2.1 Uncertainty Analysis Results 52
5.2.2 Summary of Uncertainty Analysis 59
5.4 Results for the Committee to Review Generic Requirements 61
Appendix A: Site-Specific Fitness for Duty Program Performance Data (Calendar Years 2009–2019)
Appendix B: General Inputs
Appendix C: Assumptions and Results by Regulatory Initiative
Appendix D: Costs of Subsequent Actions
Appendix E: Averted Costs
Appendix F: Alternative Specimen (Oral Fluid) Drug Testing
Figure 5-1 Industry Implementation Costs 53
Figure 5-2 Industry Operation Costs (7-Percent Discount Rate) 53
Figure 5-3 Industry Operation Costs (3-Percent Discount Rate) 54
Figure 5-4 Total (7-Percent Discount Rate) 54
Figure 5-5 Total (3-Percent Discount Rate) 55
Figure 5-8 Distribution of Additional Positive Results Projected 58
Figure 5-9 Relative Frequency of the Net Benefits of the Final Rule 58
List of Tables
Table ES-1 Cost-Benefit Comparison of Alternative 2 (Amend 10 CFR Part 26) xiii
Table 3-1 FFD Program Performance Data on Possible Impairment from Substance Use 11
Table 4-1 Range of Testing Data by Facility Type (CYs 2009–2019) 22
Table 4-2 Nominal Staffing Levels for Future Nuclear Facilities 23
Table 4-3 Suspect Specimens Collected Under Direct Observation 32
Table 5-2 Summary of Total Benefits and Costs to Industry (One-Time and Annual) 37
Table 5-3 Summary of One-Time and Annual Benefits and Costs to Industry, by Regulatory Initiative 37
Table 5-4 Projected One-Time and Annual Costs to Future Nuclear Facilities 38
Table 5-5 One-Time Implementation Costs 39
Table 5-16 Variables Used in the Uncertainty Analysis 50
Table 5-17 Uncertainty Results Descriptive Statistics 55
Table 5-18 Estimated Number of Additional Confirmed Positives per Year 57
Table 5-20 Specific CRGR Regulatory Analysis Information Requirements 61
Abbreviations and Acronyms
6-AM 6-acetylmorphine
ADAMS Agencywide Documents Access and Management System
BPTS blind performance test sample
CFR Code of Federal Regulations
COL combined license
CPI-U consumer price index for all urban consumers
CRGR Committee to Review Generic Requirements
C/V contractor/vendor
CY calendar year
dL deciliter(s)
DOT U.S. Department of Transportation
FAA Federal Aviation Administration
FFD fitness for duty
FR Federal Register
FRA Federal Railroad Administration
FTA Federal Transit Administration
HHS U.S. Department of Health and Human Services
HYC hydrocodone
HYM hydromorphone
IAEA International Atomic Energy Agency
INPO Institute of Nuclear Power Operations
LOD limit of detection
LOQ limit of quantitation
LTF licensee testing facility
MDA methylenedioxyamphetamine
MDEA methylenedioxyethylamphetamine
MDMA methylenedioxymethamphetamine
mg milligram(s)
mL milliliter(s)
MRO Medical Review Officer
MWt megawatt(s) thermal
NEI Nuclear Energy Institute
ng nanogram(s)
NLCP National Laboratory Certification Program
NPV net present value
NRC U.S. Nuclear Regulatory Commission
OMB Office of Management and Budget
ONDCP Office of National Drug Control Policy
OSHA Occupational Safety and Health Administration
OXYC oxycodone
OXYM oxymorphone
PERT program evaluation and review technique
pH a measure of the acidity or basicity of an aqueous solution
SMR small modular reactor
SSC structure, system, or component
SSNM strategic special nuclear material
U.S. United States
U.S.C. United States Code
Abstract
The U.S. Nuclear Regulatory Commission (NRC) is amending its regulations in Title 10 of the Code of Federal Regulations (10 CFR) Part 26, “Fitness for duty programs,” to align the NRC’s drug testing requirements more closely with updates made to the U.S. Department of Health and Human Services’ (HHS) “Mandatory Guidelines for Federal Workplace Drug Testing Programs” (HHS Guidelines). The final rule enhances the ability of licensees and other entities to identify additional individuals using illegal drugs and misusing legal drugs. The final rule also incorporates lessons learned from implementation of the 10 CFR Part 26 rule (published in 2008) to include enhanced methods in identifying attempts to subvert the drug testing process.
The requirements of the 10 CFR Part 26 fitness for duty (FFD) program focus, in part, on preventing and detecting impairment among personnel subject to an FFD program by providing reasonable assurance that the workplace is free of drugs and the effects of such substances. These requirements contribute to reasonable assurance that persons who have been granted unescorted access to the protected areas of NRC‑licensed facilities (i.e., operating nuclear power reactors, nuclear power reactors under construction, and Category I special nuclear material licensee facilities), who are required by a licensee to physically report to other locations (e.g., Emergency Operations Facilities, Technical Support Centers), or who have access to strategic special nuclear material or sensitive information are trustworthy and reliable and can safely and competently perform their assigned duties. These regulations also establish due process to protect individual rights.
The effectiveness of a drug testing program may weaken over time if individuals in the workplace (1) use impairing substances not included in the testing panel or (2) use products and techniques to successfully subvert the drug testing process. Program effectiveness may also weaken if the program does not incorporate technological advancements that enhance the sensitivity of drug testing. The HHS is designated as the Federal agency responsible for developing the scientific and technical guidelines for Federal employee workplace drug testing programs. The HHS is responsible for maintaining its guidelines based on the most recent research and lessons learned from Federal employee workplace and Federal agency drug testing programs. The 2017 HHS Guidelines are a national drug testing standard used by all Federal employee workplace drug testing programs (more than 100 Federal agencies) and comparable Federal agency drug testing programs that test civilians in safety- and security‑sensitive positions. The drug testing provisions in 10 CFR Part 26 should align with the national drug testing standard (i.e., the HHS Guidelines) to maintain reasonable assurance of a drug‑free workplace.
The final rule maintains the FFD program performance objectives in 10 CFR 26.23(c), to “provide reasonable measures for the early detection of individuals who are not fit to perform the duties that require them to be subject to the FFD program,” and in 10 CFR 26.23(d), to “provide reasonable assurance that the workplaces subject to this part are free from the presence and effects of illegal drugs.” The NRC staff expects that the lower testing cutoff levels, expanded drug testing panel, and enhanced subversion detection methods in the final rule will result in the detection of additional individuals (potential employees and employees of licensees) using illegal drugs, misusing legal drugs, or attempting to subvert the drug testing process. The final rule changes also may deter additional individuals using drugs from seeking employment in workplaces covered by 10 CFR Part 26, and could either deter existing employees from beginning to use drugs or encourage them to cease undetected use or seek medical assistance to address an addiction or misuse issue, or both.
This final rule contributes to a drug-free workplace by doing the following:
enhancing the capabilities to detect drugs already in the testing panel (i.e., amphetamine, cocaine, the heroin metabolite (6-acetylmorphine), and methamphetamine) and expanding the testing panel to include two amphetamine‑based Ecstasy-type drugs and four opioid drugs (hydrocodone, hydromorphone, oxycodone, and oxymorphone)
maintaining alignment with the Federal employee workplace drug testing program and those programs implemented by comparable Federal agencies that test civilians in safety- and security‑sensitive positions (e.g., U.S. Department of Transportation)
addressing trends in societal drug use that demonstrate an increasing use of amphetamines, methamphetamines, and heroin
addressing the prevalence of subversion attempts reported by the 10 CFR Part 26 drug testing programs from 2011 through 2019 (ranging from 22.1 to 39.0 percent of violations per year, by 128 to 307 individuals per year)
Enhancing the drug testing capabilities of the FFD program maintains the effectiveness of 10 CFR Part 26 by identifying additional individuals using drugs each year. The enhancements can be accomplished at low cost (i.e., an average one-time cost per site of ($2,321) and an average annual savings per site of $808). As a result, the NRC staff concludes that the improvements will maintain the FFD program performance objectives in 10 CFR 26.23(c) and (d) and that the benefit outweighs the low cost of implementation.
This document is the regulatory analysis for the final rule and the associated Regulatory Guide 5.89, “Fitness-for-Duty Programs for Commercial Power Reactor and Category I Special Nuclear Material Licensees.”
Executive Summary
The U.S. Nuclear Regulatory Commission (NRC) is amending Title 10 of the Code of Federal Regulations (10 CFR) Part 26, “Fitness for duty programs,” to accomplish three objectives:
Maintain reasonable assurance of a drug-free workplace through the enhanced detection of individuals who are not fit for duty because of illegal drug use, legal drug misuse, or an attempt to subvert the drug testing process.
Harmonize select drug testing requirements under 10 CFR Part 26 with the U.S. Department of Health and Human Services’ (HHS) “Mandatory Guidelines for Federal Workplace Drug Testing Programs” (HHS Guidelines).
Enhance donor protection and due process requirements for individuals subject to drug testing.
The HHS published updates to the HHS Guidelines in Volume 73 of the Federal Register (FR), page 71858 (73 FR 71858; November 25, 2008) (hereafter referred to as the “2008 HHS Guidelines”), and in 82 FR 7920 (January 23, 2017) (hereafter referred to as the “2017 HHS Guidelines”). The NRC has relied on the HHS Guidelines as the technical basis to establish and update the requirements in 10 CFR Part 26 for urine specimen collection, laboratory testing, and results review. In general, the NRC deviated from the HHS Guidelines only for considerations specific to the nuclear industry. When the 2008 HHS Guidelines were published, the NRC had recently issued the 10 CFR Part 26 final rule (73 FR 16966; March 31, 2008) to align with the 2004 HHS Guidelines (69 FR 19643). Therefore, the NRC determined that postponing a rulemaking to adopt the 2008 HHS Guidelines promoted regulatory stability and provided time both to collect data on the effectiveness of the 2008 FFD final rule and assess lessons learned from rule implementation, as well as to assess changes in the 2008 HHS Guidelines that became effective in October 2010. Subsequently, the HHS published the 2017 HHS Guidelines. The NRC staff has collected data on the effectiveness of the 2008 FFD rule and on the 2008 and 2017 HHS Guidelines, such that it is appropriate to revise 10 CFR Part 26 at this time. On October 25, 2019, the HHS published its 2019 HHS Guidelines (84 FR 57554) for allowing the collection and drug testing of an alternative specimen (i.e., oral fluid).
Major changes in the final rule do the following:
Add initial and confirmatory drug testing for two Schedule I amphetamine‑based Ecstasy‑type drugs1 and four Schedule II opioid drugs (i.e., oxycodone, oxymorphone, hydrocodone, and hydromorphone).
Add initial drug testing for 6-acetylmorphine (6-AM), a metabolite of the illegal drug heroin, and update the confirmatory drug testing method for 6‑AM.
Lower the initial and confirmatory drug testing cutoff levels for amphetamines (i.e., amphetamine and methamphetamine) and cocaine metabolites to increase the “window of detection”2 for these substances.
Enhance the detection of subversion attempts by strengthening the testing methods used to identify drugs and drug metabolites in urine specimens with dilute validity test results and in specimens collected under direct observation.
Permit the collection and drug testing of an oral fluid specimen as an alternative to the collection and testing of a directly observed urine specimen.
Require Medical Review Officers to evaluate the elapsed time from specimen collection to testing and exposure to high temperature, as possible causes of some invalid test results due to high solvated hydrogen ion concentration (i.e., pH).
Improve the clarity, consistency, and organization of 10 CFR Part 26 by adding and updating definitions; increase flexibility in the personnel who may monitor a donor that is hydrating during a shy-bladder situation; and enhance donor protections by providing additional instructions for same-gender observers used in observed collections and affording due process by requiring MROs to document the date and time that an oral request is received from a donor to initiate the retesting of a specimen.
In addition, the final rule addresses two issues associated with the testing of quality control samples at licensee testing facilities that were described in a March 31, 2009, “NRC Enforcement Guidance Memorandum – Dispositioning Violations of NRC Requirements for Initial Validity and Drug Tests at Licensee Testing Facilities” (EGM 09‑003). The NRC will withdraw EGM 09‑003 upon the effective date of the final rule.
Workplace Free of Drugs and the Effects of Such Substances
The general performance objective of an FFD program, as described in the original 10 CFR Part 26 final rule (54 FR 24468; June 7, 1989), “is to provide reasonable assurance that nuclear power plant personnel are reliable, trustworthy, and not under the influence of any substance, legal or illegal, or mentally or physically impaired from any cause, which in any way adversely affects their ability to safely and competently perform their duties.” This 1989 final rule also stated that an FFD program “developed under the requirements of this rule is intended to create an environment which is free of drugs and the effects of such substances.” The regulations in 10 CFR 26.23, “Performance objectives,” establish these drug‑free workplace requirements for an FFD program. Specifically, 10 CFR 26.23(c) states that an FFD program must “provide reasonable measures for the early detection of individuals who are not fit to perform the duties that require them to be subject to the FFD program,” and 10 CFR 26.23(d) states that an FFD program must “provide reasonable assurance that the workplaces subject to this part are free from the presence and effects of illegal3 drugs.” Preventing and detecting impairment among personnel subject to an FFD program by conducting drug testing provides reasonable assurance that the workplace is free of drugs and the effects of such substances. An FFD program contributes to the reasonable assurance that persons who have been granted unescorted access to the protected areas of NRC‑licensed facilities (i.e., operating nuclear power reactors, nuclear power reactors under construction, and Category I special nuclear material licensee facilities), who are required by a licensee to physically report to other locations (e.g., Emergency Operations Facilities, Technical Support Centers), or who have access to strategic special nuclear material (SSNM) or sensitive information are trustworthy and reliable and can safely and competently perform their assigned duties.
The HHS is designated as the Federal agency responsible for developing the scientific and technical guidelines for Federal employee workplace drug testing programs and is responsible for maintaining its guidelines based on the most recent research and lessons learned from Federal employee workplace and Federal agency drug testing programs. The 2017 HHS Guidelines are a national drug testing standard used by all Federal employee workplace drug testing programs (more than 100 Federal agencies4) and comparable Federal agency drug testing programs that test civilians in safety- and security-sensitive positions, such as those programs implemented by the U.S. Department of Transportation (DOT), U.S. Department of Energy, U.S. Department of Defense, and U.S. Department of Homeland Security. These tested populations transport people and hazardous materials; operate and maintain our Nation’s electrical, pipeline, and hydrodynamic infrastructure; protect property and national resources; and make decisions and execute emergency response plans that contribute to public health and safety or protection of the environment following a natural disaster or security activity.
The effectiveness of a drug testing program may weaken over time if individuals in the workplace (1) use impairing substances not included in the testing panel or (2) use products and techniques to successfully subvert the drug testing process. Program effectiveness may also weaken if the program does not incorporate technological advancements that enhance the sensitivity of drug testing. The drug testing provisions in 10 CFR Part 26 should use the national drug testing standard established by the HHS Guidelines and existing defense-in-depth methods (e.g., behavioral observation, background checks, collection site security, and specimen collections) to maintain reasonable assurance of a drug-free workplace.
The NRC analysis of annual FFD program performance data submitted by licensees and other entities under 10 CFR 26.717, “Fitness-for-duty program performance data,” demonstrates that the workplaces subject to 10 CFR Part 26 are not free from the presence and effects of drugs.
Historically, the NRC has incorporated the appropriate provisions of the HHS Guidelines into 10 CFR Part 26 to effectively use advancements in drug testing technology and detection methods to address societal changes in drug use and in the methods and techniques used to subvert the drug testing process. The NRC amended 10 CFR Part 26 in 2008 to align with the 2004 HHS Guidelines, the testing standard used at that time to test Federal employees and the majority of civilians tested by Federal agencies. However, the current drug testing panel and cutoff levels specified in 10 CFR Part 26 do not align with changes in the 2008 and 2017 HHS Guidelines. Therefore, the improvements contained in the final rule enable licensees to maintain reasonable assurance of a drug‑free workplace.
Safety Vulnerability
The final rule enhances the ability of NRC licensees and other entities to identify additional individuals using illegal drugs, misusing legal drugs, or attempting to subvert the testing process to conceal drug use and who, as a result, are determined as not fit for duty or not trustworthy and reliable, or both. Such a determination results in a denial of unescorted access to the protected areas of NRC‑licensed facilities and other locations, access to SSNM, or access to sensitive information. The identification of these individuals enhances the existing regulatory framework to prevent drug-induced impairment (i.e., acute intoxication and the consequences of recent drug use, such as withdrawal effects) from causing or contributing to human performance errors that may result in unplanned occupational exposure; personal safety issues (e.g., injuries); unplanned radiological releases; or improper operation, maintenance, or surveillance of safety-related structures, systems, or components (SSCs).
This safety outcome is consistent with the original 10 CFR Part 26 rule, which stated that “[t]he NRC cannot be confident of the individual’s ability to limit the use of addictive substances to situations that do not adversely affect plant safety” (54 FR 24470; June 7, 1989), and that “there is an underlying assumption that workers will abide by the licensee’s policies and procedures, [therefore] any involvement with illegal drugs shows that the worker cannot be relied upon to obey laws of a health and safety nature, indicating that the individual may not scrupulously follow rigorous procedural requirements with the integrity required in the nuclear power industry to assure public health and safety” (54 FR 24468; June 7, 1989).
Security Vulnerability
The final rule enhances the ability of NRC licensees and other entities to identify additional individuals determined not to be fit for duty or not to be trustworthy and reliable, or both, because of their use of illegal drugs, misuse of legal drugs, or attempts to subvert the drug testing process. A potential security vulnerability exists because persons of questionable honesty, integrity, and motive may have unescorted access authorization to enable (either physically or remotely through electronic means) a loss of SSCs and facility control, cause radiological sabotage at a commercial power reactor, or steal or divert formula quantities of SSNM from a Category I special nuclear material licensee.
A security vulnerability also exists if security personnel use illegal drugs or misuse legal drugs. Failure to maintain a robust and up-to-date FFD program could significantly challenge the effectiveness of the site insider mitigation program (10 CFR 73.55(b)(9)), security plan (10 CFR 73.55(c)), security search program (10 CFR 73.55(h)), and detection and assessment systems that include requirements to conduct surveillance, observation, and monitoring to identify tampering and to detect and deter intruders (10 CFR 73.55(i)). These requirements cannot be effectively implemented if site security personnel are not fit for duty, because many security duties and responsibilities are conducted by security officers who operate alone (i.e., individually) and, therefore, do not benefit from a team environment, second checks, or backup. As a result, a security officer who is mentally, physically, or psychologically impaired or who does not possess the characteristics of honesty, integrity, trustworthiness, and reliability cannot be relied upon to competently execute site security requirements. Furthermore, such a security officer cannot be relied upon to maintain positive control of his or her weapons, access controls, communication devices, and security-related knowledge and to make decisions safely and competently about contingency response and the use of deadly force. This argument also applies to individuals who perform the duties and responsibilities listed in 10 CFR 73.56(i)(1)(v)(B) and those who perform nonsafety- or nonsecurity-related job functions.
Identifying Subversion Attempts
The final rule enhances the ability of NRC licensees and other entities to identify additional individuals attempting to hide their drug use by subverting the drug testing process (e.g., consuming large quantities of fluid just before submitting a specimen for testing to reduce the level of a drug in his or her urine below detectable limits or submitting the urine of a nondrug‑using individual in place of his or her own specimen). This rule requires all specimens with a dilute validity test result (dilute specimens) and specimens collected under the direct observation requirements in 10 CFR 26.115(a)(1) through (a)(3) or (a)(5) (i.e., instances where a subversion attempt is suspected) to be tested to the limit of quantification, which is the lowest concentration at which the identity and concentration of a drug can be accurately established by testing. The identification of persons attempting to subvert the drug testing process is significant because this action is conclusive evidence of a lack of integrity and honesty and a willful act to refuse to comply with an NRC-required drug test. Consequently, these individuals present a potential vulnerability to the safe and secure conduct of NRC‑licensed activities.
The final rule also allows for the collection and drug testing of an oral fluid specimen as an alternative to the collection of a urine specimen under direct observation conditions.
Safety Goal Evaluation
The NRC staff estimates that the final rule will result in a substantial increase (between 16 and 29 percent) in the number of individuals identified each year using illegal drugs, misusing legal drugs, or attempting to subvert the drug testing process, as compared to the average number of positive 10 CFR Part 26 test results for calendar year (CY) 2009 through CY 2019. The NRC staff used this projected increase in the ability to detect additional individuals using drugs as the basis for meeting the substantial increase in overall protection criterion of the safety goal. The NRC staff acknowledges that only a small percentage of individuals subject to drug testing each year test positive; however, the additional number of individuals identified as a result of the final rule changes meets the substantial increase criterion based on the effects on facility safety and security that the impairment of these individuals could have.
Based on the FFD program performance information reported to the NRC and a comparison of this information to that from previous years, as well as other indicators, the commercial nuclear industry continues to effectively implement the 10 CFR Part 26 drug testing provisions, and the FFD program has directly contributed to public health and safety and the common defense and security. An NRC analysis of testing data indicates that persons potentially impaired from the use of amphetamine, cocaine, methamphetamine, and heroin (as evident from positive for‑cause and post-event test results from CY 2010 through CY 2019) continue to be identified and removed from having protected area access at NRC-licensed facilities. Enhancing the ability to detect additional amphetamine, cocaine, heroin, and methamphetamine drug users strengthens the drug testing program in areas in which the annual FFD program performance data indicate impacts related to human performance.
Benefits and Costs
The NRC staff finds that, considered together, the detection of additional drug users and the qualitative benefits of doing so continue to maintain reasonable assurance of a drug-free workplace and outweigh the low costs of the final rule. The analysis quantified benefits and costs associated with two affected attributes—industry implementation and industry operation.5 However, the NRC staff had difficulties in monetizing the benefits associated with seven affected attributes—public health (accident), occupational health (accident), offsite property, onsite property, regulatory efficiency, safeguards and security considerations, and other considerations. The “other considerations” attribute includes public perception, workplace productivity, workplace safety, and improved protection of individual rights. The NRC staff performed a qualitative assessment of these attributes, which is consistent with the Commission’s direction in the staff requirements memorandum, “SECY-14-0087—Qualitative Consideration of Factors in the Development of Regulatory Analyses and Backfit Analyses,” dated March 4, 2015 (NRC, 2015). Because the staff could not rigorously quantify and monetize the benefits, it could not perform a quantified comparison of costs and benefits. However, for example, preventing the shutdown of a single reactor unit for 1 day as a result of the actions of an impaired individual would far exceed the estimated annual benefit to industry of the final rule changes.
The regulatory analysis resulted in the following key findings:
Benefits. The direct benefit of this rule is to enhance the effectiveness of NRC‑required FFD drug testing programs by identifying additional individuals using illegal drugs, misusing legal drugs, or attempting to subvert the drug testing process. The NRC staff estimates that the final rule will result in an estimated increase of between 16 and 29 percent per year in individuals testing positive for drugs or identified attempting to subvert the drug testing process. The final rule also improves regulatory efficiency by aligning elements of 10 CFR Part 26 with changes in the 2008 and 2017 HHS Guidelines and by applying lessons learned from implementation of the NRC’s 2008 FFD final rule by licensees and other entities. A more robust drug testing program also may deter additional individuals using drugs from seeking employment for positions subject to 10 CFR Part 26 and incentivize those in regulated positions to cease drug use or seek medical assistance to address an addiction or misuse issue, or both. While this analysis quantifies the benefit of identifying additional individuals using drugs, it cannot monetize the safety and security benefits of identifying these additional individuals, beyond training costs that are averted because the individuals are not given access. The NRC staff recognizes that a licensee or other entity will incur additional costs to replace an employee who is identified as using illegal drugs, misusing legal drugs, or attempting to subvert the drug testing process. While this analysis does not quantify these costs, they represent an additional benefit of identifying these individuals before they gain access to the facility. Regulatory efficiency is also gained by clarifying ambiguous rule language and providing additional regulatory flexibility.
Total Costs and Savings to Industry. The final rule is estimated to result in a total one‑time cost of approximately ($136,936), followed by a total annual savings of approximately $47,650. The net present value of these savings is approximately $418,356 using a 7‑percent discount rate and approximately $692,799 using a 3‑percent discount rate over the average remaining reactor license period of 24 years. These savings include averted industry training costs as a result of pre-access testing of approximately $370,539 annually, which provides a benefit of between $4.32 million using a 7-percent discount rate and $6.45 million using a 3‑percent discount rate.
Average Costs and Savings per Site. The industry would incur a one‑time average cost per site of ($2,321), followed by an average annual savings of $808.
Oral Fluid Testing Alternative. For observed collection conditions, the final rule provides the option to collect and drug test an oral fluid specimen instead of a urine specimen. For those licensees that choose to take advantage of this alternative, the NRC estimates an industry savings of approximately $6,665 per year—a savings of about $30 per test. This alternative also provides the non-quantified benefit of enhanced protection of donor privacy rights by avoiding the practice of urine specimen collection under direct observation.
Impacts to Future Power Reactor and Fuel Facility Licensees. The final rule will result in negligible implementation costs to future licensees because they will create FFD policies, procedures, and training programs after the final rule is in effect. A new microreactor or small modular reactor (SMR) licensee is expected to incur an annual incremental operating benefit of between $13 and $163 due to the smaller workforce anticipated during construction and operation. A new large nuclear facility or special nuclear material licensee is expected to incur an annual incremental operating benefit of $808, which is comparable to the estimated benefits for licensees of currently operating nuclear power reactors.
Uncertainty Analysis. The simulation analysis shows that the estimated mean benefit for this rule is $0.74 million, with 90‑percent confidence that the total cost is between ($1.14 million) and $3.10 million assuming a 7‑percent discount rate. The variations in the NRC FFD amphetamines positive test rate and the total number of drug tests performed by FFD programs on an annual basis drive the largest variation in costs.
Decision Rationale
The final rule maintains the FFD program performance objectives in 10 CFR 26.23(c), to “provide reasonable measures for the early detection of individuals who are not fit to perform the duties that require them to be subject to the FFD program,” and in 10 CFR 26.23(d), to “provide reasonable assurance that the workplaces subject to this part are free from the presence and effects of illegal drugs.” The final rule accomplishes these objectives by (1) enhancing the detection of individuals who are not fit for duty because of illegal drug use, legal drug misuse, or an attempt to subvert the drug testing process, (2) harmonizing select drug testing requirements under 10 CFR Part 26 with the 2008 and 2017 HHS Guidelines, and (3) enhancing FFD program donor protection and due process requirements for individuals subject to drug testing.
While the full benefit of identifying additional drug-using individuals cannot be monetized, the detection of these individuals supports the safety and security goals discussed above as well as ensures the achievement of the goal of the drug testing program (i.e., provide reasonable assurance that the workplaces subject to this part are free from the presence and effects of drugs). Table ES-1 shows, from a quantitative standpoint, that the rule alternative is a cost‑effective way of achieving incremental improvements in the detection of illegal drug use, legal drug misuse, and attempts to subvert the drug testing process. Note that Table ES‑1 presents the net present value results for the 24-year time period of the analysis, while it presents the estimated benefit in the detection of additional drug users by regulatory initiative on an annual basis.
Table ES-1 Cost-Benefit Comparison of Alternative 2 (Amend 10 CFR Part 26)
Regulatory Initiative |
7% Net Present Valuea (24-year
time period |
Estimated Benefit (Annual Basis) |
Enhance detection of existing paneled drugs by lowering cutoff levels (amphetamine, cocaine, methamphetamine) |
($185,898) |
45 additional positive results (i.e., 23 amphetamines positives and 22 cocaine positives) |
Expand testing panel to include initial testing of 6‑AM (and revise confirmatory testing cutoff level) |
($935,375) |
22 additional positive results |
Expand initial and confirmatory testing panels to include four opioid drugs (hydrocodone, hydromorphone, oxycodone, oxymorphone) |
($1,829,243) |
89 additional positive results |
Expand initial and confirmatory testing panels to include Ecstasy-type drugs (MDMA, MDA) |
($702,980) |
5 additional positive results |
Enhance detection of subversion attempts by requiring special analyses testing of dilute specimens and specimens collected under direct observation |
($109,322) |
16 additional positive results (8 positives from dilute specimens and 8 positives from suspect specimens) |
One-time costs to sites to change policies, procedures, and conduct training to incorporate all drug testing program changes |
($136,936) |
Required activities to implement drug testing changes at laboratories and inform all subject employees of drug testing program changes |
Averted training costs (pre‑access testing) |
$4,318,110 |
Historically, pre-access testing accounts for 67 percent of positive test results each year
Individuals testing positive before completion of training will result in savings to licensees and other entities |
Total Industry Results |
$418,356 |
176 additional positive results per year (22‑percent increase) and additional non-quantified benefits |
Average Benefit (Cost) per Siteb |
$7,091 |
|
a Net present value is the discounted present value of an alternative’s future stream of cash flows.
b Section 4.2.2 discusses the number of FFD program sites.
c The 176 additional positive results represents the mean value for the estimated increase in positive results, as reflect in Table 5-18, “Estimated Number of Additional Confirmed Positives per Year”.
The final rule does not impose modifications or additions to existing structures, components, equipment, designs, or organizations. To comply with the rule changes, licensees will update existing FFD program policies and procedures, conduct training, revise contracts with HHS‑certified laboratories and blind performance test sample providers, perform mandatory special analyses testing on some specimens, and modify the drug testing panel.
The NRC staff concludes that the final rule is projected to result in a 176 additional individuals identified each year (i.e., a 22 percent increase) using illegal drugs, misusing legal drugs, or attempting to subvert the drug testing process. This is a substantial increase in the overall protection of public health and safety and the common defense and security. This conclusion is based on the following:
The FFD program performance data received by the NRC from CY 2011 to CY 2019, which show the increases in the positive test rates as summarized in Appendix B.
The changes to the drug testing panel are broad based (i.e., the cutoff levels for multiple substances are being lowered and additional substances are being added) and address trends in FFD program performance data.
Aligning 10 CFR Part 26 with the 2017 HHS Guidelines ensures that the NRC FFD drug testing program is consistent with this national drug testing standard implemented by all comparable safety- and security-sensitive workforces tested in the United States (e.g., Federal employee workplace drug testing programs, other Federal agency programs that drug test civilians such as the DOT).
The detection of drugs in the workplace subject to 10 CFR Part 26 testing is a proactive, risk‑informed FFD strategy. Between 2009 and 2019, approximately 67 percent of individuals who tested positive for drugs or alcohol each year were identified before they receive unescorted access authorization (i.e., at pre-access testing).
The analysis of net benefits (i.e., benefits minus costs) shows that the final rule is cost-beneficial at $418,356 using a 7-percent discount rate. This net benefit is achieved because of averted training costs. If the averted training savings are not included, then the remaining six of the seven regulatory initiatives that comprise the rule are not cost-beneficial because the benefits could not be fully quantified (see Table ES‑1). If the rule is adopted, the safety and security value that the Commission assigns to detecting 176 additional individuals using drugs must be greater than ($3.90 million) (mean value), using a 7‑percent discount rate for the net costs for these six regulatory initiatives result to be positive.
The NRC staff concludes that the rule is justified in view of the substantial increase in the detection of additional individuals using drugs, as shown in Table ES‑1, and that, overall, the rule provides a net savings resulting from averted training costs.
1. Introduction
This document presents the regulatory analysis of the U.S. Nuclear Regulatory Commission’s (NRC’s) amendments to the fitness for duty (FFD) requirements in Title 10 of the Code of Federal Regulations (10 CFR) Part 26, “Fitness for duty programs,” and the associated Regulatory Guide 5.89, “Fitness-for-Duty Programs for Commercial Power Reactor and Category I Special Nuclear Material Licensees.”
The objectives of the rulemaking are to (1) maintain reasonable assurance of a drug‑free workplace through the enhanced detection of individuals who are not fit for duty because of illegal drug use, legal drug misuse, or an attempt to subvert the drug testing process, (2) harmonize select drug testing requirements under 10 CFR Part 26 with those established by the 2008 and 2017 U.S. Department of Health and Human Services’ (HHS) “Mandatory Guidelines for Federal Workplace Drug Testing Programs,” published on November 25, 2008, in Volume 73 of the Federal Register (FR), page 71858 (73 FR 71858) (hereafter referred to as the “2008 HHS Guidelines”), and on January 23, 2017, at 82 FR 7920 (hereafter referred to as the “2017 HHS Guidelines”) and implemented by other Federal agencies, and (3) enhance donor protection and due process requirements for individuals subject to drug testing. In support of these three objectives, the final rule also improves the clarity, organization, and flexibility of 10 CFR Part 26 rule language.
This introduction contains two sections. Section 1.1 provides background information, and Section 1.2 presents the statement of the problem and the objectives for the final rule.
1.1 Background
The regulations at 10 CFR Part 26 contain the NRC’s requirements for the FFD programs of licensees and other entities (also referred to in this document as “licensees” or “affected entities”0). The regulations focus, in part, on preventing and detecting impairment among personnel subject to an FFD program by providing reasonable assurance that the workplace is free of drugs and the effects of such substances.
The general performance objective of an FFD program, as described in the original 10 CFR Part 26 final rule (54 FR 24468; June 7, 1989), “is to provide reasonable assurance that nuclear power plant personnel are reliable, trustworthy, and not under the influence of any substance, legal or illegal, or mentally or physically impaired from any cause, which in any way adversely affects their ability to safely and competently perform their duties.” This 1989 final rule also states that an FFD program “developed under the requirements of this rule is intended to create an environment which is free of drugs and the effects of such substances” (54 FR 24468; June 7, 1989). The regulations at 10 CFR 26.23, “Performance objectives,” establish these drug‑free workplace requirements.
The drug‑free workplace performance objectives contribute to the ability to provide reasonable assurance that persons who have been granted unescorted access to the protected areas of NRC‑licensed facilities (i.e., operating nuclear power reactors, nuclear power reactors under construction, and Category I special nuclear material licensee facilities), who are required by a licensee to physically report to other locations (e.g., Emergency Operations Facilities, Technical Support Centers), or who have access to SSNM or sensitive information are trustworthy and reliable and can safely and competently perform their assigned duties.
