1188ss13
1188ss13.docx
TSCA Section 5 Premanufacture Review of New Chemical Substances and Significant New Use Rules for New and Existing Chemical Substances
OMB: 2070-0038
Supporting Statement for an Information Collection Request (ICR)
Under the Paperwork Reduction Act (PRA)
EXECUTIVE SUMMARY
Identification of the Information Collection – Title and Numbers
Title: TSCA Section 5 Premanufacture Review of New Chemical Substances and Significant New Use Rules for New and Existing Chemical Substances
EPA ICR No.: 1188.13
OMB Control No.: 2070-0038
Docket ID No.: EPA-HQ-OPPT-2021-0660
Abstract
This information collection request addresses the reporting and recordkeeping requirements associated with the new chemicals review and regulatory program and the existing chemicals program administered by EPA under section 5 of the Toxic Substances Control Act (TSCA), as amended in 2016 by the Frank R. Lautenberg Chemical Safety for the 21st Century Act (the “Lautenberg Act”) (15 U.S.C. 2604).
With this ICR, EPA is consolidating the paperwork activities and related estimates for burden hours and costs that are currently approved under the following two Information Collection Requests (ICRs):
“TSCA Section 5(a)(2) Significant New Use Rules for Existing Chemicals” (identified by EPA ICR No. 1188.12 and OMB Control No. 2070-0038), which is currently approved through July 31, 2022; and
“Premanufacture Review Reporting and Exemption Requirements for New Chemical Substances and Significant New Use Reporting Requirements for Chemical Substances” (identified by EPA ICR No.0574.18 and OMB Control No. 2070-0012), which is currently approved through December 31, 2022.
Originally identified as a new ICR and assigned EPA ICR No. 2702.01 (OMB Control No. 2070-NEW), after discussions with the Office of Management and Budget (OMB), EPA subsequently determined that the consolidated ICR should be submitted as a revision to the existing ICR approved under OMB Control No. 2070-0038. The consolidation ICR is now identified as EPA ICR No. 1188.13 and, with the exception of a change in its title, this otherwise involves minimal changes to the ICR itself.
This consolidated ICR covers the information collection activities associated with new chemical submissions and with Significant New Use Rules (SNURs) for both new and existing chemicals under TSCA. The information for required notifications includes chemical identity, use and exposure information, test information and descriptions of other information related to the effects on health and the environment relating to the manufacture, processing, distribution in commerce, use and disposal of the new chemical or the significant new use of the existing chemical substance.
TSCA section 5 requires that any person who proposes to manufacture (which includes import) a “new chemical” (i.e., a chemical not listed on the TSCA section 8(b) Inventory) must provide a premanufacture notice (PMN) or an exemption application to EPA at least 90 days prior to commencing manufacture of that chemical and that EPA review such notice and take action as appropriate. EPA considers certain genetically engineered microorganisms to be chemical substances for purposes of the notification requirements found in TSCA section 5; the 90-day notice for microorganisms is a Microbial Commercial Activity Notice (MCAN).
Under TSCA section 5, EPA is authorized to determine that a use of a chemical substance is a significant new use and promulgate a significant new use rule (SNUR). In certain instances, persons may opt to pursue that significant new use, in which case they must submit a notice and undergo a review. For such circumstances, TSCA section 5 requires a significant new use notice (SNUN) from any person who proposes to manufacture or process a chemical for a use that is determined by EPA to be a “significant new use.” TSCA section 5 requires EPA to make one of five possible risk determinations before the conclusion of its review of the submitted notices regarding whether the manufacture, processing, distribution in commerce, use and/or disposal of new chemical substances or significant new uses.
TSCA section 5 requires EPA to make determinations before the conclusion of its review of the submitted notices regarding risk to human health and the environment from the manufacture, processing, distribution in commerce, use and/or disposal of new chemical substances or significant new uses. EPA’s determination on a chemical substance or new use will dictate how and to what extent the chemical’s manufacture, processing, distribution, use, and/or disposal may be restricted. If EPA fails to make a timely determination, fees may be refunded; however, nothing relieves EPA of its obligation to make a determination. EPA requires that the submitter of a PMN or MCAN inform EPA when non-exempt commercial manufacture of the substance in question actually begins by submitting a Notice of Commencement; EPA would then add the new chemical substance to the TSCA section 8(b) Inventory.
Persons who intend to export a substance identified in a proposed or final SNUR are subject to the export notification provisions of TSCA section 12(b), and regulations that interpret TSCA section 12(b) appear at 40 CFR part 707 and the associated paperwork activities and burdens are approved under OMB Control No. 2070-0030, ICR entitled “Notification of Chemical Exports - TSCA Section 12(b),” identified by EPA ICR No. 0795.16.
Existing chemicals are chemicals that are already listed on the TSCA Inventory and therefore “existing chemical SNURs” are generally written to require notice for significant new uses of chemicals that are already in commerce.
Summary Total Burden and Costs
Information Collection (IC) Activities |
Annual Number of Responses |
Total Annual Burden Hours |
Total Annual Costs |
PMN |
289 |
72,394 |
$20,622,994 |
SNUN |
13 |
1,421 |
$774,564 |
MCAN |
14 |
4,296 |
$1,069,533 |
TME |
0 |
0 |
$0 |
LVE/LoREX |
305 |
33,345 |
$9,531,511 |
TERA |
2 |
1,052 |
$99,261 |
Tier I |
4 |
515 |
$43,751 |
Tier II |
1 |
129 |
$15,638 |
Polymer |
109 |
2,398 |
$194,962 |
Research & Development |
200 |
3,849 |
$327,149 |
Instant Photographic Film Articles |
1 |
17 |
$6,162 |
Bona Fide1 |
183 |
6,175 |
$533,793 |
Section 5(e) Orders with Triggered Testing1 |
20 |
3,705 |
$3,560,790 |
Section 5(e) Orders with Pended Testing/ 5(f) Orders with No Testing1 |
33 |
2,186 |
$171,763 |
NOC1 |
169 |
2,868 |
$221,985 |
CDX Registration |
2942 |
1942 |
$150,844 |
Existing Chemical Activities |
5 |
168 |
$30,114 |
Total for Respondents |
4290 |
136,292 |
$37,354,814 |
Total for the Agency |
- |
- |
$26,959,416 |
*The total number of respondents is 234 for new chemicals activities and 5 for existing chemical activities.
Supporting Statement
1. Explain the circumstances that make the collection of information necessary. Identify any legal or administrative requirements that necessitate the collection. Attach a copy of the appropriate section of each statute and regulation mandating or authorizing the collection of information.
If EPA makes a determination under TSCA section 5(a)(3) that a chemical substance or significant new use is not likely to present an unreasonable risk of injury to health or the environment, without consideration of costs or other non-risk factors, including an unreasonable risk to a potentially exposed or susceptible subpopulation identified as relevant by the Administrator under the conditions of use, then the submitter may commence manufacture (including import) or processing for the significant new use.
TSCA section 5(a) (15 U.S.C. 2604(a)(1)(B)(i)) (Attachment 1.), requires manufacturers (which includes importers) of new chemical substances to submit to the Administrator of EPA a premanufacture notice (PMN) of intent to manufacture a new chemical substance at least 90 days before manufacture begins. TSCA section 5(a)(1) also requires notification from any person who proposes to manufacture or process a chemical substance for a use that EPA has by rule determined to be a significant new use. The notice must include, insofar as known or reasonably ascertainable by the submitter, information described in TSCA section 8(a)(2) (e.g., chemical identity, use and exposure information), plus test information and descriptions of other information related to the effects on health and the environment of the manufacture, processing, distribution in commerce, use, and/or disposal of the new chemical substance. TSCA requires EPA to conduct a review of the notice, make one of five possible determinations on the notice, and take such actions as are required in association with that determination (15 U.S.C. 2604(a)(1)(B)(ii)) before manufacturing or processing of the chemical or significant new use can commence. EPA reviews the information provided in the notice and other relevant information available to EPA to evaluate the health and environmental effects of the new chemical substance and make the required determination.
TSCA section 5, as interpreted in EPA’s “Microbial Products of Biotechnology; Final Regulation under the Toxic Substances Control Act” (62 FR 17910, April 11, 1997), authorizes EPA to regulate “new” genetically engineered microorganisms. According to the 1997 final rule, “new” microorganisms are those that, through deliberate human intervention, contain genetic material from dissimilar source organisms. Specifically, “intergeneric microorganisms” are those formed by either the deliberate combination of genetic material from organisms classified in different taxonomic genera or constructed with synthetic genes that are not identical in DNA that would be derived from the same genus as the recipient microorganism. Manufacturers of these new microorganisms must submit to EPA a microbial commercial activity notice (MCAN) at least 90 days before manufacture begins. These microorganisms are subject to the same determinations and potential regulatory controls as new chemical substances.
TSCA section 5(d)(1)(B) (15 U.S.C. § 2604(d)(1)(B)) requires premanufacture notices to include all information in the submitter’s possession or control and TSCA section 5(d)(1)(C) (15 U.S.C. § 2604(d)(1)(C)) requires PMN submitters to provide other information on environmental or health effects that are known to or reasonably ascertainable by the submitter. These requirements are described in 40 CFR 720.50.
TSCA section 5(e) authorizes EPA to prohibit or limit the manufacture, processing, distribution in commerce, use and/or disposal of a new chemical substance or significant new use pending development of information sufficient to allow EPA to perform a reasoned evaluation of the health and environmental effects of the substance. EPA must issue an order under TSCA section 5(e) if the Agency determines (1) that the information available is insufficient to permit a reasoned evaluation of the health or environmental effects; (2) in the absence of sufficient information, the manufacture, processing, distribution, use, and/or disposal may present an unreasonable risk of injury to health or the environment without consideration of costs or other non-risk factors, including an unreasonable risk to a potentially exposed subpopulation identified as relevant by the Administrator under the conditions of use; or (3) the substance is or will be produced in substantial quantities and may be released to the environment in substantial quantities or there may be significant or substantial human exposure to the chemical. EPA’s actions often involve negotiation of a TSCA section 5(e) Consent Order to prohibit or limit activities associated with manufacture, processing, distribution in commerce, use and and/or disposal of the new chemical. TSCA section 5(e) Consent Orders can typically include requirements for exposure or release mitigation, testing, labeling and hazard communication, and recordkeeping.
Similarly, if EPA determines under section 5(a)(3)(A) that a chemical substance or significant new use presents an unreasonable risk of injury to health or environment, without consideration of costs or other non-risk factors, including an unreasonable risk to a potentially exposed subpopulation identified as relevant by the Administrator under the conditions of use, the Agency must regulate the chemical under section 5(f) by either (1) issuing a proposed rule under section 6(a); or (2) issuing an order to prohibit or limit the manufacture, processing, or distribution in commerce of the substance. EPA’s action can involve negotiation of a TSCA section 5(f) Consent Order with the PMN submitter.
Significant New Use Rules (SNURs) are authorized under TSCA section 5(a)(2) and EPA is required to consider whether to promulgate SNURs following issuance of section 5(e) or 5(f) orders pursuant to section 5(f)(4). Regulations providing details on EPA’s SNUR authority were promulgated at 40 CFR part 721 and at 40 CFR part 725 (Attachment 2.). A SNUR requires that any manufacturer or processor – including the PMN submitter – who intends to undertake the activities subject to the SNUR must submit to EPA a significant new use notice (SNUN). EPA must either conclude, following review of a SNUN, that the activities are not likely to present an unreasonable risk, or take appropriate action under section 5(e) or 5(f) to protect against any unreasonable risk. The review would factor in the conditions of use of the chemical specifically associated with the significant new use and, as appropriate, any other conditions of use relevant to the evaluation of the significant new use under section 5(a)(3). TSCA section 5(e) or 5(f) Orders are only binding on the original PMN submitter for that substance. Consequently, after issuing a section 5 Order, EPA generally promulgates a SNUR that requires notice to EPA by any manufacturer or processor who wishes to manufacture or process the chemical in a way other than described in the terms and conditions contained in the Order. TSCA section 5(f)(4) requires EPA to either initiate a SNUR rulemaking or explain its reasons for not doing so following action under section 5(e) or 5(f).
For existing chemical SNURs, TSCA section 5(a)(2) provides EPA with the authority to designate by rule significant new uses of existing chemical substances for which notice is required. The Administrator must consider the following factors in determining a significant new use of an existing chemical substance: 1) The projected volume of manufacturing and processing of a chemical substance; 2) The extent to which a use changes the type or form of exposure of human beings or the environment to a chemical substance; 3) The extent to which a use increases the magnitude and duration of exposure of human beings or the environment to a chemical substance; and 4) The reasonably anticipated manner and methods of manufacturing, processing, distribution in commerce, use and/or disposal of a chemical substance.
After proposal and finalization of a SNUR, a person who intends to manufacture (including import) or process a significant new use of a chemical substance covered by a SNUR must notify EPA of the intent via a Significant New Use Notice (SNUN) at least 90 days prior to commencing the new use. The required notice must be submitted electronically, via the Central Data Exchange (CDX), using the Agency’s e-PMN software. Within 90 days, EPA must either conclude, following review of a SNUN, that the activities are not likely to present an unreasonable risk to human health or the environment, or take appropriate action under TSCA section 5(e) or 5(f) to protect against any unreasonable risk to human health or the environment. The review would factor in the conditions of use of the chemical specifically associated with the significant new use and, as appropriate, any other conditions of use relevant to the evaluation of the significant new use under TSCA section 5(a)(3). If the Agency has not made a determination within 90 days, it may extend the evaluation period by up to 90 days with good cause.
For new chemicals and existing chemicals, if EPA makes a determination under TSCA section 5(a)(3)(C) that a chemical substance or significant new use is not likely to present an unreasonable risk of injury to health or the environment, without consideration of costs or other non-risk factors, including an unreasonable risk to a potentially exposed or susceptible subpopulation identified as relevant by the Administrator under the conditions of use, then the submitter may commence manufacture of the chemical substance or manufacture or processing for the significant new use, notwithstanding the remainder of the review period. In addition, for new chemicals, the Administrator shall make public a statement of the “not likely” finding in the Federal Register, in accordance with TSCA section 5(g). The same reporting requirements that apply to PMNs also apply to SNUNs, and EPA has the same authorities under TSCA section 5(e) and 5(f) to evaluate and regulate the SNUR chemical during the notice review period.
EPA may also grant certain exemptions from the PMN, SNUN, and MCAN requirements of TSCA section 5. These exemption rules reduce reporting requirements, thereby providing relief to submitters from the burdens of the full PMN reporting requirements.
Exemptions
EPA may also grant certain exemptions from the PMN, SNUN, and MCAN requirements of TSCA section 5, including the following. These exemption rules reduce reporting requirements, thereby providing relief to submitters from the burdens of the full PMN reporting requirements.
Test-Marketing Exemption (TME)
Under TSCA section 5(h)(1), persons may apply for an exemption from the requirements of TSCA section 5 for test-marketing purposes. EPA may grant the exemption if it finds that the test-marketing activities described by the applicant will not present an unreasonable risk of injury to health or the environment including an unreasonable risk to a potentially exposed or susceptible subpopulation identified by the Administrator for the specific conditions of use identified in the application. The applicant must provide the information necessary to make this finding and EPA must grant or deny the exemption within 45 days. If EPA grants the exemption, it may impose appropriate restrictions on the test-marketing activities. See 40 CFR 720.38 and 725.370.
Research and Development Exemption (R&D)
TSCA section 5(h)(3) exempts from PMN reporting small quantities of chemical substances manufactured only for research and development purposes. Persons using this exemption must have their research overseen by a technically qualified individual and must notify any person involved in the research of any risk. See 40 CFR 720.36. Small quantities of genetically modified microorganisms manufactured solely for research and development purposes are also exempt when additional criteria are met as described in 40 CFR 725.235, activities conducted inside a structure, and 40 CFR 725.238 and 239, activities conducted outside a structure.
