NSQAP Data Submission Portal Biochemical (Proficiency Te
NCEH DLS Laboratory Quality Assurance Programs
Att 3m-II. NSQAP DataPortal_BioPT
NSQAP Data Submission Portal Biochemical (Proficiency Testing) PT (Private)
OMB: 0920-1389
 NSQAP
PT Program Information Collection
NSQAP
PT Program Information Collection
Form
Approved OMB
No. 0920-xxxx
Exp.
Date xx/xx/20xx
CDC estimates the average
public reporting burden for this collection of information as 45
minutes per response, including the time for reviewing instructions,
searching existing data/information sources, gathering and
maintaining the data/information needed, and completing and
reviewing the collection of information. An agency may not conduct
or sponsor, and a person is not required to respond to a collection
of information unless it displays a currently valid OMB Control
Number. Send comments regarding this burden estimate or any other
aspect of this collection of information, including suggestions for
reducing this burden to CDC/ATSDR Information Collection Review
Office, 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN:
PRA (0920-xxxx).

Step 1 – Select PT Program Setup (select PT program/grouping of analytes)

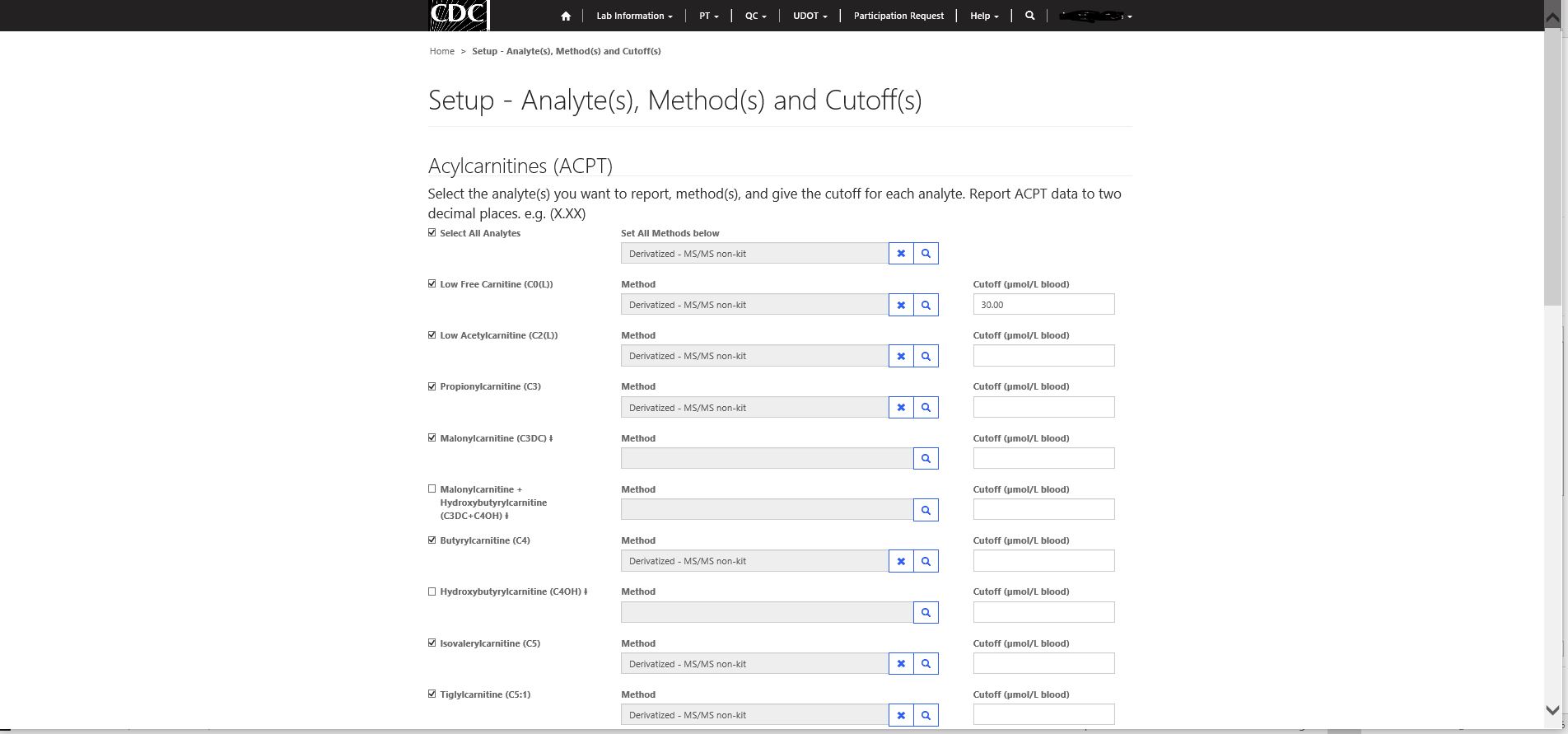
Step 2 – Selecting analytes to report, analytical method(s) and cutoffs
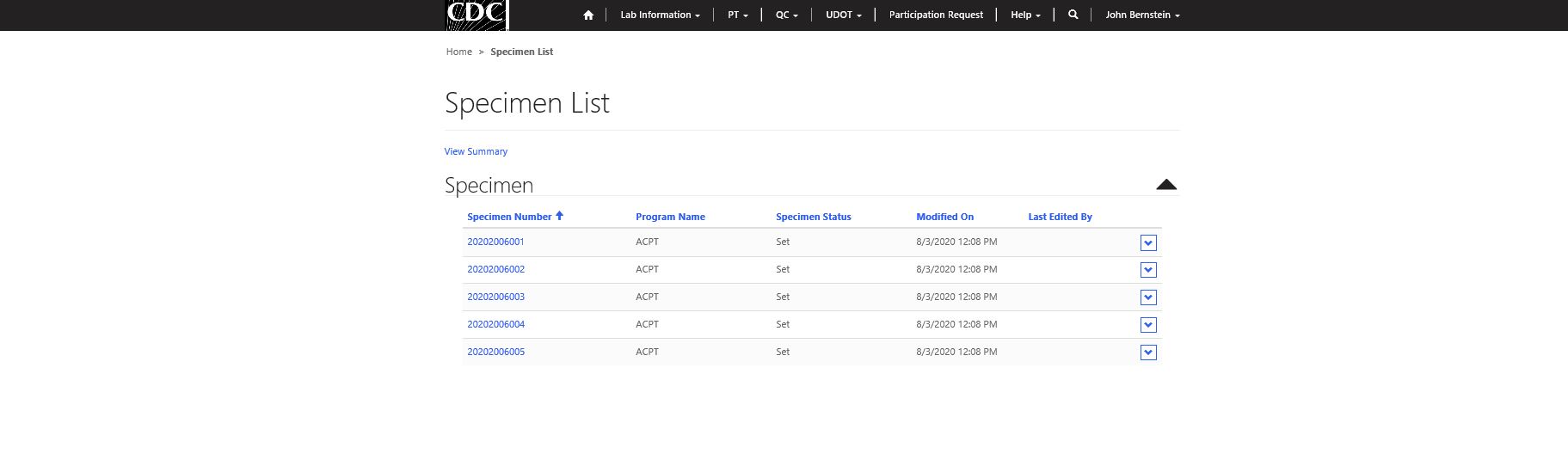
Step 3 – Select specimen for data entry
Step 4 – Analytic result and clinical assessment data entry.

| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Bernstein, John (CDC/ONDIEH/NCEH) |
| File Modified | 0000-00-00 |
| File Created | 2022-09-19 |
© 2026 OMB.report | Privacy Policy