The NRC issued a significant revision to the original 1989 FFD rule (54 FR 24468; June 7, 1989) in a final rule published on March 31, 2008 (73 FR 16966), that incorporated elements of the 2004 HHS Guidelines (69 FR 19643; April 13, 2004). The 2008 revision to the FFD requirements had several objectives. The revision enhanced the effectiveness of FFD programs by applying advancements in drug and alcohol testing technologies and lessons learned from licensees’ implementation of the 1989 FFD rule. It also improved the efficiency of FFD regulations by eliminating unnecessary requirements and by harmonizing the NRC’s original FFD rule with other Federal drug testing rules and guidelines. Furthermore, it improved the consistency between FFD requirements and the access authorization requirements established in 10 CFR 73.56, “Personnel access authorization requirements for nuclear power plants,” as supplemented by NRC orders to nuclear power plant licensees dated January 7, 2003, thereby strengthening regulatory assurance that persons of questionable integrity, honesty, trustworthiness, and reliability are not granted unescorted access authorization to the protected areas of commercial nuclear power plants and Category I special nuclear material licensee facilities, to SSNM, or to sensitive information. In addition, the 2008 FFD final rule helped to protect the privacy and other rights (including due process) of individuals subject to the NRC FFD requirements, and it established clear and enforceable requirements for the management of worker fatigue.
NRC Fitness for Duty Program and the HHS Guidelines
The HHS is designated as the Federal agency responsible for developing the scientific and technical guidelines for Federal employee workplace drug testing programs. The HHS is responsible for maintaining its guidelines based on the most recent research and lessons learned from Federal employee workplace and Federal agency drug testing programs. The 2017 HHS Guidelines establish a legal framework to conduct drug testing that provides reasonable assurance of privacy, drug test accuracy and precision, and custody and control of specimens collected and tested. It also provides for due process to individuals subject to drug testing. The 2017 HHS Guidelines can be viewed as the national standard for drug testing based on use by all Federal employee workplace drug testing programs, prevalence of use by Federal agency drug testing programs of civilians in safety- and security-sensitive positions, and use by the private sector.
The NRC has relied on the HHS to establish the technical requirements for urine specimen collection, testing, and evaluation and has deviated from the HHS Guidelines only for considerations specific to the nuclear industry. One goal of the 2008 FFD final rule was to “update and enhance the consistency of 10 CFR Part 26 with advances in other relevant Federal rules and guidelines, including the HHS Guidelines and other Federal drug and alcohol testing programs (e.g., those required by the U.S. Department of Transportation [DOT]) that impose similar requirements on the private sector” (73 FR 16970; March 31, 2008). On November 25, 2008, nearly 8 months after publication of the NRC’s 2008 FFD final rule, the HHS issued the 2008 HHS Guidelines (73 FR 71858), which incorporated advancements in drug testing technologies to improve the detection of drugs. The 2008 HHS Guidelines became effective on October 1, 2010. The NRC’s 10 CFR Part 26 regulation predates and does not fully reflect this subsequent revision of the HHS Guidelines.
Following publication of the 2008 HHS Guidelines, the NRC held four public meetings, on February 24, 2009 (NRC, 2009a); June 24, 2009 (NRC, 2009d); October 11, 2011 (NRC, 2011b); and September 11, 2013 (NRC, 2013b), to review the changes in the 2008 HHS Guidelines and to discuss the potential impacts on the NRC FFD drug testing requirements. Based on external stakeholder feedback and an NRC staff assessment, the NRC staff elected to forego another 10 CFR Part 26 rulemaking so soon after publishing the 2008 FFD final rule. This decision helped promote regulatory stability and allowed time for the NRC staff to evaluate the effectiveness of Federal agency programs implementing the revised 2008 HHS Guidelines since October 2010. Additionally, it allowed time for the NRC and licensees and other entities to learn lessons from implementing the 2008 FFD final rule. During these public meetings, representatives from the commercial nuclear power industry expressed support for revising 10 CFR Part 26 to (1) incorporate select provisions from the 2008 HHS Guidelines, (2) enhance the detection of illegal drug use and misuse of prescription drugs, and (3) enhance the methods to identify attempts to subvert the drug testing process.
Subsequently, the HHS published the 2017 HHS Guidelines in January 2017 (82 FR 7920). The NRC held a public meeting on November 7, 2019 (NRC, 2019), to provide an opportunity for the NRC staff and external stakeholders to exchange information on the proposed rule to update the FFD testing requirements and to discuss and solicit feedback on the draft regulatory analysis, draft regulatory guidance, and specific requests for comments in the proposed rule. Based on external stakeholder feedback and an NRC staff assessment, the NRC elected to incorporate the changes in the 2008 and 2017 HHS Guidelines into the NRC FFD drug testing requirements. The 2017 HHS Guidelines provide for the testing of four prescription opioid pain relievers (i.e., hydrocodone (HYC), hydromorphone (HYM), oxycodone (OXYC), and oxymorphone (OXYM)), the removal of methylenedioxyethylamphetamine (MDEA), raising the lower pH cutoff from 3 to 4 for identifying adulterated specimens, and requiring medical review officer (MRO) requalification training and reexamination at least every 5 years after initial MRO certification. On October 25, 2019, the HHS published its 2019 HHS Guidelines (84 FR 57554) for allowing the collection and drug testing of an alternative specimen (i.e., oral fluid).
1.2 Statement of the Problem and U.S. Nuclear Regulatory Commission Objectives for the Rulemaking
The 2017 HHS Guidelines (82 FR 7920; January 23, 2017) modified the advancements in drug testing technologies established by the 2008 HHS Guidelines to enhance the detection of drug use within the Federal employee workplace. The NRC did not incorporate these revisions into the 2008 FFD final rule (73 FR 16966; March 31, 2008), which was published earlier. Therefore, the drug detection and deterrence provisions in 10 CFR Part 26 are not equivalent to those in the 2008 and 2017 HHS Guidelines.
Consequently, the 10 CFR Part 26 drug testing program does not conform with (1) the workplace drug testing programs implemented by more than 100 Federal agencies0 that test Federal employees, (2) other Federal agency programs that drug test civilians such as those implemented by the DOT, U.S. Department of Energy, U.S. Department of Defense, and U.S. Department of Homeland Security, and (3) programs run by private entities that use the 2017 HHS Guidelines as a technical basis for their drug testing programs. These tested populations transport people and hazardous materials (e.g., motor carriers, aviation, railroad, public transit, and maritime workers); operate and maintain our Nation’s electrical, oil and gas pipeline, and hydrodynamic infrastructure; protect property and national resources; and make decisions and execute emergency response plans that contribute to public health and safety or protection of the environment following a natural disaster or security activity.
Because some individuals seeking employment in or already working in the commercial nuclear workforce may use illegal drugs or misuse legal drugs, or both, this rule focuses on enhancing the identification of those individuals using illegal drugs whose potential impairment could result in unsafe or unsecure conditions at NRC-licensed facilities. Granting or maintaining access authorization to these individuals represents a safety vulnerability because drug-induced impairment may cause or contribute to human performance errors that may result in unplanned occupational exposure; personal safety issues; unplanned radiological releases; or improper operation, maintenance, or surveillance of safety- or security-related structures, systems, or components (SSCs). Additionally, granting or maintaining unescorted access authorization to these individuals also presents a security vulnerability because the use of illegal drugs, misuse of legal drugs, and subversion of the 10 CFR Part 26 drug testing program are indicators that an individual is not trustworthy and reliable. An individual exhibiting these characteristics cannot be granted unescorted access authorization0 (either physically or electronically) because granting access challenges the defense in depth afforded by the FFD authorization requirements in 10 CFR Part 26 and access authorization requirements in 10 CFR Part 73, “Physical protection of plants and materials.”
The first objective of this rulemaking is to maintain reasonable assurance of a drug‑free workplace at licensee facilities through the enhanced detection of individuals who are not fit for duty because of illegal drug use, legal drug misuse, or attempts to subvert the drug testing process. Enhancing the detection of additional individuals using drugs also includes strengthening the methods used to identify individuals attempting to subvert the drug testing process, which is a lesson learned from implementing the current 10 CFR Part 26 rule.
The second objective of this rulemaking is to harmonize select drug testing requirements under 10 CFR Part 26 with the 2008 and 2017 HHS Guidelines. Updating 10 CFR Part 26 with the testing improvements in the HHS Guidelines aligns the NRC’s FFD program with this national drug testing standard and, therefore, enhances licensees’ ability to maintain reasonable assurance that the workplace is free of drugs and the effects of such substances.
The third objective is to enhance donor protection and due process requirements for individuals subject to drug testing by (1) adding instructions for same-gender observers who perform an observed collection when a trained collector of the same gender as the donor is not available, (2) requiring the limit of quantitation (LOQ)0 for special analyses testing of drugs and testing for adulterants (an added measure of testing accuracy), (3) adding an MRO review of invalid test results of high pH (9.0 to 9.5), and (4) requiring the MRO to document the date and time an oral request was received from a donor to initiate the retesting of a specimen.
In support of these three objectives, the final rule also improves the clarity, organization, and flexibility of 10 CFR Part 26 rule language.
2. Identification and Preliminary Analysis of Alternative Approaches
The NRC staff considered the following three alternatives to address the regulatory problem identified in Section 1.2:
Alternative 1: Take No Action
Alternative 2: Amend 10 CFR Part 26
Alternative 3: Address Issues Without Rulemaking
2.1 Alternative 1: Take No Action
The take no action alternative is to maintain the status quo. This alternative is the regulatory baseline from which the other alternatives are measured. Under the take no action alternative, the NRC would not amend the current FFD regulations, and licensees and other entities would continue to comply with the existing requirements in 10 CFR Part 26. As a result, the 10 CFR Part 26 drug testing provisions would not include the drug testing advancements and donor protections in the 2008 or 2017 HHS Guidelines nor conform with the other Federal agency testing programs that follow them.
Because the NRC requires all licensees to use HHS‑certified laboratories for confirmatory specimen testing, specimens submitted by licensees and other entities must be treated differently than the specimens submitted by more than 100 Federal agency employee workplace drug testing programs. Laboratories would continue to segregate the 10 CFR Part 26 specimens from all other Federal agency specimens because of the different testing parameters (e.g., drug testing panel and cutoff levels, initial testing protocol for heroin, calibrators and controls used for assays) and would have to maintain amended procedures and training.
Under the take no action alternative, the NRC would not require licensees to test for additional substances or use lower cutoff levels to test for existing drugs and drug metabolites in the testing panel. Currently, 10 CFR 26.31(d) provides licensees and other entities with the flexibility to use lower testing cutoff levels than specified by rule for the NRC‑required drug testing panel or to test for additional drugs, or both. However, no licensee or other entity testing program has incorporated the use of the lower testing cutoff levels or tests for the additional substances included in the 2008 or 2017 HHS Guidelines. Following the second public meeting held during rulemaking activities in 2009, the Nuclear Energy Institute submitted a letter on May 31, 2009 (NRC, 2009e), detailing the results of a survey it had conducted of its members and stating the following:
While many of the respondents are in favor of expanding the panel, all companies responding to the survey responded that they would change their panel only [sic] if the NRC mandated the expansion of the panel to the 7 drugs specified in the HHS Guidelines. The reason is that many of the companies have had to negotiate with bargaining units on the drug testing process and expansion of the panel by the company without a mandate within the rule would subject the panel to the negotiation process and not guarantee its adoption.
Regardless of whether this final rule is issued, the NRC will continue to inform the public about 10 CFR Part 26 FFD program performance to maintain the public’s trust. The NRC publishes data on the NRC Web site about domestic operating events, including significant FFD policy violations or programmatic failures, drug and alcohol testing errors, and indicators of programmatic weaknesses (i.e., 24‑hour and 30‑day reportable events under 10 CFR 26.719, “Reporting requirements”). The agency also provides analysis, trending, and summary of annual FFD program performance data submitted under 10 CFR 26.717, “Fitness‑for‑duty program performance data,” through the publication of the NRC’s Summary of Fitness for Duty Program Performance Reports (NRC, 2017). This information also is used to inform NRC oversight programs.
In 2009, the NRC developed (with input from industry) and implemented a voluntary electronic reporting (e‑reporting) system to submit 10 CFR 26.717 information. This enhanced data collection method has led to the NRC’s receipt of much more precise, detailed, and uniform information on site‑specific performance. The NRC staff has used these data throughout this analysis. The NRC also regularly consults with regulatory partners (e.g., HHS, DOT, Office of National Drug Control Policy) to assess the effectiveness of the 2008 and 2017 HHS Guidelines, societal changes in drug use, and the prevalence of products in the marketplace to enable test subversion and sample adulteration. The agency periodically provides this information to the NRC inspectors assigned to commercial power reactors and Category I special nuclear material licensees during training sessions. Collectively, these efforts have enhanced oversight of existing licensee and other entity FFD programs. However, FFD programs and NRC oversight programs cannot benefit under the current regulations from the enhancement in the effectiveness of the laboratory testing methods or the choice of drugs included in the testing panel (i.e., the aspects of Alternative 2 that are estimated to result in the majority of Alternative 2’s quantified benefit).
Lastly, not pursuing rulemaking at this time would not incorporate lessons learned from implementation of the 2008 FFD final rule that would improve the efficiency of the regulatory framework and enhance the detection of subversion attempts.
By definition, this alternative has no incremental benefits or costs, as it does not change the status quo.
2.2 Alternative 2: Amend 10 CFR Part 26
This alternative resolves the problem described in Section 1.2 about the current 10 CFR Part 26 rule and its implementation. The requirements for licensee FFD programs focus on preventing and detecting impairment among personnel subject to an FFD program by providing reasonable assurance that the workplace is free of drugs and the effects of such substances. This alternative enhances the detection of individuals who are not fit for duty because of illegal drug use, legal drug misuse, or an attempt to subvert the drug testing process. Specifically, this final rule aligns the NRC’s drug testing requirements in 10 CFR Part 26 more closely with those specified in the 2008 and 2017 HHS Guidelines that are used by more than 100 Federal employee workplace drug testing programs and comparable Federal agency drug testing programs that test civilians in safety‑ and security-sensitive positions. This rule also incorporates lessons learned from implementation of the 2008 FFD final rule and enhances donor protection and due process requirements for individuals subject to drug testing.
The NRC staff performed a comprehensive review and comparison of 10 CFR Part 26 and the 2008 HHS Guidelines to identify the specific 10 CFR Part 26 provisions that should be revised. The NRC staff also analyzed the DOT testing policies in 49 CFR Part 40, “Procedures for transportation workplace drug and alcohol testing programs,” and the technical and policy issues identified during implementation of the 2008 FFD final rule. These efforts resulted in a list of potential changes to 10 CFR Part 26 (NRC, 2011a), which the NRC staff presented to stakeholders in a series of public meetings to elicit feedback to further inform the decisionmaking process on potential regulatory changes.
Based on the results presented in Section 5 of this document, the NRC staff expects that the revisions to 10 CFR Part 26 will substantially enhance safety and security at NRC‑licensed facilities by identifying approximately 22 percent more individuals (potential employees and employees of licensees and other entities) each year using illegal drugs, misusing legal drugs, or attempting to subvert the drug testing process. The changes to the drug testing program (e.g., lower testing cutoff levels, expanded drug testing panel, subversion detection methods) also may deter additional individuals using drugs from seeking employment in 10 CFR Part 26 regulated workplaces, and may incentivize those already in regulated positions to cease undetected use or seek medical assistance to address an addiction or misuse issue.
The final rule also improves regulatory efficiency (e.g., by adding and updating definitions, incorporating lessons learned from implementation of the 2008 FFD final rule, increasing flexibility) and enhances donor protection and due process requirements (e.g., by adding instructions for same‑gender observers who perform an observed collection when a trained collector of the same gender as the donor is not available, requiring the LOQ for special analyses testing of drugs and testing for adulterants, adding a provision for MRO review of invalid test results due to high pH values (9.0 to 9.5)).
2.3 Alternative 3: Address Issues Without Rulemaking
Under this alternative, the NRC staff would not amend 10 CFR Part 26. This alternative differs from the Take No Action alternative (Alternative 1) because it would attempt to address FFD concerns through other means, such as a new regulatory guide, generic communications, stakeholder meetings, NRC inspections, or other agency initiatives, or a combination of approaches.
This alternative is not desirable for the following reasons:
This alternative would not address all identified issues (see Section 1.2 of this document), because the resolutions for many issues, such as inconsistencies with the 2008 or 2017 HHS Guidelines, require changes to 10 CFR Part 26.
This alternative would not incorporate comments from affected entities received by the NRC staff at public meetings that advocate promulgating rule changes to update the drug testing panel, testing methodologies, and evaluation criteria to help assure integrity, accuracy, sensitivity, and due process (NRC, 2009a; NRC, 2009d; NRC, 2009e; NRC, 2011b; and NRC, 2013b).
This alternative would not address an NRC “Enforcement Guidance Memorandum—Dispositioning Violations of NRC Requirements for Initial Validity and Drug Tests at Licensee Testing Facilities,” EGM 09‑003, dated March 31, 2009 (NRC, 2009c), which describes inconsistencies in terminology associated with the testing of quality control samples at licensee testing facilities (LTFs).
This alternative likely would result in inconsistencies in FFD program implementation. Under this alternative, affected entities could choose to commit to all, none, or a portion of the guidance, which could lead to inconsistent implementation across the industry and challenge regulatory effectiveness. However, as stated in the discussion of Alternative 1, 10 CFR 26.31(d) currently provides licensees with the flexibility to test for additional drugs or to use lower testing cutoff levels than required by 10 CFR Part 26, or both, but no FFD program has incorporated the changes in the 2008 or 2017 HHS Guidelines. In addition, variability in drug testing programs could lead to additional burden on the NRC staff to assess and address compliance issues, answer questions from licensees, and answer questions from personnel subject to FFD program testing (especially for individuals, such as outage workers, who work for a variety of licensee programs).
3. Safety Goal Evaluation
A safety goal evaluation determines whether a regulatory requirement should not be imposed generically on nuclear power plants because the residual risk is already acceptably low. The 1989 FFD rule addressed the significance of drug and alcohol testing on public health and safety by stating the following (54 FR 24468, June 7, 1989):
The Commission is taking this action to significantly increase assurance of public health and safety. The scientific evidence is conclusive that significant detriments in cognitive and physical task performance result from intoxication due to illicit drug abuse, as well as the use and misuse of legal substances. Given the addictive and impairing nature of certain drugs, while recognizing that the presence of drug metabolites does not necessarily relate directly to a current impaired state, the presence of drugs does strongly suggest the likelihood of past, present, or future impairment affecting job activities. In addition, the NRC believes that the reliability, integrity, and trustworthiness of persons working within nuclear power plants is important to assure public health and safety.
The calendar year (CY) 2013 performance report (NRC, 2014), summarizes the performance of the FFD drug testing program and states the following:
Based on the fitness-for-duty (FFD) performance information reported to the NRC and a comparison of this information to previous years data and other indicators, the commercial nuclear industry continues to effectively implement the Part 26 drug and alcohol (D&A) provisions and FFD program results have directly contributed to public health and safety and the common defense and security. The data indicates no adverse trends6; persons under the influence of illicit drugs and/or alcohol are being identified and removed from the protected area (PA) of NRC‑licensed facilities; and, persons of questionable trustworthiness and reliability are being identified through aggressive testing methods (e.g., limit‑of‑detection testing, lower cutoffs, and effective monitoring during specimen collections). Industry identification and communication of program weaknesses, lessons learned, and corrective actions demonstrate commitment to improved performance and a drug‑free work environment.
6 An adverse trend is one in which the NRC would evaluate the necessity to undertake a scalable response based on the severity or significance of the trend. NRC response could include, but not be limited to: inspection, issuance of guidance, licensing, or rulemaking.
The NRC evaluated FFD program performance data and trends in year-over-year increases in the positive test rate for amphetamines and a significant number of subversion attempts from CY 2011 through CY 2019. The NRC also performed a risk-informed assessment of the substances addressed in this rulemaking by evaluating the prevalence of these substances in tests performed when potential impairment from substance use is identified (for-cause tests) and after adverse safety events (post-event tests). For-cause testing, as described in 10 CFR 26.31(c)(2), is required when observed behavior, physical condition, or credible information, or a combination, indicates the potential for substance use. Post-event testing is required after certain workplace safety events, as described in 10 CFR 26.31(c)(3), which include but are not limited to events that cause death, days away from work, restricted work, medical treatment beyond first aid, loss of consciousness, radiation exposure or release in excess of regulatory limits, or actual or potential substantial degradations of the plant safety level.
Table 3-1 presents the NRC’s assessment of FFD program performance data from CY 2011 through CY 2019 on for-cause and post-event testing violations (i.e., drug positive results, identified subversion attempts). This table presents the number of individuals who tested positive for any of the drugs the final rule modifies by lowering the testing cutoff levels or improving the testing methods, and improved detection of subversion attempts. Any individual that tested positive for more than one substance appears in the table row titled “multiple substances.” The assessment eliminated alcohol positive results because the final rule does not include changes to the testing for this substance.
Table 3-1 FFD Program Performance Data on Possible Impairment from Substance Use
Performance Data |
2011 |
2012 |
2013 |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
For-Cause |
|||||||||
Total results (drug & alcohol positives & subversions) |
66 |
65 |
80 |
83 |
87 |
75 |
79 |
76 |
67 |
Total results (drug positives & subversions) |
27 |
19 |
30 |
36 |
43 |
42 |
39 |
39 |
28 |
Test Results Associated with Rule Changes (Panel of Drugs or Subversions) |
|||||||||
Amphetaminesa |
2 |
4 |
2 |
2 |
17 |
10 |
8 |
6 |
3 |
Cocaine |
3 |
- |
3 |
3 |
8 |
5 |
5 |
15 |
1 |
Opioidsb |
- |
- |
- |
- |
4 |
1 |
2 |
2 |
- |
Multiple substancesc |
1 |
1 |
3 |
3 |
5 |
3 |
3 |
2 |
1 |
Subversions |
6 |
7 |
7 |
8 |
11 |
11 |
14 |
9 |
13 |
Percent affected |
44% (12 of 27) |
64% (12 of 19) |
50% (15 of 30) |
44% (16 of 36) |
72% (31 of 43) |
71% (30 of 42) |
82% (32 of 39) |
79% (31 of 39) |
64% (18 of 28) |
Post-Event |
|||||||||
Total results (drug & alcohol positives & subversions) |
7 |
7 |
5 |
13 |
17 |
13 |
11 |
2 |
4 |
Total results (drug positive & subversions) |
6 |
7 |
4 |
11 |
15 |
11 |
11 |
2 |
4 |
Test Results Associated with Rule Changes (Panel of Drugs or Subversions) |
|||||||||
Amphetamines |
1 |
- |
1 |
2 |
2 |
3 |
3 |
- |
- |
Cocaine |
- |
1 |
2 |
1 |
6 |
1 |
1 |
- |
1 |
Opioids |
2 |
- |
- |
- |
1 |
2 |
- |
- |
- |
Multiple substances |
- |
- |
- |
- |
- |
- |
- |
- |
- |
Subversions |
- |
2 |
- |
4 |
1 |
2 |
3 |
- |
1 |
Percent affected |
50% (3 of 6) |
43% (3 of 7) |
75% (3 of 4) |
64% (7 of 11) |
60% (8 of 15)d |
73% (8 of 11) |
64% (7 of 11) |
0% |
50% (2 of 4) |
a Amphetamines results include amphetamine and methamphetamine.
b Opioid results include 6-acetylmorphine (6-AM), codeine, and morphine.
c Any combination of amphetamines, cocaine, or opioids.
d One post-event test in 2015 was a subversion attempt, and the donor’s observed specimen was positive for amphetamine and methamphetamine. This event was counted only once in the “percentage affected” tally.
Source: This table presents testing event data reported using NRC Form 890, “Single Positive Test Form.”
The for-cause testing results show that between 44 and 82 percent of positive drug tests and subversion attempts for this test category each year were for the drugs updated in the final rule. For post-event testing, 0 to 75 percent of the positive drug test results and subversion attempts for this test category each year were associated with the drugs updated by this final rule.
The NRC staff estimates that once the final rule is implemented, an additional 176 individuals using drugs or attempting to subvert the testing process will be detected per year. This represents an estimated 22‑percent increase in detection over the average number of individuals from CY 2009 through CY 2019 with a positive drug test result or identified as attempting to subvert a test. The estimated benefits in detection apply to seven qualitatively analyzed attributes described in Section 4.1: public health (accident), occupational health (accident), offsite property, onsite property, regulatory efficiency, safeguards and security considerations, and other considerations (public perception, workplace productivity, workplace safety, and improved protection of individual rights). The final rule accomplishes this by lowering the testing cutoff levels and improving the methods of detection for existing drugs in the testing panel (amphetamine, cocaine, methamphetamine, and heroin) and adding six impairing substances to the testing panel (hydrocodone, hydromorphone, methylenedioxymethamphetamine (MDMA), methylenedioxyamphetamine (MDA), oxycodone, and oxymorphone). Enhanced testing capabilities and an expanded testing panel may improve the early identification of additional individuals using drugs (i.e., pre-access, random, and followup tests) instead of by tests performed as a result of possible impairment or a safety event (i.e., for-cause and post-event tests). The dominant safety effect of this rule is to maintain reasonable assurance of a workplace free of impairing drugs and the effects of such substances (both illegal drugs and the misuse of legal drugs).
4. Evaluation of Benefits and Costs
This section examines the benefits and costs estimated to result from this rulemaking when compared to Alternative 1 (Take No Action alternative). Section 4.1 identifies attributes expected to be affected by the rulemaking. Section 4.2 describes how the NRC staff analyzed benefits and costs.
4.1 Identification of Affected Attributes
This section identifies the factors within the public and private sectors that the regulatory alternatives discussed in Section 2 are expected to affect. These factors are classified as “attributes” using the list of potential attributes provided in Chapter 5 of NUREG/BR-0058, “Regulatory Analysis Guidelines of the U.S. Nuclear Regulatory Commission,” draft Revision 5, issued February 2020 (NRC, 2020a). Each of the following 10 attributes is quantified when possible, and an uncertainty analysis is performed to report benefit and cost estimate confidence levels and to identify those variables that most affect the variation in the results distribution:
Public Health (Accident): The final rule reduces the risk to public health by helping to prevent events that may initiate or contribute to accidents or transients that could result in radiological releases to the environment. The changes reduce this public health risk by identifying additional individuals who may be impaired by their use of illegal drugs or misuse of legal drugs, thereby enabling licensees to deny or remove unescorted access authorization from these persons. This licensee action not only prevents individuals using drugs from being granted or maintaining unescorted access to the protected areas of NRC-licensed facilities, to SSNM, or to sensitive information, it also prevents these individuals from conducting the safety- and security-sensitive duties and responsibilities described in 10 CFR 26.4, “FFD program applicability to categories of individuals.” If individuals are impaired during the conduct of these activities, they have a higher potential to initiate accidents and transients as a result of human performance errors.
The NRC established a strong link between the FFD-related authorization provisions in 10 CFR Part 26 and the physical protection access authorization requirements described in 10 CFR Part 73. This relationship between FFD and access authorization strengthens the defense in depth associated with the enhanced ability to identify individuals using drugs who are not fit for duty or are not trustworthy and reliable, or both. As described in the original 10 CFR Part 26 rule (54 FR 24470, June 7, 1989)—
The NRC believes that the reliability, integrity, and trustworthiness of persons working within nuclear power plants are important to assure public health and safety. The granting of a license is based on the assumption that workers will abide by the licensees’ policies and procedures in all areas. Indications of lack or reliability, integrity, or trustworthiness, therefore, even so far as they pertain to off-site behaviors, are relevant to the NRC’s need to assure that nuclear power plants are operated safely.
The NRC further discussed these positions in the 2008 FFD final rule (73 FR 16971; March 31, 2008):
Part 26 and the access authorization requirements [of Part 73] each contain provisions that require establishing the trustworthiness and reliability of personnel before granting unescorted access authorization to the protected area of nuclear power plants.
Consequently, unless the NRC FFD program is robust in the identification of these individuals, security and safety vulnerabilities could exist because individuals with questionable motives may have unescorted access authorization.
The identification of additional individuals with confirmed positive test results leads not only to the denial of their unescorted access to that licensee’s facility in accordance with the site FFD program (see 10 CFR 26.75, “Sanctions”), but it also addresses these security and safety vulnerabilities at other commercial power reactor facilities. This occurs, in part, because denial of authorization information is shared with other NRC licensees, and these licensees must meet the authorization requirements described in both 10 CFR Part 26 and 10 CFR Part 73 before granting unescorted access authorization to any individual who was previously found to be in violation of a licensee’s FFD policy. Therefore, this program provision provides assurance that individuals of questionable honesty and integrity do not represent a safety or security concern at a different facility without adjudication by the licensee reviewing official.
Occupational Health (Accident): The final rule reduces the risk that occupational health is adversely affected by radiological releases and workplace mishaps, events, or occurrences. Risk reduction is accomplished by identifying additional individuals using drugs who are subject to the 10 CFR Part 26 drug testing requirements.
The identification of additional individuals who are not fit for duty facilitates licensee action to prevent drug-induced impairment from causing or contributing to human performance errors that may result in unplanned occupational radiation exposure; personal safety issues; or improper operation, maintenance, or surveillance of safety- and security-related SSCs. This outcome also assures that timely and effective actions will be initiated in response to accidents, transients, environmental conditions, and security threats and that human performance during these exigent situations will not degrade with time because of substance-induced impairment or withdrawal symptoms.
Although nonradiological occupational health is not within the scope of the NRC’s regulatory authority, a beneficial consequence of the 10 CFR Part 26 drug testing program is that it provides assurance that individuals are fit for duty. As described in 10 CFR 26.23(d) and (b), the FFD program must, in part, “[p]rovide reasonable assurance that the workplaces subject to this part are free from the presence and effects of illegal drugs” and “that individuals are not under the influence of any substance, legal or illegal, or mentally or physically impaired from any cause, which in any way adversely affects their ability to safely and competently perform their duties,” respectively.
Consequently, the identification of additional persons not fit for duty through the conduct of drug testing and the subsequent denial of unescorted access authorization to these individuals reduces the risk of occupational health (radiological and nonradiological) accidents.
Offsite Property: The final rule reduces the risk that radiological releases affect offsite property by identifying additional individuals impaired from using illegal drugs or misusing legal drugs among persons applying for unescorted access and those already granted unescorted access to an NRC-licensed facility. Identifying additional individuals using drugs reduces the risk of accidents and security incidents resulting from impairment that could adversely affect offsite property.
Onsite Property: The rule reduces the risk of damage to onsite property by identifying additional individuals impaired by using illegal drugs or misusing legal drugs among individuals applying for unescorted access and those already granted unescorted access to an NRC-licensed facility. Identifying additional individuals using drugs reduces the risk of accidents and security incidents resulting from impairment that could adversely affect onsite property.
Industry Implementation: The final rule requires licensees to revise their policies, procedures, training, and contracts with HHS-certified laboratories and blind performance test sample (BPTS) suppliers. Licensees that use an LTF also train Laboratory Technicians on the drug testing panel changes and validate the updated drug testing assays. Any implementation costs for HHS-certified laboratories would be included in the specimen testing costs charged to licensees and other entities (see “Industry Operation” below). The increased detection of impaired individuals reduces the risk of accidents and security incidents resulting from that impairment. Section 5.1.1 and Appendix C provide the quantitative analysis of this attribute.
Industry Operation: The final rule results in an increase in the cost to test each specimen because the testing panel includes more drugs. The changes to the drug testing panel also result in an increase in the number of individuals identified as using illegal drugs or misusing legal drugs and the number of 10 CFR Part 26 actions that each licensee must take subsequent to a positive drug test result or a confirmed subversion attempt. However, the increased detection of impaired individuals reduces the risk of accidents and security incidents resulting from that impairment. The final rule results in savings during pre-access testing from averted training costs associated with additional individuals testing positive because of the rule changes.0 Sections 5.1.2 through 5.1.6 and Appendix C provide the quantitative analysis of this attribute.
Regulatory Efficiency: The final rule results in improved regulatory efficiency by achieving better consistency and less redundancy with select drug testing procedures in the 2008 HHS Guidelines, as well as better internal consistency within 10 CFR Part 26. The final rule also harmonizes some of the NRC’s definitions with those in the 2017 HHS Guidelines, prevents dual regulation of HHS‑certified laboratories in the areas of laboratory personnel and procedures, and clarifies ambiguous or imprecise regulatory language in 10 CFR Part 26 (such as the changes to the 10 CFR Part 26 definitions). Lastly, the changes improve the protections afforded to individuals by requiring certain drug tests to be evaluated to the LOQ instead of to the limit of detection (LOD) and requiring the MRO to perform an additional review of an invalid test result due to high urine pH. These donor protection changes improve regulatory efficiency by reducing the potential for appeals associated with FFD policy violations and any subsequent followup NRC inspections.
Safeguards and Security Considerations: The final rule increases the ability of affected entities to identify additional individuals who are not trustworthy and reliable by enhancing the detection of illegal drug use, legal drug misuse, and attempts to subvert the drug testing process. The changes also enhance deterrence through the training of subject personnel on the rule changes. This could occur because the requirements in 10 CFR 26.29, “Training,” necessitate the communication of the panel of drugs to be tested, the drug testing cutoffs, required sanctions, and licensee actions that are taken if an individual violates the licensee’s FFD policy.
The benefit of the final rule related to safeguards and security considerations is reflected qualitatively in the “Public Trust” topic within the “Other Considerations” attribute.
Other Considerations
Public Perception: The final rule changes provide the public with additional assurance that the NRC is addressing potential safety concerns that could result from worker use of impairing drugs and security concerns by identifying individuals who display or demonstrate characteristics of not being fit for duty, or not being trustworthy and reliable, or both. Furthermore, the rule changes more closely align 10 CFR Part 26 with existing Federal agency drug testing programs for individuals in positions analogous to those covered by the NRC FFD testing program. These Federal agency drug testing programs include, but are not limited to, those implemented by over 100 Federal agencies that test Federal employees, and comparable Federal agencies testing civilians in safety- and security-sensitive positions. An example of such a comparable Federal agency is the DOT, with testing for airline pilots, armed security guards, bus drivers, rail and transit engineers, and commercial truck drivers hauling hazards materials. Parity with all comparable Federal agency drug testing programs improves public perception of the effectiveness of 10 CFR Part 26.
Public Trust: The final rule changes strengthen the defense-in-depth regulatory framework associated with the identification of individuals using illegal drugs, misusing legal drugs, or attempting to subvert the testing process and who are determined not to be fit for duty, or not to be trustworthy and reliable, or both. Therefore, the changes reinforce the link between the FFD‑related authorization provisions in 10 CFR Part 26 and the physical protection access authorization requirements in 10 CFR Part 73. This rule addresses these safety and security vulnerabilities and should boost public trust, because once unescorted access authorization is denied, an individual cannot act as an insider threat to challenge the safe and secure operation of the facility and the transportation of SSNM, the safety and security of licensee employees and C/Vs, or the safeguarding of sensitive information.