TSCA Section 5(h)(4) Exemptions
TSCA section 5(h)(4) authorizes EPA to exempt any person from the provisions of TSCA section 5 if EPA determines, upon application and by rule, that the chemical substance will not present an unreasonable risk of injury to health or the environment when manufactured, processed, distributed, used or disposed of under the exemption. To date EPA has promulgated four rules under this section for traditional chemical substance exemptions and three rules for exemptions specific to microbial products of biotechnology:
Low Volume Exemption (LVE) - This exemption applies to substances manufactured in quantities of 10,000 kilograms or less per year; submitters may request that EPA evaluate their exemption at a lower production volume level, to which the submitter would be legally bound. See 40 CFR 723.50.
Low Release/Low Exposure (LoREX) - This exemption applies to certain chemical substances that meet strict human exposure and environmental release criteria to ensure that these substances will not present an unreasonable risk. See 40 CFR 723.50.
Polymer Exemption - This exemption applies to polymers that comply with certain chemical characterizations and that therefore will not present an unreasonable risk of injury to health or the environment. See 40 CFR 723.250.
Instant Photographic Film Articles Exemption - This exemption applies to chemical substances used in or for the manufacture or processing of instant photographic and peel-apart film articles. See 40 CFR 723.175.
TSCA Experimental Release Application (TERA) - This exemption applies to research and development activities that result in intentional environmental releases of intergeneric microorganisms. EPA may grant the exemption if it finds that the activities described by the applicant will not present an unreasonable risk of injury to health or the environment. The applicant must provide the information necessary to make this finding and EPA must grant or deny the exemption within 60 days. If EPA grants the exemption, it may impose appropriate restrictions on the activities described in the notice. See 40 CFR 725.250.
Tier I Exemption - This exemption applies to certain microorganisms subject to physical containment and control technologies. EPA has developed specific criteria for the host microorganism, introduced genetic material, and containment technology to ensure that the microorganism will not present an unreasonable risk. See 40 CFR 725.400.
Tier II Exemption - This exemption applies to the same microorganisms subject to a Tier I exemption without specified physical containment and control technologies. EPA may grant the exemption if it finds that the physical containment and control technologies described by the applicant will control releases of and exposure to the microorganism such that the microorganism will not present an unreasonable risk of injury to health or the environment. The applicant must provide the information necessary to make this finding and EPA must grant or deny the exemption within 45 days. If EPA grants the exemption, it may impose appropriate restrictions on the activities described in the notice. See 40 CFR 725.428.
Finally, under TSCA section 26(b) manufacturers and processors pay fees for PMNs, MCANs, certain PMN exemption applications and notices, and SNUNs submitted under TSCA sections 5(a) and (h) to help defray the cost of administering. The following are fee amounts for all submissions received on or after October 1, 2018:
$16,000 for single and consolidated PMN, SNUN and MCAN submitters;
$2,800 for each PMN, SNUN, or MCAN submitted by a small business concern;
$940 for each LVE, LoREX, TME, TERA, Tier II, or photographic film article exemption notice submitted by a small business concern;
$4,700 for each LVE, LoREX, TME, TERA, Tier II, or photographic film article exemption notice submitted by all other respondents.
Persons subject to TSCA fee requirements must complete fee payments electronically using the Department of Treasury’s Pay.gov electronic collection payment services. The paperwork activities required by the fee rule are presented in another ICR approved under OMB Control No. 2070-0208 (EPA ICR No. 2569.04).
2. Indicate how, by whom, and for what purpose the information is to be used. Except for a new collection, indicate the actual use the Agency has made of the information received from the current collection
New Chemicals
TSCA requires EPA to review submitters’ section 5 notices and make an affirmative finding on the safety of new chemical substances or significant new uses of chemicals (identified by EPA in rulemaking) before they are manufactured for non-exempt commercial purposes. To make a reasoned evaluation of the potential risk to human health or the environment associated with new chemicals and significant new uses, EPA needs information on each chemical’s structure and properties, manufacturing process, worker exposure, environmental release, production volume, potential industrial, commercial, and consumer use, and potential toxicity related to the substance. EPA needs sufficient information to enable Agency scientists to identify substances with analogous chemical structures and properties, with similar manufacturing processes and with similar uses. The Agency reviews available information to evaluate the toxicity of the chemical and estimate potential exposure to the substance to assess the potential risks to human health or the environment.
A chemical is considered to be a “new” chemical if it is not listed in the TSCA section 8(b) Inventory of chemicals manufactured or processed in the United States. The Inventory includes both public and confidential information. Chemicals appear in the public portion of the Inventory by name if the company manufacturing the chemical does not claim the name of the chemical to be confidential. Chemicals whose names are claimed confidential are identified in the public portion of the Inventory by an accession number and a generic name. The specific chemical name of a confidential chemical appears only in the confidential portion of the Inventory, which is not available to the public.
A company that demonstrates a bona fide intent to manufacture a chemical substance that does not appear by a specific name in the public portion of the Inventory may ask EPA whether the substance is included in the confidential portion of the Inventory (i.e., to determine whether the substance would be considered new and therefore subject to the TSCA section 5 notice requirements). Similarly, a person who demonstrates a bona fide intent to manufacture or process a chemical substance which is described by a generic chemical name in a SNUR, may ask whether their substance is subject to the requirements of the SNUR. EPA will respond to such an inquiry only if the Agency determines that the company has demonstrated a bona fide intent to manufacture or import the substance. Reporting provisions found at 40 CFR 720.25, 721.11 or 40 CFR 725.15 require additional information from a submitter so as to encourage the submission only of bona fide inquiries that reflect serious intent.
EPA requires submitters of PMNs and “bona fide notices” to provide a specific chemical identity for the substance for which a notice is made, based on a Chemical Abstracts (CA) Index name or a CA preferred name. This requirement reduces delays caused by incorrect or ambiguous chemical identity, expedites the Agency’s ability to perform Inventory searches, and saves Agency resources spent on naming submitters’ substances.
Since a company’s initial intention to manufacture a substance or microorganism may change after making a PMN or MCAN submission, EPA requires companies to notify the Agency when manufacture (which includes import) begins by submitting a Notice of Commencement of Manufacture or Import (NOC) (see 40 CFR 720.102 and 725.190). Submitters specify in the NOC whether commencement occurred via domestic manufacture or importation and the address of the site(s) of first manufacture. This information is essential to the Agency as a compliance mechanism. The information requirements for NOC reporting also assist in identifying cases in which submitters have mistakenly reported the wrong case number in the NOC, or erroneously listed a substance that is very different from that which they intended to commence manufacture. In addition, the reporting requirements provide submitters an opportunity to update information that may no longer be correct or appropriate as reported in the notice. Finally, the NOC results in EPA adding that substance to the TSCA Inventory.
EPA requires the use of a specific reporting form (EPA Form No. 7710-56) for NOCs submitted for new chemicals in accordance with 40 CFR 720.102 (see Attachment 6). Although submission of an NOC is also required for new microorganisms, a specific reporting form is not required to be used; however, the required contents of such an NOC are described in 40 CFR 725.190. The use of a standard form for new chemicals leads to greater efficiency by assisting EPA in readily identifying the type of notice, providing uniformity in recording responses in EPA databases, and providing manufacturers a format to assure that important information is not inadvertently omitted in their submissions. Before EPA required the use of a standard NOC form, a significant number of NOCs created difficulty because they were not recognized by the Agency as a NOC or they contained confusing, missing or unnecessary information. These problems resulted in a waste of time and resources for both submitters and EPA personnel who must prepare or review these notices. The required use of a standard reporting form also reduces EPA processing time for NOCs.
On a monthly basis, EPA publishes for the public in the Federal Register information summarizing the content of each PMN and test market exemption application received, including the specific or generic name of the chemical substance and the proposed use(s), as required by TSCA section 5(d)(2). This monthly publication also includes a list of the PMNs for which EPA has received NOCs.
In order to be responsive to inquiries from Congress, the press and the public, EPA also periodically compiles certain information such as the number of notices submitted and their disposition.
The recordkeeping requirements for PMNs, MCANs, exemption applications, SNUNs and SNURs are necessary for EPA enforcement purposes. As part of its enforcement program, EPA conducts inspections to review the records of TSCA section 5 submitters to ensure that the information submitted in the notice was correct, that the submitter did not begin manufacture or processing before EPA made a determination, and that, for PMN chemicals or MCAN microorganisms, the notice of commencement was submitted when domestic manufacture or import began. The Agency also expects manufacturers’ or processors’ chemical substances subject to SNURs to ensure that they are not doing so in violation of the SNUR. The recordkeeping requirements for exemptions are necessary for enforcement purposes as well. EPA conducts inspections to ensure that the information submitted in the aforementioned applications is true and that the person holding the exemption is complying with any restrictions EPA imposed when it granted the exemption.
Existing Chemicals
TSCA requires EPA to review submitters’ notices and make an affirmative finding on the safety of significant new uses of chemicals (identified by EPA in rulemaking) before they are manufactured (including imported) for non-exempt commercial purposes. To make a reasoned evaluation of the potential risk to human health or the environment associated with significant new uses, EPA needs information on each chemical’s structure and properties; manufacturing process; worker exposure; environmental release; production volume; potential industrial, commercial, and consumer use; and potential toxicity related to the substance. EPA needs sufficient information to enable Agency scientists to identify substances with analogous chemical structures and properties, with similar manufacturing processes, and with similar uses. The Agency reviews available data to evaluate the toxicity of the chemical and estimate potential exposure to the substance to assess the potential risks to human health or the environment.
For both new and existing chemicals, users of the information are EPA employees located primarily in the Office of Pollution Prevention and Toxics (OPPT), within the Office of Chemical Safety and Pollution Prevention (OCSPP), and in the Office of Enforcement and Compliance Assurance (OECA), and Core TSCA Regional Coordinator Inspectors. EPA staff within the various divisions of OPPT use this information to review and evaluate the health and environmental effects of new chemicals and significant new uses of existing chemicals, and to recommend and implement regulatory actions if warranted. OCSPP employees in the Regional Offices and OECA employees in Headquarters and in the Regions use TSCA section 5 information for compliance monitoring and enforcement purposes.
3. Describe whether, and to what extent, the collection of information involves the use of automated, electronic, mechanical, or other technological collection techniques or other forms of information technology, e.g., permitting electronic submission of responses, and the basis for the decision for adopting this means of collection. Also describe any consideration of using information technology to reduce burden.
EPA developed e-PMN software for use in preparing and submitting TSCA section 5 notices such as SNUNs (for new and existing chemicals) and support documents electronically to the Agency via CDX and updated the e-PMN software in 2015 so that it operates more efficiently as a “cloud” software system rather than as a downloadable software system (Attachment 3.). EPA’s CDX is the point of entry on the Environmental Information Exchange Network (Exchange Network) for environmental data submissions to the Agency. CDX provides the capability for submitters to access their data through the use of web services. CDX enables EPA and participating program offices to work with stakeholders–including State, tribal, and local governments and regulated industries–to enable streamlined electronic submission of data via the Internet. For more information about CDX, go to https://cdx.epa.gov/. The use of CDX for submission of TSCA section 5 notices and support documents is consistent with the Government Paperwork Elimination Act (GPEA, Title XVII of Public Law 105-277) that requires Federal agencies to provide electronic submission, maintenance, or disclosure of information, when practicable as a substitute for paper.
4. Describe efforts to identify duplication. Show specifically why any similar information already available cannot be used or modified for use for the purposes described in Item 2 above.
New Chemicals
EPA is the only federal agency that regularly collects information on new chemical substances as defined under TSCA section 3 (in instances where chemical substances also have drug or cosmetic uses, the Food and Drug Administration would have jurisdiction over activities associated with those uses.) Therefore, the information submitters provide in a PMN or MCAN cannot be obtained elsewhere. However, information previously submitted to EPA need not be resubmitted if the following conditions are met: the information was submitted with no claims of confidentiality and the PMN (or other TSCA section 5 notice) identifies the office or person to whom the information was submitted and the date of the submission.
Existing Chemicals
EPA is the only federal agency that collects information on significant new uses of chemical substances. A notification of an intent to engage in a significant new use serves two functions: as a notice, and as a document that contains information about a chemical substance and potential exposures to that substance.
The notification element is unique to SNURs and therefore not obtainable elsewhere. The chemical information aspect will also contain unique information. Only the person who intends to commence a significant new use of a chemical substance will know the potential for human and environmental exposures to that substance, the quantity intended to be manufactured (including imported) or processed, and the manner in which the person will engage in the significant new use.
A person submitting a significant new use notice is not required to develop test data. However, the person must submit data that are known to or reasonably ascertainable by that person. For published data the submitter need only provide a literature citation (40 CFR 720.50(d)(3)(ii)). For existing chemicals that are related to the chemical substance that is the subject of the SNUR (e.g., impurities, byproducts), neither the published data nor a literature citation need be submitted. Also, notices need not include information previously submitted to EPA (unless the previously submitted information was claimed confidential, in which case it must be resubmitted).
5. If the collection of information impacts small businesses or other small entities, describe the methods used to minimize burden.
All business, regardless of size, must comply with the requirements of TSCA section 5. However, OPPT has taken a number of steps intended to minimize the burden placed on small business. For instance, TSCA section 26(d) established an Assistance Office to provide technical and other nonfinancial assistance to manufacturers (including importers) and processors of chemical substances and mixtures. This office has established a toll-free hotline, performs on-site field visits and consultations, and has hired a contractor to assist small businesses, free of charge, in complying with TSCA requirements. Small businesses also pay a lower fee for SNUN submissions.
The reporting and recordkeeping requirements associated with TSCA section 5 are applicable to all affected entities, regardless of size of business. However, EPA provides specialized assistance to respondents, particularly to small entities. TSCA section 26(d) established the TSCA Assistance Office, now known as the Stakeholder Engagement Branch, to provide technical and other non-financial assistance to manufacturers and processors of chemical substances. This office has established a TSCA Hotline to assist small businesses complying with TSCA rules. It provides material such as copies of Federal Register notices, advisories, and other information on request. In addition, currently “small business concerns” submit a reduced fee for notices and exemptions where such fees apply. Under the 2018 user fee rule, for PMNs, SNUNs, and MCANs, small business concerns submit a fee of $2,800 (rather than $16,000) and for LVE, LoREX, TME, TERA, Tier II, and photographic film article exemptions, a fee of $940 (rather than $4,700).
Moreover, EPA has taken certain steps to minimize the reporting burden associated with complying with this collection for all respondents. For example, the information technology used by EPA includes bibliographic databases that reference scientific literature and databases containing previously-submitted chemical information. These databases allow EPA to exempt submitters from needlessly providing already-published data or resubmitting previously submitted information (unless the previously submitted information was claimed confidential).
Also, as discussed previously, EPA has issued several TSCA section 5 exemption rules that reduce PMN reporting requirements thereby providing relief to submitters from the burden of responding to the full PMN/MCAN requirements.
Finally, EPA provides the services of pre-notice communications coordinators and other personnel to assist persons in a comprehensive manner for purposes of notice preparation prior to submission. For instance, for new chemical substances a PMN submitter may, upon consultation with the pre-notice communication coordinator, prepare one “consolidated notice” for up to six chemical substances if they are similar in physicochemical structure and use and share common test data or other information. Pre-notice communication coordinators respond to other pre-notice inquiries that may pertain to the full scope of the TSCA section 5 regulations.
6. Describe the consequence to Federal program or policy activities if the collection is not conducted or is conducted less frequently, as well as any technical or legal obstacles to reducing burden.
The frequency of the submission of information under TSCA section 5 is not under the Agency’s control. Manufacturers of new chemical substances must submit a PMN, SNUN, or MCAN at least 90 days prior to anticipated manufacturing or distribution of the substance for non-exempt commercial use. For existing chemicals, manufacturers submit a SNUN. Submissions of exemption applications must be submitted within 45 or 30 days prior to anticipated manufacturing or distribution of the substance for non-exempt commercial use. For both new and existing chemical substances, information is provided to EPA on an as-needed basis, initiated by submitters. Subsequent reporting would only be required if EPA determined that a specific use of a substance not included in the notice under review constituted a significant new use. Less frequent collection would mean submitters not being required to provide notice to EPA at all, which is contrary to the statutory direction. Without the notice and included information, EPA would be unable to administer the TSCA new chemical review requirements and would be unable to carry out its statutory mandate to protect the public from unreasonable risks to health and the environment.