Worker Productivity: Affected licensees may accrue benefits from using the expanded drug testing panel and the increased testing sensitivities, which could result in deterring additional individuals from using the drugs included in the NRC testing panel. A beneficial outcome is that these changes result in improved workforce productivity, reduced employee turnover, and reduced absenteeism related to the health effects associated with drug use and possible addiction (ONDCP, 2011). The effects of productivity losses caused by undetected drug use0 could have direct and indirect effects on operating costs. Furthermore, the impact of employee drug use is a problem that extends beyond the drug-using employee. Coworkers may have to work harder, redo work, or cover a shift for a coworker because of a fellow employee’s absence (ONDCP, 2011). In addition, enhancing the detection of illegal drug use, legal drug misuse, and subversion attempts may deter individuals using drugs from seeking employment at a facility with a drug testing program and existing employees from starting to use drugs. It may also encourage existing employees to seek medical assistance to address an addiction or misuse issue, which could result in a lower turnover rate for individuals possessing requisite skills, knowledge, and experience who contribute to the safe and secure operation of the NRC-licensed facility. With a lower turnover rate, licensees may accrue benefits from reduced expenditures to recruit, hire, and train replacement employees (Boushey and Glynn, 2012; Tracey and Hinkin, 2008).
Improved Protection of Individual Rights: Individuals subject to 10 CFR Part 26 may accrue benefits from the revised MRO review procedures for invalid test results due to high pH values and from clearer requirements describing MRO actions when a donor requests testing of Bottle B or a retest of a single specimen and the specimen is unavailable. Additionally, workers may accrue benefits from the change to use the LOQ instead of the LOD in various test scenarios. The LOQ reliably detects and quantifies an analyte (the substance tested), whereas the LOD reliably detects an analyte but does not precisely quantify it. This change provides an additional measure of accuracy in the testing process. The changes improve consistency with the HHS Guidelines, provide additional protection of individual rights, and may reduce the number of potential appeals of drug testing results (10 CFR 26.39, “Review process for fitness-for-duty policy violations”).
The staff does not expect this rulemaking to affect the attributes of public health (routine), occupational health (routine), NRC implementation and operation, other government, general public, improvements in knowledge, antitrust considerations, and environmental considerations.
4.2 Analytical Methodology
This section describes the process used to evaluate benefits and costs associated with the identified alternatives. The benefits include any desirable changes in affected attributes (e.g., monetary savings, improved safety, improved security), while the costs include any undesirable changes in affected attributes (e.g., monetary costs, increased exposures).
Of the nine affected attributes discussed in Section 4.1, two could be evaluated on a quantitative basis—industry implementation and industry operation. Quantitative analysis requires a baseline characterization of the affected universe (see Table 5‑16 and Appendices B and C to this document), including the characterization of factors such as the number of affected entities, the nature of the activities being conducted, and the types of systems and procedures that licensees implement or no longer implement if the final rule alternative is chosen. Affected entities differ from each other in a variety of ways, such as FFD program management (e.g., specific to a site, or centrally managed at a corporate office by a licensee that owns multiple sites) and testing laboratories used (e.g., LTF, HHS‑certified laboratory). As a result, affected entities may respond to the final rule changes in different ways. Sections 4.2.1 through 4.2.6 present the analytical data and assumptions used in the quantitative analysis of these attributes, which the NRC staff then used in performing the uncertainty analysis contained in Section 5.2.
The analysis relies on non-quantitative techniques for the remaining seven affected attributes (public health (accident), occupational health (accident), offsite property, onsite property, regulatory efficiency, safeguards and security considerations, and other considerations (which include public perception, workplace productivity, workplace safety, and improved protection of individual rights)). Non-quantitative techniques are used because monetizing the full impact of each attribute is not possible or practical. Monetizing the impact of these attributes requires the estimation of factors such as the frequency of accidents and other safety- and security-related events caused by drug‑induced impairment and the consequences of such events. These data do not exist. However, improving the detection of individuals who use impairing drugs supports the general performance objectives in 10 CFR 26.23(c), to “provide reasonable measures for the early detection of individuals who are not fit to perform the duties that require them to be subject to the FFD program,” and in 10 CFR 26.23(d), to “provide reasonable assurance that the workplaces subject to this part are free from the presence and effects of illegal drugs.” Sections 4.2.1 through 4.2.6 describe the analytical method and assumptions used in the quantitative and non-quantitative analyses of these attributes. Appendices B through D present the analysis calculations, unit costs, data sources, and assumptions used.
To estimate the costs associated with the evaluated alternative, the NRC staff used a work breakdown approach to deconstruct the activities for each requirement. For each required activity, the NRC staff further subdivided the work across labor categories (e.g., FFD Manager, Facility Worker). The NRC staff estimated the necessary level of effort for each required activity and labor rates for personnel performing these activities to develop cost estimates.
The NRC staff gathered data to develop levels of effort and unit cost estimates. The NRC staff applied several cost estimation methods in this analysis. The NRC staff used professional knowledge and judgment to estimate some of the costs and benefits. Additionally, the NRC staff used an engineering buildup method, solicitation of licensee input, and extrapolation techniques to estimate costs and benefits. The engineering buildup method used a step‑by‑step, bottom-up description of task requirements and estimated resources for labor, materials, and other direct costs to estimate a total cost. The NRC staff also consulted subject matter experts within and outside of the agency to develop inputs used in the analysis. For example, the NRC staff collected industry wage data, costs for specimen testing, and other inputs for this analysis.
The NRC staff extrapolated to estimate the cost for some activities, relying on past or current costs to estimate the future cost of similar activities. For example, to estimate the cost to conduct testing for two Ecstasy-type drugs and four Schedule II opioid drugs (i.e., hydrocodone, hydromorphone, oxycodone, and oxymorphone) at an HHS‑certified laboratory, the NRC staff used the testing costs published by the DOT in its final rule aligning 49 CFR Part 40 with the 2008 HHS Guidelines (75 FR 49850; August 16, 2010) and increased that cost based on operational data for current drug testing costs and the projected number of future positive test results. However, for steps for which the NRC staff has no data, the NRC staff estimated the level of effort based on similar steps in the process for which data are available.
To evaluate the effect of uncertainty in the model, the NRC staff employed a Monte Carlo simulation, which is an approach to uncertainty analysis in which input variables are expressed as distributions. The simulation was run 5,000 times, and values were chosen at random from the distributions of the input variables provided in Section 5.2 of this document. The result is a distribution of values for the output variable of interest. The Monte Carlo simulation makes it possible to determine the input variables that have the greatest effect on the value of the output variable. Section 5.2 gives a detailed description of the Monte Carlo simulation methods and the results.
4.2.1 Baseline for Analysis
This regulatory analysis measures the incremental impacts of the rulemaking alternative relative to a baseline that reflects the anticipated behavior if the NRC undertakes no other regulatory action (Alternative 1, Take No Action alternative). As part of the regulatory baseline used in this analysis, the NRC staff assumes licensee compliance with existing NRC regulations. Section 5.1 presents the estimated incremental costs and benefits of the rule relative to this baseline.
4.2.2 Affected Entities (Sites and Fitness for Duty Programs)
For use in this analysis, the NRC staff created the following groupings based on how the alternative affects NRC licensees:
FFD Program Sites:0 The analysis modeled 59 sites covered by the 10 CFR Part 26 FFD program requirements, including 51 power reactor sites,0,0 5 corporate offices, 2 Category I special nuclear material licensees, and 1 C/V that maintains its own FFD program. Appendix A to this document contains site-specific FFD program performance data supporting this quantification. The analysis includes Diablo Canyon Units 2 and 3, until the site permanently ceases power operations in 2024 and 2025, respectively. The net result is that beginning in CY 2026, the analysis models 58 sites with FFD programs, which includes 50 operating power reactor sites.
FFD Programs: The analysis models 24 FFD programs for the 59 sites covered by 10 CFR Part 26. FFD programs are based on corporate ownership. If a corporate entity operates multiple sites, the entity will maintain one FFD program for all its sites (see Appendix A to this document).
Drug Testing Laboratories: Each licensee and other entity may choose to conduct initial urine specimen testing at an LTF; then, it must conduct confirmatory testing at an HHS‑certified laboratory. Alternatively, the licensee or entity may conduct all urine testing at an HHS-certified laboratory. The analysis models that 56 sites will conduct all urine testing at HHS-certified laboratories and that 3 sites will use an LTF for initial testing and an HHS‑certified laboratory for confirmatory testing.
Future Nuclear Facilities: Recently, there has been an increasing emphasis on the development of small and medium reactors with capacities that range between 1.5 megawatts electric to several hundred megawatts per unit. The designs of these reactors incorporate features to make them simpler and quicker to build, operate, inspect, maintain, and repair relative to existing facilities. These features will result in smaller workforces to construct and operate these new facilities and fewer personnel who are subject to FFD testing than existing nuclear power plants or nuclear fuel facilities. Information collected on projected staffing estimates for various small modular designs (IAEA, 2001) shows that these new facilities could have staffing levels lower than for existing like-sized units, in the range of a 30- to 40-percent reduction (IAEA, 2001; Reuters, 2018). Staffing levels for small units could be even less, limited to a minimum number required to support safe operations and security regardless of power level.
4.2.3 Cost and Benefit Calculations
This section describes the method used to estimate the quantifiable costs and benefits:
All licensees and other entities subject to 10 CFR Part 26 comply with the existing regulatory requirements. Therefore, this analysis only presents the incremental costs associated with the final rule changes.
The total industry cost or benefit associated with each rule requirement is calculated using the following five-step approach:
Step 1: Estimate the average incremental cost or benefit per affected entity (i.e., a site or FFD program) to comply with the new requirement (e.g., the cost to conduct initial urine drug testing for two Ecstasy-type drugs). The use of average incremental cost or benefit per entity is a simplification, with some affected entities incurring higher or lower costs.
Step 2: Estimate the number of times an affected entity would incur the incremental cost or benefit associated with the new requirement in a year (e.g., how many individuals will be drug tested at each site).
Step 3: Estimate the number of affected entities that would incur the incremental cost or receive the benefit associated with the new requirement in a year.
Step 4: Estimate the number of years the incremental cost or benefit would be incurred.
Step 5: Multiply the outcomes of Steps 1 through 4.
Not all final rule requirement changes apply to all 59 sites or all 24 FFD programs. For example, some changes only impact the three sites that conduct initial drug and validity testing at LTFs and not the 56 sites that only use HHS‑certified laboratories to conduct all drug and validity testing. Appendix C presents the differences in the cost calculations by final rule requirement.
The average cost per site to comply with each rule requirement is calculated by dividing the total industry cost or benefit per requirement by the total number of affected sites. While the average cost or benefit per site does not present the potential variability for an estimated value based on facility type (e.g., corporate office, Category I special nuclear material licensee facility, operating power reactor), the NRC staff believes that this is a reasonable measure to present the potential impact to the nuclear industry of each rule change for the following four reasons:
Operating power reactors constitute the majority of sites in the analysis
(86 percent of sites; 51 of 59 sites).
The rule changes (beyond the implementation activities in the initial year of the rule associated with policy updates, contract revisions, and training) only pertain to conducting drug tests and the associated positives that result from those tests. Therefore, the impact of the rule is directly dependent on the number of individuals tested at each site and the resulting positive tests. Typically, a multiunit nuclear power reactor site will use a larger workforce than a single-unit site. However, the workforce at any site is affected by plant outages because of the additional workers brought on site. Appendix A provides site‑specific testing data from CY 2009 through CY 2019.
The number of positive test results may vary from year to year at a site. Possible reasons for changes in the positive testing rate at a site might include changes in the characteristics of the workforce (e.g., age of workers, job duties, and employment types), number of new hires, or changes in the availability of illegal drugs in the local area. For example, the analysis of FFD program performance data has identified that C/Vs, on average, have a higher rate of positive test results (i.e., approximately 3.7 times more) than licensee employees (NRC, 2014).0 In outage years at a site, it is typical to see an increase in the number of positive results because of the surge in the number of short-term contractors used to support the outage.
The size of the workforce at the two Category I special nuclear material licensees, five corporate offices, and one C/V (Institute of Nuclear Power Operations (INPO)) is much smaller and more stable than at operating power reactor sites or at power reactor construction sites because these sites do not experience periodic workforce surges for refueling outages or new construction. Drug use is also very low, as presented in Appendix A. As a result, the NRC staff anticipates that these types of facilities will incur costs lower than the average per site.
Testing Data by Facility Type: To evaluate variability among facility types, the NRC staff analyzed testing data for CY 2009 through CY 2019 and calculated the average number of tests performed and the average number of positive results for each of the sites. Table 4-1 summarizes the results of the site-specific testing data analysis. Appendix A includes the site‑specific testing data summarized in this table.
Table 4-1 Range of Testing Data by Facility Type (CYs 2009–2019)
-
Facility Type
Number
of Units
No. of Tests per Year
No. of Positives per Year
Minimum
(10%)
Maximum
(90%)
Average
Minimum
(10%)
Maximum
(90%)
Average
Power Reactor—Operating
All
1,157
3,804
2,442
4.0
25.0
14.4
1
805
2,704
1,713
2.0
20.0
10.5
2
1,785
3,802
2,725
8.0
28.0
16.2
3
2,703
4,628
3,845
14.0
27.7
20.2
Power Reactor—Constructiona
3,277
15,829
8,525
56.0
344.0
168.5
Corporate Office
291
717
515
0
2.0
1.0
Category I Special Nuclear Material licensee facility
491
886
766
0
4.0
1.9
C/V (INPO)
264
367
310
0
1.0
0.4
a The power reactor—construction category reflects test data from Vogtle Units 3 and 4, which is expected to be completed by November 2022. The construction of V.C. Summer Units 2 and 3 was halted on July 31, 2017.
Operating power reactors have the largest variability in the number of tests conducted by facility type. This variability primarily depends on the number of reactors at the site (e.g., one to three units), although an analysis of the data in Appendix A shows that a single-unit site may perform more tests annually than a two‑unit site, and a two‑unit site may conduct more tests annually than a three‑unit site.
Variability in the workforce size at a reactor construction site depends on the stage of construction. The NRC FFD program performance data for CY 2009 through CY 2019 reflect construction on Vogtle Units 3 and 4, which began in CY 2009 and is expected to be completed in November 2021 and November 2022, respectively.0
Reactor construction sites have the largest number of positive tests of any facility type. Reactor construction sites primarily rely on C/V personnel, and the positive testing rates for these workers have been higher than comparable C/V workforces used at operating power reactor sites (including during outages) (see Appendix A).
New Nuclear Facility Staffing Levels: To evaluate the effect of the rule changes on nuclear facilities constructed after the effective date of the rule, the NRC staff estimated the facility staffing levels, which are directly correlated to the amount of testing performed. Table 4-2 provides nominal staffing levels for future nuclear facilities.
Table 4-2 Nominal Staffing Levels for Future Nuclear Facilities
Facility Type |
Unit Rating (MWt) |
Nominal Operating Staffing Level Subject to 10 CFR Part 26a |
||
Minimum (10%) |
Maximum (90%) |
Average |
||
Microreactor |
≤ 20 |
10b |
40c |
25 |
Small reactor |
20 < x < 1000 |
40 |
240 |
140 |
Medium reactor |
1,000 ≤ x < 2,000 |
210 |
420 |
315 |
Large reactor |
≥ 2,000 |
Same staffing level as current reactors |
||
Category I special nuclear material licensee facility |
-- |
Same staffing level as current Category I special nuclear material licensee facilities |
||
a Staffing levels are nominal for illustrative purposes and are intended to provide an order of magnitude‑level estimate of the impact of the final rule.
b Some microreactor units are intended for minimal operational staffing, with an option for unattended operation and monitoring by a centralized, regional support facility. The NRC staff assumes five shifts with two persons per shift.
c The assumed staffing of an operating shift is four persons, which includes the shift supervisor, the chief reactor control engineer, a mechanic for mechanical operating systems, and an electrician for instrumentation and control systems. Assuming five shifts plus support staff including engineers, technicians, and administrative staff results in 40 persons.
Analysis Horizon: Licensees incur costs and savings over a 24-year time period, the average remaining license term of the 59 sites0 included in the analysis. The time period that each site operates is dependent on the term of the operating license and whether the licensee chooses to operate the site for the duration of the licensed period. The average life term is based on the following assumptions:
The licensee for each operating nuclear power reactor is known or assumed to apply for and receive a 20-year license extension beyond the original 40-year licensed term. In addition, the analysis accounts for a second 20-year license extension for 21 units whose licensees have either received this extension or have publicly announced their intention to apply for this license extension.0
Each Category I special nuclear material licensee is assumed to request and receive operating license extensions to support the possession, use, and manufacture of nuclear material. As these facilities provide nuclear material for noncommercial nuclear power reactors, the NRC staff assumed that their operations would continue (assumed at 63 years) independent of activities associated with civilian nuclear power reactors.
Base Year: The base year for this analysis is CY 2022. The NRC staff assumes that the final rule is effective in CY 2022. One-time implementation costs are assumed to be incurred in CY 2022. Ongoing and annual costs of operations related to the alternatives are assumed to begin in CY 2023, unless otherwise stated. Calculated benefits and costs are discounted into 2022 dollars.
Discounting of Costs and Savings: The costs or savings incurred in each year of the analysis are discounted at 7‑percent and 3‑percent discount rates as compared to the base year. These discount rates are in accordance with NUREG/BR-0058, draft Revision 5. Section 5.1 presents these results.
Cost/Benefit Inflators: To evaluate the costs and benefits consistently, the analysis inputs are put into base year dollars. The most common inflator is the consumer price index for all urban consumers (CPI-U), developed by the U.S. Department of Labor, Bureau of Labor Statistics. The formula to determine the amount in base year dollars is as follows:
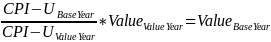
Sign Conventions: The sign convention used in this analysis is that all favorable consequences for the alternative are positive and all adverse consequences for the alternative are negative. Negative values are shown using parentheses (e.g., negative $500 is displayed as ($500)).
Labor Rates: In estimating the incremental costs of the alternatives, the analysis uses hourly labor rates that include salary, fringe benefits (e.g., paid leave and health benefits), and overhead (e.g., payroll costs). Table 5-16 presents the labor rates used for the uncertainty analysis, and Appendix B presents the labor rates (in 2022 dollars) for the base case.
4.2.4 Incremental Requirements in the Final Rule
The NRC quantitatively evaluated the impacts of the following six rule changes relative to the baseline described in Section 4.2.1:
Lowered initial and confirmatory drug testing cutoff levels for amphetamines and cocaine metabolites.
The final rule updates the cutoff levels for initial drug testing, listed in 10 CFR 26.133, “Cutoff levels for drugs and drug metabolites,” and 10 CFR 26.163(a)(1), and for confirmatory drug testing, listed in 10 CFR 26.163(b)(1), to conform with the changes to Section 3.4 of the 2008 and 2017 HHS Guidelines as follows:
lowers the initial drug testing cutoff level for cocaine metabolites by 50 percent (from 300 nanograms (ng) per milliliter (mL) to 150 ng/mL)
lowers the initial drug testing cutoff level for amphetamines by 50 percent (from 1,000 ng/mL to 500 ng/mL)
lowers the confirmatory drug testing cutoff level for cocaine metabolites by 33 percent (from 150 ng/mL to 100 ng/mL)
lowers the confirmatory drug testing cutoff levels for amphetamine and methamphetamine by 50 percent (from 500 ng/mL to 250 ng/mL)
Significantly lowering the drug testing cutoff levels for amphetamines and cocaine metabolites increases the timeframe in which these drugs can be identified after use (i.e., the window of detection). Increasing the window of detection increases the number of individuals identified with urine specimens containing amphetamines or cocaine metabolites, or both. Increased detection of amphetamines and cocaine use provides a higher degree of assurance that persons subject to 10 CFR Part 26 testing are not using these drugs and contributes to a licensee determination of whether each individual is fit for duty and trustworthy and reliable.0
Expanded initial drug testing panel to include 6-AM and revised confirmatory drug testing cutoff level for 6‑AM.
The final rule adds testing for 6-AM to the initial drug testing panel in 10 CFR 26.31(d)(1) and 10 CFR 26.405(d). It also makes conforming changes to the substances for initial testing listed in 10 CFR 26.133 and 10 CFR 26.163(a)(1), and for confirmatory drug testing listed in 10 CFR 26.163(b)(1). These changes ensure that 10 CFR Part 26 is consistent with Section 3.4 of the 2008 and 2017 HHS Guidelines.
The final rule revises the list of substances to be tested as follows:
includes initial drug testing for 6-AM with a 10-ng/mL testing cutoff level
eliminates the requirement to conduct confirmatory drug testing of 6-AM only when the confirmatory drug test result for morphine exceeded 2,000 ng/mL (if initial testing for 6-AM is positive, confirmatory testing for 6-AM is to proceed independent of the morphine concentration)
The enhanced testing capability enables the identification of additional instances of heroin use (6-AM is a metabolite of heroin). Enhancing the detection of 6-AM is important given the increased prevalence of heroin use among individuals performing safety-sensitive duties in other sectors of the economy and the adverse effect of these illegal drugs on persons in the workplace.0 In addition, improved testing for 6-AM could deter additional individuals from seeking employment in 10 CFR Part 26 regulated workplaces.
Expanded initial and confirmatory drug testing panels to include Ecstasy drugs.
The final rule adds testing for the Ecstasy-type drugs MDMA and MDA to the drug testing panel in 10 CFR 26.31(d)(1) and 10 CFR 26.405(d), to the substances for initial drug testing listed in 10 CFR 26.133 and 10 CFR 26.163(a)(1), and to the substances for confirmatory drug testing listed in 10 CFR 26.163(b)(1). Conforming changes add MDA and MDMA to the annual statistical summary reporting requirements for HHS‑certified laboratories in 10 CFR 26.169(h)(3). These changes ensure that 10 CFR Part 26 is consistent with Section 3.4 of the 2008 HHS Guidelines, with the exception of not listing MDEA, which the HHS subsequently removed from the list of authorized test analytes in the 2017 HHS mandatory guidelines (82 FR 7920, January 23, 2017).
The final rule revises the list of substances to be tested as follows:
includes initial drug testing for MDMA and MDA with a 500-ng/mL testing cutoff level
includes confirmatory drug testing for MDMA and MDA with 250-ng/mL testing cutoff levels.
Testing for these additional substances enables the identification of a greater range of illegal drugs that could impair human performance. Ecstasy is added to the drug testing panels because of its adverse effects on persons in the workplace. Testing for these substances also may deter additional individuals from seeking employment in 10 CFR Part 26 regulated workplaces.
Expanded initial and confirmatory drug testing panels to include the opioid drugs hydrocodone, hydromorphone, oxycodone, and oxymorphone.
The final rule adds testing for four additional Schedule II drugs of the Controlled Substances Act (i.e., oxycodone, oxymorphone, hydrocodone and hydromorphone) to the drug testing panel in 10 CFR 26.31(d)(1) and 10 CFR 26.405(d). Hydrocodone, hydromorphone, oxycodone, and oxymorphone are added to the substances for initial drug testing listed in 10 CFR 26.133 and 10 CFR 26.163(a)(1) and to the substances for confirmatory drug testing listed in 10 CFR 26.163(b)(1). Conforming changes add these four opioids to the annual statistical summary reporting requirements for HHS‑certified laboratories in 10 CFR 26.169(h)(3). These changes ensure that 10 CFR Part 26 is consistent with Section 3.4 of the 2017 HHS Guidelines (82 FR 7920, January 23, 2017).
The final rule revises the list of substances to be tested as follows:
includes initial drug testing for hydrocodone and hydromorphone with a 300‑ng/mL testing cutoff level
includes initial drug testing for oxycodone and oxymorphone with a 100-ng/mL testing cutoff level
includes confirmatory drug testing for hydrocodone, hydromorphone, oxycodone, and oxymorphone with 100-ng/mL testing cutoff levels
Testing for these four opioid pain relievers enables the detection of impairing drugs whose use has been increasing in society and that could impair human performance. The final rule adds hydrocodone, hydromorphone, oxycodone, and oxymorphone to the drug testing panels because of their prevalence of use and potential adverse effects on persons in the workplace. Testing for these substances also may deter additional individuals from seeking employment in 10 CFR Part 26 regulated workplaces.
Required special analyses testing of dilute specimens and specimens collected during suspected subversion attempts.
Existing regulations in 10 CFR 26.163(a)(2) provide licensees with the option to conduct special analyses testing on any urine specimen with a dilute validity test result (i.e., a creatinine concentration greater than or equal to 2 milligrams (mg) per deciliter (dL) but less than 20 mg/dL). Special analyses testing consists of conducting confirmatory drug testing to the LOD for any drug with an initial test result (i.e., immunoassay response) equal to or greater than 50 percent of the testing cutoff level.
The final rule includes three changes:
requires special analyses testing for any drug in a dilute specimen with an initial drug test result that is equal to or greater than 40 percent of the testing cutoff level
expands special analyses testing to circumstances in which a subversion attempt is suspected during the specimen collection process (e.g., if the initial specimen is out of the expected temperature range, the second specimen collected under direct observation would be subject to the special analyses provisions)
use of the LOQ instead of the LOD as the level at which confirmatory drug testing is to be conducted
These three changes enhance the detection of individuals using illegal drugs or misusing legal drugs, or both, in circumstances when the urine specimens provided do not present normal physiological characteristics. The 2008 and 2017 HHS Guidelines do not address special analyses testing, but the final rule changes are based on industry experience (i.e., high industry adoption of the voluntary 10 CFR 26.163(a)(2) special analyses testing of dilute specimens and the additional dilute positive test results identified each year) and feedback received from HHS-certified laboratories in implementing the 2008 FFD final rule.0
Allowing for the collection and testing of an alternative specimen (oral fluid).
The final rule allows for the collection and drug testing of an oral fluid specimen as an alternative to the collection of a urine specimen under direct observation collection conditions.
The NRC staff developed equations to estimate costs and savings using available data and described any assumptions used, when necessary. Appendices B, C, and D document this analysis, including the specific per‑site or per‑FFD‑program cost assumptions used to quantify costs and savings.
The final rule also includes the following changes, which result in either no or negligible incremental costs to licensees but lead to some benefits as discussed below:
The final rule adds and revises definitions in 10 CFR Part 26 to improve consistency with the definitions in the 2008 HHS Guidelines and also to improve internal consistency in 10 CFR Part 26. These administrative changes are estimated to result in negligible incremental costs and could result in savings. The changes improve regulatory efficiency, in part by promoting clear and unambiguous communications.
The final rule replaces the LOD with the LOQ as the decision point for determining whether a specimen contains an adulterant or is invalid (i.e., a valid test result cannot be determined) based on the possible presence of a halogen or an oxidizing adulterant. This entails minor procedural changes with negligible incremental costs. The change to LOQ enhances the protection afforded to individuals subject to validity testing because the test result reliably identifies and quantifies the substance tested.
The final rule clarifies the procedures for observed urine specimen collections, as well as specimen quantity, altered specimens, and refusals to test. These changes clarify existing procedures, and the NRC staff therefore expects incremental costs to be negligible. The changes enhance consistency with the 2008 and 2017 HHS Guidelines and allow for increased flexibility in licensee implementation of the rule.
The final rule permits additional licensee or other entity staff at the collection site to observe a donor in the hydration process. An individual enters the hydration process after the initial unsuccessful attempt to provide a urine specimen of adequate volume for testing (i.e., a shy bladder). Currently, the specimen collector must remain with the donor for the duration of the hydration period (a maximum of 3 hours) and not conduct an additional collection until the collection process for the hydrating individual is completed. The final rule adds flexibility to the collection process by permitting the original specimen collector to conduct additional collections while the hydrating donor is observed by another specimen collector or individual who has been instructed on required responsibilities (a hydration monitor). The NRC does not collect data on the incidence of shy‑bladder events and therefore is unable to estimate potential savings associated with the additional flexibility provided in the final rule.
The final rule eliminates the 6-month in-service limit for BPTSs and allows BPTS suppliers to specify the shelf life of sample lots. The option to specify shelf-life duration adds flexibility to the rule and does not impose any incremental costs because current practice is acceptable. The change enhances consistency with the 2008 and 2017 HHS Guidelines, which do not require similar in‑service limits on BPTS lots.
The final rule removes 10 CFR 26.155, “Laboratory personnel,” and paragraphs (b) through (e) of 10 CFR 26.157, “Procedures,” which repeat requirements contained in the HHS Guidelines that the National Laboratory Certification Program (NLCP) verifies in order for a laboratory to achieve and maintain HHS certification. Eliminating dual regulation of an HHS-certified laboratory (a private entity) reduces the regulatory burden on licensees.
The final rule address issues described in an enforcement guidance memorandum dated March 31, 2009 (NRC, 2009c) on the testing of quality control samples at licensee testing facilities.
The final rule establishes a required MRO review for invalid validity test results due to high pH values between 9.0 and 9.5. This review will result in some incremental effort on the part of the MRO (e.g., about an hour per occurrence to review specimen handling conditions), but the cost will be incurred infrequently because an invalid specimen test result is a rare event. Therefore, the total cost of the change will be small. This change enhances FFD program integrity and the protection of individual rights.
The final rule requires the MRO to document a donor’s verbal request to test Bottle B of a split specimen or retest a single specimen. This change ensures that the donor’s request is documented and confirms that a request is made in a timely manner (i.e., within 3 business days of MRO notification, as permitted under 10 CFR 26.165(b)(2)). This change enhances consistency with the 2008 and 2017 HHS Guidelines, increases transparency of the testing process, and affords due process to the donor.
The final rule requires the testing of any specimen collected during a post-event testing situation in which a testing refusal is determined during the collection process. Previously, any specimen collected could be discarded as the rule did not include a requirement for specimen testing. To improve the root‑cause evaluation process associated with accidents, testing of any urine specimen collected will be required to ensure that all available information is obtained to support the evaluation of human performance associated with the event. Because post‑event testing situations are rare, and an event in which a donor provides a specimen and then refuses to cooperate with the collector after providing the specimen is even rarer, the incremental cost associated with this rule change is negligible (i.e., the cost of testing a specimen for an infrequent event).
4.2.5 Data Sources
The analysis used the following data sources:
Affected entities: The determination of 59 affected entities, also called sites in the analysis, is based on the FFD program performance information reported to the NRC under 10 CFR 26.717. The analysis does not include data for any site that already has entered decommissioning or that has announced early plant closure and would cease operations before or during CY 2022 and no longer be subject to 10 CFR Part 26.0
Site-specific drug and alcohol testing data: Appendix A to this document presents the NRC FFD program performance data on the total number of drug tests conducted as well as the total number of positive, adulterated, substituted, and refusal to tests results by site for CY 2009 through CY 2019. The NRC staff used the average of 11 years of testing data, which accounts for several outage cycles for each operating power reactor.
Workforce to receive training on policy changes: Each site reports its workforce subject to 10 CFR Part 26 random testing in its annual FFD program performance report submission to the NRC as required by 10 CFR 26.717. This information is the best source available to the NRC on the size of the workforce that would require training on the rule changes. The NRC’s analysis of FFD program performance report data from CY 2009 through CY 2019 determined that the average workforce size subject to 10 CFR Part 26 testing annually is 92,356.
NRC drug testing information: The summary of FFD program performance reports for CY 2009 through CY 2015 (NRC, 2017) and FFD program performance data received for CY 2016 through CY 2019 (i.e., the agency has received and evaluated the data but has not yet published the summary report) are the sources of NRC licensee and other entity drug testing data used in the analysis. In the base case estimate, the NRC staff used the 11‑year average of data from CY 2009 through CY 2019 for the following:
number of drug tests conducted annually = 92,356
positive test rate for amphetamines = 0.066 percent
positive test rate for cocaine = 0.083 percent
Future nuclear facilities (test results): The NRC staff modeled the drug tests to be performed and the positive results to be expected during the operation of future nuclear facilities that commence operations after the effective date of the final rule.
Percent change in positive rates (amphetamines and cocaine): The changes in positive rates are based on an NRC analysis of MRO-verified drug test results from CY 2010 and CY 2011 for three DOT modal administrations (i.e., Federal Aviation Administration (FAA), Federal Transit Administration (FTA), and Federal Railroad Administration (FRA)). Use of MRO-verified results is important because Schedule II drugs can be legally prescribed to treat a medical condition (e.g., amphetamines may be prescribed to treat attention deficit disorder), and so the results could be downgraded to a negative result upon medical doctor review. Use of MRO-verified results ensures that the detection improvements model illegal drug use and not legitimate prescription use. The staff used 1 year of DOT testing data to limit the potential differences in substance use between the NRC- and DOT-covered workforces. The NRC applied the change in the positive testing rates in the year that the lower cutoff levels were implemented in the DOT testing program to the existing average positive testing rates for amphetamines and cocaine in the 10 CFR Part 26 testing program.
The NRC staff assumes that positive laboratory test results for amphetamines will be confirmed as illegal drug use or legal drug misuse by an MRO 75 percent of the time.
The NRC staff assumes that all positive laboratory test results for cocaine will be confirmed as illegal drug use or legal drug misuse by an MRO. It is unlikely that an individual subject to 10 CFR Part 26 would have recently been subject to a medical procedure for which cocaine might have been used (e.g., nasal or throat surgery, an intubation procedure) and then returned to work before the medical condition had resolved and the individual was able to physically return to work.
Positive rates for the expanded opioid panel: The NRC used the NRC FFD program performance test results for amphetamine positives as a proxy for the projected confirmed positive test rate for the expanded opioid panel. This decision is partially informed by the DOT’s HHS-certified laboratory drug test results after the DOT implemented the 2017 HHS Guidelines changes. The estimated increase in positive results for the expanded opioid panel is based, in part, on the incremental changes in the MRO‑verified positive testing rates in the year after the DOT implemented the HHS Guidelines changes (i.e., change in positive rates from 2017 to 2018 for opioids).
projected confirmed positive test rate for expanded opioid panel = 0.066 percent
Positive rates for 6-AM and Ecstasy-type drugs: The NRC used the DOT’s HHS‑certified laboratory drug test results after the DOT implemented the 2008 HHS Guidelines changes to inform the NRC’s expected positive rates for new drugs in the test panel. MRO verification for these test results is unnecessary because each is a Schedule I drug (i.e., an illegal drug with no medical use permitted in the United States). Unlike the positive test results for amphetamines and cocaine, these data do not permit analysis by DOT modal administration. Therefore, even though these positive rates are low, they may be higher than the analogous worker populations reflected in the three DOT modal administrations evaluated for amphetamines and cocaine. The analysis uses the average annual positive rates from CY 2010 through CY 2018:
6-AM = 0.016 percent
Ecstasy-type drugs = 0.004 percent
Specimen testing costs: The analysis used input from stakeholders received during and after public meetings held during rulemaking activities, and the estimated specimen testing costs published in Federal Register notices issued by the HHS and the DOT when testing changes were implemented. These data also were supplemented by the NRC staff’s professional judgment, when necessary. Appendix B lists the data sources for these inputs.