7. Explain any special circumstances that require the collection to be conducted in a manner:
requiring respondents to report information to the agency more often than quarterly;
requiring respondents to prepare a written response to a collection of information in fewer than 30 days after receipt of it;
requiring respondents to submit more than an original and two copies of any document;
requiring respondents to retain records, other than health, medical, government contract, grant-in-aid, or tax records, for more than three years;
in connection with a statistical survey, that is not designed to produce valid and reliable results that can be generalized to the universe of study;
requiring the use of a statistical data classification that has not been reviewed and approved by OMB;
that includes a pledge of confidentiality that is not supported by authority established in statute or regulation, that is not supported by disclosure and data security policies that are consistent with the pledge, or which unnecessarily impedes sharing of data with other agencies for compatible confidential use; or
requiring respondents to submit proprietary trade secrets, or other confidential information unless the agency can demonstrate that it has instituted procedures to protect the information's confidentiality to the extent permitted by law.
Recordkeeping for new chemical substances is for five years.
8. If applicable, provide a copy and identify the date and page number of publication in the Federal Register of the agency’s notice, required by 5 CFR 1320.8(d), soliciting comments on the information collection prior to submission to OMB. Summarize public comments received in response to that notice and describe actions taken in response to the comments. Specifically address comments received on cost and hour burden.
Describe efforts to consult with persons outside EPA to obtain their views on the availability of data, frequency of collection, the clarity of instructions and recordkeeping, disclosure, or reporting format (if any), and on the data elements to be recorded, disclosed, or reported.
Consultation with representatives of those from whom information is to be obtained or those who must compile records should occur at least once every 3 years - even if the collection of information activity is the same as in prior periods. There may be circumstances that may preclude consultation in a specific situation. These circumstances should be explained.
Additionally, under 5 CFR 1320.8(d)(1), OMB requires agencies to consult with potential ICR respondents and data users about specific aspects of ICRs before submitting an ICR to OMB for review and approval. In accordance with this regulation, EPA submitted questions to several interested parties via email Attachment 10. The individual entities contacted were:
American Chemistry Council
American Cleaning Institute
The Household & Commercial Products Association
Enzyme Technical Association
Independent Petroleum Association of America
American Fuel & Petrochemical Manufacturers
Plastics Industry Association
Society of Chemical Manufacturers & Affiliates
American Petroleum Institute
A copy of EPA’s consultation to the above potential respondents and the response received are in Attachment 10 and are available in the docket. EPA did not receive any comments following consultation.
EPA received a comment in response to the previously provided 60-day public review opportunity (86 FR 73277) (FRL-9156-01-OCSPP).
The comment was received from the American Chemistry Council, a trade association representing chemical manufacturers and others in the chemical industry. The commenter presented four issues in their comment, some of which provide feedback on the new chemicals review process, rather than the ICR. Specifically, the commenter request that the Agency consider updating the guidance document entitled “Points to Consider When Preparing TSCA New Chemical Notifications” to better reflect additional information regarding the established processes for submitters to provide additional information and amendments to submissions. The commenter noted the Agency should ensure that its reviews are focused on evaluating potential for human health and environmental risk and not only on hazard. The commenter stated that additional clarification regarding: the process for when and how amendments to submissions are requested; if there is a time limit or limit to the number of amendments allowed during the PMN or SNUN process; and if there is an opportunity to establish a clearer schedule between the “Initial Chemistry Review,” the “Chemical Review and Search Strategy,” the Structure Activity Team (SAT) and the regulatory decision making. The second comment concerning new chemicals review, requested that EPA better articulate how the collection of information related to manufacturing, formulation, and application for PMN and SNUN submissions along with exposure and environmental release data is used in the development of exposure scenarios and characterizing risk.
The commenter stated that EPA should consider updating its Supporting Statement to better reflect the actual estimated delay cost for PMNs and SNUNs resulting from delays in the submission review process. The commenter noted that EPA is not accurately capturing the resulting loss of profits by manufacturers due to the long periods of Agency review. As noted, EPA should provide additional detail in how delay costs are calculated, including if there is a set delay timeframe that is being used to calculate the delay costs (e.g., X dollars in loss of profit per day times the number of days of the delay in excess of the original review timeline).
The commenter also noted that submitters continue to face technical issues when submitting information via CDX.
EPA appreciates the comments concerning the new chemicals review process.
The “Points to Consider When Preparing TSCA New Chemical Notifications” is an evolving document and EPA is considering updating it at some point in the future. EPA appreciates the suggestions made on what to update regarding this document.
In general, EPA will review all data submitted with the PMN application and will determine if the data is suitable for hazard or risk assessment purposes. In the absence of suitable submitted data, determinations may be based on analogue data, conservative assumptions, or model estimates. OPPT has developed several exposure assessment methods, databases, and predictive models to help in predicting what happens to industrial chemicals when they are used and released to the environment; and how workers, the general public, consumers and the aquatic ecosystems may be exposed to chemicals. The results of an exposure assessment are combined with a hazard (toxicity) assessment to predict the potential of an industrial chemical to cause risk to humans or the environment. If a potential for risk may exist, OPPT will examine these new chemical notices in greater detail and regulate them if necessary.
Regarding burden estimates relating to delays of PMN or other submissions, EPA’s methodology is presented in pages 45-48 of “Regulatory Impact Analysis of Amendments to Regulations for TSCA section 5.” The delay cost is defined as the net present value of the profit stream before and after delay, calculated using the following equation:
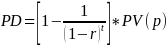
Where
PD = present value of profits delayed to PMN delay
t = time (probably in months) attributable to PMN delay
r = average real rate of return (specified in the same units of time as t) for new chemicals
PV(p) = present value (discounted at r) of profit stream associated with the average new chemical.
The above formula represents the cost of the profit stream delayed by the PMN process, rather than actual profits foregone. EPA appreciates the feedback on the burden estimates of PMN delays and will consider it if and when any revision to its methodology is undertaken.
EPA appreciates the comments concerning CDX and continues to update CDX with technological enhancements to improve the submission process. The Agency also has a dedicated CDX help desk to resolve issues facing submitters.
9. Explain any decision to provide any payment or gift to respondents, other than remuneration of contractors or grantees.
Not applicable.
10. Describe any assurance of confidentiality provided to respondents and the basis for the assurance in statute, regulation, or agency policy. If the collection requires a system of records notice (SORN) or privacy impact assessment (PIA), those should be cited and described here.
Much of the required premanufacture information may be considered by the submitter to be a trade secret, proprietary, or “confidential business information” (CBI). However, TSCA mandates that EPA require the submission of such information because it is essential for providing a basis to determine unreasonable risk. EPA cannot draw conclusions or make assumptions concerning toxicological effects and potential risks without examining physicochemical structure, methods of production, byproducts, potential uses, exposure information, etc. The Agency is required by TSCA section 5(d)(2) to publish a Federal Register notice that identifies the chemical substance, lists its uses or intended uses and describes test information. Congress included these provisions to allow active public participation in the review process.
The Agency’s policies allow public involvement while preserving confidentiality. Amended TSCA section 14(a) prohibits disclosure of trade secret information publicly when the requirements of TSCA section 14(c) are met. Also, TSCA section 14(b) allows disclosure of health and safety studies, including underlying information, unless these studies disclose confidential process or mixture information. Under 40 CFR 720.85 and 720.87 (See also 40 CFR part 2), when the specific chemical identity or use information are claimed confidential, the Agency requires the submitter to provide generic descriptions for inclusion in Federal Register notices and the public file. Additionally, the submitter must provide a “sanitized” copy of all health and environmental effects information, with confidential information deleted, for placement in the public docket. Within the Agency, only personnel with the required clearance may handle TSCA CBI.
As amended by the Lautenberg Act, TSCA section 14(c)(3) requires that any CBI claims be substantiated concurrently with the submission of the information, except that CBI claims described in TSCA section 14(c)(2) are not subject to substantiation. Based on its experience, EPA expects that most information included in TSCA section 5 notices will be claimed CBI pursuant to TSCA section 14(c)(3). To implement this new requirement for substantiation, the Agency will generally allow a PMN submitter to substantiate or correct a previously submitted substantiation for a period of 30 days from the date the PMN submitter made a CBI claim. If properly substantiated, the Agency will continue to stringently prevent unauthorized disclosure of CBI. These procedures apply to CBI submitted by manufacturers as well as CBI generated by EPA staff in the course of their review. Access to CBI is restricted to persons who need the information for their work. No one is allowed access to CBI without first undergoing instruction on procedures for handling CBI. Special procedures have been instituted to restrict access to computerized CBI and to ensure that such information is secure. These procedures are detailed in the “TSCA CBI Protection Manual,” October 2003. EPA believes these procedures protect confidential business information while providing the public with as much information as possible.
Information provided in a significant new use notice may receive confidential treatment consistent with the requirements of TSCA. TSCA section 14 allows a manufacturer (including importer) or processor to designate submitted information as confidential business information (CBI). The Agency has developed a comprehensive system to prevent the unauthorized disclosure of CBI. This system includes procedures for logging CBI in and out of designated locked file cabinets, for photocopying and transmitting CBI, and for restricting confidential information only to personnel with CBI security clearance. No one is allowed access to CBI until they have received instructions for handling CBI.
EPA will ensure secure transmission of SNUN data submitted through CDX via the Transport Layer Security (TLS) protocol. TLS is a widely used approach for securing Internet transactions and is endorsed by the National Institute of Standards and Technology (NIST) for protecting data sent over the Internet. See NIST Special Publication 800–52 Revision 1, “Guidelines for the Selection, Configuration, and Use of Transport Layer Security (TLS) Implementations, (dated August 2019)”. (Attachment 4.) In addition, e-PMN software enables the SNUN submitter to electronically sign, encrypt, and submit reports which EPA subsequently provides back to the submitter as an unaltered copy of record. This assures the submitter that the Agency has received exactly what the submitter sent to EPA.
Any information being sent via CDX is transmitted using secure technologies to protect CBI. The e-PMN software encrypts PMN submissions using a Federal Information Processing Standards (FIPS)-compliant encryption module. The encryption module employs a public key algorithm which converts readable text into encrypted text. This public key is downloaded from CDX to the e-PMN software, and the corresponding private key is sent to EPA’s New Chemical System (NCS). The encryption remains while the submission is transmitted via CDX to NCS. The file can be decrypted only with the NCS's private key when it has reached its final destination. The NCS is the only party that possesses the private key, which converts the encrypted text back into readable text.
The same processes would be used for all correspondence returning to the submitter. The NCS and e-PMN software are also provided with a set of public and private keys, so that correspondence containing any potential confidential business information will remain encrypted during transmission via CDX and can be opened only by the submitter within the e-PMN software.
Additionally, special procedures also restrict access to computerized CBI. These security measures apply to CBI submitted by manufacturers as well as CBI generated by EPA staff in the course of their review.
11. Provide additional justification for any questions of a sensitive nature, such as sexual behavior and attitudes, religious beliefs, and other matters that are commonly considered private. This justification should include the reasons why the agency considers the questions necessary, the specific uses to be made of the information, the explanation to be given to persons from whom the information is requested, and any steps to be taken to obtain their consent.
Not applicable. The information does not include responses to questions of a sensitive nature.
12. Provide estimates of the hour burden of the collection of information. The statement should:
Indicate the number of respondents, frequency of response, annual hour burden, and an explanation of how the burden was estimated. Unless directed to do so, agencies should not conduct special surveys to obtain information on which to base hour burden estimates. Consultation with a sample (fewer than 10) of potential respondents is desirable. If the hour burden on respondents is expected to vary widely because of differences in activity, size, or complexity, show the range of estimated hour burden, and explain the reasons for the variance. Generally, estimates should not include burden hours for customary and usual business practices.
If this request for approval covers more than one form, provide separate hour burden estimates for each form and aggregate the hour burdens.
Provide estimates of annualized cost to respondents for the hour burdens for collections of information, identifying and using appropriate wage rate categories. The cost of contracting out or paying outside parties for information collection activities should not be included here. Instead, this cost should be included under ‘Annual Cost to Federal Government’.
Respondents or entities potentially affected by this ICR are chemical manufacturers (defined by statute to include importers) and processors, e.g., entities identified by the North American Industrial Classification System (NAICS) codes 325, Chemicals and Allied Products Manufacturers, and 324, Petroleum Refining.
Information submitted under this collection must include, insofar as it is known to or reasonably ascertainable by the submitter, information described in TSCA section 8(a)(2) (i.e., chemical identity, use, and exposure data), as well as test data, and descriptions of other data related to the effects on health and the environment of the manufacture, processing, distribution in commerce, use and/or disposal of the chemical substance (TSCA section 5(d)). After receipt of a notice, EPA has 90 days (extendable to 180 days) to evaluate the notice’s content.
Only those persons who intend to engage in a significant new use of a chemical substance must submit notice of their intentions to EPA. According to 40 CFR 721.1(c), persons submitting a SNUN must comply with the same notice requirements and EPA regulatory procedures as persons submitting a PMN, including submission of test data on health and environmental effects as described in 720.50. SNUNs must be on EPA Form No. 7710–25, generated using e-PMN software, and submitted to the Agency in accordance with the procedures set forth in 721.25 and 720.40. E-PMN software is available electronically (Attachment 3).
This section presents estimates of the cost and burden associated with the recordkeeping and reporting requirements for significant new use rules for existing chemicals under TSCA section 5(a)(2). The methodology used to estimate the recordkeeping costs, reporting costs, and burden for this ICR renewal is largely based on previous experience with SNURs and is consistent with the analysis presented in the supporting statement prepared for the previous ICR. This ICR is updated from the previous ICR to account for the changes in burden resulting from updated assumptions and corrections to prior analyses.
To comply with the regulation, manufacturers (including importers) must complete the activities listed in Table 1. Table 1 also provides a cross-walk of the related Information Collection that corresponds to each activity.
Table 1: Cross-walk between Industry Activities and Related Information Collections (ICs)
Activity |
Description |
Related IC(s) |
Chemical verification |
When a SNUR is published, companies must review the rule to verify whether a chemical they manufacture (including import) is subject to the rule. |
Chemical Verification |
Rule familiarization |
Site staff must familiarize themselves with the requirements of the rule. This entails reading the rule, understanding the various reporting and administrative requirements, and determining the manner in which the reporting requirements will be met. |
Rule Familiarization |
CDX registration, electronic signature agreement, and Pay.gov account set up |
Before submitting a SNUN, all respondents must register with CDX. In addition, respondents must complete an Electronic Signature Agreement form, which is signed, dated, and either submitted electronically or mailed back to EPA, and register for a Pay.gov account. |
CDX Registration Activities |
Preparation of reports (form completion and form submission) |
Site staff must collect all information required for a SNUN and submit an electronic SNUN form. Firms must also keep records supporting their submissions. |
Prepare and Submit Report, and Maintain Records |
Notifying customers |
Manufacturers (including importers) and processors must notify customers of the SNUR such as by annotating an SDS. |
Customer Notification |
To assist in the evaluation, EPA encourages submitters to contact an EPA specialist through a pre-notice consultation to ensure that the submitter understands EPA’s review process of the information helpful to make a determination regarding the chemical. EPA also issued a guidance document, entitled “Points to Consider When Preparing TSCA New Chemical Notifications,” to inform and assist submitters planning to prepare PMNs, SNUNs (including for existing chemicals), and exemption applications for Agency review (Attachment 5.).
Data Items - Reporting Requirements
Premanufacture Notices - Premanufacture notices required by TSCA section 5 must include certain information to the extent known to or reasonably ascertainable by the submitter. This information is defined in TSCA sections 5(d)(1) and 8(a)(2) and 40 CFR 720.45. Specific information includes the following:
Common or trade name, chemical identity and molecular structure of the chemical in question;
Categories or proposed categories of use of the chemical;
Estimate of the total amount of such chemical to be manufactured or processed, including the amount to be manufactured or processed for each use category;
Description of the byproducts resulting from the manufacture, processing, use and/or disposal of the chemical;
Estimate of the total number of individuals who will be exposed to the chemical in their places of employment, and the duration of such exposure; and
Manner or method of the disposal of the chemical.