Special analyses testing of specimens collected under direct observation (suspect specimens): E-reported FFD program performance data provide detailed information on each subversion attempt. Table 4-3 presents information on the total number of subversion attempts confirmed in CY 2011 through CY 2019, the number of subversion attempts confirmed through the testing of specimens collected under direct observation, the percentage of subversion attempts determined through the testing of specimens collected under direct observation, and the percentage of all specimens collected each year that are suspect specimens collected under direct observation and that test positive.
Table 4-3 Suspect Specimens Collected Under Direct Observation
Year |
Total Number of Subversion Attempts |
Number of Subversion Attempts Confirmed by Testing of Specimens Collected under Direct Observation |
Percentage of Subversion Attempts Confirmed Through the Testing of Specimens Collected under Direct Observation |
Percentage of Total Specimens Collected Each Year that Are Suspect Specimens Collected under Direct Observation and Test Positive |
2011 |
128 |
42 |
32.8% |
0.030% |
2012 |
159 |
56 |
35.2% |
0.036% |
2013 |
145 |
44 |
30.3% |
0.029% |
2014 |
187 |
63 |
33.7% |
0.038% |
2015 |
232 |
81 |
34.9% |
0.050% |
2016 |
305 |
119 |
39.0% |
0.077% |
2017 |
305 |
93 |
30.5% |
0.063% |
2018 |
298 |
72 |
24.2% |
0.049% |
2019 |
307 |
68 |
22.1% |
0.052% |
Special analyses testing of dilute specimens: Beginning in CY 2013, changes to the e‑reporting forms permitted the uniform collection of data on the number of dilute specimens subject to special analyses testing. Additionally, the number of dilute specimens that tested positive during special analyses testing has been collected uniformly in the e-reporting system since CY 2011. Over 90 percent of licensees and other entities have voluntarily adopted the optional special analyses testing of dilute specimens in 10 CFR 26.163(a)(2).
Appendices B, C, and D provide more information on the assumptions and data sources used in the analysis.
4.2.6 Assumptions
The NRC staff made the following assumptions to quantify the costs and benefits of the rule alternative:
The NRC estimates of the expected positive testing rates are based on DOT drug tests performed from CY 2010 through CY 2018 for three DOT modal administrations (i.e., FAA, FTA, and FRA). These testing data represent a comprehensive set of annual drug testing results (approximately 483,000 tests per year) for a federally regulated industry (the transportation industry) with safety- and security-sensitive positions comparable to those in the commercial nuclear industry.
The estimated increase in positive results for amphetamines, cocaine, and the expanded opioid panel is based on the incremental changes in the MRO-verified positive testing rates in the year after the DOT implemented the HHS Guidelines changes (i.e., change in positive rates from 2010 to 2011 for amphetamines and cocaine, and the change in positive rate from 2017 to 2018 for opioids). For heroin and Ecstasy-type drugs, the analysis modeled the detection of these drugs by taking the average annual unverified laboratory positive test results reported by the DOT from October 2010 (when the DOT began implementing the 2008 HHS Guidelines) through CY 2015, which is the extent of DOT data available. While these testing data are unverified, these drugs are Schedule I drugs (i.e., no legitimate medical use in the United States).
The NRC evaluated 11 years of site-specific 10 CFR Part 26 FFD program performance testing data (CY 2009 through CY 2019) to establish the baseline estimates of tested populations and positive testing rates for substances evaluated in the regulatory analysis. The NRC also used the data from this period to determine expected variation in certain inputs in order to establish realistic ranges for use in the uncertainty analysis.
The NRC used 10 CFR Part 26 FFD program performance testing data as the basis to forecast the future positive testing rates for 6-AM, amphetamines, cocaine, dilute specimens, and suspect specimens (subversion attempts). The site-specific FFD program performance data in Appendix A reflect the total tests performed and total positive test results per year (includes all drug and alcohol positives and subversion attempts). These data were used to model the number of tests performed at each facility type, as well as the number of testing violations reported each year.
The 10 CFR Part 26 FFD program positive test rates for amphetamines, methamphetamines, and cocaine could be higher than reported because of the prevalence of attempts to subvert the drug testing process. The model forecasts detection improvements using the average positive rate for each substance from CY 2010 through CY 2019. Because approximately two-thirds of those identified as subverting a test do not submit a specimen for testing (average 155 individuals per year), the drug(s) in a donor’s body cannot be detected and captured in the total results for the year.
Appendices B, C, and D provide details the assumptions used in the analysis. Section 5.2 presents the inputs and results of the uncertainty analysis.
5. Results
This section organizes the analytical results into four sections. Section 5.1 presents results on the benefits and costs of the final rule. Section 5.2 evaluates the uncertainties in the benefit and cost estimate and identifies those uncertain variables that most affect the variation in the results. Section 5.3 addresses the disaggregation results for each of the regulatory initiatives that comprise the final rule. Section 5.4 describes the information required for review by the Committee to Review Generic Requirements (CRGR).
5.1 Benefits and Costs of the Final Rule
This section discusses the benefits and costs estimated for the rule (as summarized in Tables 5-1 and 5-2) and for each quantifiable regulatory initiative contained in the rule (as summarized in Table 5-3). Sections 5.1.7 through 5.1.10 describe the qualitatively evaluated attributes in the analysis. The NRC staff performed a qualitative assessment of these attributes, which is consistent with the Commission’s direction in SECY-14-0087 (NRC, 2015).
The final rule (Alternative 2) will result in an estimated net benefit of between $418,356 and $692,799, at 7-percent and 3-percent discount rates, respectively. These costs are associated with two affected attributes—industry implementation and industry operation. These numbers include averted training costs (i.e., quantified benefits) to industry operation associated with additional individuals testing positive during pre‑access drug testing.
Appendix C provides details on the industry’s incremental activities required under the final rule and estimates the one-time and annual costs associated with these activities. This analysis considers the potential costs associated with required sanctions resulting from additional positive test results. The regulations in 10 CFR 26.75(e) require that a first positive drug or alcohol test result must lead to termination of the individual’s unescorted access authorization for at least 14 days. For a second positive drug or alcohol test result, 10 CFR 26.75(e) requires a 5‑year denial of access.0
The NRC staff assumes that Alternative 2 results in qualitative benefits in the attributes of public health (accident), occupational health (accident), offsite property, onsite property, regulatory efficiency, safeguards and security considerations, and other considerations, which include public perception, public trust, workplace productivity, workplace safety, and improved protection of individual rights.
As benefits, the NRC staff estimates that the rule results in a 22‑percent increase in the number of individuals identified each year using illegal drugs, misusing illegal drugs, or attempting to subvert the testing process and who would be determined not to be fit for duty or not trustworthy and reliable, or both. The rule maintains the existing FFD program performance objectives in 10 CFR 26.23(c), to “provide reasonable measures for the early detection of individuals who are not fit to perform the duties that require them to be subject to the FFD program,” and in 10 CFR 26.23(d), to “provide reasonable assurance that the workplaces subject to this part are free from the presence and effects of illegal drugs.” The NRC analysis of annual FFD program performance data submitted to the NRC by licensees and other entities demonstrates that the workplaces subject to 10 CFR Part 26 testing are not free from the presence and effects of illegal drugs.
Licensees and other entities also may recognize a variety of other benefits, such as those associated with the following types of activities:
Permanent denial: If an individual is identified as having subverted the testing process, the individual will be permanently denied access under 10 CFR 26.75(b). As a result, the entire industry benefits from no longer incurring the potential risk of this individual working at any sites or any of the associated costs.
Second chance policy and follow‑up testing: Licensees may provide a second chance to their employees who test positive for a drug (they generally do not do so for C/V workers). As a result, each of these individuals who successfully receives treatment and returns to the workforce will be subject to a 10 CFR Part 26 follow‑up testing program. If pre-access testing detects drug use by the individual, then the cost of conducting follow‑up testing on an individual is averted.
The rule changes also improve regulatory efficiency through regulatory and compliance improvements, including harmonizing definitions and procedures with those described in the 2008 and 2017 HHS Guidelines, eliminating dual regulation of HHS-certified laboratories, and clarifying ambiguous or imprecise regulatory language in 10 CFR Part 26.
Table 5-1 Summary of Overall Benefits and Costs (Quantitative and Qualitative), Alternative 2 (Amend 10 CFR Part 26)
Benefits (Costs) |
Qualitative Benefits and Costs |
Industry Implementation ($0.14 million) Industry Operation $0.56 million using a 7% discount rate $0.83 million using a 3% discount rate Total Net Costs $0.42 million using a 7% discount rate $0.69 million using a 3% discount rate |
Benefits Estimated 22‑percent increase in detection of individuals using drugs or attempting to subvert the drug testing process. This equates to an average of 176 individuals per year or 4,235 individuals over the 24-year period of the analysis.
Public Health (Accident): Identifying additional individuals using drugs and denying them unescorted access authorization reduces the risk that public health is affected by an accident resulting from human performance issues associated with drug‑induced impairment.
Occupational Health (Accident): Identifying additional individuals using drugs and denying them unescorted access authorization reduces the risk that occupational health is affected by an accident resulting from human performance issues associated with drug‑induced impairment.
Offsite Property: Identifying additional individuals using drugs and denying them unescorted access authorization reduces the risk that offsite property is affected by an accident resulting from human performance issues associated with drug-induced impairment.
Onsite Property: Identifying additional individuals using drugs and denying them unescorted access authorization reduces the risk that onsite property is affected by radiological releases resulting from human performance issues associated with drug‑induced impairment.
Regulatory Efficiency: Harmonizing definitions and procedures with those in the 2008 and 2017 HHS Guidelines, addressing dual regulation of HHS‑certified laboratories, clarifying ambiguous rule language, providing additional regulatory flexibility in 10 CFR Part 26, and enhancing donor due process provisions improve regulatory efficiency.
Safeguards and Security Considerations: Increasing assurance that individuals are trustworthy and reliable by enhancing the detection and deterrence of illegal drug use, legal drug misuse, and attempts to subvert the drug testing process improves safeguards and security.
Other Considerations: The deterrent effect of a drug testing program provides benefits to industry in that it helps dissuade additional individuals using illegal drugs and misusing legal drugs from seeking employment in 10 CFR Part 26 regulated workplaces. Industry benefits from fewer drug users in the workforce may include increased worker productivity, fewer sick days, less turnover in positions, reduced number of job‑related accidents, reduced number of disability claims, and reduced likelihood of equipment damage as a result of personnel impaired from the use or abuse of drugs. |
Table 5-2 Summary of Total Benefits and Costs to Industry (One-Time and Annual)
Industry Total |
Average per Sitea |
|
||||
One-Time Benefit (Cost) |
Annual Benefit (Cost) |
7% NPV |
3% NPV |
One-Time Benefit (Cost) |
Annual Benefit (Cost) |
|
|
||||||
($136,936) |
$47,650 |
$418,356 |
$692,799 |
($2,321) |
$808 |
|
a Average cost per site calculated by dividing the total industrywide cost by the number of sites (59).
Note: Table results are stated in 2022 dollars.
Table 5-3 Summary of One-Time and Annual Benefits and Costs to Industry, by Regulatory Initiative
Industry Total |
Average per Sitea |
||
Annual Benefit (Cost) |
7% NPV |
3% NPV |
Annual Benefit (Cost) |
Costs to implement drug testing program changes (one-time policy, procedure, and training) |
|||
- |
($136,936) |
($136,936) |
- |
|
|||
($15,952) |
($185,898) |
($277,775) |
($270) |
|
|||
($80,265) |
($935,375) |
($1,397,666) |
($1,360) |
|
|||
($156,968) |
($1,829,243) |
($2,733,312) |
($2,660) |
|
|||
($60,323) |
($702,980) |
($1,050,415) |
($1,022) |
|
|||
($9,381) |
($109,322) |
($163,353) |
($159) |
|
|||
$370,539 |
$4,318,110 |
$6,452,255 |
$6,280 |
TOTAL |
|||
$47,650 |
$418,356 |
$692,799 |
$808 |
AVERAGE NET PRESENT VALUE BENEFIT PER SITE |
|||
|
$7,091 |
$11,742 |
|
|
|||
Alternative Specimen (Oral Fluid) Drug Testingb |
|||
$6,665 |
$77,671 |
$116,059 |
$113 |
a Average cost per site is calculated by dividing the total industrywide cost by the number of sites (59).
b The total benefit (cost) for the final rule does not include the forecasted benefits from the alternative specimen drug testing method (i.e., oral fluid versus urine) for an observed collection because each licensee can choose whether or not to use this alternative.
Note: Table results are stated in 2022 dollars.
Table 5-4 provides the projected one-time and annual costs to future nuclear facilities based on the projected operations workforce detailed in Table 4-2.
Table 5-4 Projected One-Time and Annual Costs to Future Nuclear Facilities
Facility Type |
Projected Benefits and (Costs) by Facility Type |
|
One-Time Benefit (Cost) |
Annual Benefit (Cost)a |
|
Microreactorb |
Negligible |
$13 |
Small reactorb |
Negligible |
$72 |
Medium reactorb |
Negligible |
$163 |
Large reactor |
Negligible |
$808 |
Category I special nuclear material licensee facility |
Negligible |
$808 |
a Annual cost per facility type is calculated by dividing the worker population per site for each type of facility by the average number of workers subject to the 10 CFR Part 26 FFD program per site and then multiplying by the industry average annual site cost or benefit.
b The NRC staff assumes that microreactors and small and medium reactors would proportionally benefit from averted training costs as a result of pre-access testing.
Note: Table results are stated in 2022 dollars.
Sections 5.1.1 through 5.1.6 discuss the quantified one-time costs and annual costs associated with each of the regulatory initiatives, which can be quantified. Sections 5.1.7 through 5.1.10 present further discussion on qualitatively evaluated elements in the analysis. Appendices B, C, and D provide the specific inputs and calculations used in the development of the summary results presented in the tables in this section.
5.1.1 One-Time Policy, Procedure, and Training Costs
The regulatory initiatives impact FFD program policies, procedures, and training. Specifically, licensees need to update FFD program policies and procedures to account for the new drug testing protocols and inform individuals who are covered by the FFD program of the changes in policies and procedures. In addition, the rule changes require each FFD program to update its contracts—up to two with its HHS-certified laboratories (the primary and secondary laboratories0) and one with its BPTS supplier to reflect the new drug testing criteria. Additionally, sites using LTFs for initial drug testing need to train Laboratory Technicians on the new protocols and validate the immunoassays that change because of lower cutoff levels and the inclusion of additional substances in the testing panel.
The NRC staff assumes that each licensee pursues the least cost approach to implement the rule changes. With respect to informing individuals already subject to an FFD program about the changes in the FFD program policies and procedures, the analysis estimates that 95 percent of sites incorporate this information into the annual refresher training required by 10 CFR 26.29(c) and post information at the collection sites and on bulletin boards. This approach does not result in an incremental change in costs of training individuals on the FFD policy changes because the refresher training already includes time to update individuals on changes in the FFD program from the previous training. However, the NRC staff does estimate that the remaining 5 percent of sites will distribute information on FFD program changes outside the annual refresher training process and provide each individual with a summary of the FFD policies and procedures to read and sign an acknowledgment of receipt of the information.0
In summary, the one-time costs include the following:
one-time cost to industry = ($136,936)0
one-time average cost per site = ($2,321)
Table 5-5 summarizes the one-time costs by implementation activity for industry.
Table 5-5 One-Time Implementation Costs
Affected Entity |
Implementation Activity |
Base Estimate Cost (Undiscounted, 2022 dollars) |
Industry |
Update policies and procedures |
($24,912) |
Inform employees of policy change |
($72,873) |
|
Revise contract with the primary HHS-certified laboratory |
($12,336) |
|
Revise contract with the backup HHS-certified laboratory |
($12,336) |
|
Revise contract with BPTS supplier |
($6,168) |
|
Train LTF technicians |
($2,131) |
|
Validate drug testing assays at LTF |
($6,180) |
|
Total for all sites |
($136,936) |
|
Average cost per site |
($2,321) |
Future Nuclear Facilities: The NRC staff estimates negligible incremental rule implementation costs for nuclear facilities constructed and operated after the rule effective date because each activity listed in Table 5-5 is included in the initial development of FFD program policies and procedures and the delivery of staff training. Furthermore, the initial contract with the primary and backup HHS-certified laboratories would include the updates to the drug testing panel amended by the rule.
5.1.2 Lowered Initial and Confirmatory Drug Testing Cutoff Levels for Amphetamines and Cocaine Metabolites
Lowering the testing cutoff levels for amphetamines and cocaine metabolites increases the timeframe (i.e., the window of detection) in which these drugs can be detected in an individual’s urine specimen after use. As a result, the NRC staff anticipates that the use of lower testing cutoffs will increase the number of individuals who test positive for amphetamines and cocaine metabolites. Licensees will incur the costs associated with confirmatory testing and subsequent actions taken when an individual tests positive (i.e., on the part of the FFD program staff, the MRO, and the donor). These incremental costs are estimated as follows:
total annual cost to industry = ($15,952)
average annual cost per site = ($270)
In making these changes to maintain reasonable assurance of a drug-free workplace, the NRC staff estimates that this regulatory initiative results in 45 additional confirmed positive test results, as presented in Table 5-6. Therefore, lowering the testing cutoff levels for amphetamines and cocaine metabolites provides additional assurance that persons who are using illegal drugs or misusing legal drugs will be identified and denied unescorted access authorization than under the current 10 CFR Part 26 framework. Appendices B and C provide additional information on the estimated increase in positive test results.
Table 5-6 Additional Amphetamines and Cocaine Positives from Lower Testing Cutoff Levels (Estimated Total for All Sites)
Substance |
Projected Number of Additional Confirmed Positive Test Results per Year |
Amphetamines |
23 |
Cocaine |
22 |
Future Nuclear Facilities: The NRC staff estimates the projected number of additional confirmed positives for each future nuclear facility site testing for amphetamines and cocaine at lower testing cutoff levels by multiplying the expected number of tests performed by these facilities by the average positive test rate for the subject drug at existing facilities. Table 5-7 presents the projected number of confirmed positive test results by facility type.
Table 5-7 Additional Amphetamines and Cocaine Positives from Lower Testing Cutoff Levels by Future Nuclear Facilities
Facility Type |
Projected Number of Additional Confirmed Positive Test Results Annually by Nuclear Facility Type per Site |
|
Amphetamines |
Cocaine |
|
Microreactor |
0.01 |
0.01 |
Small reactor |
0.03 |
0.03 |
Medium reactor |
0.08 |
0.07 |
Large reactor |
0.39 |
0.37 |
Category I special nuclear material licensee facility |
0.39 |
0.37 |
5.1.3 Expanded Initial Drug Testing Panel to Include 6-AM and Revised Confirmatory Testing Cutoff Level for 6-AM
Licensees incur costs to conduct initial testing of each urine specimen for 6-AM (the metabolite of the illegal drug heroin), which increases the number of urine specimens identified as containing 6-AM. Licensees also will incur costs associated with any specimens that test positive on confirmatory testing and the subsequent actions taken when an individual tests positive (i.e., on the part of the FFD program staff, the MRO, and the donor). These incremental costs are estimated as follows:
total annual cost to industry = ($80,265)
average annual cost per site = ($1,360)
In making these changes to maintain reasonable assurance of a drug-free workplace, the NRC staff estimates that this regulatory initiative will result in an additional 22 confirmed positive test results per year, as presented in Table 5‑8. Therefore, expanding the initial drug testing panel to include 6-AM and revising the confirmatory testing cutoff level for 6-AM provides additional assurance that persons who are using the illegal drug heroin will be identified and denied unescorted access authorization than under the current 10 CFR Part 26 framework. Appendices B and C provide additional information on the estimated increase in positive test results.
Table 5-8 Additional
6-AM Positive Results from Drug Testing Panel
Changes
(Estimated Total for All Sites)
Substance |
Projected Number of Additional Confirmed Positive Test Results per Year |
6-AM |
22 |
Future Nuclear Facilities: The NRC staff estimates the number of additional confirmed 6-AM positive results for each future nuclear facility site testing for 6-AM by multiplying the expected number of tests performed by these facilities by the average positive test rate for 6-AM at existing facilities. Table 5-9 presents the projected number of confirmed positive test results by facility type.
Table 5-9 Additional
6-AM Positive Results from Drug Testing Panel Changes
by Future
Nuclear Facilities
Facility Type |
Projected Number of Additional 6-AM Confirmed Positive Test Results Annually by Nuclear Facility Type per Site |
Microreactor |
0.01 |
Small reactor |
0.03 |
Medium reactor |
0.07 |
Large reactor |
0.37 |
Category I special nuclear material licensee facility |
5.1.4 Expanded Initial and Confirmatory Drug Testing Panels to Include Ecstasy
Licensees will incur costs to conduct initial testing of each urine specimen for Ecstasy-type drugs MDMA and MDA. Licensees also will incur costs associated with any specimens that test positive on confirmatory testing and the subsequent actions taken when an individual tests positive (i.e., on the part of the FFD program staff, the MRO, and the donor). These incremental costs are estimated as follows:
total annual cost to industry = ($60,323)
average annual cost per site = ($1,022)
In making these changes to maintain reasonable assurance of a drug-free workplace, the NRC staff estimates that this regulatory initiative results in an additional seven confirmed positive test results per year, as presented in Table 5-10. As a result, this change provides additional assurance that persons who are using illegal drugs are identified and denied unescorted access authorization than under the current 10 CFR Part 26 framework. Appendices B and C provide additional information on the estimated increase in positive test results.
Table 5-10 Projected Ecstasy Positive Results from Expanded Drug Testing Panel (Estimated Total for All Sites)
Substance |
Projected Number of Confirmed Positive Test Results per Year |
Ecstasy |
5 |
Future Nuclear Facilities: The NRC staff estimates the number of additional confirmed Ecstasy positive results for each future nuclear facility site testing for Ecstasy by multiplying the expected number of tests performed by these facilities by the average positive test rate for Ecstasy at existing facilities. Table 5-11 presents the projected number of confirmed positive test results by facility type.
Table 5-11 Projected
Ecstasy Positive Results from Expanded Drug Testing Panel
by
Future Nuclear Facilities
Facility Type |
Projected Number of Ecstasy Confirmed Positive Test Results Annually by Nuclear Facility Type per Site |
Microreactor |
0.001 |
Small reactor |
0.01 |
Medium reactor |
0.02 |
Large reactor |
0.09 |
Category I special nuclear material licensee facility |
0.09 |
5.1.5 Expand the Initial and Confirmatory Drug Testing Panels to Include Four Opioids
Licensees will incur costs to conduct initial and confirmatory testing of each urine specimen for four opioid pain relievers—hydrocodone, hydromorphone, oxycodone, and oxymorphone—and the subsequent actions taken when an individual tests positive (i.e., on the part of the FFD program staff, the MRO, and the donor). These incremental costs are estimated as follows:
total annual cost to industry = ($156,968)
average annual cost per site = ($2,660)
In making these changes to maintain reasonable assurance of a drug-free workplace, the NRC staff estimates that this regulatory initiative results in an additional 89 confirmed positive test results per year, as presented in Table 5-12. As a result, this change provides additional assurance that persons who are using illegal drugs are identified and denied unescorted access authorization than under the current 10 CFR Part 26 framework. Appendices B and C provide additional information on the estimated increase in positive test results.
Table 5-12 Projected Positive Results from Expanded Opioid Drug Testing Panel (Estimated Total for All Sites)
Substance |
Projected Number of Confirmed Positive Test Results per Year |
Opioid drugs (hydrocodone, hydromorphone, oxycodone, oxymorphone) |
89 |
Future Nuclear Facilities: The NRC staff estimates the number of additional confirmed opioid positive results for each future nuclear facility site testing by multiplying the expected number of tests performed by these facilities by the average positive test rate for opioids at existing facilities. Table 5-13 presents the projected number of confirmed positive test results by facility type.
Table 5-13 Projected
Positive Results from Expanded Opioid Drug Testing Panel
by
Future Nuclear Facilities
Facility Type |
Projected
Number of Additional Opioid Confirmed Positive Test Results
Annually |
Microreactor |
0.02 |
Small reactor |
0.13 |
Medium reactor |
0.30 |
Large reactor |
1.51 |
Category I special nuclear material licensee facility |
1.51 |
5.1.6 Required Special Analyses Testing of Dilute Specimens and Specimens Collected during Suspected Subversion Attempts
Licensees will incur costs to conduct mandatory special analyses testing of dilute specimens (presently 10 CFR 26.163(a)(2) provides licensees with the option to conduct this testing, and all licensees instituted this testing policy as of CY 2014). Licensees also will incur incremental costs to conduct special analyses testing of specimens collected under direct observation (i.e., specimens collected during suspected subversion attempts). These special analyses requirements result in incremental improvement, with additional costs associated with the newly required confirmatory testing and subsequent actions associated with additional positive test results (i.e., on the part of the FFD program staff, the MRO, and the donor). These incremental costs are estimated as follows:
total annual cost to industry = ($9,381)
average annual cost per site = ($159)
In making these changes to maintain reasonable assurance of a drug-free workplace, the NRC staff estimates that this regulatory initiative will result in an additional 16 confirmed positive test results, as presented in Table 5-14.0 Therefore, this change provides additional assurance that persons who are using illegal drugs, misusing legal drugs, or attempting to subvert the drug testing process will be identified and denied unescorted access authorization than under the current 10 CFR Part 26 framework. Appendices B and C provide additional information on the estimated increase in positive test results.
Table 5-14 Additional
Positive Results from Special Analyses Testing of Dilute
and
Subversion Specimens (Estimated Total for All Sites)
Specimen Type |
Projected Number of Additional Confirmed Positive Test Results per Year |
Dilute |
8 |
Subversion Specimens |
8 |
Future Nuclear Facilities: The NRC staff estimates the number of additional positive results from special analyses testing of dilute and subversion specimens for each future nuclear facility site performing such testing by multiplying the expected number of tests performed by these facilities by the average positive test rate for special analyses testing at existing facilities. Table 5-15 presents the projected number of confirmed positive test results by facility type.
Table 5-15
Additional Positive Results from Special Analyses Testing of Dilute
and Subversion Specimens by Future Nuclear Facilities
Facility Type |
Projected
Number of Additional Confirmed Positive Test Results Annually
from Special Analyses Testing |
|
Dilute Specimens |
Suspect Specimens |
|
Microreactor |
0.002 |
0.002 |
Small reactor |
0.01 |
0.01 |
Medium reactor |
0.03 |
0.03 |
Large reactor |
0.14 |
0.14 |
Category I special nuclear material licensee facility |
0.14 |
0.14 |
5.1.7 Averted Costs
The NRC estimates that the rule will result in savings (i.e., averted costs) to licensees and other entities associated with training during the in-processing of licensee employees and C/Vs. Pre-access testing accounts for approximately 67 percent of positive test results each year. As a result, if an individual tests positive for a drug during pre-access testing, any remaining training not completed by that individual at the time the confirmed positive test result is received results in a savings to the licensee or other entity because the individual would immediately be denied unescorted access authorization for failing the required FFD drug test. Appendices D and E provide additional information.
These incremental savings (averted costs) are estimated as follows:
total annual savings to industry = $370,539
average annual savings per site = $6,280
The projected savings associated with the final rule are based on the estimated increase in the number of individuals testing positive each year, as accounted for by the projected number of additional confirmed positives detected, and the subsequent averted training costs. Appendix F provides additional information.
5.1.8 Alternative Specimen (Oral Fluid) Drug Testing
In the final rule, 10 CFR 26.83(b) provides licensees and other entities with the option to collect and test an oral fluid specimen instead of a urine specimen for any of the observed specimen collection conditions under 10 CFR 26.115(a)(1) through (a)(3) and (a)(5). Testing of an oral fluid specimen must be performed at an HHS-certified laboratory.
The NRC staff estimated the net benefits (costs) of the oral fluid specimen option by calculating the costs of the alternative evaluation process and subtracting those costs from the costs to collect and test urine specimens under the same conditions. The majority of observed collections performed each year pertain to two types of potential subversion attempts identified during the specimen collection process: the donor’s urine specimen is outside the acceptable temperature range (10 CFR 26.115(a)(2)), and donor conduct is observed indicating an attempt to subvert the testing process (10 CFR 26.115(a)(3)). The annual FFD program performance reports include event-specific data on these testing events.
The analysis assumes that all licensees and other entities use the alternative evaluation process to avoid observed urine collection and to benefit from the lower costs for this collection method. Appendix F provides additional information.
The incremental savings (averted costs) from using this option are estimated as follows:
total annual savings to industry = $6,665
average annual savings per site = $113
average savings per test = $30
Use of this voluntary alternative evaluation process has no effect on the number of confirmed positive test results.
5.1.9 Workplace Free of Drugs and the Effects of Such Substances
The rule will maintain the FFD program performance objectives in 10 CFR 26.23(c), to “provide reasonable measures for the early detection of individuals who are not fit to perform the duties that require them to be subject to the FFD program,” and in 10 CFR 26.23(d), to “provide reasonable assurance that the workplaces subject to this part are free from the presence and effects of illegal drugs.” Based on the analysis of annual FFD program performance data submitted to the NRC by licensees and other entities, the workplaces subject to 10 CFR Part 26 testing are not free from the presence and effects of illegal drugs.
The effectiveness of a drug testing program may erode over time if the workforce uses impairing substances not in the testing panel, if individuals use products and techniques to successfully subvert the drug testing process, and if testing programs do not use technological advancements that enhance drug testing sensitivity. Therefore, the drug testing provisions in 10 CFR Part 26 should remain at least as effective as the national drug testing standard (i.e., the 2017 HHS Guidelines) and should apply defense-in-depth requirements (e.g., behavioral observation, background checks, collection site security, and specimen collections) to maintain reasonable assurance of a drug-free workplace.
The 2017 HHS Guidelines are a national drug testing standard used by all Federal employee workplace drug testing programs (over 100 Federal agencies0) and comparable Federal agency drug testing programs that test civilians, such as those programs implemented by the DOT, U.S. Department of Defense, U.S. Department of Energy, and U.S. Department of Homeland Security. The HHS is responsible by law0 to maintain its guidelines based on the most recent research and lessons learned from Federal employee workplace drug testing programs and from implementation of the HHS Guidelines by HHS‑certified laboratories and private entities. The HHS also revises its guidelines to address findings and observations from the NLCP and in response to expert and public review.
The NRC historically has incorporated appropriate provisions of the HHS Guidelines into 10 CFR Part 26 in order to apply advancements in drug testing technology and detection methods, address societal changes in drug use, and align the methods and techniques used to identify subversion of the drug testing process with a standard used for testing Federal employees and the majority of civilians tested by Federal agencies. The drug testing panel and cutoff levels specified in 10 CFR Part 26 are currently not in alignment with the 2008 or 2017 HHS Guidelines.
5.1.8 Safety Vulnerability
The final rule will enhance the identification of additional individuals subject to 10 CFR Part 26 who are using illegal drugs, misusing legal drugs, or attempting to subvert the testing process and who are determined not to be fit for duty or not to be trustworthy and reliable, or both. Such a determination results in a denial of unescorted access to the protected areas of NRC‑licensed facilities and other locations and a denial of access to SSNM or sensitive information. Of the approximately 176 additional individuals determined to be using drugs as a result of this final rule, 118 individuals will be identified by pre-access testing, preventing each from entering an NRC-licensed facility or accessing information and potentially challenging safety.0 The remaining 58 individuals will be identified after being granted authorization (i.e., identified during random, for-cause, followup, or post-event testing), during the performance of safety- and security-sensitive duties as described in 10 CFR 26.4.
The identification of these 58 individuals performing safety- and security-sensitive duties enhances the existing regulatory framework to prevent drug-induced impairment (both acute intoxication and the consequences of recent drug use, such as withdrawal effects) from causing or contributing to human performance errors that may result in consequences to the safe operation of a licensed facility. For example, an impaired individual could introduce or fail to identify latent failures during maintenance, surveillance, modification, or operation of safety- and security-related SSCs, and these failures could contribute to an unplanned occupational exposure, personal safety issues, unplanned radiological releases, an accident, or a transient.
Similarly, the labor categories of individuals identified as testing positive for drugs include licensed operators, supervisors, and managers whose job performance includes facility operations; responding to accidents, transients, and fires; directing the workforce; and staffing the Emergency Operations Facility and Technical Support Center upon execution of the site emergency plan. Consequently, any programmatic assurance that helps ensure that the workforce is fit for duty reduces the safety vulnerability.
This safety outcome is consistent with the original 10 CFR Part 26 rule (54 FR 24468; June 7, 1989), which stated “[t]he NRC cannot be confident of the individual’s ability to limit the use of addictive substances to situations that do not adversely affect plant safety” (54 FR 24470), and that “there is an underlying assumption that workers will abide by the licensee’s policies and procedures, [therefore] any involvement with illegal drugs shows that the worker cannot be relied upon to obey laws of a health and safety nature, indicating that the individual may not scrupulously follow rigorous procedural requirements with the integrity required in the nuclear power industry to assure public health and safety” (54 FR 24468).
5.1.9 Security Vulnerability
The final rule will lead to the identification of additional individuals determined not to be fit for duty or not to be trustworthy and reliable, or both, because of their use of illegal drugs, misuse of legal drugs, or attempts to subvert the drug testing process. This will strengthen the defense‑in‑depth regulatory framework provided by the authorization requirements in 10 CFR Part 26, Subpart C, “Granting and Maintaining Authorization,” and 10 CFR Part 73 for both commercial power reactors and Category I special nuclear material licensees.
This security vulnerability is reduced, in part, because once unescorted access authorization is denied, the individual cannot act as an insider threat—an important security determination linked to the conduct of drug testing. To help identify an insider threat, as required by 10 CFR 73.55(b)(1), commercial power reactor licensees “shall establish and maintain a physical protection program...which will have its objective to provide high assurance that activities involving special nuclear material are not inimical to the common defense and security and do not constitute an unreasonable risk to the public health and safety.” One requirement that helps achieve this general performance objective is the provision in 10 CFR 73.55(b)(9) that licensees shall establish, maintain, and implement an insider mitigation program (Regulatory Guide 5.77, “Insider Mitigation Program” (NRC, 2009b). This program, as described in 10 CFR 73.55(b)(9)(i), “must monitor the initial and continuing trustworthiness and reliability of individuals granted or retaining unescorted access authorization to a protected or vital area, and implement defense-in-depth methodologies to minimize the potential for an insider to adversely affect, either directly or indirectly, the licensee’s capability to prevent significant core damage and spent fuel sabotage.” The insider mitigation program shall also include, in part, elements from the FFD program described in 10 CFR Part 26. Consequently, the regulatory framework establishes a strong link between the FFD-related authorization provisions in 10 CFR Part 26 and the physical protection access authorization requirements described in 10 CFR Part 73.