In addition, the submitter must provide any test data in the submitter’s possession that indicate the health or environmental effects of the chemical, and a description of any other data known to the submitter concerning the health or environmental effects of the chemical. The specific information requirements are spelled out in 40 CFR part 720, on the PMN reporting form itself, and in the Instruction Manual for Reporting under the TSCA §5 New Chemicals Program (Attachment 3).
To facilitate the review of chemicals, EPA has developed a PMN reporting form (EPA Form 7710-25; Attachment 6). This form is required for reporting new chemicals under TSCA section 5(a)(1) and is also required for submitting Significant New Use Notices (SNUNs). By supplying the information specified in the form, submitters do not incur the burden of providing information unnecessary for EPA’s review. Therefore, use of the form lessens the burden on companies by reducing uncertainty, minimizing the need for additional contact with EPA, and allowing companies to establish procedures for meeting reporting requirements.
EPA has limited the level of detail of information required in the PMN form to that necessary for EPA to conduct an initial review of a chemical. However, submitters may include additional or optional information in their notices that they believe EPA should consider in its review. For example, submitters may identify pollution prevention techniques being employed by the submitter that may be relevant to the Agency’s risk assessment. EPA encourages submitters to provide information on the benefits of the new substance in comparison to existing chemical substances, information on the substitutes, and any additional information available to them on waste management techniques.
The existing PMN form is not appropriate for reporting of new microorganisms in MCANs since the form was designed with traditional chemical substances in mind. The submitter will be able to provide the information required by the regulations in a format of his or her own choosing. Submitters of MCANs are required to use the e-PMN software to generate a finalized “header” sheet, called EPA Form 6300-07, TSCA Biotechnology Notice for Online Submissions. The form simply requires contact information, the addition of any attachments, and a signature page (Attachment 7.).
The e-PMN form also includes a User Fee Payment Identity Number field to enable the Agency to match more easily a particular user fee with a particular notice submission. A User Fee Payment Identity Number is required and may be a wire transfer number, or a “Pay.gov” transaction number used to transmit the user fee. This information is presently in the submitter’s possession.
Another information element on the PMN form is optional and consists simply of the e-mail addresses for the principals listed on the Submitter Identification section of the PMN form. This information helps facilitate electronic communications with the proper point of contact from the submitting entity.
Exemption Applications - Applications for exemptions from premanufacture or microbial commercial activity notice requirements have additional information requirements, as follows:
Test-Marketing Exemption (TME) (40 CFR 720.38) - Since April 6, 2010, TME submitters have been required to use the e-PMN software to generate finalized submissions using either Form 7710-25 or a cover letter and attached information. The test-marking exemption rule states that applicants should provide the following information: (1) all existing health and environmental effects data on the chemical or a discussion of toxicity based on structure-activity relationships and relevant data on chemical analogues; (2) the maximum quantity of the chemical substance that the applicant will manufacture for test-marketing purposes; (3) the maximum number of persons who may be provided the chemical substance during test-marketing; (4) the maximum number of persons who may be exposed to the chemical substance as a result of test-marketing, including information regarding the duration and route of such exposure; and (5) a description of the test-marketing activity, including its length and how it can be distinguished from full-scale commercial production and research and development. The Agency retains the right to determine that an application contains insufficient information to make an evaluation. Any person who receives a test-marketing exemption must retain documentation of any information in the exemption application and documentation of their compliance with any restrictions imposed by EPA when it granted the application. This information must be retained for five years from the final date of manufacture or import under the exemption.
Research and Development Exemption (R&D) (40 CFR 720.36) - A manufacturer using this exemption must notify all persons in its employ, or to whom it distributes the chemical substance and who are involved in any way in the research, of any risk to health associated with the chemical substance.
TSCA section 5(h)(4) Exemptions - For the low volume exemption (LVE) (40 CFR 723.50), submitters are required to submit their exemption on the PMN form (generated using the e-PMN software) to ensure that the Agency has adequate information to make a determination that these substances will not present an unreasonable risk. Statements describing exposure and release controls, site, and use in an exemption application are legally binding and enforceable.
For the low exposure/low release exemption (LoREX) (40 CFR 723.50), submitters are required to submit their exemption on the PMN form (generated using the e-PMN software). The LoREX exemption encourages the use of pollution prevention practices through the development of manufacturing, processing and use techniques that minimize exposure to workers, consumers, the general public and the environment. As with the low volume exemption, site, use, exposure and release controls identified in the notice are binding.
The polymer exemption rule (40 CFR 723.250) - requires the submission of a post-manufacture report to EPA. A simple one-page annual report is required to be submitted to the Agency no later than January 31 of the year subsequent to initial manufacture under the terms of the exemption. The report must include company identity information including the name and telephone number of a technical contact and the number of exempt substances for which manufacture commenced during the preceding year. These reports are not subject to the electronic reporting requirements of the e-PMN rule.
Instant photographic film articles exemption notices (under 40 CFR 723.175) – there must, at a minimum, identification by the manufacturer and the new chemical substance. Applicants must submit an exemption notice when manufacture begins and comply with certain requirements to limit exposure to the chemical. Applicants must retain certain records for 30 years from the final date of manufacture.
TSCA experimental release application (TERA) (under 40 CFR 725.250) - applies to research and development activities that result in intentional environmental releases of new microorganisms. Applicants are required to include adequate information in their exemption application so that the Agency can make a determination as to whether the microorganism will present an unreasonable risk. Submitters must follow the conditions described in the TERA as well as any conditions of EPA’s TERA approval.
Tier 1 exemption (40 CFR 725.424) - applies to certain new microorganisms subject to physical containment and control technologies. EPA has developed specific criteria for the host microorganism, introduced genetic material, and containment technology to ensure that the microorganism will not present an unreasonable risk. Applicants must notify EPA 10 days before manufacture, certifying compliance with the exemption criteria and include the site of manufacture or import.
Tier II exemption (40 CFR 725.428) - applies to the same microorganisms subject to a Tier I exemption; however, the applicant must provide adequate information on its proposed physical containment and control technologies in order for EPA to evaluate the exemption. If EPA grants the exemption, it may impose appropriate restrictions on the activities described in the notice.
Submitters of biotechnology notices (i.e., MCANs, TERAs, Tier I exemptions, and Tier II exemptions) - are required to use the e-PMN software to generate a finalized “header” sheet, called EPA Form 6300-07, TSCA Biotechnology Notice for Online Submissions. The form requires contact information, the addition of any attachments, and a signature page.
Notices of Commencement - Under 40 CFR 720.102 and 725.190, EPA requires companies to notify the Agency by submitting a Notice of Commencement (NOC) when non-exempt commercial manufacture (including importation) of a new chemical begins. Required reporting information includes the following:
Specific chemical identity of the chemical, and a generic chemical name if the specific name is considered confidential;
Premanufacture notice number assigned by EPA;
Date that manufacture commenced;
Address of the site where manufacture commenced;
Name and address of the submitting company, the name of the authorized official signing the NOC, the name and telephone number of a technical contact person; and
Clear indication of what information, if any, is to be considered confidential.
For traditional chemicals regulated under 40 CFR part 720, NOCs must be submitted to EPA using the NOC form (EPA Form 7710-56)(Attachment 8). Since the effective date of the e-PMN rule, submitters are required to use the e-PMN software to generate a finalized submission using Form 7710-56. The submitter must provide the NOC to EPA on, or no later than 30 calendar days after, the day manufacture (including importation) began. The existing NOC form is not appropriate for reporting of new microorganisms since the form was designed with traditional chemical substances in mind. Thus, under 40 CFR 725.190 the submitter may provide information in a format of his or her own choosing when reporting an NOC for a new microorganism.
Bona Fides - To determine whether a chemical substance is on the confidential portion of the TSCA Inventory, submitters of bona fide inquiries under 40 CFR 720.25 and 40 CFR 725.15 are required to provide the specific chemical identity of the substance in question, a signed statement that the submitter intends to manufacture that substance, a description of the research and development activity conducted on that substance, a description of the intended use of the substance, infrared spectrum data to identify the substance, the estimated date on which the company intends to submit a PMN, the address of the facility where manufacturing or processing will occur, and a description of the manufacturing process. A similar procedure is followed for companies wishing to obtain confidential information for a SNUR, including chemical identity, and terms of the SNUR (see 40 CFR 721.11).
To determine whether a microorganism is on the confidential portion of the TSCA Inventory, submitters of bona fide inquiries under 40 CFR 725.15 are required to provide the taxonomic designations, pertinent genotypic and phenotypic information, a signed statement that the submitter intends to manufacture that microorganism, a description of the research and development activity conducted on that substance, a description of the intended use of the substance, and an indication of whether a related microorganism was previously reviewed by EPA to the extent known by the submitter. Bona fides are also subject to the e-PMN rule electronic reporting requirements.
User Fees – There is a TSCA section 26(b) rule in place at 40 CFR part 700 that requires manufacturers and processors to pay fees for PMNs, MCANs, certain PMN exemption application notices, and SNUNs submitted under TSCA sections 5(a) and (h), and requires a limited amount of additional information to be submitted with the section 5 notice. This information includes certification that the firm is a “small business concern” (if applicable), a certification statement that the submitter remitted the appropriate fee, and the placement of corresponding identifying numbers both on the PMN form and the fee remittance. A final rule (and associated ICR) that established the TSCA-associated user fees was issued to implement the Lautenberg Act amendments to TSCA became effective October 18, 2018 (83 FR 52694).
Information Items - Recordkeeping Requirements
Under 40 CFR 720.78(a), TSCA section 5 notice submitters must keep the following information or data for five years from the date of commencement of manufacture or processing: documentation of information in the notice (e.g., sources of information provided in the notice); production volume for the first three years of production; the date of commencement, plus documentation of this information; and “other data” described in the notice, as required by 40 CFR 720.50(b).
Recordkeeping requirements under SNURs require persons who manufacture or process a substance subject to significant new use reporting to maintain records indicating their compliance with certain methods of manufacture or processing. Recordkeeping requirements apply to SNURs for which compliance can be monitored by recordkeeping or SNUR notice (SNUN) submission under TSCA section 5(a)(2). For example, upon occasion EPA may determine that a specific set of exposure controls will adequately mitigate risks to workers by a specific chemical substance. In such cases, EPA may determine, by rule, that the failure to utilize such controls constitutes a significant new use. However, those persons employing the controls identified in the SNUR are not required to report to EPA. In order to demonstrate to EPA inspectors that they are properly employing worker exposure controls (to avoid SNUR notification requirements), manufacturers or processors will likely maintain some record of their compliance. In instances such as those described above, EPA would require that records be kept documenting the establishment and implementation of procedures to ensure that the exposure controls were in place. These records aid inspectors in EPA’s compliance monitoring program during their visits to plants where substances subject to SNUR requirements are manufactured or processed. EPA does not consider recordkeeping that indicates compliance with a SNUR to be burdensome. Information contained in these records is not submitted to EPA. Therefore, the costs of keeping such records should be minimal.
There are also recordkeeping requirements for persons subject to section 5 orders (most often consent orders) containing exposure controls. Depending on the facts of each case, submitters must keep records in connection with the use of the exposure controls including one or more of the following: (1) documentation of manufacture volumes of the PMN substance, with associated dates of manufacture; (2) documentation of the names and addresses of all persons outside the site of manufacture to whom the submitter directly sells or transfers the substance, with associated dates of transfer; (3) documentation of the establishment and implementation of personal protective equipment program; (4) documentation of chemical protective clothing imperviousness testing; (5) documentation of the hazard communication program; (6) copies of labels; (7) copies of material safety data sheets; (8) documentation of compliance with industrial, commercial and consumer use limitations; and (9) documentation of compliance with disposal and release to water limitations.
Respondent Activities
In responding to the reporting and recordkeeping requirements outlined in this document, respondents will engage in the following activities:
Read regulatory requirements and provisions;
Determine which provisions are applicable to their activities;
Gather information necessary to meet the requirements;
Substantiate any claims of confidential business information;
Register with CDX;
Use the e-PMN software for notifications including SNUNs;
Submit information to EPA, as necessary;
Comply with any restrictions EPA may impose upon completion of review of their submission; and
Maintain any necessary records.
Estimating Respondent Burden
Number of Significant New Use Notices Projected
During the years 2019 through December 2021, EPA proposed and finalized existing chemical SNURs under TSCA section 5(a)(2). EPA expects to promulgate up to 12 existing chemical SNURs between 2022-2024, for an average of four existing chemical SNURs per year. Therefore, EPA is estimating an average of three to four existing chemical SNURs per year (9-12 SNURS) under TSCA section 5(a)(2) during the period covered by this ICR.
EPA may receive SNUNs as a result of SNURs. SNUNs cover SNURs that are issued for both the new chemical and existing chemical programs. EPA reviewed the number of SNUNs received between 2019 and 2021 and determined that only one of the SNUNs received resulted from promulgation of an existing chemical SNUR. Therefore, this ICR conservatively estimates an average of one SNUN received per year as a result of existing chemical SNURs, with a single firm impacted per SNUN submitted.
Table 2: Anticipated Number of Existing Chemical SNURs and SNUNs
Year |
Anticipated Number of SNURs |
Anticipated Number of SNUNS |
First Year |
4 |
1 |
Second Year |
4 |
1 |
Third Year |
4 |
1 |
Three Year Totals |
12 |
3 |
Alternative Responses
The burden associated with a SNUR could involve a number of possible industry responses. That is, when a SNUR is promulgated, a firm seeking to engage in a new use for a subject chemical has five options regarding possible courses of action that may generate burden:
The company could submit a SNUN and could provide information in the SNUN to demonstrate that the new use would not be likely to present unreasonable risk to health or the environment under the conditions of use. This option would be chosen by any company wishing to initiate a new use covered by the provisions of the SNUR.
In the event that a significant new use is described as the failure to establish and implement programs for providing for the use of specific measures to control worker exposure to or release of substances, a company can request an equivalency determination. This option would be chosen if a manufacturer (including importer) had reason to believe that there may be alternative methods not considered by EPA that provide equivalent or superior protection from worker exposure or environmental release of the subject chemical.
The company can comply with the SNUR, ensuring that all provisions of the SNUR are implemented regarding the planned use of the subject chemical.
The company can request review of the SNUR for possible modification or revocation.
The company may simply decide to forgo the new use, avoiding regulatory compliance activities altogether.
Additionally, under current regulations at 40 CFR 721.5(a)(2), all manufacturers (including importers) and processors of chemicals subject to SNURs are subject to certain requirements regardless of whether they engage in a significant new use unless certain information can be demonstrated1. However, without prior knowledge of chemicals which would be the subject of future SNURs, estimating the number of potentially affected entities subject to 40 CFR 721.5(a)(2) is not possible.
The following section estimates the cost and burden of submitting a SNUN (option 1) and then discusses the other options qualitatively.
Burden Estimates
TSCA section 5(a)(2) imposes two requirements on industry: first, manufacturers (including importers) and processors of chemicals must choose among the options mentioned above. This section presents estimates of submitting SNUNs (i.e., the first option) and then briefly discusses the other four options. Second, manufacturers (including importers) and processors of chemicals covered by SNURs will incur burden and costs associated with notifying customers of the SNUR requirements. Therefore, they must first determine if their chemical is subject to the SNUR and then must determine how to notify their customers.
Chemical Verification
When a SNUR is published, companies must review the rule to verify whether their chemical substance is subject to the rule. EPA distinguishes between SNURs that regulate a specific chemical and SNURs that regulate a chemical formula, understood as a set of distinct chemicals that share a common chemical structure. EPA expects to promulgate up to 12 existing chemical SNURs during the three-year period covered by this ICR, covering an average of 4.33 chemicals and 0.83 chemical formulas per SNUR. EPA estimates that an average of 0.17 hours (10 minutes) of technical labor time per chemical is used to verify that a chemical is subject to a SNUR and 0.50 hours (30 minutes) to verify that a chemical formula is subject to a SNUR. This is equivalent to 1.14 hours ([0.17 hours/chemical x 4.33 chemicals/SNUR] + [0.50 hours/chemical x 0.83 chemical formulas/SNUR] = 1.14 hours).
Rule Familiarization
Staff at firms that manufacture or process chemicals subject to the SNUR must become familiar with the SNUR and its various requirements. In EPA’s best professional judgment, rule familiarization is estimated to be equivalent to the burden for companies to become familiar with the Premanufacture Notification Electronic Reporting Rule, which requires the mandatory electronic reporting of SNUNs and other TSCA section 5 notices: 0.55 hours of technical labor and 0.27 hours of managerial labor, as described in the Economic Analysis of the Premanufacture Notification Electronic Reporting Final Rule (EPA, 2009a).
Table 3: Rule Familiarization Burden for SNUR Submitters
Activity |
Clerical Hours |
Technical Hours |
Managerial Hours |
Total Hours |
Rule familiarization |
0.00 |
0.55 |
0.27 |
0.82 |
Some burden estimate totals may not calculate due to rounding of unit burden estimates
CDX Registration, CDX Electronic Signature, and Pay.gov Account Setup
First-time submitters of any section 5 notice (including Premanufacture Notices (PMNs), Significant New Use Notices (SNUNs), Test Market Exemption (TME) applications, Low Volume Exemption (LVE) notices, Low Exposure/Low Release (LoREX) exemption notices, Biotechnology Notices for genetically modified microorganisms, Notices of Commencement of Manufacture or Import (NOCs), and support documents to section 5 notices) are required to register their company and key users with the CDX reporting tool, deliver a CDX electronic signature to EPA, and establish and use a Pay.gov E-payment account. These activities are only required for first-time submitters of any section 5 notice. It is not known how many submitters of SNUNs from existing chemical SNURs will be first-time submitters of any section 5 notices; therefore, EPA conservatively assumes that all submitters will incur these costs. These activities are estimated to require the following burden, based on the estimates presented in the Economic Analysis of the Premanufacture Notification Electronic Reporting Final Rule (EPA, 2009a):
CDX registration: EPA estimates that companies will spend approximately 0.18 hours per employee to register with CDX, and that an average of four technical staff members and one manager will need to register for each company, totaling approximately 0.92 hours of burden per company.
CDX electronic signature: EPA estimates that companies will spend 0.25 hours preparing, submitting, and filing an electronic signature agreement (Authentication of Identity) form to EPA per employee. This burden will apply to four technical staff members and one manager per company, totaling 1.25 hours of burden per company. In addition, EPA estimates that a manager will spend an additional 0.50 hours accessing, preparing, and submitting verification forms (Verification of Authorization) for all authorized submitters to EPA. The total burden incurred by companies submitting and then verifying electronic signature agreements is 1.75 hours. Note that this burden does not include any additional time required to contact EPA’s CDX help desk to notify a change of submitter status, should one occur.
Payment via Pay.gov account: EPA estimates that one manager per company will spend approximately 0.13 hours setting up a Pay.gov ID account, logging into the system, finding the appropriate form, and filling it out. This burden does not include the time required to click ‘submit’ on the form and wait for payment processing.
Table 4: CDX Registration, CDX Electronic Signature, and Pay.gov Account Setup Burden for First-Time Submitters
Activity |
Clerical Hours |
Technical Hours |
Managerial Hours |
Total Hours |
CDX registration |
0.00 |
0.73 |
0.18 |
0.92 |
CDX electronic signature |
0.00 |
1.00 |
0.75 |
1.75 |
E-payment (Pay.gov ID) |
0.00 |
0.00 |
0.13 |
0.13 |
Total |
0.00 |
1.73 |
1.07 |
2.80 |
Some burden estimate totals may not calculate due to rounding of unit burden estimates
Submitting a SNUN
When submitting a SNUN, individuals at different occupational levels must spend time on the required recordkeeping and reporting activities. SNUN submitters are required to gather and submit information regarding the data elements identified in the applicable SNUN reporting form. The methodology and calculations assume that the employee responsible for collecting, filling out, and submitting the requested information has a reasonable level of familiarity with the company and knowledge of operations at the site. It is assumed that, for most entities, these tasks are similar to other employee duties that require familiarity with EPA, state, and other federal agency requests for chemical information and do not require additional familiarization or training beyond the basic rule familiarization described above.
Estimates of the burden of completing a SNUN form are based on the burden of completing a PMN submission, since the data requirements are the same and the same form is used for both. EPA has harmonized estimates of the reporting and recordkeeping burden related to the submission of both new and existing chemical SNUNs. The reporting and recordkeeping burden for Existing Chemical SNUNs is estimated to be 91.68 hours and is based on the 1994 Regulatory Impact Analysis of Amendments (RIA) to Regulations for TSCA Section 5 Premanufacture Notifications (EPA, 1994) and has been adjusted to reflect burden reductions resulting from the 2009 final PMN Electronic Reporting (ePMN) Rule that requires the electronic submission of all TSCA section 5 notices. Electronic submission of SNUN forms is expected to remove all clerical burden and reduce the recordkeeping burden associated with preparing and submitting a SNUN (EPA, 2009a). In addition, electronic submission is expected to generate an additional 0.18 hours of technical burden associated with the completion of the User Fee Payment Identification Number and email address data elements on the electronic SNUN form.
Alternative Options
Should a company choose to request an equivalency determination (i.e., the second option), or review for modification/revocation (i.e., the fourth option), EPA estimates that a data collection and preparation effort similar to that of a SNUN would be required, and thus the burden is estimated to range up to 91.68 hours for these alternatives, the same as for submitting a SNUN.
In complying with a SNUR, a company would incur costs to ensure all provisions of the SNUR were implemented at the subject facility (i.e., the third option). Since the nature of such provisions will vary depending on the significant new uses identified in each respective SNUR, estimating burden at this time is not possible. In addition to costs of implementation, firms choosing this option will have minor costs associated with keeping records that document compliance with SNUR conditions for avoiding a Significant New Use. Such recordkeeping requirements may involve copying and filing relevant records, including those related to: category of use and marketing, and production volume. Records would typically be required to be maintained for five years from the date of their creation. Previous existing chemical SNUR ICRs have estimated recordkeeping requirements to be five percent of the reporting burden for a certain activity (EPA, 2009b). Per-activity burden is taken from the midpoint estimated burden of each section of the SNUN form (EPA, 1994). The recordkeeping burden per significant new use is estimated to range from 0.01 hours (0.25 * 0.05) for keeping records of trade names and chemical synonyms to 0.76 hours (15.25 * 0.05) for keeping records of sites controlled by others. The total recordkeeping burden per firm will depend on the significant new use(s) identified.
The final alternative for a company considering a significant new use of a chemical that is subject to a SNUR is to forgo the new use (i.e., the fifth option). In carrying out such a response, the company would incur no direct regulatory burden or costs.
Customer Notification
Manufacturers (including importers) and processors of chemical substances subject to SNURs must notify recipients of such chemicals of the SNUR or verify that knowledge of the SNUR has been otherwise acquired by recipients, or that the recipients are unable to engage in significant new uses, if they do not wish to submit a SNUN. Since it is not expected that all such entities will have complete knowledge of all uses of any chemicals subject to a SNUR, and because filing a SNUN could require significantly more burden, it is assumed that manufacturers (including importers) and processors will most often choose to notify their customers of SNUR regulatory activities. As this notification may be accomplished by simply annotating a Safety Data Sheet (SDS), EPA estimates the associated burden to be about one hour of a technical employee's time per manufacturer or processor per chemical and three hours per manufacturer or processor per chemical formula. EPA estimates that each SNUR will cover approximately 4.33 chemicals and 0.83 chemical formulas. Furthermore, EPA assumes that there are two manufacturers (including importers) or processors per chemical.2 Therefore, the notification burden per SNUR is estimated to be 13.67 hours per SNUR ([1.00 hours/chemical x 4.33 chemicals/SNUR x 2.00 manufacturers] + [3.00 hours/chemical x 0.83 chemical formulas/SNUR x 2.00 manufacturers] = 13.67 hours).
Summary of Unit Burdens
The following table summarizes the burden associated with the activities required under a SNUR, under compliance option 1.
Table 5: Summary of Unit Burdens
Collection Activity |
Estimated Burden Hours |
Chemical verification (per SNUR) |
1.14 |
Rule Familiarization (per company) |
0.82 |
CDX registration, electronic signature, and Pay.gov account set-up (per company) |
2.80 |
SNUN preparation, submission, and recordkeeping (per report) |
91.68 |
Notifying customers (per SNUR) |
13.67 |
Estimating Respondent Costs
The unit costs of filing a SNUN are estimated by monetizing the labor time spent preparing the SNUN and then adding any fixed costs associated with filing a SNUN. This section derives these unit costs.
Wages
Wage and fringe benefit data for each labor category (e.g., managerial, professional/technical, and clerical labor) are taken from the U.S. Bureau of Labor Statistics (BLS) Employer Costs for Employee Compensation (ECEC) Supplementary Tables (BLS 2021a). In the BLS report, wages are represented by the “wages and salaries” cost component and fringe benefits are represented by “total benefits” (Attachment 7).
Overhead costs are assumed to equal 20% of the sum of wages plus fringe benefits. This loading factor is described in Handbook on Valuing Changes in Time Use Induced by Regulatory Requirements and Other U.S. EPA Actions (EPA 2020d) and is reflective of multiplier values used in prior EPA RIAs and ICRs that are based on industry- and occupation-specific overhead rates affected by EPA regulations. This overhead loading factor is multiplied by the total compensation (wages plus fringe benefits). For example, the June 2021 fully loaded wage for professional/technical labor is ($45.59 + $22.80) * 1.2 = $82.06. Fully loaded costs for managerial and clerical labor are calculated in a similar manner. The calculated overhead costs (20% of the total compensation) are shown in Table 6 as well as the total hourly loaded wages.
Table 6: Derivation of Loaded Wage Rates for the Private Manufacturing Sector in 2021$
Labor
Category/ |
Date |
Wage ($/hour) |
Fringe Benefit |
Total Compensation |
Overhead as % of Total Compensationb |
Overhead |
Hourly Loaded Wagesc |
|||||||
(a) |
(b) |
(c) =(b)+(a) |
(d) |
(e)=(c)*(d) |
(f)=(c)+(e) |
|||||||||
Managerial |
||||||||||||||
BLS ECEC, Private Manufacturing industries, “Mgt, Business, and Financial” |
Jun-21 |
$54.68 |
$24.83 |
$79.51 |
20% |
$15.9014.99 |
$95.41 |
|||||||
Professional / Technical |
||||||||||||||
BLS ECEC, Private Manufacturing industries, “Professional and related“ |
Jun-21 |
$45.59 |
$22.80 |
$68.38 |
20% |
$13.68 |
$82.06 |
|||||||
Clerical |
||||||||||||||
BLS 0ECEC, Private Manufacturing industries, “Office and Administrative Support” |
Jun-21 |
$21.35 |
$9.79 |
$31.14 |
20% |
$6.23 |
$37.33 |
|||||||
Footnotes |
||||||||||||||
a Source: Employer Costs for Employee Compensation Historical Supplementary Tables, National Compensation Survey: December 2006 –June 2021 (U.S. Bureau of Labor Statistics, 2021a). |
||||||||||||||
b An overhead rate of 20% is used based on assumptions in Handbook on Valuing Changes in Time Use Induced by Regulatory Requirements and Other U.S. EPA Actions (EPA 2020d) |
||||||||||||||
c Wage data are rounded to the closest cent in this analysis. |
||||||||||||||
Table 7: CDX Registration, CDX Electronic Signature, and Pay.gov Account Setup Cost for First-Time Submitters
Activity |
Clerical Labor ($37.33/hour) |
Technical Labor ($82.06/hour) |
Managerial Labor ($95.41/hour) |
Total Labor Cost (2021$) |
CDX registration |
$0.00 |
$59.90 |
$17.17 |
$77.08 |
CDX electronic signature |
$0.00 |
$82.06 |
$71.56 |
$153.62 |
Mailing cost |
|
$2.79 |
||
E-payment (Pay.gov ID) |
$0.00 |
$0.00 |
$10.66 |
$10.66 |
Total |
$0.00 |
$141.96 |
$99.39 |
$244.15 |
Table 8: Unit Reporting Cost Estimates, Associated with Filing a SNUN by Labor Category
Activity |
Clerical Labor ($37.33/hour) |
Technical Labor ($82.06/hour) |
Managerial Labor ($95.41/hour) |
Total Labor Cost (2021$) |
SNUN preparation, submission, and recordkeeping |
$0.00 |
$6,047 |
$1,717 |
$7,764 |
User fee |
|
$16,000 |
||
Total |
$0.00 |
$6,047 |
$1,717 |
$23,767 |
Some burden estimate totals may not calculate due to rounding of unit burden estimates
Alternative Responses
Five alternative responses to any particular SNUR could be chosen by firms planning to engage in significant new uses of subject chemicals. Although EPA has not projected or quantified how frequently these alternatives might be selected, the unit costs associated with each option are discussed briefly below.
The estimated burden of requesting an equivalency determination (the second option) or review for modification/revocation (the fourth option) was judged to be similar to filing the SNUN; thus, total costs including the EPA user fee were estimated to be $23,767. However, the firm may incur additional costs in developing the data necessary to justify the alternative. This option will be preferable to compliance with the SNUR if the total cost of obtaining EPA approval of a request is less than the costs of SNUR compliance.
Firms choosing to comply with a SNUR (the third option), will incur costs to ensure all provisions of the SNUR were implemented at the subject facility and to implement recordkeeping. The costs of implementing provisions at a facility were not quantified for this ICR. Recordkeeping is expected to range from 0.01 hours to 0.76 hours for each significant new use. All recordkeeping activities are expected to be conducted by clerical staff; therefore, recordkeeping costs range from $0.37 to $28.37 per significant new use. The total recordkeeping burden per firm will depend on the significant new use(s) identified.
Customer Notification
EPA assumes that the customer notification requirement will be handled by technical labor. Section 5 of this analysis assumes a total burden of 13.67 hours of technical labor, supposing that there are two manufacturers (including importers) or processors per chemical3. Given the loaded wage of technical laborers is $82.06, the cost per SNUR is $1,121 [($82.06 per hour)*(13.67 hours per SNUR)].
Summary
Table 9 summarizes the unit costs estimated in this section. Reviewing a SNUR to verify that a chemical is included is estimated to cost $93.55 per SNUR and notifying customers is $1,121 per SNUR. The cost of completing and submitting a SNUN is approximately $23,764. EPA estimates the costs associated with rule familiarization to be $74.63 per company, and CDX registration activities to be $244.15 per company.
Table 9: Summary of Unit Costs
Collection Activity |
Estimated Cost |
Chemical verification (per SNUR) |
$93.55 |
Rule familiarization (per company) |
$74.63 |
CDX registration, electronic signature, and Pay.gov account set-up (per company) |
$244.15 |
SNUN preparation, submission, and recordkeeping* |
$23,767 |
Notifying customers (per SNUR) |
$1,121 |
* Includes $16,000 user fee per SNUN.
Total Burden and Costs to Industry
This section provides estimates of the total burden and costs imposed by the TSCA section 5(a) requirements. These estimates can be divided into five categories: chemical verification, rule familiarization, completing and submitting SNUNs, CDX registration activities, and notifying customers.
The total cost and burden imposed on industry by TSCA section 5(a)(2) requirements can be calculated by multiplying the unit burden and cost estimates by the expected number of SNURs, SNUNs, and firms. As noted above, this analysis assumes that EPA will promulgate 4 SNURs and receive 1 SNUN per year. Table 10 presents the annual burden and cost to industry. EPA estimates the total annual industry burden of existing chemical SNUR action is 168 hours and $30,114.