An insider threat is an individual who cannot be trusted or relied upon to follow licensee policies and procedures or Federal regulations designed, implemented, and maintained to protect public health and safety, promote the common defense and security, and protect the environment. An insider threat could physically or remotely (through electronic means) cause inoperable safety- or security-related SSCs, a loss of facility control, radiological sabotage at a commercial power reactor, or the theft or diversion of formula quantities of SSNM from a Category I special nuclear material licensee. Additionally, individuals who use illegal drugs may be co-opted or subverted by adversaries.
The original 10 CFR Part 26 rule (54 FR 24470; June 7, 1989) states the following:
The NRC believes that the reliability, integrity, and trustworthiness of persons working within nuclear power plants is important to assure public health and safety. The granting of a license is based on the assumption that workers will abide by the licensees’ policies and procedures in all areas. Indications of lack or reliability, integrity or trustworthiness, therefore, even so far as they pertain to off-site behaviors, are relevant to the NRC’s need to assure that nuclear power plants are operated safely.
The NRC further discussed these positions in the 2008 FFD final rule (73 FR 16971; March 31, 2008):
Part 26 and the access authorization requirements [of 10 CFR Part 73] each contain provisions that require establishing the trustworthiness and reliability of personnel before granting unescorted access authorization to the protected area of nuclear power plants.
Consequently, the FFD program objective to identify individuals using illegal drugs reduces a potential security vulnerability. The failure to identify security personnel who use illegal drugs or misuse legal drugs could significantly challenge the effectiveness of the site insider mitigation program (10 CFR 73.55(b)(9)); security plan (10 CFR 73.55(c)); security search program (10 CFR 73.55(h)); and the detection and assessment systems that include requirements to conduct surveillance, observation, and monitoring to identify tampering and to detect and deter intruders (10 CFR 73.55(i)). These requirements cannot be implemented effectively if site security personnel are not fit for duty. This is important because many security duties and responsibilities are conducted by security officers who operate alone (i.e., individually) and therefore do not benefit from a team environment, second checks, or backup. As a result, a security officer who is mentally, physically, or psychologically impaired or who does not possess the characteristics of honesty, integrity, trustworthiness, and reliability cannot be relied upon to competently execute site security requirements.
5.1.10 Improve Subversion Detection
The final rule strengthens the methods used to identify persons attempting to subvert the drug testing process. The rule requires all suspect urine specimens to be tested to the LOQ, which is the lowest concentration at which the identity and concentration of a drug can be established accurately. This change increases the licensees’ ability to identify individuals attempting to hide their drug use through subversive techniques or temporary abstention from drug use. The NRC staff estimates that approximately 16 of the additional 176 individuals each year will be identified as attempting to subvert the drug testing process (8 additional individuals with dilute specimens and 8 additional individuals with suspect specimens). An attempt to subvert the drug testing process is a willful act by an individual to refuse to comply with an NRC-required drug test (see 10 CFR 50.5, “Deliberate misconduct”; 10 CFR 26.89(c); and 10 CFR 26.825, “Criminal penalties”). Consequently, these individuals present a potential security vulnerability to the safe and secure conduct of NRC-licensed activities. LOQ testing is consistent with the reasonable assurance performance objectives in 10 CFR 26.23 as the rule proactively resolves a known hazard, leverages a testing method used in HHS‑certified laboratories, and achieves these improvements at low incremental cost.
5.2 Uncertainty Analysis
To determine the robustness of the costs and net benefits (i.e., benefits minus costs) of the rule, the NRC staff examined how the industry and the NRC costs change as a result of uncertainties associated with the NRC staff’s analytical assumptions, input data, and worker drug use behavior. As mentioned in Section 4.2, the NRC staff used Monte Carlo simulation to examine the impact of uncertainty on the estimated net benefits of the rule. These Monte Carlo simulations were performed using the @RISK® software program.0
Monte Carlo simulations involve introducing uncertainty into the analysis by replacing the point estimates of the variables used to estimate base case costs and benefits with probability distributions. By defining input variables as probability distributions instead of as point estimates, the researcher can effectively model the effect of uncertainty on the results of the analysis (i.e., the net benefits).
The probability distributions chosen to represent the different variables in the analysis were bounded by the range‑referenced input, DOT and FFD historical data, and the NRC staff’s professional judgment. When defining the probability distributions for use in the Monte Carlo simulation, summary statistics are needed to characterize the distributions. These summary statistics include the minimum, most likely, and maximum values of a program evaluation and review technique (PERT) distribution;0 the minimum and maximum values of a uniform distribution; and the specified integer values of a discrete population.
For the majority of uncertain variables, the NRC staff used the PERT distribution to reflect the relative spread and skewness of the distribution defined by the three estimates. If the likelihood of the result was judged to be equally likely within a range, the data were modeled using a uniform distribution defined by the low and high values. In a few cases, the NRC staff used a discrete distribution to model possible outcomes and their likelihood, such as the number of sites using an LTF or an HHS-certified laboratory.
Table 5-16 identifies the data elements, the distribution and summary statistic, and the mean value of the distribution that the NRC staff used in the uncertainty analysis.
Table 5-16 Variables Used in the Uncertainty Analysis
Data Element |
Distribution |
Low Estimate |
Base Case |
High Estimate |
Regulated Universe |
||||
Number of sites using an LTF |
Discrete |
0 |
3 |
3 |
Number of sites only using an HHS-certified laboratory |
Discrete |
56 |
56 |
59 |
NRC FFD Program Data |
||||
Number of workers subject to a 10 CFR Part 26 FFD program |
PERT |
77,020 |
93,187 |
104,370 |
Number of drug tests conducted per year under 10 CFR Part 26 |
PERT |
107,786 |
134,399 |
153,461 |
Average percentage of total positive, adulterated, substituted, and refusal to test results occurring at pre‑access testing |
PERT |
64.4% |
67.2% |
69.6% |
Hourly Wage Rates (Dollars per Hour) |
||||
Clerical |
PERT |
$22.70 |
$23.84 |
$24.97 |
Facility Worker (weighted average, licensee employees and C/V workers) |
PERT |
$67.97 |
$70.18 |
$75.23 |
FFD Manager |
PERT |
$37.84 |
$48.59 |
$54.58 |
FFD Staff |
PERT |
$36.47 |
$40.52 |
$48.63 |
LTF Laboratory Technician |
PERT |
$36.15 |
$40.17 |
$48.20 |
LTF Laboratory Supervisor |
PERT |
$60.78 |
$67.53 |
$81.04 |
Legal |
PERT |
$121.56 |
$135.07 |
$162.08 |
MRO |
PERT |
$113.52 |
$151.36 |
$189.20 |
Industry Implementation—Training |
||||
Number of sites distributing a summary of FFD program rule changes to employees outside of routine training |
Uniform |
0% |
|
10% |
Cost of LTF training materials (per LTF) |
PERT |
$400 |
$500 |
$800 |
Number of Laboratory Technicians per LTF |
PERT |
2 |
2 |
3 |
Industry Operations—FFD Drug Testing Costs |
||||
Initial testing for one additional drug at an LTF |
PERT |
$1.50 |
$3.24 |
$7.06 |
Initial and confirmatory drug testing, HHS-certified laboratory (sites using an LTF for initial testing) |
PERT |
$16.00 |
$30.00 |
$37.00 |
Initial and confirmatory drug testing (sites only using an HHS-certified laboratory) |
Uniform |
$9.75 |
|
$16.00 |
Testing for 6-AM (sites only using an HHS-certified laboratory) |
Uniform |
$0.26 |
|
$0.52 |
Testing for expanded opioid panel drugs (HYC, HYM, OXYC, OXYM) (sites only using an HHS-certified laboratory) |
PERT |
$0.30 |
$0.60 |
$0.75 |
Testing for Ecstasy-type drugs (sites only using an HHS-certified laboratory) |
Uniform |
$0.09 |
|
$0.18 |
Special analyses testing at an HHS-certified laboratory |
PERT |
$0.00 |
$7.75 |
$15.00 |
Industry Operations—FFD Drug Testing Rates |
||||
Opioid: 6-AM |
||||
Projected confirmed positive test rate |
Uniform |
0.010% |
|
0.022% |
Amphetamines |
||||
FFD current confirmed positive test rate |
PERT |
0.033% |
0.067% |
0.095% |
Projected percent increase in confirmed positive test rate |
PERT |
0.00% |
39.38% |
62.35% |
Projected percentage of additional positive results that will confirm positive after MRO interview with donor |
PERT |
50% |
75% |
75% |
Cocaine |
||||
FFD current confirmed positive test rate |
PERT |
0.064% |
0.083% |
0.104% |
Projected increase in positive test rate |
PERT |
11.60% |
18.38% |
32.85% |
Ecstasy-Type Drugs (MDMA, MDA) |
||||
Projected confirmed positive test rate |
Uniform |
0.002% |
|
0.006% |
Cost of BPTSs (MDMA/MDA) |
PERT |
$57.00 |
$60.00 |
$63.00 |
Expanded Panel Opioids (OXYC, OXYM, HYC, HYM) |
||||
Projected confirmed positive test rate |
PERT |
0.033% |
0.067% |
0.095% |
Cost of BPTSs (OXYC/OXYM; HYC/HYM) |
PERT |
$57.00 |
$60.00 |
$63.00 |
Dilute Specimens and Specimens Collected during Suspected Subversion Attempts |
||||
Average annual percentage of specimens tested that are dilute and special analyses testing is performed |
PERT |
0.222% |
0.381% |
0.501% |
Average annual percentage of specimens tested that are dilute and test positive on special analyses testing |
PERT |
0.001% |
0.006% |
0.013% |
Average annual percentage of specimens tested that are determined to be a subversion attempt and that test positive (suspect specimens that test positive on special analyses testing) |
PERT |
0.029% |
0.047% |
0.077% |
Projected percent increase in confirmed positive test rate for specimens collected under direct observation |
Uniform |
0% |
|
25% |
Labor Following a Laboratory Positive Test Result or Subversion Event |
||||
MRO subsequent action labor hours |
PERT |
0.25 |
0.75 |
1.00 |
Alternative Specimen (Oral Fluid) Collection and Testing |
||||
Testing of an oral fluid specimen at an HHS-certified laboratory |
PERT |
$18.00 |
$20.00 |
$22.00 |
Oral fluid collection time (donor) |
PERT |
0.25 hour |
0.33 hour |
0.50 hour |
Oral fluid collection time (collector) |
PERT |
0.25 hour |
0.33 hour |
0.50 hour |
Annual number of identified subversion attempts |
PERT |
128 |
230 |
307 |
Percentage of subversion attempts confirmed through the testing of specimens collected under direct observation |
PERT |
22.1% |
31.4% |
39.0% |
Direct Observation Collection of Urine Specimens |
||||
Urine collection time (donor) |
PERT |
0.50 hour |
1.00 hour |
3.00 hour |
Urine collection time (collector) |
PERT |
0.50 hour |
1.00 hour |
3.00 hour |
Future Power Reactor or Fuel Facility Staffing |
||||
Microreactor operations staffing level |
Uniform |
10 |
|
40 |
Small reactor operations staffing level |
Uniform |
40 |
|
240 |
Medium reactor operations staffing level |
Uniform |
210 |
|
420 |
The NRC staff performed the Monte Carlo simulation by repeatedly recalculating the results 5,000 times. For each analysis iteration, the values identified in Table 5-16 were chosen randomly from the probability distributions that define the input variables. The staff recorded the value of the output variables for each iteration and used these resulting output variable values to define the resultant probability distribution.
For each figure below, Monte Carlo simulations were run with the key variables changed to assess the effects on costs. The cost distributions illustrated in Figures 5-1 through 5-5 represent the incremental costs for Alternative 2 from the regulatory baseline of Alternative 1 (Take No Action alternative). As shown in Figure 5-1, no part of the industry implementation cost curve is net beneficial. The industry operations costs shown in Figures 5-2 and 5-3 have a 73.5-percent likelihood that these costs are cost-beneficial. The total industry costs shown in Figures 5-4 and 5-5 have about a 70-percent likelihood of being net beneficial based on the projected averted training costs resulting from the identification of new personnel who fail their pre-access drug test during site in-processing.
Figure 5-1 Industry Implementation Costs
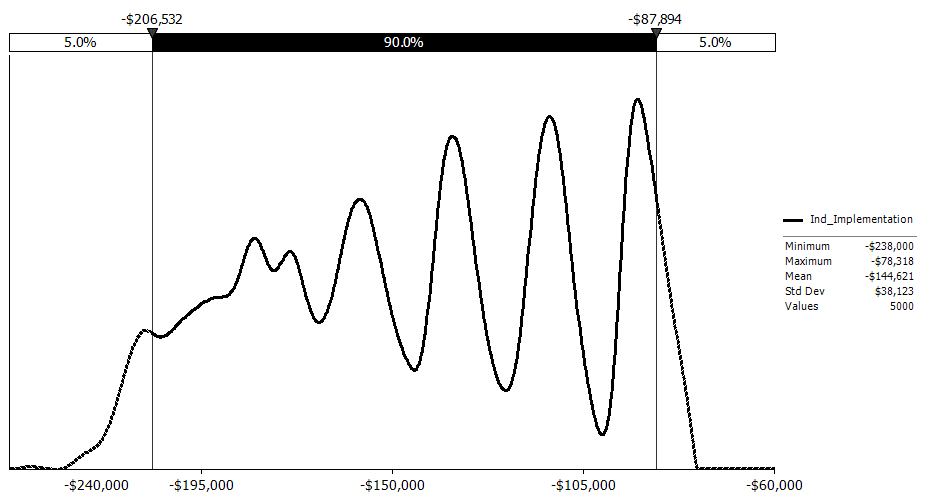
Figure 5-2 Industry Operation Costs (7-Percent Discount Rate)
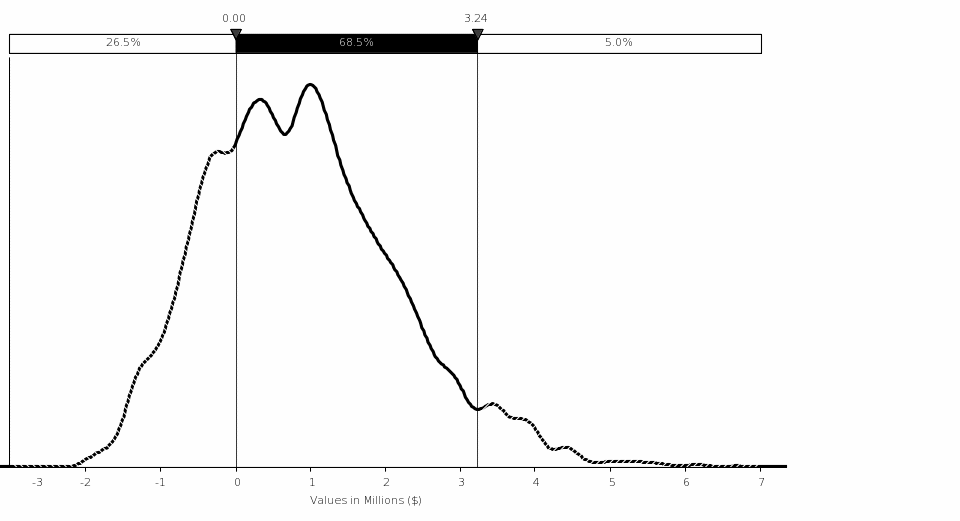
Figure 5-3 Industry Operation Costs (3-Percent Discount Rate)
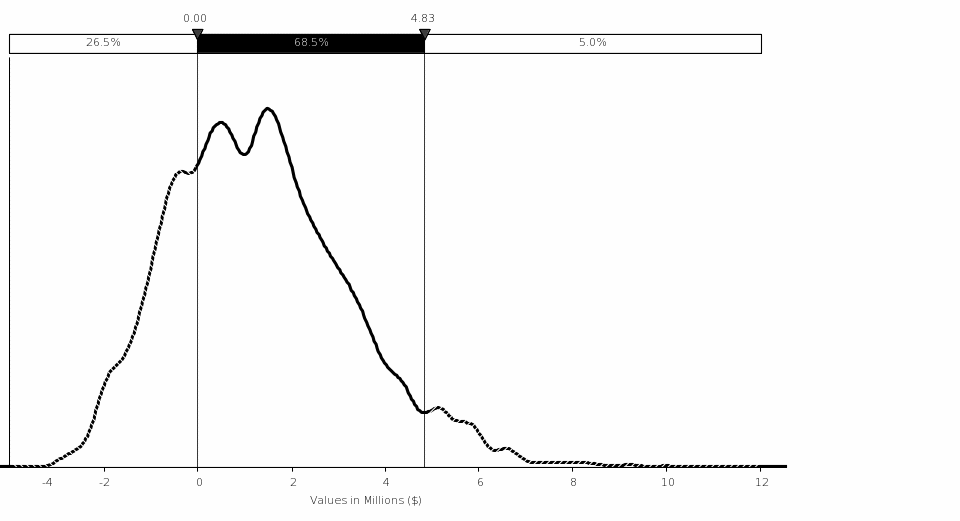
Figure 5-4 Total (7-Percent Discount Rate)
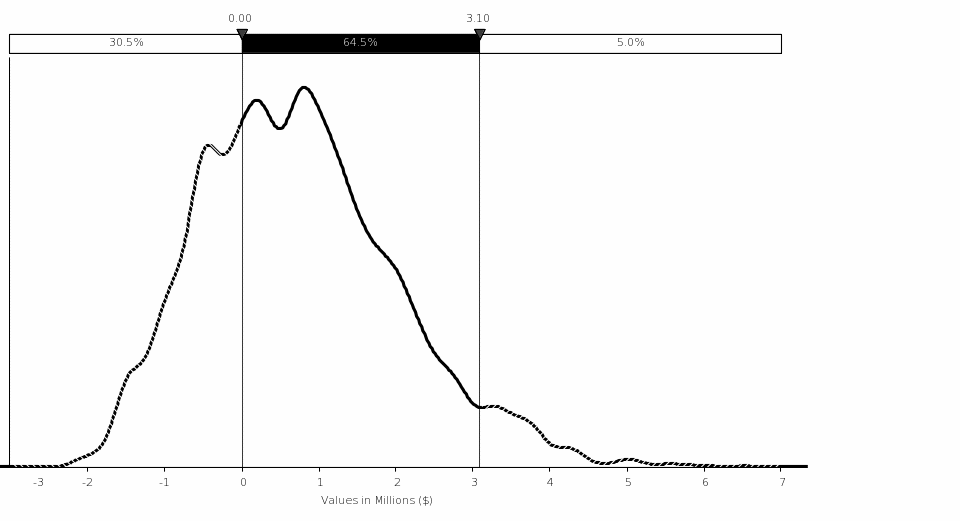
Figure 5-5 Total (3-Percent Discount Rate)
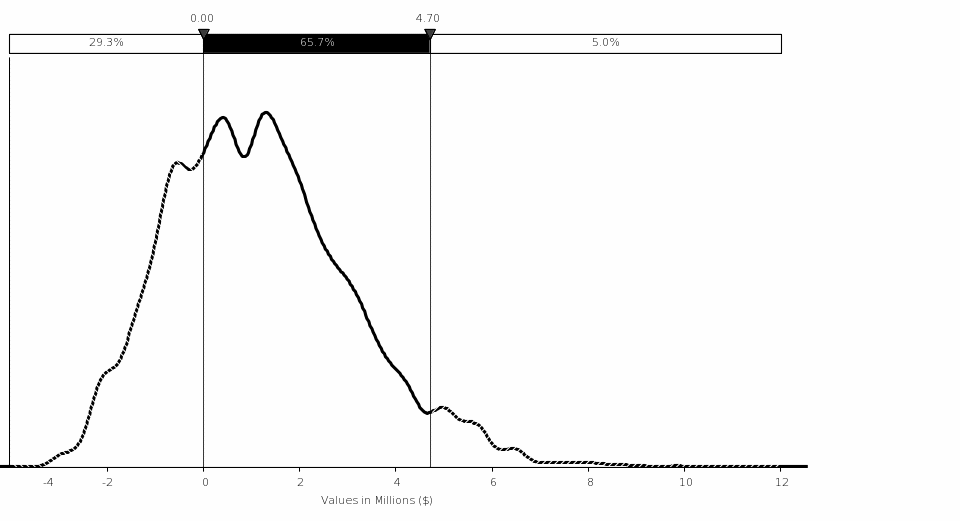
Table 5-17 presents the key statistical results for the uncertainty analysis, including the 90‑percent confidence interval in which the net benefits fall between the 5 and 95 percentile values.
Table 5-17 Uncertainty Results Descriptive Statistics
Uncertainty Result |
Uncertainty Results (2022 Million Dollars) |
||||||
Min |
Mean |
Mode |
Median |
Max |
0.05 |
0.95 |
|
Industry Implementation |
($0.24) |
($0.14) |
($0.14) |
($0.14) |
($0.08) |
($0.21) |
($0.09) |
Industry Operation (7% discount rate) |
($2.19) |
$0.89 |
$0.79 |
$0.79 |
$6.81 |
($0.99) |
$3.24 |
Industry Operation (3% discount rate) |
($3.27) |
$1.32 |
$1.19 |
$1.19 |
$10.17 |
($1.48) |
$4.83 |
Total (7% discount rate) |
($2.38) |
$0.74 |
$0.64 |
$0.64 |
$6.67 |
($1.14) |
$3.10 |
Total (3% discount rate) |
($3.46) |
$1.18 |
$1.04 |
$1.04 |
$10.03 |
($1.63) |
$4.70 |
By examining the range of the resulting output distribution in Table 5-17 and Figures 5-4 and 5‑5, it is possible to discuss the potential costs and benefits of the rule more confidently. Figure 5-4 shows that the final rule is cost-beneficial for 69.5 percent of the simulations when using a 7‑percent discount rate, and Figure 5-5 shows that the final rule is cost-beneficial for 70.7 percent of the simulations when using a 3-percent discount rate.
In addition to estimating the probability distributions for the net benefits of the rule, the NRC staff used the Monte Carlo simulation to determine the variables with the greatest impact on the resulting net benefits. Variables shown to have a large effect on the resulting net benefits may deserve more attention and scrutiny than variables shown to have a small or minimal effect.
To estimate the effect of each variable on the net benefits, the NRC staff performed a regression, with the net benefits modeled as the dependent variable and the inputs as the independent variables. The result of this regression is called a tornado diagram, and it represents in vertical order the variables with the greatest influence on the net benefits. The tornado diagram also displays the resulting impact on the calculated mean value for each of the input variables. Figure 5‑6 presents the tornado diagram for the total cost of the rule using a 7‑percent discount factor. Similarly, Figure 5-7 presents the tornado diagram for the total cost of the rule using a 3‑percent discount factor.
Examining the tornado diagrams provides insight into which inputs have the largest impacts on the results of this quantitative analysis. Figure 5-6 shows that the parameters having the greatest impact on the net benefits of the rule when using a 7‑percent discount factor are the uncertainties associated with the current NRC FFD amphetamines positive test rate and the total number of NRC FFD program annual tests. The influence of a variable on the output is not only a function of the value of that variable but also of the spread of its distribution. In Figure 5‑7, using a 3‑percent discount factor, the same parameters appear in the same ranked order as in Figure 5‑6.
Figure 5-6 Key Variables Whose Uncertainty Drives the Largest Impact on Costs (7‑Percent Net Present Value)
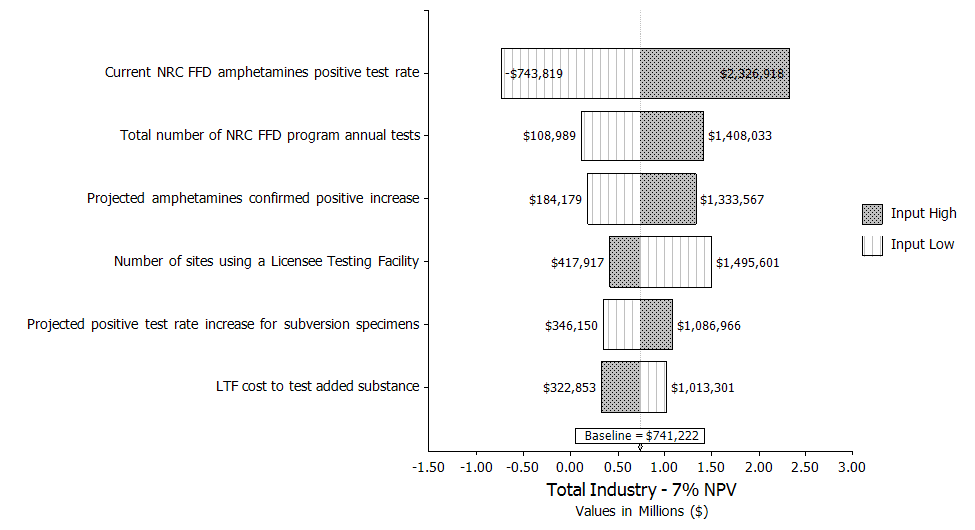
Inputs
ranked by effect on output mean
Figure 5-7 Variables Whose Uncertainty Drives the Largest Impact on Costs (3‑Percent Net Present Value)
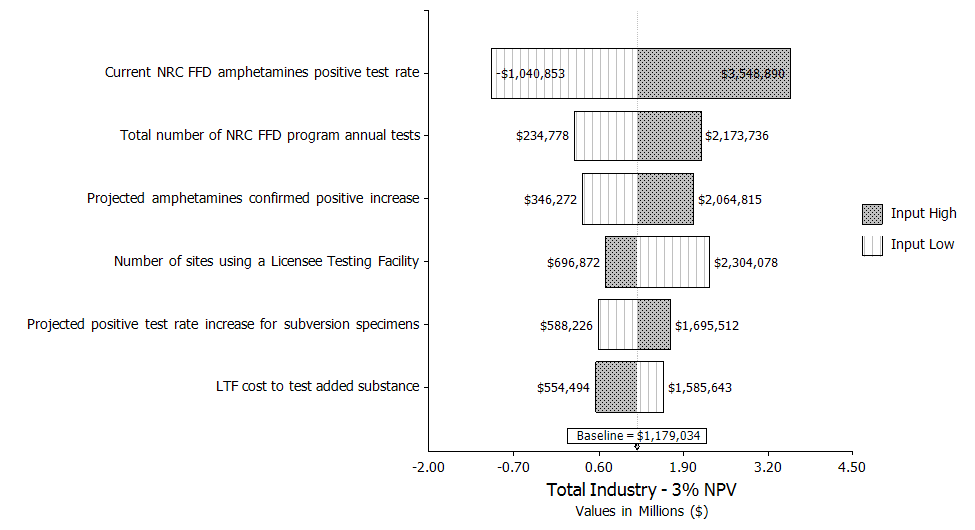
Table 5-18 presents the range of additional positive results that are estimated to be detected if the rule is implemented. These estimates of additional positive results are based on the uncertainty estimate inputs and distributions in Table 5-16 and reflect the uncertainties associated with using historical DOT and NRC test results data to forecast future FFD test results.
Table 5-18 Estimated Number of Additional Confirmed Positives per Year
Substance |
Min |
Mean |
Mode |
Median |
Max |
0.05 |
0.95 |
6-AM |
12 |
22 |
21 |
21 |
33 |
14 |
29 |
Amphetamines |
2 |
23 |
21 |
22 |
52 |
9 |
37 |
Cocaine |
10 |
22 |
21 |
21 |
43 |
14 |
31 |
Ecstasy-type drugs (MDMA, MDA) |
2 |
5 |
6 |
5 |
9 |
3 |
8 |
Opioid drugs (HYC, HYM, OXYC, OXYM) |
72 |
89 |
90 |
89 |
102 |
79 |
98 |
Dilute |
2 |
8 |
7 |
8 |
17 |
4 |
13 |
Subversion specimens |
0 |
8 |
8 |
8 |
24 |
1 |
17 |
Total |
99 |
176 |
175 |
175 |
280 |
125 |
233 |
a The totals are statistics from the total confirmed positives distribution, which may not match the sum of the statistical values from the individual substance confirmed positive distribution curves.
Figure 5-8 presents the distribution of additional positive results above the 11‑year average of 790 positives per year that is projected if this rule is implemented. Based on this distribution, the expected increase in positives is 22 percent, or an additional 176 positives per year, with 90‑percent confidence of an increased positive rate between 18.9 and 25.9 percent.
Figure 5-8 Distribution of Additional Positive Results Projected
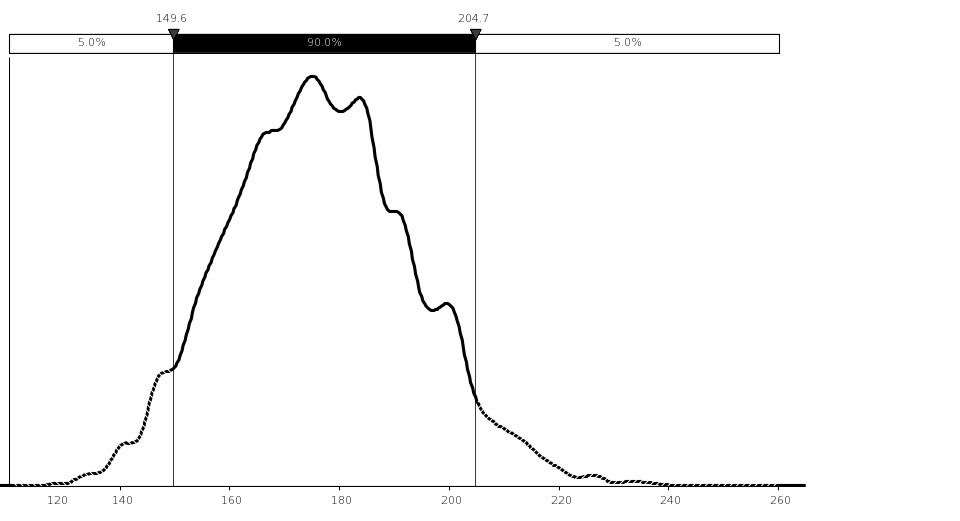
Figure 5-9 presents three plots that summarize the distribution of the undiscounted net benefits, the net benefits discounted at 3 percent, and net benefits discounted at 7 percent. As illustrated by this figure, the rule has a positive monetized net benefit for approximately 71 percent of all simulations and for all analyzed discount rates.
Figure 5-9 Relative Frequency of the Net Benefits of the Final Rule
Undiscounted
3
Percent NPV
7
Percent NPV


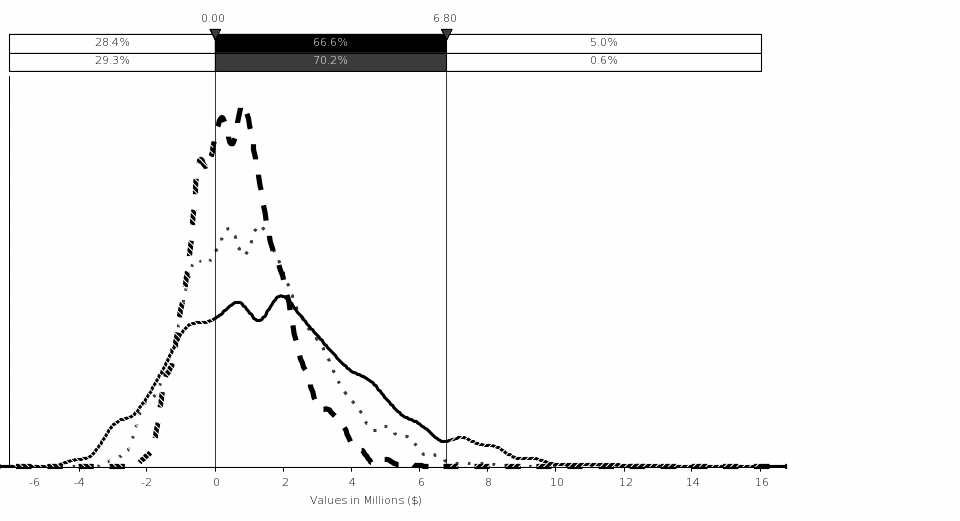
Figure 5-9 also displays the sensitivity of the uncertainty analysis to the discount rates used for the net benefits (i.e., benefits minus costs) of the rule based on 5,000 simulations. By allowing uncertain assumptions and inputs to vary across a distribution, the results are no longer static and instead are spread across a range with varying degrees of certainty.
The simulation analysis results in Table 5-17 show that the estimated mean cost for this rule is $0.74 million, with 90‑percent confidence that the cost is between ($1.14 million) and $3.10 million using a 7‑percent discount rate with a projected increase of 22 percent in positive test results.
The NRC projections use 11 years of 10 CFR Part 26 FFD program test results that are supplemented with DOT drug testing data of a similar DOT population for projecting the detection of additional positives for new drugs added to the NRC initial and confirmatory testing panels.
The NRC staff assessed the variables that have the largest impact on total costs (and averted costs) for the final rule. As shown in Figures 5‑6 and 5‑7, the two largest uncertainties in cost are associated with the current NRC FFD amphetamines positive test rate and the total number of NRC FFD program annual tests. The remaining variables have lesser impacts on the total cost of implementing the rule.
As illustrated in Figure 5-9, variation in the key variables results in cost distributions that range from ($4.87 million) to $14.5 million from the regulatory baseline of Alternative 1 (Take No Action alternative) when accounting for different discount factors.
5.3 Disaggregation
In order to implement the guidance in Section 4.3.2, “Criteria for the Treatment of Individual Requirements,” of NUREG/BR-0058, draft Revision 5 (NRC, 2018b), the NRC staff performed a screening review to determine whether any of the individual requirements (or set of integrated requirements) of the rule are unnecessary to achieve the objectives of the rulemaking. The NRC staff concludes that each of the rule changes are necessary to achieve one or more of the objectives of the rulemaking, as described in Section 1.2 and summarized in Table 5-19. The objectives of the rulemaking are achieved by maintaining reasonable assurance of a drug‑free workplace through the improved detection of persons who are not fit for duty because of illegal drug use or legal drug misuse; harmonizing select drug testing requirements under 10 CFR Part 26 with those implemented by the 2008 and 2017 HHS Guidelines and other Federal agencies; and improving the clarity, organization, and flexibility of the 10 CFR Part 26 rule language.