Table 10: Estimated Existing Chemicals Annual Respondent Burden and Cost
Information Collection Activity |
Total Burden per Activity (hours) |
Total Cost per Activity (2021$) |
Total Number of Units Annually |
Total Annual Burden (hours) |
Total Annual Cost (2021$) |
Chemical verification (per SNUR) |
1.14 |
$93.55 |
4 SNURs |
4.56 |
$374.20 |
Rule familiarization (per firm) |
0.82 |
$74.63 |
4.67 Firms |
3.81 |
$349 |
CDX registration, electronic signature, Pay.gov set-up (per firm) |
2.80 |
$244.15 |
4.67 Firms |
13.08 |
$1,140 |
Preparation, submission, and recordkeeping for SNUN (per report) |
91.68 |
$23,767 |
1 SNUN |
91.68 |
$23,767 |
Notifying customers (per SNUR) |
13.67 |
$1,121 |
4 SNURs |
54.68 |
$4,484 |
Total Existing Chemicals-Related Industry Burden and Cost |
168 |
$30,114 |
|||
Some burden estimate totals may not calculate due to rounding of unit burden estimates
Table 11 presents the total burden and cost to industry over the three-year ICR period. EPA estimates the total annual industry burden of existing chemical SNUR action to be 504 hours and $90,341.
Table 11: Estimated Total Respondent Burden and Cost, Three Year Totals
Information Collection Activity |
Total Burden per Activity (hours) |
Total Cost per Activity (2021$) |
Total Number of Units |
Total Burden (hours) |
Total Cost (2021$) |
Chemical verification (per SNUR) |
1.14 |
$94 |
12 SNURs |
13.68 |
$1,123 |
Rule familiarization (per firm) |
0.82 |
$75 |
14.01 Firms |
11.44 |
$1,045 |
CDX registration, electronic signature, Pay.gov set-up (per firm) |
2.80 |
$244 |
14.01 Firms |
39.23 |
$3,421 |
Preparation, submission, and recordkeeping for SNUN (per report) |
91.68 |
$23,767 |
3 SNUNs |
275.05 |
$71,301 |
Notifying customers (per SNUR) |
13.67 |
$1,121 |
12 SNURs |
164.04 |
$13,452 |
Total Industry Burden and Cost |
504 |
$90,341 |
|||
Some burden estimate totals may not calculate due to rounding of unit burden estimates
Table 12 presents the annual burden by collection activity. Chemical verification is expected to have a total burden of 2.28 hours annually. Notifying consumers is expected to have a total burden of 127.65 hours annually. Companies are expected to incur a total burden of 3.81 hours for rule familiarization, 13.08 hours for CDX registration activities and a total of 89.91 hours for SNUN preparation (including submission and recordkeeping), each year.
Table 12: Annual Information Collection Tally for ICR Reporting Period
Information Collection |
No. of Respondents |
No. of Responses / Respondent |
Responses Subtotal |
Annual Burden Hours per Response |
Annual Burden Hours per Activity |
Per Firm Activities |
|||||
Rule familiarization |
4.67 |
1 |
4.67 |
0.82 |
3.81 |
CDX registration activities |
4.67 |
1 |
4.67 |
2.80 |
13.08 |
|
4.67 |
1 |
4.67 |
0.92 |
4.28 |
|
4.67 |
1 |
4.67 |
1.75 |
8.17 |
|
4.67 |
1 |
4.67 |
0.13 |
0.62 |
Preparation, submission, and recordkeeping for SNUN |
4.67 |
0.21 |
0.98 |
91.68 |
89.91 |
Subtotal |
119.87 |
||||
Per SNUR Activities |
|||||
Chemical verification |
2 |
1 |
2 |
1.14 |
2.28 |
Notifying customers (per SNUR) |
4.67 |
2 |
9.34 |
13.67 |
127.65 |
Subtotal |
129.93 |
||||
Total |
249.80 |
||||
Some burden estimate totals may not calculate due to rounding of unit burden estimates
Table 13 presents the total burden hours for the ICR period, organized by information collection. Chemical verification is expected to result in a total burden of 6.83 hours over the three-year ICR period. Notifying consumers is expected to result in a total burden of 382.94 hours over the three-year ICR period. Companies are expected to incur at total of 11.44 hours in burden for rule familiarization, 39.23 hours for CDX registration activities and a total of 269.74 hours for SNUN preparation, submission and recordkeeping over the three-year ICR period.
Table 13: Total Information Collection Tally for ICR Reporting Period
Information Collection |
No. of Respondents |
No. of Responses / Respondent |
Responses Subtotal |
Total Burden Hours per Response |
Total Burden Hours Subtotal |
|||
Per Firm Activities |
||||||||
Rule familiarization |
14.01 |
1 |
14.01 |
0.82 |
11.44 |
|||
CDX registration activities |
14.01 |
1 |
14.01 |
2.80 |
39.23 |
|||
|
14.01 |
1 |
14.01 |
0.92 |
12.84 |
|||
|
14.01 |
1 |
14.01 |
1.75 |
24.52 |
|||
|
14.01 |
1 |
14.01 |
0.13 |
1.87 |
|||
Preparation, submission, and recordkeeping for SNUN |
14.01 |
0.21 |
2.94 |
91.68 |
269.74 |
|||
Subtotal |
359.64 |
|||||||
Per SNUR Activities |
||||||||
Chemical verification |
6 |
1 |
6 |
1.14 |
6.83 |
|||
Notifying customers (per SNUR) |
14.01 |
2 |
28.02 |
13.67 |
382.94 |
|||
Subtotal |
389.77 |
|||||||
Total |
749.41 |
|||||||
Some burden estimate totals may not calculate due to rounding of unit burden estimates
This analysis covers submissions of PMNs, SNUNs, MCANs, and associated exemption applications: TME, R&D, LVE/LoREX, instant photographic film articles, TERAs, and Tier I and II exemption applications. It also covers submission of NOCs and Bona Fide notifications, and the burden associated with implementation of TSCA section 5(e) and 5(f) consent order restrictions. Since the last renewal of this ICR, the Frank R. Lautenberg Chemical Safety for the 21st Century Act, amending TSCA, was signed into law. Manufacturers (including importers) must complete the activities outlined in Table 14. Burden and cost calculations are based on the assumption that EPA will receive approximately 1,343 TSCA section 5 notices each year, based on the average annual number of notices submitted to EPA between Fiscal Year FY 2015 and FY 2017 (except for polymers, which are based on FY 2016 and FY 2017 data) and adjusted for a decrease in the number of PMNs, SNUNs, and MCANs expected to be submitted by Industry as a result of higher fees (83 FR 52694, October 17, 2018; see Table 14 for average number of annual responses). The fees for LVE, LoREX, TME, TERA, Tier II, and photographic film articles similarly increased, but EPA does not anticipate a decrease in these submissions (due to an anticipated shift away from PMNs toward these exemptions).
Estimating Respondent Burden
The burden to respondents includes: (1) reporting burden for submission of PMNs, SNUNs, MCANs, exemption notices, and implementation of TSCA section 5(e) and 5(f) consent order restrictions such as the use of exposure controls and/or performing toxicity testing; (2) marking any information as confidential and substantiating any claims of confidentiality in a separate statement; (3) recordkeeping burden associated with notice submissions, consent orders, exposure controls and toxicity testing; and (4) burden associated with CDX registration activities.
Burden Associated with Reporting
The total respondent reporting and third-party notification burden associated with this information collection is estimated to total 136,292 hours. This burden estimate is calculated by multiplying the number of each type of notice that EPA expects to receive by the corresponding hours of reporting burden and summing across the notice types.
Number of Notices. The number of notices expected to be submitted annually for each of the submission types is found in Table 14. Expected numbers are mostly based on the average number of submissions EPA received between FY 2019 and FY 2021 rounded up to the nearest whole number. The number of post manufacture reports claiming the polymer exemptions are expected to be submitted for approximately 119 chemicals each year, based on FY 2019-F0Y 2021 data. For R&D exemptions, firms are not required to submit any data to EPA to meet the exemption requirements; however, they must meet certain procedural requirements. EPA maintains the estimate from the previous ICR and assumes that approximately 200 chemicals will meet the requirements for the R&D exemption each year. Additionally, EPA has not received any Instant Photographic Film Articles notifications in many years, but maintains a count of 1 notification per year for analytical purposes. The number of and basis for each type of notice is presented in Table 14.
Table 14: Types of Notices and Average Responses
Type of Notice |
Average Annual Responses |
Basis1 |
PMN |
289 |
FY 2019-FY2021 |
SNUN |
13 |
FY 2019-FY2021 |
MCAN |
14 |
FY 2019-FY2021 |
Exemptions |
||
TME |
0 |
FY 2019-FY2021 |
LVE/LoREX |
305 |
FY 2019-FY2021 |
TERA |
2 |
FY 2019-FY2021 |
Tier I |
4 |
FY 2019-FY2021 |
Tier II |
1 |
FY 2019-FY2021 |
Polymer |
109 |
FY 2019-FY2021 |
Research & Development |
200 |
EPA’s best professional judgement |
Instant Photographic Film Articles |
1 |
EPA’s best professional judgement |
Bona Fide |
183 |
FY 2019-FY2021 |
Section 5(e)/5(f) Order Test |
20 |
FY 2019-FY2021 |
Non-Testing Section 5(e)/5(f) Order |
33 |
FY 2019-FY2021 |
NOC |
169 |
FY 2019-FY2021 |
Total |
1,343 |
|
Burden hours
Burden hours for each type of notice were estimated in previous analyses, beginning with EPA's 1994 Regulatory Impact Analysis of Amendments to Regulations for TSCA section 5 Premanufacture Notifications and 2009 Economic Analysis of the e-PMN final rule.
Based on the number of substantive questions and data elements applicable to post-market submissions, and in keeping with the burden estimates developed in EPA’s 2018 Information Collection Request for Chemical Data Reporting, EPA estimates that CBI substantiation will be associated with an additional 16.245 burden hours for each notice.
EPA also issued an information document entitled “Points to Consider When Preparing TSCA New Chemical Notifications” (Attachment 5) to inform and assist submitters planning to prepare PMNs, SNUNs, and exemption applications for Agency review. EPA estimates that it will take submitters 1.4 hours of managerial and technical burden per notice to read through and familiarize themselves with the document. This burden applies to PMN, SNUN, MCAN, TME, LVE/LoREX, TERA, and Tier I/Tier II notices. EPA also estimates an additional burden of 10 managerial and technical hours per notice for submitters planning to prepare PMNs.
This ICR also considers the time and resources expended by submitters of PMNs to undertake pre-notification consultations as well as post-notification communications with EPA. EPA estimates an additional burden of 5 managerial hours and 10 technical hours per PMN for pre-notification consultation. The 10 technical hours is used for review of prenotice submissions across all relevant branches in OPPT and then in consultation with the PMN submitter. The 5 managerial hours consists of facilitating and assigning appropriate professionals to address issues raised in the prenotice materials. This additional burden is only incurred by some submitters, so this ICR estimates that this burden applies only to 20 percent of PMNs submitted. Similarly, for PMNs in particular, EPA estimates that submitters expend time in engaging in post-notification communications with EPA, consisting of an estimated 97.5 managerial and technical hours.
These changes and the reporting burden estimates associated with them have been incorporated into this ICR supporting statement.
The burden hours for each type of notice are described below:
PMNs: The base hours for respondent reporting burden for a full electronic PMN submission are expected to average 109.33 respondent burden hours. Additionally, EPA estimates an average of 141.17 additional hours per PMN submission, as discussed further in Table 15 below. This yields a total of 250.498 hours per PMN.
SNUNs, LVEs and LoREXs: The hours for respondent reporting burden for SNUNs, LVEs, and LoREX are expected to average 109.33 respondent burden hours per notice. The derivation of the burden hours for these types of notices (exclusive of the burden hours for CBI substantiation).
MCANs: The respondent burden for an MCAN is estimated to average of 305.85 hours.
TERAs: The respondent burden for a TERA is estimated to average 524.85 hours.
Tier I or Tier IIs: The respondent burden for a Tier I or Tier II exemption is estimated to average 127.85 hours.
Polymer Exemptions: The respondent burden for submission of a polymer exemption post-manufacture annual report is estimated to average 18.50 hours.
R&D Exemptions: The respondent burden to meet the procedural requirements for an R&D exemption is estimated to average 18.75 hours.
Instant Photographic Film Article: The minimum amount of information that is required to be contained in an exemption notification for instant photographic film articles is the identity of the manufacturer and the new chemical substance. The burden associated with preparing and submitting this type of exemption notification is 16.75 hours.
Bona Fides: The respondent burden for submission of a bona fide notice is estimated to average 32.75 hours.
TSCA section 5(e) and 5(f) Consent Orders: The respondent burden for consent orders ranges from 41.25 to 167.75 hours per submission, depending on whether or not testing data are submitted. For TSCA section 5(e) consent orders where testing data are submitted, EPA assumes testing is contracted out to a laboratory and burden associated with testing requirements represents the time that personnel from the submitting firm would spend overseeing the testing.
NOCs: The respondent burden for submission of a NOC is estimated to be 16.85 hours.
Table 15: Reporting Burden
Type of Notice |
Average Annual Responses |
Average Reporting Hours per Response |
Average Annual Burden |
|
PMN1,2 |
289 |
250.498 |
72,394 |
|
SNUN1,2 |
13 |
109.328 |
1,421 |
|
MCAN1,2 |
14 |
305.845 |
4,282 |
|
Exemptions: |
||||
LVE/LoREX1 |
305 |
109.328 |
33,345 |
|
TERA1 |
2 |
524.845 |
1,050 |
|
Tier I1 |
4 |
127.845 |
511 |
|
Tier II1 |
1 |
127.845 |
128 |
|
Polymer3 |
109 |
18.495 |
2,016 |
|
Research & Development4 |
200 |
18.745 |
3,749 |
|
Instant Photographic Film Articles4 |
1 |
16.745 |
17 |
|
Bona Fide1 |
183 |
32.745 |
5,992 |
|
Section 5(e) Orders with Triggered Testing1 |
20 |
167.745 |
3,355 |
|
Section 5(e) Orders with Pended Testing/ 5(f) Orders with No Testing1 |
33 |
41.245 |
1,361 |
|
NOC1 |
169 |
16.845 |
||
Total |
1,343 |
|
132,468 |
|
Footnotes |
||||
1 |
Based on the average number of submissions EPA received between FY 2015 and FY 2017, rounded to the nearest whole number. |
|||
2 |
The estimated average number of PMNs, SNUNs, and MCANs are expected to decrease as indicated in in the final rule Fees for the Administration of the Toxic Substances Control Act (2018) (83 FR 52694). |
|||
3 |
Based on the average number of submissions EPA received between FY 2016 and FY 2017, rounded up to the nearest whole number. |
|||
4 |
Based on EPA professional judgment and industry data. |
|||
Development of Burden Estimate Associated with PMNs
The unit submission costs for TSCA section 5 PMNs has been developed over the course of several rulemakings, discussed here in some detail. The PMN burden is also used as the basis for estimating the burden for other notices (PMN, SNUN, TME, and LVE/LoREX), because each of these notices requires the submission of a complete PMN form.
The burden estimate began with EPA’s (1994) Regulatory Impact Analysis of Amendments to Regulations for TSCA section 5 Premanufacture Notifications and EPA’s (2009) Economic Analysis of the Premanufacture Notification Electronic Reporting (e-PMN) final rule.
The PMN submission time estimates are derived from two documents, which we refer to using the following terms:
“The 1994 RIA”: Regulatory Impact Analysis of Amendments to Regulations for TSCA Section 5 Premanufacture Notifications.
“The 2009 EA”: The Economic Analysis of the e-PMN final rule.
The 1994 RIA was the first to estimate the PMN submission time, and gives a range of low/high estimates for each element of the PMN form.4 When these estimates are summed for the entire PMN form, they result in a range of 12 to 14 Clerical hours, 67 to 80 technical hours, and 16 to 20 managerial hours, for a total of 95 to114 total hours per PMN (see Table 16).