Revised Requirement |
Improve Detection |
Align Requirements |
Individual Rights and Lessons Learned |
Lower drug testing cutoff levels for amphetamine, cocaine, and methamphetamine |
X |
X |
|
Expand initial drug testing panel to include 6-AM and revise confirmatory testing cutoff level for 6-AM |
X |
X |
|
Expand
testing panel to include Ecstasy-type drugs |
X |
X |
|
Expand testing panel to include HYC, HYM, OXYC, and OXYM |
X |
X |
|
Require special analyses testing of dilute specimens and specimens collected during suspected subversion attempts |
X |
|
X |
Add and revise definitions to improve consistency with definitions in the 2008 HHS Guidelines |
|
X |
X |
Replace the LOD with the LOQ as the decision point in special analyses testing and adulterant testing of specimens |
|
X |
X |
Clarify procedures for observed collections of urine specimens, specimen quantity, altered specimens, and refusal to test situations |
|
X |
X |
Permit use of additional qualified staff beyond the specimen collector to observe a donor in the hydration process initiated after the donor’s initial inability to provide a urine specimen of adequate volume for testing (i.e., a shy bladder) |
|
|
X |
Eliminate 6-month in-service requirement for BPTSs and permit the suppliers to specify the shelf life |
|
X |
X |
Eliminate dual regulation of HHS‑certified laboratories by removing documentation requirements for laboratory personnel and procedures that are already contained in the 2008 HHS Guidelines and verified in the HHS laboratory certification process |
|
|
X |
Address issues associated with the testing of quality control samples at licensee testing facilities described in an enforcement guidance memorandum (NRC, 2009c) |
|
|
X |
Enhance donor protection by requiring MRO review of specimens with invalid validity test results due to high pH values (between 9.0 and 9.5) |
|
X |
X |
Enhance donor protection and the transparency of the retesting process by requiring the MRO to document an oral request made by a donor for a second laboratory to test Bottle B of a split specimen or to retest an aliquot of a single specimen |
|
X |
X |
Require retention of any specimen collected during a post-event testing (even if the donor refuses to complete the test after providing a specimen) to enhance the root‑cause evaluation process associated with accidents |
X |
|
|
5.4 Results for the Committee to Review Generic Requirements
This section addresses the regulatory analysis information requirements for rulemaking actions or NRC staff positions that are subject to CRGR review. Information called for by the CRGR charter (NRC, 2018a) is presented in this regulatory analysis or in the Federal Register notice for the final rule. As a reference aid, Table 5-20 provides a cross-reference between the relevant information and its location in this document or the Federal Register notice.
Table 5-20 Specific CRGR Regulatory Analysis Information Requirements
CRGR Procedures (NRC, 2018b) |
Information Item To Be Included in a Regulatory Analysis Prepared for CRGR Review |
Where Item Is Discussed |
Appendix B, (i) |
Proposed generic requirement or staff position as it is proposed to be sent out to licensees. |
Rule text in Federal Register notice for the final rule. |
Appendix B, (ii) |
Draft papers or other documents supporting the requirements or staff positions. |
Federal Register notice for the final rule. |
Appendix B, (iii) |
The sponsoring office's position on each proposed requirement or staff position as to whether the proposal would modify requirements or staff positions, implement existing requirements or staff positions, or relax or reduce existing requirements or staff positions. |
Regulatory Analysis, Section 5.1. |
Appendix B, (iv) |
The proposed method of implementation. |
Regulatory Analysis, Section 7. |
Appendix B, (vi) |
Identification of the category of power reactors, new reactors, or nuclear materials facilities or activities to which the proposed generic requirement or staff position is applicable. |
Regulatory Analysis, Section 4.2.2. |
Appendix B, (vii)‑(ix) |
If the proposed action involves a power reactor backfit and the exceptions at 10 CFR 50.109(a)(4) are not applicable, the items required at 10 CFR 50.109(c) and the required rationale at 10 CFR 50.109(a)(3) are to be included. For proposed generic relaxations or decreases in current requirements or staff positions, provide a determination along with the rationale that (a) the public health and safety and the common defense and security would be adequately protected if the proposed relaxations were implemented and (b) the cost savings attributed to each action would be significant enough to justify the action. |
Backfitting and issue finality assessment. Federal Register notice for the final rule. |
Appendix B, (xvi) |
Preparation of an assessment of how the proposed action relates to the Commission’s Safety Goal Policy Statement (51 FR 30028; August 21, 1986). |
Regulatory Analysis, Section 3. |
6. Decision Rationale
This analysis is based on the qualitative consideration of the benefits resulting from seven affected attributes (i.e., public health (accident), occupational health (accident), offsite property, onsite property, regulatory efficiency, safeguards and security considerations, and other considerations, which include public perception, workplace productivity, workplace safety, and improved protection of individual rights). The NRC staff performed a qualitative analysis because of the difficulties associated with monetizing these seven affected attributes as well as the full benefit to industry operations that results from the detection each year of additional individuals using illegal drugs, misusing legal drugs, or subverting the testing process. For example, monetizing the impact of these attributes requires estimating factors such as the frequency and consequences of accidents and other safety- or security-related events (e.g., an insider threat) caused by drug‑induced impairment, and the benefits of deterring additional individuals using drugs from seeking employment in positions that require testing under 10 CFR Part 26.
The NRC staff was able to quantify the costs resulting from two other affected attributes (industry implementation and industry operation). Relative to Alternative 1 (Take No Action alternative), the staff estimates the final rule will result in an incremental benefit to industry of approximately $0.42 million total present value over a 24-year period assuming a 7-percent discount rate, or approximately $0.69 million over the same period assuming a 3‑percent discount rate. The cost includes a one-time industry implementation cost of ($136,936) (averaging $2,321 per site) and annual industry operations savings of $808 per site, which includes a projected $6,280 in averted training costs.0 The NRC is not expected to incur any incremental costs resulting from these rule changes. NRC costs to complete the final rule (i.e., analyze public comments, hold public meeting(s), and develop the final rule) and to issue regulatory guidance are sunk costs and do not affect future decisions.
Because the NRC staff cannot monetize the benefit of an additional 22-percent increase (by approximately 176 individuals) each year in the number of individuals identified as using illegal drugs, misusing legal drugs, or attempting to subvert the drug testing process, a net cost‑beneficial determination is not meaningful. However, the NRC staff concludes that the final rule has merit relative to the nonmonetized benefit of identifying additional individuals using illegal drugs, misusing legal drugs, or attempting to subvert the drug testing process each year. The rule benefits public health and safety and the common defense and security at a low average cost per site0 for the following reasons:
The final rule will enhance FFD program effectiveness (i.e., detection) by identifying additional individuals each year determined not to be fit for duty or not to be trustworthy and reliable, or both, because of illegal drug use, legal drug misuse, or attempts to subvert the drug testing process, which benefits public health and safety and the common defense and security by reducing safety and security vulnerabilities.
The final rule will improve regulatory effectiveness and efficiency through regulatory and compliance improvements. Updating 10 CFR Part 26 to be consistent with the 2017 HHS Guidelines (82 FR 7920; January 23, 2017) will improve the effectiveness of the 10 CFR Part 26 drug testing provisions by aligning it with a national drug testing standard used by all Federal employee workplace drug testing programs (more than 100 Federal agencies) and by comparable Federal agency drug testing programs that test civilians in safety- and security-sensitive positions. Alignment with the 2017 HHS Guidelines ensures that the drug testing provisions in 10 CFR Part 26 continue to be scientifically and technically sound, reduces administrative burden on licensees and HHS‑certified laboratories, and helps maintain the public trust.
A more robust drug testing program may deter individuals from seeking employment in 10 CFR Part 26 regulated positions by doing the following:
Expanding the drug testing panel and lowering the testing cutoff levels for select drugs. Lowering the testing cutoff levels for amphetamine, cocaine metabolites, and methamphetamine increases the timeframe (i.e., the window of detection) in which these drugs can be detected in an individual’s body after use. This reduces the likelihood that individuals can subvert the testing process through temporary abstinence from a drug. Adding 6-AM to the initial testing panel and revising the confirmatory testing cutoff improves the testing method to identify use of the illegal drug heroin. Expanding the initial and confirmatory testing panels to include hydrocodone, hydromorphone, MDMA, MDA, oxycodone, and oxymorphone improves the trustworthiness and reliability of the workforce by enabling the identification of individuals using illegal drugs or misusing legal drugs and the resulting denials of unescorted access authorization.
Requiring and expanding special analyses testing. Requiring special analyses testing on dilute specimens and expanding special analyses testing to specimens collected under direct observation reduces the likelihood that individuals can subvert the testing process. Similarly, using the LOQ instead of the LOD as the level at which confirmatory drug testing is to be conducted increases the assurance provided by special analyses testing by adding a level of precision to the testing method. These changes enhance the detection of drugs in specimens that do not present normal physiological characteristics. The identification of additional persons using illegal drugs, misusing legal drugs, or attempting to subvert the drug testing process improves the trustworthiness and reliability of the workforce by denying unescorted access authorization to these individuals.
Enhancing FFD program integrity and protection of individual rights. By adding MRO review procedures for invalid validity test results due to high pH values and clarifying the requirements for MRO actions when a donor requests the testing of a Bottle B specimen or a retest of a single specimen, the final rule enhances consistency with the 2008 HHS Guidelines, FFD program integrity, and the protection of individual rights. Requiring the use of the LOQ instead of the LOD as the decision point for validity testing protocols for dilute and adulterated specimens also enhances the protection of individual rights because the LOQ adds a level of precision to the testing method.
Enhancing protection of individual rights by allowing for alternative specimens. Allowing for the collection and testing of an alternative specimen (oral fluid) instead of the collection of an observed urine specimen enhances the protection of individual rights by avoiding the practice of urine specimen collection under direct observation and providing a method with a lower cost than urine specimen testing.
Improving regulatory efficiency between 10 CFR Part 26 and other related Federal rules and guidelines. The final rule improves regulatory efficiency by (1) harmonizing select 10 CFR Part 26 definitions and drug testing procedures with those described in the 2008 and 2017 HHS Guidelines, (2) clarifying ambiguous or imprecise regulatory language in 10 CFR Part 26, such as the terminology related to quality control samples, and applying lessons learned during implementation of the 2008 FFD final rule, and (3) eliminating dual regulation of HHS-certified laboratories (private entities) and reducing the regulatory burden on licensees by removing select 10 CFR Part 26 requirements also included in the 2008 and 2017 HHS Guidelines that the NLCP verifies in order for a laboratory to achieve and maintain HHS certification.
Enhancing root‑cause analysis in post-event testing situations associated with a refusal to test determination at the collection site. Under the current rule, if a refusal to test is determined during the specimen collection process, any specimen(s) obtained from the donor are discarded. The final rule requires the retention and testing of any specimen collected during post-event situations in which a refusal to test determination was made at the collection site. This change will enhance the ability of the licensee or other entity to determine whether substance use could have been a contributing factor to an accident.
The analysis of net benefits (i.e., benefits minus costs) shows that the final rule is cost‑beneficial at $0.74 million using a 7-percent discount rate. This net benefit is achieved because of averted training costs. If the averted training savings are not included, then the remaining six of the seven regulatory initiatives that comprise the rule are not cost-beneficial because the benefits could not be fully quantified. If the rule is adopted, the safety and security value that the Commission assigns to detecting 22‑percent more individuals using drugs must be greater than ($3.90 million ) (mean value), using a 7 percent discount rate for the net costs for these six regulatory initiatives result to be positive.
The NRC staff concludes that the benefit of the improvements in the final rule to maintain the FFD program performance objectives in 10 CFR 26.23(c), to “provide reasonable measures for the early detection of individuals who are not fit to perform the duties that require them to be subject to the FFD program,” and in 10 CFR 26.23(d), to “provide reasonable assurance that the workplaces subject to this part are free from the presence and effects of illegal drugs” outweighs the low cost of implementation.
7. Implementation
The NRC regulatory instrument for implementing the recommended action is to amend select provisions of 10 CFR Part 26 through rulemaking and to develop a regulatory guide to describe a method that is acceptable to the NRC for 10 CFR Part 26 implementation.
There are always concerns with timing, especially during the spring and fall when power plants process approximately 800 to 1,200 workers to support their outages. Typically, a nuclear power plant licensee establishes a blackout period for the 2 months before and after an outage to provide plant personnel time to prepare for and recover from the significant surge in activity to support an outage (e.g., no policy or procedure changes, no training). As such, licensees and other entities need to have sufficient time to revise site policies and procedures; conduct training; revise contracts with HHS-certified laboratories and BPTS suppliers; provide input and revise the training course used industrywide; and modify, test, and install updates to licensee’s FFD software that incorporates data fields for expanded panels and other modifications in order to meet the amended requirements of this rule. Based on these schedule restrictions and the work necessary to comply with this final rule, the NRC staff proposes the following schedule:
Publication of the final rule and associated guidance: CY 2022
Effective date of the final rule: 30 days after publication in the Federal Register
Compliance date of the final rule: CY 2023 (1 year from the publication date of the final rule)
The NRC staff does not expect the implementation schedule to result in a cumulative impact on affected entities because (1) no other pending 10 CFR Part 26 regulatory actions exist that impact the site professionals responsible for implementing the rule requirements and (2) the schedule provides adequate time to modify FFD policy, procedures, contracts, training, and FFD‑related software systems. This implementation schedule also enables the NRC staff to finalize updates to NRC inspector guidance.
8. References
10 CFR Part 26. Code of Federal Regulations, Title 10, “Energy,” Part 26, “Fitness for duty programs.”
10 CFR Part 50. Code of Federal Regulations, Title 10, “Energy,” Part 50, “Domestic licensing of production and utilization facilities.”
10 CFR Part 52. Code of Federal Regulations, Title 10, “Energy,” Part 52, “Licenses, certifications, and approvals for nuclear power plants.”
10 CFR Part 73. Code of Federal Regulations, Title 10, “Energy,” Part 73, “Physical protection of plants and materials.”
49 CFR Part 40. Code of Federal Regulations, Title 49, “Transportation,” Part 40, “Procedures for transportation workplace drug and alcohol testing programs.” transportation workplace drug and alcohol testing programs.”
51 FR 30028, August 21, 1986. U.S. Nuclear Regulatory Commission, “Policy Statement on Safety Goals for the Operation of Nuclear Power Plants,” August 4, 1986. Corrected and reprinted at Federal Register, Vol. 51, No. 162, pp. 30028–30035, http://www.nrc.gov/reading-rm/doc-collections/commission/policy/51fr30028.pdf.
52 FR 38077, October 14, 1987. U.S. Nuclear Regulatory Commission, “Commission Policy Statement on Deferred Plants,” Federal Register, Vol. 52, No. 198, pp. 38077–38080.
54 FR 24468, June 7, 1989. U.S. Nuclear Regulatory Commission, “Fitness for Duty Programs,” Federal Register, Vol. 54, No. 108, pp. 24468–24508.
69 FR 19643, April 13, 2004. U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, “Mandatory Guidelines for Federal Workplace Drug Testing Programs,” Federal Register, Vol. 69, No. 71, pp. 19643–19673.
73 FR 16966, March 31, 2008. U.S. Nuclear Regulatory Commission, “Fitness for Duty Programs,” Federal Register, Vol. 73, No. 62, pp. 16966–17235.
73 FR 71858, November 25, 2008. U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, “Mandatory Guidelines for Federal Workplace Drug Testing Programs,” Federal Register, Vol. 73, No. 228, pp. 71858–71907.
75 FR 49850, August 16, 2010. U.S. Department of Transportation, “Procedures for Transportation Workplace Drug and Alcohol Testing Programs,” Federal Register, Vol. 75, No. 157, pp. 49850–49864.
82 FR 7920, January 23, 2017. U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, “Mandatory Guidelines for Federal Workplace Drug Testing Programs,” Federal Register, Vol. 82, No. 13, pp. 7920–7970.
84 FR 57554, October 25, 2019. U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, “Mandatory Guidelines for Federal Workplace Drug Testing Programs—Oral/Fluid,” Federal Register, Vol. 84, No. 207, pp. 57554–57600.
Boushey, H., and Glynn, S.J. (Boushey and Glynn, 2012), Center for American Progress, “There Are Significant Business Costs to Replacing Employees,” Washington DC, November 16, 2012, https://cdn.americanprogress.org/wp-content/uploads/2012/11/16084443/CostofTurnover0815.pdf.
Controlled Substances Act, Pub. L. No. 91-513, 84 Stat. 1236, 21 U.S.C. § 812, October 27, 1970.
International Atomic Energy Agency (IAEA, 2001), “Staffing Requirements for Future Small and Medium Reactors (SMRs) Based on Operating Experience and Projections,” IAEA‑TECDOC‑1193, January 2001.
Office of National Drug Control Policy (ONDCP, 2011), “How Illicit Drug Use Affects Business and the Economy,” Executive Office of the President, May 24, 2011, https://obamawhitehouse.archives.gov/sites/default/files/ondcp/Fact_Sheets/effects_of_drugs_on_economy_jw_5-24-11_0.pdf.
Reuters Events (Reuters, 2018), “NuScale Targets SMR Staff Costs Below Nuclear Industry Average,” Nuclear Energy Insider, May 2, 2018, https://www.reutersevents.com/nuclear/nuscale-targets-smr-staff-costs-below-nuclear-industry-average.
Tracey, J.B., and Hinkin, T.R. (Tracey and Hinkin, 2008), “Contextual Factors and Cost Profiles Associated with Employee Turnover,” Cornell University School of Hotel Administration, The Scholarly Commons, Ithaca, NY, February 2008,
U.S. Nuclear Regulatory Commission (NRC, 2009a), “Meeting Summary of a Public Meeting to Discuss the Impact of New Department of Health and Human Services Guidelines on Title 10 Code of Federal Regulations Part 26,” summary of public meeting held February 24, 2009, summary dated March 17, 2009, ADAMS Accession No. ML090771060.
U.S. Nuclear Regulatory Commission (NRC, 2009b), “Insider Mitigation Program,” Regulatory Guide 5.77, March 2009, ADAMS Accession No. ML15219A609.
U.S. Nuclear Regulatory Commission (NRC, 2009c), “Enforcement Guidance Memorandum—Dispositioning Violations of NRC Requirements for Initial Validity and Drug Tests at Licensee Testing Facilities,” EGM 09‑003, March 31, 2009, ADAMS Accession No. ML090760728.
U.S. Nuclear Regulatory Commission (NRC, 2009d), “Summary of Public Meeting to Discuss the Potential Impact of the Revised U.S. Department of Health and Human Services Drug Testing Guidelines on U.S. Nuclear Regulatory Commission Fitness for Duty Requirements,” summary of public meeting held June 24, 2009, summary dated July 14, 2009, ADAMS Accession No. ML091910511.
U.S. Nuclear Regulatory Commission (NRC, 2009e), “Summary of Public Meeting to Discuss the Potential Impact of the Revised U.S. Department of Health and Human Services Drug Testing Guidelines on U.S. Nuclear Regulatory Commission Fitness for Duty Requirements,” Enclosure C, “NEI Memo Containing Results of Industry Survey,” enclosure dated May 31, 2009, summary dated July 14, 2009, ADAMS Accession No. ML091910511.
U.S. Nuclear Regulatory Commission (NRC, 2011a), “NRC Staff-Proposed Changes for Direct Final Rulemaking 10 CFR Part 26: Proposed Amendment to Incorporate Selected Provisions of the November 25, 2008, U.S. Department of Health and Human Services’ Mandatory Guidelines for Federal Workplace Drug Testing (i.e., HHS Guidelines),” October 10, 2011, ADAMS Accession No. ML112980140.
U.S. Nuclear Regulatory Commission (NRC, 2011b), “Summary of the October 11, 2011, Public Meeting to Discuss Staff Proposed Changes to Title 10 of the Code of Federal Regulations, Part 26, Based on the U.S. Department of Health and Human Services Guidelines for Drug Testing,” summary of public meeting held October 11, 2011, summary dated November 14, 2011, ADAMS Accession No. ML112980110.
U.S. Nuclear Regulatory Commission (NRC, 2013a), “Regulatory Basis: Proposed Rulemaking to Amend 10 CFR Part 26, ‘Fitness for Duty Programs,’ based on Select Provisions of the 2008 HHS Guidelines,” May 3, 2013, ADAMS Accession No. ML13066A703.
U.S. Nuclear Regulatory Commission (NRC, 2013b), “Summary of the September 11, 2013, Category 2 Public Meeting to Discuss NRC Staff-Proposed Changes to Title 10 of the Code of Federal Regulations, Part 26, Based on the U.S. Department of Health and Human Services’ Guidelines for Drug Testing,” summary of public meeting held September 11, 2013, summary dated October 29, 2013, ADAMS Accession No. ML13290A236.
U.S. Nuclear Regulatory Commission (NRC, 2014), “Summary of Fitness for Duty Program Performance Reports for Calendar Year 2013,” September 3, 2014, ADAMS Accession No. ML14246A440.
U.S. Nuclear Regulatory Commission (NRC, 2015), “Staff Requirements—SECY‑14‑0087—Qualitative Consideration of Factors in the Development of Regulatory Analyses and Backfit Analyses,” SRM-SECY-14-0087, March 4, 2015, ADAMS Accession No. ML15063A568.
U.S. Nuclear Regulatory Commission (NRC, 2017), “Summary of Fitness for Duty Program Performance Reports for Calendar Year 2015,” November 13, 2017, ADAMS Accession No. ML17313A337. This report is the most recent available; it and the reports for previous years are available at http://www.nrc.gov/reactors/operating/ops-experience/fitness-for-duty-programs/performance-reports.html.
U.S. Nuclear Regulatory Commission (NRC, 2018a), “Charter: Committee to Review Generic Requirements,” Revision 9, June 2018, ADAMS Accession No. ML17355A532.
U.S. Nuclear Regulatory Commission (NRC, 2018b), “Committee to Review Generic Requirements Procedures and Internal Administrative Process, Revision 9,” June 2018, ADAMS Accession No. ML17355A533.
U.S. Nuclear Regulatory Commission (NRC, 2019), “Public Meeting to Discuss the Proposed Rule Associated with the Fitness for Duty Drug Testing Requirements Rulemaking,” summary of public meeting held November 7, 2019, summary dated December 5, 2019, ADAMS Accession No. ML19336A003.
U.S. Nuclear Regulatory Commission (NRC, 2020a), “Regulatory Analysis Guidelines of the U.S. Nuclear Regulatory Commission,” NUREG/BR-0058, draft Revision 5, February 13, 2020, ADAMS Accession No. ML19261A277.
U.S. Nuclear Regulatory Commission (NRC, 2020b), “2020–2021 Information Digest,” NUREG‑1350, Volume 32, August 2020, ADAMS Accession No. ML20282A632.
U.S. Nuclear Regulatory Commission (NRC, 2022), “Fitness-for-Duty Programs for Commercial Power Reactor and Category I Special Nuclear Material Licensees,” Regulatory Guide 5.89, September 2022, ADAMS Accession No. ML20143A034
SUBJECT: Regulatory Analysis for the 10 CFR Part 26 Fitness for Duty Drug Testing Requirements Final Rule [Docket ID NRC-2009-0025], dated Month date, 2021
ADAMS Accession Numbers: Package: ML21111A017; Regulatory Analysis: ML21111A026 *via email
OFFICE |
NMSS/REFS/RASB/TL* |
QTE* |
NMSS/REFS/RRPB/RS |
NMSS/REFS/RRPB/BC |
NAME |
FSchofer |
JDougherty |
GLappert |
IBerrios |
DATE |
5/24/2021 |
6/4/2021 |
6/17/2021 |
6/23/2021 |
OFFICE |
NMSS/REFS/RASB/BC |
NSIR/DPCP/RSB/BC |
NMSS/REFS/D |
NMSS/DFM/D |
NAME |
CBladey |
ABowers (LCubellis for) |
JTappert |
SHelton (CRegan for) |
DATE |
6/29/2021 |
6/28/2021 |
7/7/2021 |
7/7/2021 |
OFFICE |
NRR/DRO/D |
NSIR/DPCP/D |
NSIR/D |
NMSS/D |
NAME |
CMiller |
SAtack (BThomas for) |
MGavrilas (CErlanger for) |
JLubinski |
DATE |
7/7/2021 |
7/7/2021 |
7/15/2021 |
7/20/21 |
OFFICE |
OCFO/D |
OCIO/GEMS/FLICB/ICT/CO |
OGC (NLO) |
NMSS/REFS/RRPB/PM |
NAME |
CJohnson (RAllwein for) |
DCullison |
HBenowitz |
SSchneider |
DATE |
7/14/2021 |
8/11/2021 |
8/09/2021 |
8/16/2012 |
OFFICE |
NRR/D |
EDO |
|
|
NAME |
AVeil |
MDoane |
|
|
DATE |
8/27/2021 |
09/15/21 |
|
|
OFFICIAL RECORD COPY
Appendices
[Page Intentionally Blank]
Appendix A: Site‑Specific Fitness for Duty Program Performance Data (Calendar Years 2009–2019)
(Table sorted by Facility Type, then Units, and then Facility)
Facility Type |
FFD Program |
Facility |
Units |
2009 Total |
2010 Total |
2011 Total |
2012 Total |
2013 Total |
2014 Total |
2015 Total |
2016 Total |
2017 Total |
2018 Total |
2019 Total |
Average |
||||||||||||
Tested |
Positive |
Tested |
Positive |
Tested |
Positive |
Tested |
Positive |
Tested |
Positive |
Tested |
Positive |
Tested |
Positive |
Tested |
Positive |
Tested |
Positive |
Tested |
Positive |
Tested |
Positive |
Tested |
Positive |
||||
Corporate Office |
Duke Energy |
Duke Energy |
1 |
337 |
0 |
373 |
1 |
402 |
0 |
443 |
1 |
475 |
0 |
612 |
2 |
489 |
0 |
336 |
0 |
298 |
1 |
324 |
2 |
289 |
0 |
398 |
0.6 |
Southern Nuclear |
Southern Nuclear |
1 |
656 |
1 |
716 |
1 |
781 |
2 |
717 |
1 |
691 |
2 |
649 |
1 |
610 |
2 |
621 |
3 |
562 |
0 |
479 |
0 |
446 |
2 |
630 |
1.4 |
|
Tennessee Valley Authority (TVA) |
TVA |
1 |
649 |
1 |
787 |
1 |
557 |
0 |
250 |
3 |
585 |
2 |
590 |
0 |
591 |
0 |
584 |
1 |
343 |
0 |
218 |
1 |
177 |
0 |
485 |
0.8 |
|
Xcel Energy |
Xcel Energy |
1 |
160 |
0 |
225 |
2 |
293 |
1 |
311 |
0 |
370 |
0 |
399 |
1 |
411 |
1 |
485 |
0 |
512 |
4 |
507 |
0 |
544 |
1 |
383 |
0.9 |
|
Exelon |
Exelon |
2 |
444 |
0 |
431 |
2 |
459 |
0 |
525 |
0 |
580 |
0 |
537 |
0 |
565 |
2 |
616 |
1 |
1,092 |
5 |
1,047 |
0 |
1,164 |
6 |
678 |
1.5 |
|
Contractor/ Vendor |
Institute of Nuclear Power Operations (INPO) |
INPO |
1 |
81 |
0 |
348 |
1 |
367 |
0 |
362 |
0 |
380 |
1 |
324 |
1 |
317 |
0 |
347 |
0 |
314 |
0 |
307 |
0 |
264 |
1 |
310 |
0.4 |
Category I Special Nuclear Material |
BWX Technologies (BWXT) |
BWXT, Lynchburg, VA |
1 |
710 |
2 |
765 |
0 |
747 |
1 |
852 |
1 |
847 |
0 |
830 |
0 |
749 |
2 |
478 |
0 |
469 |
1 |
519 |
0 |
488 |
0 |
678 |
0.6 |
Nuclear Fuel Services (NFS) |
NFS, Erwin, TN |
1 |
849 |
1 |
866 |
2 |
874 |
4 |
858 |
3 |
790 |
3 |
747 |
2 |
887 |
3 |
811 |
5 |
824 |
3 |
920 |
4 |
978 |
4 |
855 |
3.1 |
|
Reactor |
Ameren UE |
Callaway |
1 |
1,005 |
3 |
1,766 |
6 |
1,924 |
7 |
924 |
3 |
1,840 |
8 |
2,044 |
18 |
1,155 |
4 |
1,672 |
8 |
2,241 |
11 |
1,079 |
5 |
1,635 |
9 |
1,571 |
7.5 |
Exelon |
Clinton |
1 |
1,265 |
11 |
1,958 |
8 |
1,743 |
13 |
755 |
3 |
2,018 |
11 |
952 |
3 |
1,540 |
4 |
806 |
5 |
2,779 |
5 |
1,503 |
6 |
2,346 |
13 |
1,606 |
7.5 |
|
Energy Northwest |
Columbia |
1 |
3,209 |
29 |
1,494 |
6 |
3,835 |
32 |
1,171 |
2 |
2,083 |
23 |
1,354 |
7 |
2,262 |
22 |
1,260 |
8 |
2,144 |
16 |
1,233 |
14 |
1,925 |
12 |
1,997 |
15.5 |
|
Nebraska Public Power District |
Cooper |
1 |
2,478 |
12 |
1,070 |
2 |
1,681 |
10 |
2,173 |
13 |
793 |
4 |
1,734 |
4 |
695 |
2 |
1,854 |
12 |
661 |
0 |
1,518 |
6 |
637 |
2 |
1,390 |
6.1 |
|
Energy Harbor |
Davis-Besse |
1 |
863 |
3 |
2,662 |
9 |
2,903 |
15 |
1,545 |
3 |
1,867 |
10 |
3,017 |
14 |
970 |
6 |
1,941 |
9 |
828 |
2 |
1,598 |
13 |
904 |
1 |
1,736 |
7.7 |
|
Detroit Edison |
Fermi 2 |
1 |
2,550 |
1 |
2,922 |
19 |
1,625 |
9 |
2,855 |
15 |
1,842 |
10 |
3,030 |
15 |
3,446 |
24 |
1,910 |
20 |
2,696 |
13 |
2,883 |
22 |
1,469 |
3 |
2,475 |
13.7 |
|
Entergy Nuclear |
FitzPatrick |
1 |
829 |
2 |
2,135 |
10 |
757 |
3 |
2,078 |
12 |
802 |
2 |
2,288 |
10 |
750 |
4 |
1,178 |
7 |
2,129 |
11 |
2,167 |
15 |
566 |
3 |
1,425 |
7.2 |
|
Entergy Nuclear |
Grand Gulf |
1 |
1,202 |
2 |
2,080 |
18 |
2,427 |
19 |
5,314 |
22 |
1,230 |
11 |
2,380 |
15 |
1,213 |
9 |
2,438 |
18 |
1,824 |
7 |
3,277 |
34 |
2,172 |
13 |
2,323 |
15.3 |
|
Duke Energy |
H.B. Robinson |
1 |
734 |
3 |
1,596 |
10 |
1,368 |
7 |
2,458 |
16 |
2,771 |
15 |
1,266 |
3 |
1,353 |
7 |
1,181 |
2 |
1,993 |
9 |
2,307 |
24 |
583 |
1 |
1,601 |
8.8 |
|
Xcel Energy |
Monticello |
1 |
2,452 |
11 |
1,234 |
8 |
3,329 |
17 |
1,019 |
5 |
2,794 |
18 |
835 |
4 |
1,199 |
3 |
615 |
1 |
1,055 |
3 |
493 |
5 |
961 |
6 |
1,453 |
7.4 |
|
Entergy Nuclear |
Palisades |
1 |
2,019 |
7 |
2,060 |
24 |
893 |
8 |
1,855 |
22 |
1,083 |
7 |
1,894 |
7 |
1,827 |
8 |
803 |
10 |
1,387 |
12 |
1,972 |
15 |
776 |
6 |
1,506 |
11.5 |
|
Energy Harbor |
Perry |
1 |
2,512 |
12 |
1,126 |
2 |
2,066 |
16 |
1,192 |
5 |
2,561 |
19 |
1,265 |
3 |
2,738 |
19 |
980 |
4 |
2,150 |
27 |
689 |
3 |
2,103 |
25 |
1,762 |
12.3 |
|
Exelon |
R.E. Ginna |
1 |
1,890 |
30 |
933 |
11 |
1,306 |
2 |
1,217 |
15 |
778 |
1 |
1,035 |
10 |
1,091 |
10 |
684 |
2 |
1,456 |
4 |
1,187 |
14 |
622 |
4 |
1,109 |
9.4 |
|
Entergy Nuclear |
River Bend |
1 |
2,083 |
16 |
1,632 |
13 |
1,421 |
5 |
1,054 |
8 |
2,184 |
11 |
1,078 |
10 |
1,745 |
9 |
1,512 |
12 |
2,738 |
18 |
1,539 |
11 |
2,188 |
19 |
1,743 |
12.0 |
|
NextEra Energy |
Seabrook |
1 |
2,628 |
19 |
1,050 |
6 |
2,021 |
18 |
2,293 |
19 |
848 |
3 |
1,597 |
10 |
2,164 |
33 |
712 |
2 |
1,533 |
11 |
1,496 |
18 |
751 |
12 |
1,554 |
13.7 |
|
Duke Energy |
Shearon Harris |
1 |
1,114 |
3 |
2,460 |
12 |
1,128 |
0 |
1,943 |
6 |
1,870 |
6 |
1,481 |
4 |
1,848 |
12 |
2,139 |
13 |
984 |
4 |
1,346 |
7 |
1,463 |
8 |
1,616 |
6.8 |
|
Dominion Generation |
V.C. Summer 1 |
1 |
1,667 |
13 |
1,112 |
4 |
1,792 |
11 |
2,016 |
11 |
1,867 |
16 |
2,781 |
28 |
3,058 |
20 |
2,095 |
11 |
2,434 |
19 |
1,872 |
10 |
980 |
4 |
1,970 |
13.4 |
|
Entergy Nuclear |
Waterford |
1 |
1,623 |
15 |
1,475 |
7 |
1,451 |
11 |
2,918 |
30 |
930 |
8 |
1,511 |
21 |
1,881 |
25 |
1,228 |
8 |
2,055 |
18 |
2,146 |
21 |
1,766 |
14 |
1,726 |
16.2 |
|
Reactor (continued) |
Wolf Creek |
Wolf Creek |
1 |
2,117 |
5 |
1,246 |
1 |
2,667 |
17 |
1,756 |
7 |
3,286 |
8 |
2,017 |
9 |
2,414 |
9 |
2,032 |
2 |
965 |
1 |
1,777 |
9 |
1,556 |
7 |
1,985 |
6.8 |
Entergy Nuclear |
Arkansas Nuclear One |
2 |
2,309 |
14 |
2,628 |
14 |
2,820 |
25 |
2,407 |
16 |
3,182 |
32 |
2,331 |
23 |
3,804 |
35 |
3,061 |
21 |
3,013 |
47 |
3,939 |
35 |
2,693 |
31 |
2,926 |
26.6 |
|
Energy Harbor |
Beaver Valley |
2 |
2,924 |
21 |
2,149 |
11 |
2,129 |
9 |
3,391 |
19 |
2,736 |
12 |
2,683 |
9 |
3,322 |
16 |
2,381 |
4 |
1,839 |
10 |
2,292 |
14 |
1,765 |
12 |
2,510 |
12.5 |
|
Exelon |
Braidwood |
2 |
3,511 |
33 |
2,510 |
17 |
2,053 |
9 |
3,013 |
15 |
2,491 |
5 |
1,804 |
8 |
2,484 |
21 |
2,677 |
10 |
3,234 |
11 |
2,334 |
18 |
1,786 |
11 |
2,536 |
14.4 |
|
Duke Energy |
Brunswick |
2 |
2,311 |
16 |
2,603 |
10 |
2,697 |
15 |
2,779 |
17 |
3,789 |
18 |
3,546 |
13 |
3,637 |
21 |
3,011 |
14 |
2,282 |
17 |
2,561 |
14 |
2,368 |
22 |
2,871 |
16.1 |
|
Exelon |
Calvert Cliffs |
2 |
2,343 |
10 |
2,305 |
14 |
2,225 |
13 |
2,504 |
13 |
2,463 |
15 |
2,231 |
8 |
2,433 |
20 |
2,429 |
16 |
1,800 |
14 |
1,566 |
13 |
2,096 |
14 |
2,218 |
13.6 |
|
Duke Energy |
Catawba |
2 |
2,976 |
14 |
2,670 |
16 |
2,453 |
16 |
3,054 |
20 |
3,007 |
17 |
2,091 |
11 |
3,033 |
19 |
2,406 |
13 |
1,309 |
3 |
2,153 |
12 |
1,999 |
12 |
2,468 |
13.9 |
|
Vistra Energy |
Comanche Peak |
2 |
2,248 |
15 |
2,274 |
6 |
3,119 |
16 |
2,351 |
10 |
2,490 |
8 |
3,837 |
17 |
2,514 |
13 |
2,301 |
11 |
3,444 |
16 |
2,117 |
12 |
2,157 |
8 |
2,623 |
12.0 |
|
Indiana Michigan Power |
D.C. Cook |
2 |
4,337 |
52 |
4,017 |
30 |
3,565 |
22 |
3,012 |
17 |
4,482 |
29 |
3,493 |
15 |
3,369 |
18 |
5,122 |
25 |
3,021 |
15 |
3,368 |
21 |
3,988 |
37 |
3,798 |
25.5 |
|
Pacific Gas & Electric |
Diablo Canyon |
2 |
4,731 |
28 |
3,105 |
14 |
2,973 |
17 |
2,826 |
14 |
2,937 |
10 |
3,486 |
25 |
3,238 |
14 |
2,822 |
9 |
2,684 |
10 |
2,732 |
8 |
3,087 |
14 |
3,147 |
14.8 |
|
Southern Nuclear |
E.I. Hatch |
2 |
2,823 |
7 |
3,187 |
31 |
3,592 |
47 |
3,114 |
18 |
3,205 |
17 |
3,078 |
23 |
3,506 |
27 |
3,194 |
32 |
2,761 |
15 |
2,758 |
15 |
2,868 |
12 |
3,099 |
22.2 |
|
Southern Nuclear |
Joseph M. Farley |
2 |
2,513 |
29 |
3,968 |
43 |
3,724 |
39 |
2,681 |
42 |
2,797 |
11 |
1,935 |
16 |
1,969 |
11 |
2,764 |
31 |
2,395 |
15 |
2,000 |
12 |
2,635 |
9 |
2,671 |
23.5 |
|
Exelon |
LaSalle |
2 |
2,440 |
9 |
2,698 |
18 |
3,270 |
11 |
2,829 |
9 |
2,360 |
11 |
2,583 |
5 |
2,998 |
14 |
3,132 |
14 |
1,779 |
9 |
1,726 |
18 |
2,436 |
12 |
2,568 |
11.8 |
|
Exelon |
Limerick |
2 |
2,526 |
16 |
2,599 |
16 |
3,049 |
23 |
3,622 |
23 |
2,751 |
24 |
2,551 |
9 |
2,480 |
15 |
2,355 |
12 |
2,741 |
13 |
1,990 |
8 |
1,859 |
12 |
2,593 |
15.5 |
|
Duke Energy |
McGuire |
2 |
2,703 |
17 |
2,536 |
16 |
4,370 |
18 |
3,568 |
6 |
2,965 |
10 |
4,198 |
19 |
2,713 |
14 |
2,502 |
11 |
3,035 |
15 |
2,293 |
10 |
1,687 |
16 |
2,961 |
13.8 |
|
Dominion Generation |
Millstone |
2 |
2,206 |
9 |
2,206 |
16 |
2,917 |
25 |
2,403 |
7 |
2,384 |
19 |
3,526 |
22 |
3,139 |
28 |
2,428 |
11 |
3,271 |
26 |
2,185 |
15 |
2,081 |
20 |
2,613 |
18.0 |
|
Exelon |
Nine Mile Point |
2 |
2,520 |
20 |
3,132 |
31 |
2,552 |
13 |
3,141 |
24 |
2,678 |
16 |
2,256 |
16 |
2,650 |
12 |
2,295 |
15 |
1,746 |
5 |
1,930 |
11 |
1,984 |
13 |
2,444 |
16.0 |
|
Dominion Generation |
North Anna |
2 |
1,828 |
12 |
3,085 |
14 |
2,031 |
6 |
2,121 |
14 |
2,305 |
12 |
2,269 |
5 |
2,267 |
9 |
3,048 |
7 |
1,905 |
6 |
1,997 |
7 |
2,793 |
15 |
2,332 |
9.7 |
|
Exelon |
Peach Bottom |
2 |
3,075 |
21 |
2,912 |
14 |
3,802 |
19 |
3,643 |
18 |
4,123 |
19 |
3,836 |
14 |
4,051 |
22 |
2,187 |
14 |
1,728 |
11 |
2,336 |
15 |
1,831 |
9 |
3,048 |
16.0 |
|
NextEra Energy |
Point Beach |
2 |
2,340 |
4 |
2,214 |
5 |
4,831 |
12 |
1,290 |
3 |
1,260 |
0 |
1,771 |
8 |
1,311 |
4 |
1,351 |
4 |
1,798 |
6 |
929 |
2 |
998 |
4 |
1,827 |
4.7 |
|
Xcel Energy |
Prairie Island |
2 |
1,663 |
9 |
1,625 |
5 |
1,260 |
6 |
2,057 |
9 |
2,822 |
11 |
1,824 |
4 |
1,940 |
6 |
1,579 |
4 |
1,275 |
5 |
1,550 |
8 |
1,108 |
8 |
1,700 |
6.8 |
|
Exelon |
Quad Cities |
2 |
2,247 |
19 |
2,476 |
17 |
2,014 |
10 |
2,111 |
11 |
2,242 |
19 |
1,854 |
10 |
1,715 |
9 |
1,608 |
7 |
2,024 |
11 |
1,502 |
14 |
1,766 |
14 |
1,960 |
12.8 |
|
TVA |
Sequoyah |
2 |
2,916 |
18 |
2,974 |
22 |
2,849 |
20 |
5,048 |
28 |
2,660 |
14 |
1,942 |
14 |
2,231 |
14 |
2,020 |
12 |
1,526 |
8 |
2,641 |
23 |
2,449 |
20 |
2,660 |
17.5 |
|
STP Nuclear |
South Texas Project |
2 |
2,672 |
17 |
2,757 |
8 |
3,082 |
15 |
2,302 |
17 |
2,629 |
17 |
2,428 |
13 |
3,109 |
13 |
2,474 |
17 |
2,467 |
20 |
2,983 |
17 |
2,438 |
17 |
2,667 |
15.5 |
|
NextEra Energy |
St. Lucie |
2 |
2,525 |
17 |
4,534 |
26 |
5,204 |
22 |
4,887 |
14 |
2,809 |
13 |
2,504 |
15 |
3,362 |
11 |
2,514 |
8 |
2,306 |
13 |
3,187 |
19 |
2,264 |
12 |
3,281 |
15.5 |
|
Dominion Generation |
Surry |
2 |
2,069 |
17 |
2,147 |
18 |
2,744 |
48 |
2,306 |
19 |
1,520 |
15 |
1,869 |
8 |
3,004 |
19 |
1,535 |
9 |
1,673 |
10 |
2,205 |
25 |
1,511 |
20 |
2,053 |
18.9 |
|
Talen Energy |
Susquehanna |
2 |
3,167 |
14 |
3,324 |
13 |
3,327 |
8 |
2,914 |
9 |
2,985 |
11 |
3,435 |
15 |
3,026 |
16 |
3,224 |
11 |
2,806 |
16 |
2,698 |
16 |
2,399 |
20 |
3,028 |
13.5 |
|
NextEra Energy |
Turkey Point |
2 |
3,813 |
19 |
3,827 |
20 |
4,718 |
21 |
8,216 |
40 |
2,247 |
10 |
2,904 |
10 |
1,908 |
8 |
2,050 |
9 |
2,377 |
21 |
1,852 |
14 |
1,989 |
9 |
3,264 |
16.5 |
|
Southern Nuclear |
Vogtle 1 & 2 |
2 |
2,774 |
30 |
2,837 |
26 |
3,856 |
21 |
3,284 |
57 |
2,605 |
27 |
3,749 |
34 |
3,282 |
49 |
2,651 |
28 |
3,625 |
15 |
2,552 |
32 |
2,040 |
13 |
3,023 |
30.2 |
|
Reactor (continued) |
TVA |
Watts Bar |
2 |
4,799 |
19 |
6,506 |
40 |
5,918 |
26 |
5,628 |
38 |
4,477 |
27 |
5,244 |
34 |
4,259 |
24 |
1,472 |
8 |
2,769 |
17 |
2,655 |
12 |
1,831 |
8 |
4,142 |
23.0 |
TVA |
Browns Ferry |
3 |
3,313 |
16 |
4,958 |
17 |
3,607 |
9 |
4,713 |
25 |
3,922 |
27 |
3,897 |
22 |
2,746 |
17 |
4,372 |
18 |
3,305 |
23 |
4,653 |
34 |
3,134 |
21 |
3,875 |
20.8 |
|
Duke Energy |
Oconee |
3 |
3,742 |
22 |
3,309 |
21 |
2,643 |
16 |
3,443 |
14 |
3,106 |
15 |
3,792 |
28 |
2,841 |
15 |
2,685 |
12 |
2,097 |
14 |
2,186 |
14 |
1,856 |
13 |
2,882 |
16.7 |
|
Arizona Public Service |
Palo Verde |
3 |
6,961 |
18 |
4,873 |
19 |
4,422 |
11 |
4,377 |
16 |
4,171 |
18 |
4,194 |
21 |
4,260 |
14 |
4,263 |
16 |
4,225 |
18 |
4,569 |
23 |
4,530 |
20 |
4,622 |
17.6 |
|
PSEG Nuclear |
Salem/ Hope Creek |
3 |
3,768 |
24 |
4,291 |
28 |
4,199 |
23 |
4,288 |
34 |
4,252 |
24 |
4,195 |
27 |
4,424 |
34 |
4,098 |
22 |
3,484 |
25 |
3,432 |
20 |
3,567 |
22 |
4,000 |
25.7 |
|
Reactor - Construction |
Southern Nuclear |
Vogtle 3 & 4 |
2 |
47 |
0 |
3,277 |
56 |
3,933 |
80 |
5,440 |
101 |
5,862 |
98 |
9,055 |
168 |
7,833 |
149 |
12,079 |
228 |
12,256 |
247 |
15,829 |
344 |
18,168 |
383 |
8,525 |
168.5 |
Totals |
100 |
137,266 |
808 |
143,035 |
858 |
152,765 |
919 |
155,617 |
963 |
140,879 |
830 |
144,638 |
891 |
142,826 |
971 |
131,408 |
847 |
132,466 |
932 |
134,103 |
1,134 |
123,914 |
1,059 |
139,902 |
928.4 |
||
Notes on Appendix A:
Site construction at Vogtle Units 3 and 4 began in calendar year (CY) 2009. Site construction is expected to be complete prior to the compliance date of the final rule.
Construction of Watts Bar Unit 2 restarted in CY 2008 and completed in CY 2015; the licensee did not report separately for the construction site.
Model Inputs |
Mean Value |
Data Source |
Final Rule—Effective Date and Scope |
||
Year rule finalized |
2022 |
U.S. Nuclear Regulatory Commission (NRC) staff assumption |
Year rule effective |
2023 |
NRC staff assumption |
Total number of fitness for duty (FFD) programs |
24 |
NRC FFD Program Performance Results Calendar Year (CY) 2019 |
Total number of sites |
59 |
NRC FFD Program Performance Results CY 2019 (see Appendix A). Total sites = 51 operating power reactor sites, 2 Category I special nuclear material licensees, 5 corporate offices, and 1 contractor/vendor (C/V). (This analysis excludes sites that are in decommissioning or sites with announced dates when their unit will permanently cease commercial operation, as described in Section 4.2.2.) |
Number of sites using a licensee testing facility (LTF) |
3 |
NRC FFD Program Performance Results CY 2019 |
Number of sites only using a U.S. Department of Health and Human Services (HHS)‑certified laboratory |
56 |
NRC FFD Program Performance Results CY 2019 |
Number of workers subject to a 10 CFR Part 26 FFD program |
92,356 |
NRC FFD Program Performance Results CYs 2009–2019. In the annual 10 CFR 26.717 FFD program performance report submitted to the NRC, each licensee or other entity reports the average number of licensee employees and C/Vs subject to random testing in the reporting year. The average of the yearly total for all FFD sites in CYs 2009–2019 is the best approximation of the total number of individuals in the workforce who require training on policy changes resulting from the rule. Adjusted for construction sites becoming operational in CYs 2021 and 2022. |
Average number of workers subject to a 10 CFR Part 26 FFD program per site |
1,565 |
Calculated from the NRC FFD Program Performance Results CYs 2009–2019 [(total number of individuals subject to random testing per year) / (total number of sites)] |
Number of drug tests conducted per year |
133,141 |
NRC FFD Program Performance Results (average of total number of tests conducted for CYs 2009–2019), adjusted for construction sites becoming operational (used operating site data at the co‑located reactors to model test results) |
Number of drug tests conducted per site per year |
2,257 |
Calculated from the NRC FFD Program Performance Results CYs 2009–2019 [(total number of drug tests conducted per year) / (total number of sites)] |
Industry Implementation (One-Time)—Hourly Wage Rates |
||
Clerical |
$23.84/hour |
Model facility data: “Inputs—Wages” (from January to May 2002) provided to the NRC by the Nuclear Energy Institute on FFD drug and alcohol testing programs.
These data were used in the regulatory impact analysis for the 10 CFR Part 26 FFD final rule (March 2008) and were converted into 2022 dollars. |
Facility Worker (weighted average of licensee and contractor/vendor workers) |
$70.65/hour |
|
FFD Manager |
$47.79/hour |
|
FFD Staff |
$41.20/hour |
|
LTF Laboratory Technician |
$40.84/hour |
|
LTF Laboratory Supervisor |
$68.66/hour |
|
Legal |
$137.32/hour |
|
Medical Review Officer (MRO) |
$151.36/hour |
|
Industry Implementation (One-Time)—Training |
||
Number of sites that distribute a summary of FFD program rule changes to employees outside of routine training |
3 |
NRC staff assumption. Based on the implementation timeframe, most licensees and other entities will incorporate training on the new FFD program requirements into existing annual training/refresher training opportunities, as well as post information at the collection sites and on bulletin boards, etc. The NRC staff estimates that between 0 and 10 percent of sites will conduct training specifically on rule changes and outside routine training. (0.05 × 59 sites = 3 sites) |
Number of FFD programs with a blind performance test sample (BPTS) supplier contract |
24 |
NRC staff assumption (all FFD programs have a contract with a BPTS supplier) |
Cost of LTF training materials |
$533.33 per LTF |
NRC staff assumption based on the 2008 10 CFR Part 26 FFD final rule regulatory analysis (March 2008) |
Number of Laboratory Technicians per LTF |
2 |
NRC staff assumption based on communications in CY 2021 with licensees using LTFs |
Industry Operations (Annual)—Costs |
||
Initial testing for one additional drug at an LTF |
$3.59 per test |
NRC staff assumption based on feedback received in CY 2021 from licensees using LTFs |
Initial and confirmatory drug testing, HHS-certified laboratory (sites using an LTF for initial testing) |
$28.83 per specimen |
NRC staff assumption based on industry feedback received in CY 2021 (weighted average of LTF testing costs for positive results from CYs 2009–2019) |
Initial and confirmatory drug testing, HHS-certified laboratory (sites only using HHS-certified laboratories) |
$12.88 per specimen |
NRC staff assumption based on industry feedback received in CYs 2020 and 2021 |
Testing for 6-AM (sites only using an HHS-certified laboratory) |
$0.39 per test |
NRC staff assumption partially informed by the 49 CFR Part 40 final rule (75 FR 49850; August 16, 2010) that aligned the U.S. Department of Transportation (DOT) drug testing panel with the 2008 HHS Guidelines (it reported an average cost per 6‑AM test as $0.26) |
Testing for Expanded Opioid Panel drugs (hydrocodone, hydromorphone, oxycodone, and oxymorphone) (sites only using an HHS-certified laboratory) |
$0.58 per test |
NRC staff assumption partially informed by industry feedback on HHS-certified test laboratory reported costs and estimated testing cost increases reported by the HHS and the DOT. The 2017 HHS Guidelines (82 FR 7931; January 23, 2017) reported an estimated cost per specimen from $0.11 to $0.30 for expanded opioid panel testing. In the DOT's 49 CFR Part 40 final rule (82 FR 52240–52241, November 13, 2017), the DOT reported an estimated increase in per specimen cost of $0.60. |
Testing for Ecstasy-type drugs (sites only using an HHS-certified laboratory) |
$0.14 per test |
NRC staff assumption partially informed by the 49 CFR Part 40 final rule (75 FR 49850; August 16, 2010) that aligned the DOT drug testing panel with the 2008 HHS Guidelines (the DOT reported an average cost per specimen to test for Ecstasy-type drugs of $0.09) |
Special analyses testing at an HHS‑certified laboratory |
$7.67 per specimen |
NRC staff assumption based on industry feedback received in CY 2021 |
Cost of subsequent actions (per positive result) |
$310.66 per test |
Cost estimate based on information in the 10 CFR Part 26 Office of Management and Budget clearance supporting statement (No. 3150‑0146) approved on April 3, 2018, as well as the NRC staff assumption on MRO review time |
Industry Operations (Annual)—Drug Testing Rates |
||
Opioid: 6-Acetylmorphine (6-AM) |
||
Projected confirmed positive test rate |
0.016% |
DOT laboratory test results Average positive rate for 6-AM (CYs 2010–2017, Jan-Jun 2018) [2010 = 0.010%; 2011 = 0.014%; 2012 = 0.016%; 2013 = 0.019%; 2014 = 0.022%, 2015 = 0.022%; 2016 = 0.021%; 2017 = 0.018%; 2018 (Jan-Jun) = 0.015%] |
Opioid: Hydrocodone/Hydromorphone/Oxycodone/Oxymorphone |
||
Projected confirmed positive test rate for expanded opioid panel |
0.066% |
NRC FFD Program Performance Results for amphetamines positives is used as a proxy for the projected confirmed positive test rate for the expanded opioid panel.
This decision is partially informed by HHS laboratory results for all DOT tests (worksheet "DOT Lab Testing Data"). Similar to amphetamines, these drugs are available legally by prescription and have a tendency to be abused. Based on these data, the NRC staff concluded that the confirmed positive test rate for amphetamines is a better indicator of initial opioid positivity. |
Projected percent increase in positive test rate in first year |
293% |
MRO-verified test results for CYs 2017 and 2018 for DOT modal administrations (Federal Aviation Administration (FAA), Federal Rail Administration (FRA), and Federal Transit Administration (FTA)). Change in average opioid positive rate for CY 2018, using CY 2017 as the baseline positive rate for comparison:
[Opioids average positive rate: 2017 = 0.028%; 2018 = 0.109%] |
Projected percentage of additional opioid positive results that will confirm positive after MRO interview with donor |
71% |
Projected percentage of additional opioid positive results that will confirm positive after MRO interview with donor |
Projected percentage of additional opioid positive results that will be negative after MRO interview with donor |
29% |
Calculated value |
Amphetamines |
||
FFD current confirmed positive test rate |
0.066% |
NRC FFD Program Performance Results Average positive rate for amphetamines (CYs 2010–2019) [2010 = 0.033%; 2011 = 0.048%; 2012 = 0.036%; 2013 = 0.053%; 2014 = 0.067%; 2015 = 0.067%; 2016 = 0.094%; 2017 = 0.095%; 2018 = 0.091%, 2019 = 0.085%] |
Projected percent increase in confirmed positive test rate |
36.65% |
MRO-verified test results for CYs 2010 and 2011 for DOT modal administrations (FAA, FRA, and FTA). Change in average amphetamines positive rate for CY 2011, using CY 2010 as the baseline positive rate for comparison (i.e., CY 2011 was the first year of lower amphetamines testing cutoff levels).
[Amphetamines average positive rate: 2010 = 0.057%; 2011 = 0.080%] |
Projected percentage of additional positive results that will confirm positive after MRO interview with donor |
71% |
NRC staff assumption based on FFD Program Performance Results on amphetamine and methamphetamine positive results |
Projected percentage of additional positive results that will be negative after MRO interview with donor |
29% |
NRC staff assumption |
Cocaine |
||
FFD current confirmed positive test rate |
0.083% |
NRC FFD Program Performance Results Average positive rate for cocaine (CYs 2010–2019) [2010 = 0.076%; 2011 = 0.071%; 2012 = 0.075%; 2013 = 0.077%, 2014 = 0.064%; 2015 = 0.094%; 2016 = 0.100%, 2017 = 0.104%; 2018 = 0.089%; 2019 = 0.077%] |
Projected percent increase in confirmed positive test rate |
19.66% |
MRO-verified test results for CYs 2010 and 2011 for DOT modal administrations (FAA, FRA, and FTA). Change in average cocaine positive rate for CY 2011, using CY 2010 as the baseline year for comparison (i.e., CY 2011 was the first year of lower cocaine testing cutoff levels).
[Cocaine average positive rate: 2010 = 0.175%; 2011 = 0.207%] |
Ecstasy-Type Drugs |
||
Projected confirmed positive test rate |
0.004% |
DOT laboratory test results (CYs 2010–2015) Average positive rate for the Ecstasy-type drugs MDMA and MDA [2010 = 0.002%; 2011 = 0.005%; 2012 = 0.004%; 2013 = 0.005%, 2014 = 0.005%; 2015 = 0.006%] |
Dilute Specimens (Special Analyses Testing) |
||
Average annual percentage of specimens tested that are dilute and special analyses testing performed |
0.375% |
NRC FFD Program Performance Results (e-reported data, data first available in CY 2013)
|
Average annual percentage of specimens tested that are dilute and test positive on special analyses testing |
0.006% |
NRC FFD Program Performance Results (e-reported data, first available in CY 2011)
|
Subversion Attempts (Special Analyses Testing of Suspect Specimens) |
||
Average annual percentage of specimens tested that are determined to be a subversion attempt and that test positive (suspect specimens that test positive on special analyses testing) |
0.049% |
The final rule requires special analyses testing in two circumstances: (1) on the second specimen collected under direct observation when the initial specimen collected exhibits unusual characteristics (e.g., temperature out of range, unusual color or odor), and (2) on the second specimen collected under direct observation when the initial specimen is reported as an invalid test result.
NRC FFD Program Performance Results (e-reported data, first available in CY 2011)
|
Project percent increase in confirmed positive test rate for specimens collected under direct observation |
12.5% |
NRC staff assumption |
MRO subsequent action labor hours |
0.71 hour |
NRC staff assumption |
Averted Training Costs—Pre-Access Testing |
||
Percentage of total positive, adulterated, substituted, and refusal to test results occurring at pre-access testing (6-year average) |
67.1% |
NRC FFD Program Performance Results (CYs 2009–2019) [2009 = 68.2%; 2010 = 68.7%; 2011 = 68.6%; 2012 = 68.8%; 2013 = 64.8%; 2014 = 67.3%; 2015 = 67.0%; 2016 = 64.7%; 2017 = 64.4%; 2018 = 69.6%; 2019 = 67.3%] |
Alternative Specimen (Oral Fluid) Collection and Testing |
||
Testing of an oral fluid specimen using an HHS-certified laboratory |
$20.00 per specimen |
NRC staff assumption based on industry feedback |
Oral fluid collection time (donor) |
0.35 hour |
NRC staff assumption. The time value is the same for the donor and collector because both individuals participate in the process for the duration of the sample collection process. |
Oral fluid collection time (collector) |
0.35 hour |
|
Annual number of identified subversion attempts |
226 |
NRC FFD Program Performance Results (CYs 2011–2019) Average number of subversion attempts identified per year |
Percentage of subversion attempts confirmed through the testing of specimens collected under direct observation |
31.1% |
NRC FFD Program Performance Results (CYs 2011–2019) Average percentage of subversion attempts confirmed through the collection and testing of a second specimen under direct observation |
Direct Observation Collection of Urine Specimens |
||
Urine collection time (donor) |
1.25 hours |
NRC staff assumption. On average, a donor must hydrate for a period of time before providing a second specimen under direct observation. The time value is the same for the donor and the collector because the donor is observed during the hydration process and the donor and collector participate in the collection process for the duration of the event. |
Urine collection time (collector) |
1.25 hours |
|
Entity-Specific Information |
||
Average remaining license term per site |
24 years |
Calculated based on license expiration date (assumes all 40-year operating power reactor licenses are extended for 20 years). Certain reactors are assumed to operate for a second 20-year term (80-year total) for those licensees that have received or have announced their intention to apply for a second license renewal). Category I special nuclear material licensees are assumed to continue to operate as long as any reactor is operating. |
New reactor license term |
60 years |
New power reactors are assumed to operate for the original 40‑year operating license and for an additional 20-year license extension. |
Inflation Rates |
||
Ratio of 2022 Annual Average CPI-U to 2006 Annual Average CPI-U |
1.35 |
U.S. Bureau of Labor Statistics (Table 24. Historical Consumer Price Index for All Urban Consumers (CPI-U): U.S. city average, all items)
[CPI-U: CY 2002 = 179.9; CY 2006 = 201.6; CY 2022 = 272.3] |
Ratio of 2022 Annual Average CPI-U to 2002 Annual Average CPI-U |
1.51 |
|
Appendix C: Assumptions and Results by Regulatory Initiative
C.1 Policy, Procedure, and Training Costs The U.S. Nuclear Regulatory Commission’s (NRC’s) final rule imposes one-time costs on industry for the following five activities:
|
||||||||||
Activity |
Labor Category |
Wage Rate or Unit Cost |
Quantity |
Benefits (Cost) |
Entities Affected |
Total Benefits (Costs) |
||||
INDUSTRY IMPLEMENTATION (ONE-TIME) |
||||||||||
(1) Update policies and procedures |
FFD Manager |
$47.79/hour |
12 hours/program |
($574) |
24 |
($13,776) |
||||
FFD Staff |
$41.20/hour |
4 hours/program |
($165) |
24 |
($3,960) |
|||||
Clerical |
$23.84/hour |
1 hour/program |
($24) |
24 |
($576) |
|||||
Legal |
$137.32/hour |
2 hours/program |
($275) |
24 |
($6,600) |
|||||
(2) Inform employees of policy change |
FFD Manager |
$47.79/hour |
4 hours/program |
($191) |
24 |
($4,584) |
||||
Clerical |
$23.84/hour |
0.5 hour/program |
($12) |
24 |
($288) |
|||||
Legal |
$137.32/hour |
0.5 hour/program |
($69) |
24 |
($1,656) |
|||||
Facility Worker |
$70.65/hour |
0.2 hour/worker and 1,565 workers per site |
($22,115) |
3 |
($66,345) |
|||||
(3) Revise contract with primary HHS-certified laboratory |
FFD Manager |
$47.79/hour |
4 hours/program |
($191) |
24 |
($4,584) |
||||
Clerical |
$23.84/hour |
2 hours/program |
($48) |
24 |
($1,152) |
|||||
Legal |
$137.32/hour |
2 hours/program |
($275) |
24 |
($6,600) |
|||||
(3) Revise contract with backup HHS-certified laboratory |
FFD Manager |
$47.79/hour |
4 hours/program |
($191) |
24 |
($4,584) |
||||
Clerical |
$23.84/hour |
2 hours/program |
($48) |
24 |
($1,152) |
|||||
Legal |
$137.32/hour |
2 hours/program |
($275) |
24 |
($6,600) |
|||||
(3) Revise contract with BPTS supplier |
FFD Manager |
$47.79/hour |
2 hour/program |
($96) |
24 |
($2,304) |
||||
Clerical |
$23.84/hour |
1 hour/program |
($24) |
24 |
($576) |
|||||
Legal |
$137.32/hour |
1 hour/program |
($137) |
24 |
($3,288) |
|||||
(4) Train LTF Technicians |
LTF Technician |
$40.84/hour |
2 hours/technician (2 technicians/LTF) |
($177) |
3 |
($531) |
||||
Training Materials |
$533 per LTF |
1 per LTF |
($533) |
3 |
($1,600) |
|||||
(5) Validate drug test assays at the LTF |
LTF Supervisor |
$68.66/hour |
5 hours per drug assay (6 drug assays per LTF) |
($2,060) |
3 |
($6,180) |
||||
Total Industry Implementation Cost |
($136,936) |
|||||||||
Average Implementation Cost Per Site |
($2,321) |
|||||||||
Calculations (totals may not add because of rounding):
Assumptions:
|
||||||||||
C.2 Lower Initial and Confirmatory Testing Cutoff Levels for Amphetamines and Cocaine The final rule revises the cutoff levels for initial testing (Title 10 of the Code of Federal Regulations (10 CFR) 26.133, “Cutoff levels for drugs and drug metabolites,” and 10 CFR 26.163(a)(1)) and for confirmatory testing (10 CFR 26.163(b)(1)) to align with Section 3.4 of the 2008 and 2017 HHS Guidelines as follows:
Lower cutoff levels for amphetamines and cocaine metabolites increase the window of detection in which these drugs can be identified in the urine specimens provided by individuals. The changes also provide additional assurance that persons will be unable to subvert the drug testing process by temporarily abstaining from using these drugs. As a result, the staff estimates the lower cutoffs will result in an increase in the number of urine specimens identified as containing amphetamines or cocaine metabolites, or both. The rule changes will improve the detection of drug users and may increase the deterrent effect of the testing program under 10 CFR Part 26, “Fitness for duty.” |
||||||||||
Activity |
Parameter |
Value |
Benefits (Cost) |
Sites Affected |
Total Benefits (Costs) |
|||||
INDUSTRY OPERATIONS (ANNUAL) |
||||||||||
Amphetamines |
||||||||||
Additional testing at HHS-certified laboratory for amphetamines positive results (sites using LTFs) |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($16) |
3 |
($48) |
|||||
FFD current positive test rate |
0.066% |
|
|
|
||||||
Projected percent increase in positive testing rate |
36.65% |
|
|
|
||||||
Initial and confirmatory drug testing, HHS-certified laboratory |
$28.83 |
|
|
|
||||||
Medical Review Officer (MRO) review of result, donor interview, and medical downgrade for valid prescription use of amphetamines (all sites) |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($35) |
59 |
($2,065) |
|||||
FFD current positive test rate |
0.066% |
|
|
|
||||||
Projected percent increase in positive test rate |
36.65% |
|
|
|
||||||
Expected percent of additional positive results that will be negative after MRO interview with donor |
29% |
|
|
|
||||||
MRO activities (1 hour per positive): review laboratory result, interview donor, and evaluate medical information from donor |
$151.36 per hour |
|
|
|
||||||
Facility Worker activities (1 hour per positive): participate in interview with MRO, obtain medical information on valid use, and provide to MRO |
$70.65 per hour |
|
|
|
||||||
Subsequent actions by FFD program personnel for additional amphetamines confirmed positive test results (all sites) |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($120) |
59 |
($7,080) |
|||||
FFD current positive test rate |
0.066% |
|
|
|
||||||
Projected percent increase in confirmed positive test rate |
36.65% |
|
|
|
||||||
Projected percent of additional positive results that will confirm positive after MRO interview with donor |
71% |
|
|
|
||||||
Cost of subsequent actions (per positive result) |
$310.66 |
|
|
|
||||||
Cocaine |
||||||||||
Additional testing at HHS-certified laboratory for cocaine positive results (sites using LTFs) |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($11) |
3 |
($33) |
|||||
FFD current positive test rate |
0.083% |
|
|
|
||||||
Projected percent increase in positive test rate |
19.66% |
|
|
|
||||||
Initial and confirmatory drug testing, HHS-certified laboratory |
$28.83 |
|
|
|
||||||
Subsequent actions by FFD program personnel for additional cocaine positive test results (all sites) |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($114) |
59 |
($6,726) |
|||||
FFD current positive testing rate |
0.083% |
|
|
|
||||||
Projected percent increase in positive test rate |
19.66% |
|
|
|
||||||
Cost of subsequent actions (per positive result) |
$310.66 |
|
|
|
||||||
Total Industry Operations Cost |
($15,952) |
|||||||||
Average Operations Cost Per Site |
($270) |
|||||||||
Calculations (totals may not add because of rounding):
Assumptions:
|
||||||||||
|
||||||||||
C.3 Expand Initial Drug Testing Panel to Include 6-AM and Revise Confirmatory Testing Cutoff Level for 6-AM
The final rule adds testing for 6‑acetylmorphine (6-AM) to the initial testing panel (10 CFR 26.31(d)(1) and 10 CFR 26.405(d)) and makes conforming changes to the substances for initial testing (10 CFR 26.133 and 10 CFR 26.163(a)(1)) and confirmatory testing (10 CFR 26.163(b)(1)). These changes align 10 CFR Part 26 with Section 3.4 of the 2008 HHS Guidelines as follows:
Conducting initial testing for an additional substance, 6-AM, enables the improved detection of the illegal drug heroin, which has been increasing in use in society. The performance of initial testing for 6-AM is estimated to result in an increase in the number of urine specimens identified as containing 6-AM. The rule change also may increase the deterrent effect of the testing program under 10 CFR Part 26. |
||||||||||
Activity |
Parameter |
Value |
Benefits (Cost) |
Sites Affected |
Total Benefits (Costs) |
|||||
INDUSTRY OPERATIONS (ANNUAL) |
||||||||||
6-AM |
||||||||||
6-AM
initial
testing
|
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($8,095) |
3 |
($24,285) |
|||||
Initial testing for one additional drug at an LTF |
$3.59 |
|
|
|
||||||
6-AM
testing |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($880) |
56 |
($49,280) |
|||||
Testing for 6-AM (sites only using an HHS‑certified laboratory) |
$0.39 |
|
|
|
||||||
Additional testing at HHS‑certified laboratory for 6‑AM positive results (sites using LTFs) |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($11) |
3 |
($33) |
|||||
Projected confirmed positive test rate |
0.016% |
|
|
|
||||||
Initial and confirmatory drug testing, HHS-certified laboratory (sites using an LTF for initial testing) |
$28.83 |
|
|
|
||||||
Subsequent
actions
by
FFD
program personnel
for
additional
6-AM positive
test
results
|
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($113) |
59 |
($6,667) |
|||||
Projected confirmed positive test rate |
0.016% |
|
|
|
||||||
Cost of subsequent actions (per positive result) |
$310.66 |
|
|
|
||||||
Total Industry Operations Cost |
($80,265) |
|||||||||
Average Operations Cost Per Site |
($1,360) |
|||||||||
Calculations (totals may not add because of rounding):
Assumptions:
|
||||||||||
C.4 Expand the
Initial and Confirmatory Drug Testing Panels to Include the
Opioids: The final rule adds four opioid pain relievers (hydrocodone (HYC), hydromorphone (HYM), oxycodone (OXYC), and oxymorphone (OXYM)) to the testing panel (10 CFR 26.31(d)(1) and 10 CFR 26.405(d)); makes conforming changes to the substances for initial testing (10 CFR 26.133 and 10 CFR 26.163(a)(1)) and for confirmatory testing (10 CFR 26.163(b)(1)); and adds these new substances to the annual statistical summary reporting requirements for HHS-certified laboratories (10 CFR 26.169(h)(3)). These changes align 10 CFR Part 26 with Section 3.4 of the 2017 HHS Guidelines. The rule revises the list of substances to be tested as follows:
Testing for the four additional opioids (i.e., expanded opioid panel) enables the detection of impairing drugs prevalently used in society. Testing for these drugs is expected to increase the number of urine specimens identified as containing HYC, HYM, OXYC, or OXYM, or a combination of these. The rule changes also may increase the deterrent effect of the testing program under 10 CFR Part 26 by including these additional substances in the testing panel. The addition of these four opioids to the testing panel also will result in an increase in the number of BPTSs that a licensee or other entity must submit to each HHS-certified laboratory that it maintains under contract. One BPTS will be submitted each quarter for OXYC/OXYM and one for HYC/HYM to meet the existing requirements in 10 CFR 26.168, “Blind performance testing.” |
|||||
Activity |
Parameter |
Value |
Benefits (Cost) |
Sites Affected |
Total Benefits (Costs) |
INDUSTRY OPERATIONS (ANNUAL) |
|||||
Hydrocodone, Hydromorphone, Oxycodone, and Oxymorphone |
|||||
Initial
testing
for expanded opioid panel |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($8,095) |
3 |
($24,285) |
Initial testing for one additional drug at an LTF |
$3.59 |
|
|
|
|
Testing for expanded opioid panel (sites only using HHS‑certified laboratories) |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($1,298) |
56 |
($72,688) |
Testing for expanded opioid panel (sites only using an HHS-certified laboratory) |
$0.58 |
|
|
|
|
Additional testing at HHS‑certified laboratory for expanded opioid panel positive results (sites using LTFs) |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($43) |
3 |
($129) |
Projected confirmed positive test rate for expanded opiate panel |
0.066% |
|
|
|
|
Initial and confirmatory drug testing, HHS‑certified laboratory (sites using an LTF for initial testing) |
$28.83 |
|
|
|
|
MRO result review, donor interview, and medical downgrade for valid prescription use of any of the four additional opioids (all sites) |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($96) |
59 |
($5,664) |
Projected confirmed positive test rate for expanded opioid panel |
0.066% |
|
|
|
|
Expected percent of additional opioid positive results that will be negative after MRO interview with donor |
29% |
|
|
|
|
MRO activities (1 hour per positive): review laboratory result, interview donor, and evaluate medical information from donor |
$151.36 |
|
|
|
|
Facility Worker activities (1 hour per positive): participate in interview with MRO, obtain medical information on valid use, and provide to MRO |
$70.65 |
|
|
|
|
Subsequent actions by
FFD
program personnel
for
additional
opioid positive
test
results
|
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($327) |
59 |
($19,293) |
Projected confirmed positive test rate for expanded opiate panel |
0.066% |
|
|
|
|
Projected percentage of additional opioid positive results that will confirm positive after MRO interview with donor |
71% |
|
|
|
|
Cost of subsequent actions (per positive result) |
$310.66 |
|
|
|
|
Activity |
Parameter |
Value |
Benefits (Cost) |
Sites Affected |
Total Benefits (Costs) |
BPTSs for expanded opioid panel (sites only using HHS‑certified laboratories) |
Number of BPTSs submitted per site per year (2 BPTSs per quarter) |
8 |
($580) |
56 |
($32,491) |
Cost per BPTS |
$60.00 |
|
|
|
|
Testing for expanded opioid panel (sites only using an HHS-certified laboratory) |
$0.58 |
|
|
|
|
Time to prepare and send a BPTS to the HHS‑certified laboratory for testing, and review the laboratory's test results |
0.25 hour |
|
|
|
|
FFD Manager wage rate |
$47.79 |
|
|
|
|
BPTSs for expanded opioid panel (sites using LTFs) |
Number
of BPTSs submitted per site per year |
8 |
($806) |
3 |
($2,419) |
Cost per BPTS test |
$60.00 |
|
|
|
|
Initial and confirmatory drug testing, HHS‑certified laboratory (sites using an LTF for initial testing) |
$28.83 |
|
|
|
|
Time to prepare and send a BPTS to the HHS‑certified laboratory for testing, and review the laboratory's test results |
0.25 hour |
|
|
|
|
FFD Manager wage rate |
$47.79 |
|
|
|
|
Total Industry Operations Cost |
($156,968) |
||||
Average Operations Cost Per Site |
($2,660) |
||||
Calculations (totals may not add because of rounding):
Assumptions:
|
|||||
C.5 Expand the Initial and Confirmatory Drug Testing Panels to Include Ecstasy The final rule adds testing for two Ecstasy-type drugs (MDMA/MDA) to the testing panel (10 CFR 26.31(d)(1) and 10 CFR 26.405(d)); makes conforming changes to the substances for initial testing (10 CFR 26.133 and 10 CFR 26.163(a)(1)) and confirmatory testing (10 CFR 26.163(b)(1)); and adds these new substances to the annual statistical summary reporting requirements for HHS-certified laboratories (10 CFR 26.169(h)(3)). These changes ensure that 10 CFR Part 26 is consistent with Section 3.4 of the 2008 and 2017 HHS Guidelines. The rule revises the list of substances to be tested as follows:
Testing for Ecstasy drugs enables the detection of additional illegal drugs that could impair employee performance. The performance of testing for Ecstasy drugs is estimated to result in an increase in the number of urine specimens identified as containing MDMA or MDA, or both. The rule changes also may increase the deterrent effect of the testing program under 10 CFR Part 26 by including these additional substances in the testing panel.