The 2009 e-PMN rule EA used the PMN submission time estimates given in the 1994 RIA, with three key modifications. First, it used the average of the ranges presented in the 1994 RIA instead of ranges. Second, it eliminated the average of 13 clerical hours due to the PMN form being submitted electronically rather than in paper form. Third, the e-PMN final rule added two data fields, and the 2009 e-PMN EA adds burden to reflect these new items. These include 10 minutes of additional technical time for the User Fee Payment Identification Number field and 1 minute of additional technical time for the email address field, for a total of 11 new minutes. Taken together, these three changes result in a total of 0 Clerical hours, 73.683 technical hours, and 18 managerial hours, for a total of 91.683 hours per PMN.
As noted in above, the 2016 TSCA amendments also added burden for CBI substantiation. Based on the number of substantive questions and data elements applicable to post-market submissions, and in keeping with the burden estimates developed in EPA’s 2017 Information Collection Request for Chemical Data Reporting, EPA estimates that CBI substantiation will be associated with a total of 16.245 additional burden hours. For Chem ID-based substantiation, this includes 9.625 hours and 4.750 managerial hours. For Non-Chem ID-based substantiation, this includes 1.550 technical hours and 0.320 managerial Hours.
Based on consideration of comments received from the TSCA NCC relevant to burden costs, this ICR now estimates an additional notice preparation burden of 25.67 technical hours and five managerial hours per PMN, for a total of 30.67 hours per PMN.
EPA’s document entitled “Points to Consider When Preparing TSCA New Chemical Notifications” (Attachment 5) was released as part of an emergency amendment to ICR 0574.17 and OMB control number 2070-0012 (83 FR 27768, June 14, 2018) to inform and assist submitters planning to prepare PMNs, SNUNs, and exemption applications for Agency review. EPA estimates that it will take submitters 1.4 hours of managerial and technical burden per notice to read through and familiarize themselves with the document. One-third of this total burden is allocated to technical labor and two-thirds to managerial labor. For PMNs in particular, EPA also estimates that familiarization with the “Points to Consider” document will require an additional 9 hours of technical burden and 1 hour of managerial burden per notice, for a total of 11.4 hours per PMN.
EPA also estimates that 20 percent of PMNs will require time for pre-notification consultation, consisting of 10 hours of technical time and 5 hours of managerial time, or 15 hours total. Converting this number to an overall average applicable to all PMNs, the expected burden for the average PMN is 2 hours of technical time, 1 hours of managerial time, and 3 hours total.
Finally, EPA estimates that PMN submitters will spend time engaging in post-notification communications with EPA, consisting of 90 hours of technical time and 7.5 hours of managerial time, for a total of 97.5 hours (see Appendix)
Burden Associated with Recordkeeping
The total respondent recordkeeping burden associated with this information collection is an estimated 1,882 hours (17). This burden estimate is calculated by multiplying the estimated recordkeeping burden associated with each type of submission, by the estimated number of submissions for each notice and summing across notice types. All recordkeeping burden estimates are taken from the Economic Analysis of the Premanufacture Notification Electronic Reporting Final Rule (EPA, 2009). It should be noted that there is no recordkeeping burden associated with submitting a full electronic PMN SNUN, LVE, or LoREX submission because the burden estimate of 91.683 hours per form already includes the burden associated with recordkeeping.5
Once a respondent presents information in an initial TSCA section 5 submission, the burden for maintaining or updating these records is minimal. The recordkeeping burden for several TSCA section 5 submissions have decreased due to the implementation of the e-PMN rule, which was finalized in 2010. EPA assumes an aggregate annualized recordkeeping burden of one hour for each PMN, SNUN, MCAN, exemption submission, or biotech submission under this new rule. This estimate is based on the recordkeeping burden associated with essential technical requirements, such as records that demonstrate that the first commercial batch of a chemical manufactured for commercial purposes under the exemption met certain eligibility criteria. The recordkeeping burden estimate for section 5(e) testing is17.5 hours. For section 5(e) and 5(f) non-testing the burden estimate is 25 hours.
The overall respondent recordkeeping burden is displayed below in Table16.
Table 16: Respondent Recordkeeping Burden
Type of Notice |
Average Annual Responses |
Hours for Recordkeeping1 |
Average Annual Burden |
|
PMN |
289 |
0 |
0 |
|
SNUN |
13 |
0 |
0 |
|
MCAN |
14 |
1 |
14 |
|
Exemptions: |
||||
LVE/LoREX |
305 |
0 |
0 |
|
TERA |
2 |
1 |
2 |
|
Tier I |
4 |
1 |
4 |
|
Tier II |
1 |
1 |
1 |
|
Polymer |
109 |
3.5 |
381.5 |
|
Research & Development |
200 |
0.5 |
100 |
|
Instant Photographic Film Articles |
1 |
0.25 |
||
Bona Fide |
183 |
1 |
183 |
|
Section 5(e) Orders with Triggered Testing1 |
20 |
17.5 |
350 |
|
Section 5(e) Orders with Pended Testing/ 5(f) Orders with No Testing1 |
33 |
25 |
825 |
|
NOC |
169 |
0.125 |
21.125 |
|
Total |
1,343 |
|
1,882 |
|
Footnote |
||||
1 |
Average recordkeeping hours per response |
|||
Burden Associated with CDX Registration
As part of EPA’s electronic reporting requirements, submitters of TSCA section 5 notices are required to register and submit information electronically with EPA’s Central Data Exchange (CDX) system. EPA estimates that companies submitting TSCA section 5 notices for the first time would incur a one-time burden to complete CDX registration activities, obtain a CDX electronic signature, and set up a Pay.gov ID account. The total burden associated with CDX registration is approximately 2.80 hours per company. EPA assumes each company will register five employees, resulting in a per-employee burden of 0.66 hours. This per-employee burden includes 0.18 hours for CDX registration, 0.35 hours for submitting and then verifying electronic signature agreements, and 0.13 hours for setting up a Pay.gov account. In FY 2019 through FY 2021, there were an average of 2,942 new registrants, of which an average of 2,284 TSCA-Authorized Officials (AO) and 658 TSCA-Support Registrants (SR) were approved via CDX for all TSCA section 5 activities. Multiplying the annual burden by the average number of new registrants results in a total annual burden of 1,942 hours (Table 17).
Table 17: Burden Associated New CDX Registration Activities
CDX Registration Burden (Hours) |
CDX E-Signature Burden (Hours) |
E-Payment
(Pay.gov ID account) |
Total
Burden for CDX Registration Activities |
Annual Number of New CDX Registrants |
Total
Annual Burden for CDX Registration |
0.18 |
0.35 |
0.13 |
0.66 |
2,942 |
1,942 |
Estimating Total Burden
Table 18. below contains the total annual respondent burden for all submissions and activities covered under this ICR. The total annual burden is estimated to be approximately 136,292 hours (132,468 reporting hours + 1,882 recordkeeping hours + 1,942 CDX registration hours). For this ICR, EPA estimated the total number of potential respondents at 234. This estimate is based on the average number of unique entities that submitted a response of any kind during FY 2019- FY 2021. EPA estimates the average number of responses per respondent as the total number of annual responses (minus any CDX registration activity) divided by the total number of respondents, which yields approximately 5.74 responses per respondent (1,343 annual responses ÷ 234 annual respondents).
Table 18: Total Annual Respondent Burden Calculation
Estimating Respondent Cost
Respondents to TSCA section 5 reporting requirements experience costs associated with (1) reporting, (2) recordkeeping, and (3) compliance with exposure controls and testing requirements included in TSCA section 5(e) and 5(f) orders, when EPA takes regulatory action. The respondent costs associated with this information collection are estimated to total $37,324,700 (Table 19).
Respondent costs for all submissions consist of three components: (1) labor costs, calculated by multiplying the estimated burden hours associated with each submission type by the appropriate labor rate; (2) delay costs, estimated as the cost of the delayed receipt of profits by chemical manufactures as a result of the submission review process, and (3) explicit costs, such as user fees or lab testing fees.
In order to estimate total respondent cost associated with TSCA section 5 submissions, an average cost per notice was first calculated for each type of notice. The cost for each notice type was calculated by summing each of the associated cost components, and then multiplying by the expected number of notice submissions. The total industry cost was calculated by summing the costs across notice types. Table 19 outlines the average cost calculations for the various types of notice submissions and presents the total respondent cost estimate.
As noted above, delay costs reflect the cost of the delayed receipt of profits by chemical manufactures as a result of the submission review process. Industry delay costs used to calculate the average cost per submission were computed using the midpoint of the low and high delay cost estimates (1993 dollars) as presented in the “Regulatory Impact Analysis of Amendments to Regulations for TSCA section 5 Premanufacture Notifications” (1994), and inflated to 2016 dollars using the Bureau of Labor Statistics’ Producer Price Index data for the Chemical Manufacturing industry.
The EPA fees (explicit costs) used to calculate the average cost per submission were taken from the 2018 fees rule: Fees for the Administration of the Toxic Substances Control Act (83 FR 82694). This rule increased fees for PMNs, SNUNs, and MCANs from $2,500 to $16,000 and fees for LVE, LoREX, TME, TERA, Tier II, and photographic film articles from $0 to $4,700. Laboratory testing costs were inflated to 2016 dollars using the Bureau of Labor Statistics (BLS) Employment Cost Index (ECI) series for private industry workers in all industries and occupations.
Table 19. Total Respondent Burden and Cost |
||||||||||||
|
Average Annual Responses |
Total Burden (Reporting + Recordkeeping) and Wage Rate by Labor Category |
Labor Costs1 |
Delay Costs2 |
Fees3 |
Avg. Cost Per Notice4 |
Total |
|||||
Managerial |
Technical |
Clerical |
||||||||||
Hours |
Wage ($) |
Hours |
Wage ($) |
Hours |
Wage ($) |
|||||||
PMN |
289 |
38.5 |
$95.41 |
211.998 |
$82.06 |
0 |
$37.33 |
$21,070 |
$34,290 |
$16,000 |
$71,360 |
$20,622,994 |
SNUN |
13 |
24 |
$95.41 |
85.328 |
$82.06 |
0 |
$37.33 |
$9,292 |
$34,290 |
$16,000 |
$59,582 |
$774,564 |
MCAN |
14 |
71 |
$95.41 |
235.345 |
$82.06 |
0.5 |
$37.33 |
$26,105 |
$34,290 |
$16,000 |
$76,395 |
$1,069,533 |
Exemptions: |
|
|||||||||||
TME |
0 |
23 |
$95.41 |
81.345 |
$82.06 |
.5 |
$37.33 |
$8,121 |
0 |
$4,700 |
$12,821 |
$0 |
LVE/LoREX |
305 |
24 |
$95.41 |
85.328 |
$82.06 |
0 |
$37.33 |
$9,292 |
$17,259 |
$4,700 |
$31,251 |
$9,531,511 |
TERA |
2 |
135 |
$95.41 |
390.345 |
$82.06 |
0.5 |
$37.33 |
$44,931 |
- |
$4,700 |
$49,631 |
$99,261 |
Tier I |
4 |
29 |
$95.41 |
99.345 |
$82.06 |
0.5 |
$37.33 |
$10,938 |
- |
- |
$10,938 |
$43,751 |
Tier II |
1 |
29 |
$95.41 |
99.345 |
$82.06 |
0.5 |
$37.33 |
$10,938 |
- |
$4,700 |
$15,638 |
$15,638 |
Polymer |
109 |
6.32 |
$95.41 |
13.425 |
$82.06 |
2.25 |
$37.33 |
$1,789 |
- |
- |
$1,789 |
$194,962 |
R&D |
200 |
5.07 |
$95.41 |
13.925 |
$82.06 |
0.25 |
$37.33 |
$1,636 |
- |
- |
$1,636 |
$327,149 |
Film Articles |
1 |
5.07 |
$95.41 |
11.925 |
$82.06 |
0 |
$37.33 |
$1,462 |
- |
$4,700 |
$6,162 |
$6,162 |
Bona Fide |
183 |
11.07 |
$95.41 |
22.675 |
$82.06 |
0 |
$37.33 |
$2,917 |
- |
- |
$2,917 |
$533,793 |
Section 5(e) Orders with Triggered Testing |
20 |
43.87 |
$95.41 |
132.625 |
$82.06 |
8.75 |
$37.33 |
$15,395 |
- |
$162,644 |
$178,039 |
$3,560,790 |
Section 5(e) Orders with Potentially Useful Information/ 5(f) Orders with No Testing1 |
33 |
24.57 |
$95.41 |
29.175 |
$82.06 |
12.5 |
$37.33 |
$5,205 |
- |
- |
$5,205 |
$171,763 |
NOC |
169 |
5.07 |
$76.67 |
11.8375 |
$77.94 |
0.0625 |
$34.99 |
$1,314 |
- |
- |
$1,314 |
$221,985 |
New CDX Registration |
2942 |
0.132 |
$76.67 |
0.528 |
$77.94 |
0 |
$34.99 |
$51 |
- |
- |
$51 |
$150,844 |
Total |
|
$37,324,700
|
||||||||||
Footnotes |
|
|||||||||||
1 |
Labor costs are calculated by multiplying burden hours by the wage rate for each labor category and summing across labor categories. |
|||||||||||
2 |
Delay costs calculated using the average of low and high estimates from “Regulatory Impact Analysis of Amendments to Regulations for TSCA section 5 Premanufacture Notifications” September 9, 1994 (RIA, 1994), updated to 2020$, using Bureau of Labor Statistics Producer Price Index data for the chemical manufacturing industry. MCANs are assumed to have the same delay costs as PMNs. |
|||||||||||
3 |
User fees charged by EPA, except where noted. |
|||||||||||
4 |
Average cost per notice is the sum of labor costs, delay costs and fees. |
|||||||||||
5 |
This figure is for a representative testing regimen consisting of 835.3110 (ready biodegradability), 850.1010, 850.1075, 850.5400 (aquatic base set), and OECD 407 (28-day repeated dose), based on an analysis of average costs for 277 testing cases and inflated to 2020$ using the Bureau of Labor Statistics (BLS) Employment Cost Index (ECI) series for all workers in private industry (Series ID CIS2010000000000I (B)). |
|||||||||||
6 |
While companies incur costs for control equipment, such costs are outside the scope of this ICR |
|||||||||||
13. Provide an estimate for the total annual cost burden to respondents or recordkeepers resulting from the collection of information. (Do not include the cost of any hour burden already reflected on the burden worksheet).
The cost estimate should be split into two components: (a) a total capital and start-up cost component (annualized over its expected useful life) and (b) a total operation and maintenance and purchase of services component. The estimates should take into account costs associated with generating, maintaining, and disclosing or providing the information. Include descriptions of methods used to estimate major cost factors including system and technology acquisition, expected useful life of capital equipment, the discount rate(s), and the time period over which costs will be incurred. Capital and start-up costs include, among other items, preparations for collecting information such as purchasing computers and software; monitoring, sampling, drilling and testing equipment; and record storage facilities.
If cost estimates are expected to vary widely, agencies should present ranges of cost burdens and explain the reasons for the variance. The cost of purchasing or contracting out information collections services should be a part of this cost burden estimate. In developing cost burden estimates, agencies may consult with a sample of respondents (fewer than 10), utilize the 60-day pre-OMB submission public comment process and use existing economic or regulatory impact analysis associated with the rulemaking containing the information collection, as appropriate.
Generally, estimates should not include purchases of equipment or services, or portions thereof, made: (1) prior to October 1, 1995, (2) to achieve regulatory compliance with requirements not associated with the information collection, (3) for reasons other than to provide information or keep records for the government, or (4) as part of customary and usual business or private practices.
There are no operation and maintenance costs associated with this collection.
14. Provide estimates of annualized cost to the Federal government. Also, provide a description of the method used to estimate cost, which should include quantification of hours, operational expenses (such as equipment, overhead, printing, and support staff), and any other expense that would not have been incurred without this collection of information. Agencies may also aggregate cost estimates from Items 12, 13, and 14 in a single table.
From the Agency’s perspective, the organizing reporting unit is “notices.” A given notice typically submitted by a single firm may pertain to a single or multiple (related) chemical substances, but to the Agency involves similar burden according to notice.