The addition of the two Ecstasy-type drugs to the testing panel also will result in an increase in the number of BPTSs that a licensee or other entity will need to submit to each HHS-certified laboratory that it maintains under contract. One BPTS is submitted each quarter to meet the existing requirements in 10 CFR 26.168. |
|||||
Activity |
Parameter |
Value |
Benefits (Cost) |
Sites Affected |
Total Benefits (Costs) |
INDUSTRY OPERATIONS (ANNUAL) |
|||||
Ecstasy |
|||||
Ecstasy
initial
testing
|
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($8,095) |
3 |
($24,285) |
Initial testing for one additional drug at an LTF |
$3.59 |
|
|
|
|
Ecstasy testing (sites only using HHS‑certified laboratories) |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($305) |
56 |
($17,080) |
Testing for Ecstasy drugs (sites only using an HHS‑certified laboratory) |
$0.14 |
|
|
|
|
Additional testing at HHS‑certified laboratory for Ecstasy positive results (sites using LTFs) |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($3) |
3 |
($9) |
Projected confirmed positive test rate |
0.004% |
|
|
|
|
Initial and confirmatory drug testing, HHS‑certified laboratory (sites using an LTF for initial testing) |
$28.83 |
|
|
|
|
BPTS for Ecstasy (sites only using HHS‑certified laboratories) |
Number of BPTSs submitted per site per year (1 BPTS per quarter) |
4 |
($288) |
56 |
($16,147) |
Cost per BPTS |
$60.00 |
|
|
|
|
Testing for Ecstasy drugs (sites only using an HHS‑certified laboratory) |
$0.14 |
|
|
|
|
Time to prepare and send a BPTS to the HHS‑certified laboratory for testing, and review the laboratory's test results |
0.25 hour |
|
|
|
|
FFD Manager wage rate |
$47.79 |
|
|
|
|
BPTS for Ecstasy (sites using LTFs) |
Number of BPTSs submitted per site per year (1 BPTS per facility per quarter) |
4 |
($403) |
3 |
($1,209) |
Cost per BPTS test |
$60.00 |
|
|
|
|
Initial and confirmatory drug testing, HHS‑certified laboratory (sites using an LTF for initial testing) |
$28.83 |
|
|
|
|
Time to prepare and send a BPTS to the HHS‑certified laboratory for testing, and review the laboratory's test results |
0.25 hour |
|
|
|
|
FFD Manager wage rate |
$47.79 |
|
|
|
|
Subsequent actions by FFD program personnel for additional Ecstasy positive test results (all sites) |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($27) |
59 |
($1,593) |
Projected confirmed positive test rate |
0.004% |
|
|
|
|
Cost of subsequent actions (per positive result) |
$310.66 |
|
|
|
|
Total Industry Operations Cost |
($60,323) |
||||
Average Operations Cost Per Site |
($1,022) |
||||
Calculations (totals may not add because of rounding):
Assumptions:
|
|||||
C.6 Special Analyses Testing of Dilute Specimens and Specimens Collected during Suspected Subversion Attempts
The regulations in 10 CFR 26.163(a)(2) provide licensees and other entities with the option to conduct special analyses testing on a donor specimen with a dilute validity test result (i.e., specimens with a creatinine concentration greater than or equal to 2 milligrams per deciliter (mg/dL) but less than 20 mg/dL). Special analyses testing consists of conducting confirmatory testing to the limit of detection (LOD) if the immunoassay response during initial drug testing is equal to or greater than 50 percent of the cutoff calibrator in a drug class.
The final rule does the following:
These changes further enhance the detection of drugs when specimens do not present normal physiological characteristics. The 2008 and 2017 HHS Guidelines do not address special analyses testing, but the final rule changes are based on industry experience and feedback received from HHS-certified laboratories in implementing the 2008 FFD final rule.
|
|||||
Activity |
Parameter |
Value |
Benefits (Cost) |
Sites Affected |
Total Benefits (Costs) |
INDUSTRY OPERATIONS (ANNUAL) |
|||||
Special Analyses Testing of Dilute Specimens |
|||||
LOQ special analyses testing at an HHS-certified laboratory (all sites) |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($65) |
59 |
($3,835) |
Average annual percentage of specimens tested that are dilute and special analyses testing performed |
0.375% |
|
|
|
|
Special analyses testing at an HHS-certified laboratory |
$7.67 |
|
|
|
|
Subsequent actions by FFD program personnel for additional dilute positive test results (all sites) |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($43) |
59 |
($2,537) |
Average annual percentage of specimens tested that are dilute and test positive on special analyses testing |
0.006% |
|
|||
Cost of subsequent actions (per positive result) |
$310.66 |
||||
Special Analyses Testing of Specimens Collected during Suspected Subversion Attempts |
|||||
LOQ special analyses testing at an HHS-certified laboratory (all sites) |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($8) |
59 |
($472) |
Average annual percentage of specimens tested that are determined to be a subversion attempt and that test positive (i.e., suspect specimens that test positive on special analyses testing) |
0.049% |
|
|
|
|
Special analyses testing at an HHS-certified laboratory |
$7.67 |
|
|
|
|
Subsequent actions by FFD program personnel for additional positive drug test results (all sites) |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
($43) |
59 |
($2,537) |
Average annual percentage of specimens tested that are determined to be a subversion attempt and that test positive (i.e., suspect specimens that test positive on special analyses testing) |
0.049% |
|
|
|
|
Projected percent increase in confirmed positive test rate |
13% |
||||
Cost of subsequent actions (per positive result) |
$310.66 |
|
|
|
|
Total Industry Operations Cost |
($9,381) |
||||
Average Operations Cost Per Site |
($159) |
||||
Calculations (totals may not add because of rounding):
Assumptions:
|
|||||
Appendix D: Costs of Subsequent Actions
Subsequent Action Labor Hours and Costs per Positive, Adulterated, Substituted, or Refusal to Test Result
“Subsequent actions” refers to the activities completed by staff of the licensee or other entity and the Medical Review Officer (MRO) following a drug or alcohol positive result, an adulterated or substituted validity test result, or refusal to test (as required by Title 10 of the Code of Federal Regulations (10 CFR) Part 26, “Fitness for duty [FFD] programs”).
Subsequent actions consist of activities performed by the licensee or other entity staff and the MRO related to the review and confirmation of a test result, communications with the donor throughout the verification and sanctioning process, and recordkeeping and reporting. For example, subsequent actions include MRO communications with the donor about the result, the communications between the MRO and the licensee about a confirmed test result (recording and reporting the result), licensee or other entity administrative actions implemented under 10 CFR 26.75, “Sanctions,” and any request by the donor to request the retesting of an aliquot of a single specimen or the testing of Bottle B of the split specimen, or to appeal of the result.
|
|||
Labor Category |
Wage Rate |
Labor Per Result |
Total Cost Per Result |
MRO |
$151.36/hour |
0.75 hour |
$113.52/result |
FFD Manager |
$47.79/hour |
2.00 hours |
$95.59/result |
FFD Staff |
$41.20/hour |
0.75 hour |
$30.90/result |
Facility Worker |
$70.65/hour |
1.00 hour |
$70.65/result |
Total |
|
4.50 hours |
$310.66/result |
Calculations:
Assumptions: |
|||
Appendix E: Averted Costs
Averted Training Costs—Pre-Access Testing The rule is estimated to result in savings (i.e., averted costs) to licensees and other entities associated with training during the in‑processing of licensee employees and contractors/vendors (C/Vs). Pre-access testing accounts for approximately 67 percent of positive test results each year. As a result, if an individual tests positive for a drug during pre-access testing, any remaining training not completed by that individual at the time of the confirmed positive test result is received results in savings because of the immediate denial of access authorization to the individual for failing the required fitness for duty drug test.
The U.S. Nuclear Regulatory Commission (NRC) staff estimated averted training costs by calculating the “Total Additional Positive Test Results Expected from the Rule Changes” and multiplying that value by the cost of labor that is averted for each positive result. |
|||||
Activity |
Parameter |
Value |
Positives Per Site |
Sites Affected |
Total Positives |
INDUSTRY OPERATIONS (ANNUAL) |
|||||
Total Additional Positive Test Results Projected from Rule Changes |
|||||
Additional 6‑AM positive results |
Number of drug tests conducted per site per year under 10 CFR Part 26, “Fitness for duty programs” |
2,257 |
0.37 |
59 |
22 |
Projected confirmed positive test rate |
0.016% |
|
|
|
|
Additional amphetamine positive results |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
0.39 |
59 |
23 |
Fitness for duty (FFD) current confirmed positive test rate |
0.066% |
|
|
|
|
Projected percent increase in positive test rate |
36.65% |
|
|
|
|
Projected percentage of additional positive results that will confirm positive after Medical Review Officer (MRO) interview with donor |
70.83% |
|
|
|
|
Additional cocaine positive results |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
0.37 |
59 |
22 |
FFD current confirmed positive test rate |
0.083% |
|
|
|
|
Projected percent increase in positive test rate |
19.66% |
|
|
|
|
Ecstasy positive results |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
0.09 |
59 |
5 |
Projected confirmed positive test rate |
0.004% |
|
|
|
|
Expanded panel opioid positive results (hydrocodone, hydromorphone, oxycodone, oxymorphone) |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
1.49 |
59 |
88 |
FFD current confirmed positive rate for opioids |
0.066% |
|
|
|
|
Additional dilute specimen positives |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
0.14 |
59 |
8 |
Average annual percentage of specimens tested that are dilute and test positive on special analyses testing |
0.006% |
|
|
|
|
Additional suspect specimen positives |
Number of drug tests conducted per site per year under 10 CFR Part 26 |
2,257 |
0.14 |
59 |
8 |
Average annual percentage of specimens that are determined to be a subversion attempt and that test positive (suspect specimens that test positive on special analyses testing) |
0.049% |
|
|
|
|
Projected percent increase in confirmed positive test rate for specimens collected under direct observation |
13% |
|
|
|
|
Total Additional Positive Test Results Projected from Rule Changes |
176 |
||||
Activity |
Parameter |
Value |
Positives Per Site |
Sites Affected |
Total Positives |
Averted Training Costs—Pre-Access Testing |
|||||
Averted training costs for new licensee employees and C/Vs with positive pre-access drug test results |
Change in number of positive results per site based on rule changes (division of “Total Additional Positive Test Results Projected from Rule Changes” by the number of sites) |
2.97/site |
$2,114 |
59 |
$370,539 |
Percentage of total positive test results occurring at pre-access testing |
67.1% |
|
|
|
|
Weighted average of total in-processing training time for a new licensee employee or C/V |
43 hours |
|
|
|
|
Average number of training hours until receipt of MRO-verified positive drug test result after collection |
28 hours |
|
|
|
|
Training time (in hours) averted for an individual with a positive drug test during in-processing. (Difference between “Weighted average of total in-processing training time for a new licensee employee or C/V” and the “Average number of training hours until receipt of MRO-verified positive drug test result after collection) |
15 hours |
|
|
|
|
Facility Worker hourly wage rate |
$70.65 |
|
|
|
|
Total Industry Operations Benefit |
$370,539 |
||||
Average Operations Benefit Per Site |
$6,280 |
||||
Calculations (totals may not add because of rounding):
Assumptions (all values based on NRC professional judgment):
Weighted average of total training days per person during in-processing = (50% x 40 hours) + (25% x 44 hours) + (25% x 48 hours) = 43 hours
|
|||||
Appendix F: Alternative Specimen (Oral Fluid) Drug Testing
Oral Fluid Specimen Drug Testing In the final rule, Title 10 of the Code of Federal Regulations (10 CFR) 26.83(b) provides licensees and other entities with the option to collect and test an oral fluid specimen instead of a urine specimen for any of the observed specimen collection conditions under 10 CFR 26.115(a)(1) through (a)(3) and (a)(5). Testing of an oral fluid specimen must be performed at a laboratory certified by the U.S. Department of Health and Human Services (HHS).
The U.S. Nuclear Regulatory Commission (NRC) staff estimated the net benefits (costs) of the oral fluid specimen option by calculating the costs of the alternative process (oral fluid) and subtracting those costs from the costs to collect and test urine specimens under the same conditions. The majority of observed collections performed each year pertain to potential subversion attempts identified during the collection process, as described under 10 CFR 26.115(a)(2), when the donor’s urine specimen is outside the required temperature range, and under 10 CFR 26.115(a)(3), when donor conduct is observed indicating an attempt to subvert the testing process. The annual fitness for duty (FFD) program performance reports include event-specific data on these testing events. This appendix models these testing events. |
|||||
Activity |
Parameter |
Value |
Annual Savings (Cost) Per Site |
Sites Affected |
Annual Savings (Cost) |
INDUSTRY OPERATIONS (ANNUAL) |
|||||
Observed Urine Specimen Collection and Testing |
|||||
Urine specimen collection and testing following a possible subversion attempt (sites using licensee testing facilities (LTFs)) |
Number of subversion attempts per year per site under 10 CFR Part 26 |
4 |
($201) |
3 |
($603) |
Percentage of subversion attempts confirmed through the testing of specimens collected under direct observation |
31.1% |
|
|
|
|
Urine specimen collection time |
1.25 hour |
|
|
|
|
Facility Worker (donor) wage rate |
$70.65/hour |
|
|
|
|
FFD Staff (observer) wage rate |
$41.20/hour |
|
|
|
|
Initial and confirmatory drug testing, HHS-certified laboratory (sites using an LTF for initial testing) |
$28.83/ specimen |
|
|
|
|
Urine specimen collection and testing following a possible subversion attempt (sites only using HHS-certified laboratories) |
Number of subversion attempts per year per site under 10 CFR Part 26 |
4 |
($182) |
56 |
($10,192) |
Percentage of subversion attempts confirmed through the testing of specimens collected under direct observation |
31.1% |
|
|
|
|
Urine specimen collection time |
1.25 hour |
|
|
|
|
Facility Worker (donor) wage rate |
$70.65/hour |
|
|
|
|
FFD Staff (observer) wage rate |
$41.20/hour |
|
|
|
|
Initial and confirmatory drug testing, HHS-certified laboratory (sites only using HHS-certified laboratories) |
$12.88/ specimen |
|
|
|
|
Observed Oral Fluid Specimen Collection and Testing |
|||||
Oral fluid specimen collection and testing following a possible subversion attempt |
Number of subversion attempts per year per site under 10 CFR Part 26 |
4 |
($70) |
59 |
($4,130) |
Percentage of subversion attempts confirmed through the testing of specimens collected under direct observation |
31.1% |
|
|
|
|
Oral specimen collection time (donor) |
0.35 hour |
|
|
|
|
Facility Worker (donor) wage rate |
$70.65/hour |
|
|
|
|
Oral specimen collection time (observer) |
0.35 hour |
|
|
|
|
FFD Staff (observer) wage rate |
$41.20/hour |
|
|
|
|
Testing of an oral fluid specimen using an HHS-certified laboratory |
$20.00/ specimen |
|
|
|
|
Net Industry Operations Annual Benefits |
$6,665 |
||||
Average Operations Annual Net Benefit Per Site |
$113 |
||||
Average Operations Annual Net Benefit Per Test |
$30 |
||||
Calculations (totals may not add because of rounding):
Assumptions:
|
|||||
1 Ecstasy-type drugs included within the scope of this rule are the Schedule I illegal drugs methylenedioxymethamphetamine (MDMA) and methylenedioxyamphetamine (MDA). A Schedule I drug or substance, as defined by the Controlled Substances Act, has a high potential for abuse, has no currently accepted medical use in treatment in the United States, and there is a lack of accepted safety for use of the drug or substance under medical supervision (21 U.S.C. § 812). A Schedule II drug or substance has a high potential for abuse, has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions, and abuse of the drug or substance may lead to severe psychological or physical dependence. Schedule II through V substances have accepted safe uses under medical supervision, pursuant to a valid prescription
2 The “window of detection” refers to the time period after use during which the established detection technologies, methodologies, and cutoff levels can identify and quantify a target drug metabolite.
3 The regulations in 10 CFR 26.5, “Definitions,” define the use of any Schedule I to V drug when not used pursuant to a valid prescription as an “illegal drug.” To improve the clarity of the discussion of the rule changes, use of a Schedule I drug is referred to as “use of an illegal drug,” while use of a Schedule II through V drug without a valid prescription is referred to as “misuse of a legal drug.”
4 The number of Federal agencies using the 2017 HHS Guidelines appears in the Office of Management and Budget (OMB) information collection’s supporting statement (OMB No. 0930-0158) filed by the Substance Abuse and Mental Health Services Administration for the “Mandatory Guidelines for Federal Workplace Drug Testing Programs” on April 28, 2017. The supporting statement is available at the OMB Web site https://www.reginfo.gov/public/do/PRAViewDocument?ref_nbr=201704-0930-001.
5 NRC implementation costs to prepare and publish the final rule and associated guidance are sunk costs, and therefore the analysis does not include them.
0 The entities subject to 10 CFR Part 26 requirements include (1) licensees authorized to possess, use, or transport formula quantities of strategic special nuclear material (SSNM) (e.g., Category I special nuclear material licensees), (2) holders of, and certain applicants for, a combined license (COL) for a nuclear power plant under the provisions of 10 CFR Part 52, “Licenses, certifications, and approvals for nuclear power plants,” (3) holders of, and certain applicants for, nuclear power plant construction permits and operating licenses under the provisions of 10 CFR Part 50, “Domestic licensing of production and utilization facilities,” and (4) contractors/vendors (C/Vs) that implement FFD programs or program elements to the extent that the licensees rely on C/V FFD programs or program elements.
0 The number of Federal agencies using the 2017 HHS Guidelines appears in the Office of Management and Budget (OMB) information collection’s supporting statement (OMB No. 0930-0158) filed by the Substance Abuse and Mental Health Services Administration for the “Mandatory Guidelines for Federal Workplace Drug Testing Programs” on April 28, 2017. The supporting statement is available at the OMB Web site https://www.reginfo.gov/public/do/PRAViewDocument?ref_nbr=201704-0930-001.
0Under 10 CFR 26.69(b), a licensee or other entity may (but is not required to) restore FFD authorization to an individual who tests positive on a drug or alcohol test, or both, after completion of the sanction under 10 CFR 26.75, “Sanctions,” satisfactory completion of any assigned treatment program (10 CFR 26.189, “Determination of fitness”), and inclusion of the individual in a follow-up testing program (10 CFR 26.31(c)(5)).
0 The limit of quantification is the lowest concentration at which the identity and concentration of a drug can be established accurately.
0 The NRC staff does not anticipate false positive results (i.e., errors in the laboratory testing process) as a result of the testing changes. Historical FFD program performance data demonstrate the rigor of the laboratory testing process and the rarity of such testing errors.
0 The rule improves the capability to detect amphetamine, cocaine, heroin, and methamphetamine drug use, and adds required testing for two Ecstasy-type drugs (MDMA, MDA) and four opioid drugs (oxycodone, oxymorphone, hydrocodone, and hydromorphone).
0 The term “FFD program site” corresponds to the term “facility,” which is used to describe licensees and other entities that are subject to the reporting requirements in 10 CFR 26.717 and that submit drug and alcohol testing data to the NRC in annual FFD program performance summary reports.
0 Vogtle Electric Generating Plant (Vogtle), Units 3 and 4, are scheduled to begin commercial operation in CY 2022 and CY 2023, respectively, and are modeled with the operating units.
0 This analysis does not include the Bellefonte Nuclear Power Station (Bellefonte) because the site does not have any operating units and new construction is delayed indefinitely. Bellefonte Units 1 and 2 are covered under the Commission Policy Statement on Deferred Plants (52 FR 38077; October 14, 1987). Additionally, four licensees with COLs for six new reactor units have no immediate plans to begin construction. One COL was issued to DTE Electric Company on May 1, 2015, for Enrico Fermi Nuclear Plant, Unit 3. Two COLs were issued to Duke Energy Carolinas, LLC, on December 19, 2016, for William States Lee III Nuclear Station, Units 1 and 2. One COL was issued to Virginia Electric and Power Company on June 2, 2017, for North Anna Power Station (North Anna), Unit 3. Two COLs were issued to Florida Power & Light Company on April 12, 2018, for Turkey Point Nuclear Generating Units 6 and 7.
0 Since testing began in 1990, the C/V positive rate for all tests conducted has ranged from 2.4 times (in 1990) to 4.5 times (in 2002) higher than that for licensee employees. For all tests conducted in 2019, C/Vs tested positive at a rate of 1.10 percent as compared to 0.26 percent for licensee employees, which is 4.3 times greater than for licensee employees.
0 The construction of V.C. Summer Units 2 and 3 began in CY 2011 but was halted on July 31, 2017. This analysis does not include this facility.
0 The NRC analyzed data on power reactors (operating, under construction) and Category I special nuclear material licensees from NUREG-1350, Volume 32, “2020–2021 Information Digest,” issued August 2020 (NRC, 2020b), which is adjusted for early plant retirement announcements.
0NextEra Energy received a second license renewal for Turkey Point Units 3 and 4 on December 4, 2019. Exelon received a second license renewal for Peach Bottom Units 2 and 3 on March 5, 2020. Dominion Energy received a second license renewal for Surry Units 1 and 2 on May 4, 2021. The NRC received an application from Dominion Energy for a second license renewal for North Anna Units 1 and 2 on June 6, 2021. The NRC received an application from Duke Energy for a second license renewal for Oconee Units 1, 2, and 3 on June 7, 2021. Duke Energy announced its intention to apply for a second license renewal for Brunswick Units 1 and 2; Catawba Units 1 and 2; H.B. Robinson Unit 2; McGuire Units 1 and 2; and Shearon Harris Unit 1. The NRC received an application from NextEra Energy for a second license renewal for Point Beach Units 1 and 2 on November 16, 2020, and NextEra Energy announced its intention to apply for a second license renewal for St. Lucie Units 1 and 2. The Tennessee Valley Authority announced its intention to apply for a second license renewal for Browns Ferry Units 1, 2, and 3. Due to the timing of production of this document, the second license renewals for Browns Ferry and St. Lucie plants have not been factored into the analysis.
0 Sections 3.8 and 3.9 of the regulatory basis for this rulemaking (NRC, 2013a) provide additional information on the technical basis for lowering the initial and confirmatory drug testing cutoff levels for amphetamines and cocaine metabolites.
0 Sections 3.3, 3.7, and 3.8 of the regulatory basis for this rulemaking (NRC, 2013a) provide additional information on the technical basis for expanding the initial drug testing panel to include 6-AM and revising the confirmatory drug testing cutoff level for 6‑AM.
0 Sections 3.11 through 3.13 of the regulatory basis for this rulemaking (NRC, 2013a) provide additional information on the technical basis for requiring special analyses testing of dilute specimens and specimens collected during suspected subversion attempts.
0As described in Section 4.2.2, this set of sites reflects the NRC’s understanding of licensees’ decommissioning plans at the time the staff prepared this regulatory analysis. The costs and benefits of the rule change if the number of facilities that decommission changes over the timeframe considered in this analysis.
0 In practice, some affected entities may take additional actions in response to positive drug test results, which may involve staffing actions such as compensating other staff for overtime to cover the assignments of the individual who committed the FFD violation or hiring and training a replacement. The NRC staff assumes that the costs associated with staffing actions in response to any additional positive drug test results each year stemming from the final rule are negligible for the following reasons. First, data collected by the NRC on existing FFD programs indicate that approximately 67 percent of positive test results occur during pre-access testing; this value varies between 64 and 70 percent annually. The NRC staff assumes that this historical trend will continue, such that 67 percent of the additional positive drug test results do not result in costs associated with staffing actions because these individuals are detected during pre-access testing. Second, an NRC analysis of FFD program performance data from CY 2010 through CY 2019 indicates that C/V staff account for an average of 75 percent of the remaining (non-pre-access) positive drug test results, and the NRC staff assumes that this historical trend will continue. Licensees typically impose a “zero tolerance” policy on C/Vs, so individuals with positive test results are immediately replaced with another C/V employee if needed. Removing the estimated positive test results associated with pre-access testing (176 positives x 67 percent = 118 positives) and C/V staff positives for non-pre-access tests ((176 tests – 118 positives = 58 positives) x 75 percent = 44 C/V positive results) leaves an estimated 14 additional licensee employee positive results per year for non-pre-access tests. For this analysis, the NRC staff assumes that these 14 additional positive test results are evenly distributed across the industry, resulting in an average of approximately 0.2 additional positive test result per site per year (14 positive results / 59 sites = 0.2 per site).
0 The NRC staff made a simplifying assumption that all FFD programs that use HHS‑certified laboratories have contracts with a primary and a backup laboratory. Not all FFD programs maintain a contract with a backup laboratory.
0 The NRC staff estimates that approximately 5 percent of sites (i.e., 3 of 59 sites) will conduct an independent training on the rule changes (in accordance with labor agreements) instead of including the information update as part of annual FFD refresher training.
0 This cost could be as high as ($6.6 million) if all sites choose to hold trainings and distribute information on FFD program changes outside of the annual refresher training required by 10 CFR 26.29(c) (i.e., if sites do not pursue the least cost approach).
0 Based on trends in subversion attempts (see NRC, 2017), attempts to subvert tests are increasing (64 percent of the sites reported at least one subversion attempt), and the majority of the attempts occur during pre-access testing (16 additional confirmed positive test results are expected to occur during pre-access testing).
0 The number of Federal agencies using the 2017 HHS Guidelines appears in the OMB information collection’s supporting statement (OMB No. 0930-0158) filed by the Substance Abuse and Mental Health Services Administration for the “Mandatory Guidelines for Federal Workplace Drug Testing Programs” on April 28, 2017. The supporting statement is available at the OMB Web site https://www.reginfo.gov/public/do/PRAViewDocument?ref_nbr=201704-0930-001.
0 Section 503 of Public Law 100–71, 5 U.S.C. Section 7301 note.
0 Most licensees impose a sanction for a pre-access positive drug test result that is more stringent than that required by 10 CFR 26.75 (i.e., the minimum NRC sanction for a first positive drug test result is a 14-day denial of unescorted access). The NRC analysis of FFD program performance result data from CY 2009 through CY 2019 indicates that approximately 67 percent of positive test results occur during pre-access testing. Therefore, the NRC staff estimates that 118 of the 176 additional positive drug test results and subversion attempts each year would be identified by pre-access testing.
0 Information about this software is available online at http://www.palisade.com.
0 A PERT distribution is a special form of the beta distribution with minimum and maximum values specified. The shape parameter is calculated from the defined most likely value. The PERT distribution is similar to a triangular distribution in that it has the same set of three parameters. Technically, it is a special case of a scaled beta (or beta general) distribution. It is generally considered superior to the triangular distribution when the parameters result in a skewed distribution, as the smooth shape of the curve places less emphasis in the direction of skew. Similar to the triangular distribution, the PERT distribution is bounded on both sides and therefore may not be adequate for some modeling purposes, such as those intended to capture tail or extreme events.
0 The NRC staff assumes that the licensee or other entity for each site will incur an average cost per requirement. This assumption is a simplification; some licensees and other entities will incur a higher or lower operations cost depending on the size of the population drug tested at the site (e.g., an operating power reactor site conducts more drug tests than a corporate office). The licensees and other entities subject to 10 CFR Part 26 include operating power reactor sites, 5 corporate offices, 2 Category I special nuclear material licensees, and 1 C/V (see Appendix A). Corporate offices, Category I special nuclear material licensees, and C/Vs use much smaller workforces than operating power reactor sites or power reactor construction sites (see Table 4-1 and Appendix A). They also do not incur periodic workforce surges as a result of changing site conditions, unlike power reactor sites (e.g., refueling outages). The final rule changes have limited impact on additional detection at other facility types given the very low number of positive results (see Table 4-1). As a result, the NRC staff anticipates improvement in detection at operating power reactor and power reactor construction sites. By using an average cost per site, the analysis overestimates the operations costs for sites with smaller workforces and underestimates the costs for sites with larger workforces, but on balance it provides a reasonable estimate of the incremental testing costs associated with the rule given that 86 percent of the sites and tested workforces (51 of 59) are at operating power reactors.
0 Each site incurs an average one-time cost of ($2,321) and an average annual savings of $808.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Benowitz, Howard |
| File Modified | 0000-00-00 |
| File Created | 2023-08-26 |
© 2026 OMB.report | Privacy Policy