In connection with administering the TSCA section 5 new chemical review and regulatory program, EPA performs the following activities:
Conducts pre-notice meetings with respondents when requested;
Reviews and makes determinations on PMN/MCAN/SNUN submissions;
Analyzes submissions for confidentiality and provides appropriate protection for confidential information;
Files and stores submissions to Agency data systems;
Proposes and implements regulatory action as appropriate
Acknowledges receipt of submissions and notifies respondents of any submission deficiencies;
Provides technical assistance to notice submitters pre- and post-submission; and
Conducts site and record inspections and performs related compliance monitoring functions.
The Agency costs associated with this information collection are estimated to total $26,959,416 (Table 20). Costs to the government include: (1) review of the chemical substance or significant new use under the conditions of use (circumstances under which the chemical is intended, known or reasonably foreseen to be manufactured, processed, distributed in commerce, used, or disposed of), (2) making a final determination on the chemical substance under TSCA section 5(a)(3), and (3) as appropriate, taking regulatory action under TSCA sections 5(e), 5(f) or 5(g).
The provisions of TSCA, as amended, result in additional TSCA section 5 Agency costs that arise primarily from the requirement to review the intended, known or reasonably foreseen activities associated with the chemical, from the requirement to make an affirmative risk determination, and from development of significant new use rules (SNURs) and orders that result from our analysis and findings under TSCA, as amended. Therefore, the Agency used the cost estimates from prior experience and prior versions of this ICR as a starting point and then added estimates for the costs of these additional responsibilities to calculate the Agency’s program costs associated with TSCA section 5 as part of the final rule: Fees for the Administration of the Toxic Substances Control Act (83 Fed. Reg. 52694) (2018).
Agency cost estimates include the costs of processing, reviewing, and making determinations, and the Agency’s costs of taking any regulatory action, such as with a SNUR or a consent order. Costs of reviewing any data that is submitted to the Agency as a result of an order is also included. Agency cost estimates for administering TSCA section 5 also include the costs associated with processing and retaining records related to NOCs. NOC costs also include the cost of registering the chemical with the Chemical Abstracts Service (CAS). These estimates of average cost per notice are based on projected FTE and extramural support needed for these actions divided by the number of notices the Agency assumes will be received each year once fees are in place. Total Agency cost estimates are then calculated as average cost per notice multiplied by the average number of notices estimated in section 6(b) of this ICR.
Table 20: Total Agency Cost Calculation
Type of Notice |
Average Annual Responses |
Avg. Cost Per Notice1 |
Total Agency Cost2 |
PMN3 |
289 |
$49,610 |
$14,337,290 |
SNUN3 |
13 |
$49,610 |
$644,930 |
MCAN3 |
14 |
$49,610 |
$694,540 |
Exemption: |
|
|
|
LVE/LoREX |
305 |
$6,800 |
$2,074,000 |
TERA |
2 |
$6,800 |
$13,600 |
Tier I |
4 |
$6,800 |
$27,200 |
Tier II |
1 |
$6,800 |
$6,800 |
Polymer |
109 |
$6,800 |
$741,200 |
R&D |
200 |
$6,800 |
$1,360,000 |
Film Articles |
1 |
$6,800 |
$6,800 |
Bona Fide4 |
183 |
- |
- |
Section 5(e) Orders with Triggered Testing4 |
20 |
- |
- |
Section 5(e) Orders with Potentially Useful Information/ 5(f) Orders with No Testing4 |
33 |
- |
- |
NOC |
169 |
$7,024 |
$1,187,056 |
New CDX Registration4 |
2942 |
- |
- |
Risk Management5 |
- |
- |
$5,866,000 |
Total |
$26,959,416 |
||
Footnotes |
|
1 |
Values derived from TSCA User Fees Technical Support Document (2018). |
2 |
Total Agency Cost per type of notice calculated as average cost per notice multiplied by average number of annual responses per type of notice. |
3 |
Average of annual responses of PMN/SNUN/MCAN based on historical activity and are expected to decline because fees were increased by the final rule titled Fees for the Administration of the Toxic Substances Control Act (2018) (83 FR. 52694). |
4 |
Agency costs associated with review of Bona Fide notices, 5(e) orders, 5(f) orders, and new CDX registrations is included in the average cost per notice for PMNs, SNUNs, MCANs, Exemptions and NOCs, as well as in the total Agency cost associated with Risk Management activities. |
5 |
Includes costs associated with taking regulatory actions under TSCA sections 5(e), 5(f), or 5(g), as well as reviewing data submitted from consent orders. |
A significant new use rule (SNUR) on an existing chemical substance is the product of a process that is designed to develop the appropriate information-gathering collection for a substance. This process has three major steps: Chemical Referral, Regulatory Selection, and Regulation Development.
Regulatory Development: Prior to the development of a rule, the recommended rulemaking approach must be reviewed by the referring office and approved by the Office Director of OPPT. If the recommendation is approved, then the rulemaking process begins.
An existing chemical SNUR is developed and, if OMB determines that the proposed and/or final rule is a significant regulatory action under Executive Order 12866, the draft rule is submitted to OMB for interagency review under Executive Order 12866 prior to proposing or promulgating the rule in the Federal Register.
EPA’s cost to review and process SNUN submissions is assumed to be represented by its costs for a larger category of similar TSCA section 5 notices that includes SNUNs. On December 21, 2020, EPA proposed revisions to the TSCA Fees Rule under the TSCA, as amended in 2016 by the Frank R. Lautenberg Act Chemical Safety for the 21st Century Act.
In revising the fees, EPA re-estimated its total annual costs for processing, reviewing, and making determinations under TSCA section 5 between fiscal years 2022 and 2024. These estimates were based on EPA payroll data, in contrast to the theoretical estimates put forward in the 2018 TSCA Fees Rule. EPA estimated its direct and indirect costs for reviewing PMNs, SNUNs, Microbial Commercial Activity Notices (MCANs) and exemptions to be $34,713,428 per year during this period. The proposed fee rule of 2020 did not provide a cost estimate per PMN, SNUN, MCAN, or exemption, as was present in the 2018 TSCA fees rule. In order to estimate the cost per PMN, SNUN, MCAN, and exemption in this document, we inflate the value of $41,000 (per PMN, SNUN, and MCAN), and the value of $5,622 (per exemption) by a factor of 1.216This yields an average Agency cost of approximately $49,610 apiece for reviewing and processing PMNs, SNUNs, and MCANs.7 Thus, processing and reviewing any SNUNs submitted due to this SNUR is also expected to cost EPA approximately $49,610. The reviewing of exemptions is expected to cost EPA approximately $6,800.
15. Explain the reasons for any program changes or adjustments reported in hour or cost burden.
This information collection combines the burdens from two previously approved ICRs, EPA ICR No.1188.12 and EPA ICR No. 0574.18. The total combined burden from these two previously approved ICRs was [192,156 + 137 = 192,293 hours]. The total burden requested for this ICR is [136,292 hours], or a decrease of [56,001hours] from the previous total burden. The difference between the current burden request and the previously approved requests is primarily due to the declining number of section 5 submissions for new chemicals. Additionally, minor adjustments were made in EPA’s estimates of the number of respondents and of the burden.
The total combined cost burden from these two currently approved ICRs is $54,542,968 [$26,752+$54,516,216], and the total cost burden requested for this ICR is [$37,354,814]. Once this ICR is approved, it will replace the existing ICRs, resulting in decrease in the estimated total cost burden of $17,188,154 [$54,542,968 - $37,354,814]. The difference between the current cost burden request and the previously approved requests are due to the consolidation of the individual ICRs when calculating the burden, as well as adjustments in EPA’s estimates of the number of respondents and of the burden. In addition to the adjustments listed above, the wage rates and material costs were revised to reflect 2020 dollars for this information collection request.
16. For collections whose results will be published, outline the plans for tabulation and publication. Address any complex analytical techniques that will be used. Provide the time schedule for the entire project, including beginning and ending dates of the collection of information, completion of report, publication dates, and other actions.
Not applicable.
17. If seeking approval to not display the expiration date for OMB approval of the information collection, explain the reasons why display would be inappropriate.
Approved previously, when EPA adopted a fully electronic reporting environment, there are no changes in the activities or reporting tool that warrant reconsideration.
18. Explain each exception to the certification statement identified in “Certification for Paperwork Reduction Act Submissions.”
EPA does not request an exception to the certification of this information collection.
SUPPLEMENTAL INFORMATION
PRA Burden Statement for Collection Instruments:
This collection of information is approved by OMB under the Paperwork Reduction Act, 44 U.S.C. 3501 et seq., and assigned OMB Control No. 2070-0038. Responses to this collection of information are mandatory for certain persons who engage in the covered activities as specified in section 5 of the Toxic Substances Control Act and EPA implementing regulations (40 CFR parts 720 through 725). An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. The public reporting and recordkeeping burden for this collection of information, which varies based on the activity, is estimated to be between 16.97 to 525.85 hours per response. Send comments on the Agency’s need for this information, the accuracy of the provided burden estimates and any suggested methods for minimizing respondent burden to the Director of the Regulatory Support Division, Office of Mission Support, U.S. Environmental Protection Agency (2821T), 1200 Pennsylvania Ave., NW, Washington, D.C. 20460. Include the OMB control number in any correspondence. Do not send the completed form to this address.
To comment on the Agency's need for this information, the accuracy of the provided burden estimates, and any suggested methods for minimizing respondent burden, including the use of automated collection techniques, EPA has established a public docket for this ICR under Docket ID Number EPA-HQ-OPPT-2021-0660, which is available at http://www.regulations.gov. This site can be used to submit or view public comments, access the index listing of the contents of the public docket, and to access those documents in the public docket that are available electronically. When in the system, select “search,” then key in the Docket ID Number identified above.
You can also provide comments to the Office of Information and Regulatory Affairs, Office of Management and Budget via http://www.reginfo.gov/public/do/PRAMain. Find this particular information collection by selecting ‘‘Currently under 30-day Review—Open for Public Comments’’ or by using the search function.
All comments received by EPA will be included in the docket without change, including any personal information provided, unless the comment includes profanity, threats, information claimed to be Confidential Business Information (CBI), or other information whose disclosure is restricted by statute. Do not submit electronically any information you consider to be CBI or other information whose disclosure is restricted by statute.
For additional information on EPA/DC services, instructions for commenting and docket access, visit https://www.epa.gov/dockets.
LIST OF ATTACHMENTS
The attachments listed below can be found in the docket for this ICR or by using the hyperlink that is provided in the list below. The docket for this ICR is accessible electronically through http://www.regulations.gov using Docket ID Number: EPA-HQ-OPPT-2021-0660.
Ref. |
Title |
1. |
Statutory Authority – TSCA Section 5, 15 U.S.C. 2406 |
2. |
Implementing Regulations in Title 40 of the CFR (links to eCFR): – 40 CFR part 720 - Premanufacture Notification – 40 CFR part 721 (Subpart A through D) – Significant New Uses of Chemical Substances – 40 CFR part 723 - Premanufacture Notification Exemptions – 40 CFR part 725 - Reporting Requirements and Review Processes for Microorganisms |
3. |
Instruction Manual for Reporting under the TSCA §5 New Chemicals Program |
4. |
|
5. |
Points to Consider When Preparing TSCA New Chemical Notifications |
6. |
EPA Form 7710-25 (Fillable on-line in CDX PMN) |
7. |
EPA Form 6300-07 (Fillable on-line in CDX BioTech) |
8. |
EPA Form 7710-56 (Fillable on-line in CDX Notice of Commencement) |
9. |
Wage Rates |
10 |
Consultations |
REFERENCES
BLS, 2006. U.S. Bureau of Labor Statistics, Employment Cost Index News Release Text: Employment Cost Index, March 2006 (April 28, 2006), at http://www.bls.gov.
BLS, 2018. U.S. Bureau of Labor Statistics. “Employer Costs for Employee Compensation Supplementary Tables: December 2006 – December 2017” Accessed March 26, 2018. https://www.bls.gov/web/ecec/ecsuphst.pdf
EPA, 1994. U.S. EPA, Office of Pollution Prevention and Toxics Regulatory Impacts Branch. Regulatory Impact Analysis of Amendments to Regulations for TSCA Section 5 Premanufacture Notifications. Washington, DC: U.S. EPA/OPPT/EETD/RIB, September 9, 1994.
EPA, 2002a. U.S. EPA, Office of Pollution Prevention and Toxics, Economic and Policy Analysis Branch. Revised Economic Analysis for the Amended Inventory Update Rule: Final Report. Washington, DC. August 2002.
EPA, 2002b. U.S. EPA, Office of Pollution Prevention and Toxics, Economic and Policy Analysis Branch, Wage Rates for Economic Analysis of the Toxics Release Inventory Program. Washington, DC: June 10, 2002.
EPA, 2007. U.S. EPA Office of Pollution Prevention and Toxics, Supporting Statement for Information Collection Request: Pre-Manufacture Review Reporting and Exemption Requirements for New Chemical Substances and Significant New Use Reporting Requirements for Chemical Substances. OMB Control Number 2070-0012, EPA Tracking Number 0574.13, December 11, 2007.
EPA, 2009a. U.S. EPA, Office of Pollution Prevention and Toxics, Economic and Policy Analysis Branch. Economic Analysis of the Premanufacture Notification Electronic Reporting Final Rule. (EPA-HQ-OPPT-2008-0296). July 13, 2009
EPA, 2009b. U.S. EPA, Office of Pollution Prevention and Toxics ICR No. 118.09. [Information Collection Request for] TSCA Section 5(a)(2) Significant New Use Rules for Existing Chemicals (Renewal). Supporting Statement for a Request for OMB Review under the Paperwork Reduction Act, February 6, 2009.
Appendix. Reference PMN Submission Labor Time Estimates (Hours per Response) |
||||||||||||||||
Activity Description |
1994 RIA Averages |
2009 EA |
2016 TSCA CBI Substantiation |
Additional Notice Preparation |
||||||||||||
Clerical |
Tech. |
Manager |
Total |
Clerical |
Tech. |
Manager |
Total |
Clerical |
Tech. |
Manager |
Total |
Clerical |
Tech. |
Manager |
Total |
|
Average |
Total |
Total |
Total |
|||||||||||||
Costs Based on 1994 RIA |
||||||||||||||||
General Instructions |
2.250 |
1.750 |
3.500 |
7.500 |
0.000 |
1.750 |
3.500 |
5.250 |
0.000 |
1.750 |
3.500 |
5.250 |
0.000 |
1.750 |
3.500 |
5.250 |
Certification |
|
|
0.500 |
0.500 |
|
|
0.500 |
0.500 |
|
|
0.500 |
0.500 |
|
|
0.500 |
0.500 |
I. General Information |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A. Submitter Information |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
B. Chemical Identity Info |
1.750 |
4.500 |
1.000 |
7.250 |
0.000 |
4.500 |
1.000 |
5.500 |
0.000 |
4.500 |
1.000 |
5.500 |
0.000 |
4.500 |
1.000 |
5.500 |
1. Impurities |
|
0.500 |
|
0.500 |
|
0.500 |
|
0.500 |
|
0.500 |
|
0.500 |
|
0.500 |
|
0.500 |
2. Synonyms |
|
0.250 |
|
0.250 |
|
0.250 |
|
0.250 |
|
0.250 |
|
0.250 |
|
0.250 |
|
0.250 |
3. Trade Identification |
|
0.250 |
|
0.250 |
|
0.250 |
|
0.250 |
|
0.250 |
|
0.250 |
|
0.250 |
|
0.250 |
4. Generic Chemical Name |
|
0.500 |
|
0.500 |
|
0.500 |
|
0.500 |
|
0.500 |
|
0.500 |
|
0.500 |
|
0.500 |
5. Byproducts |
|
0.500 |
|
0.500 |
|
0.500 |
|
0.500 |
|
0.500 |
|
0.500 |
|
0.500 |
|
0.500 |
C. Production, Import, and Use Information |
1.500 |
|
2.500 |
4.000 |
0.000 |
|
2.500 |
2.500 |
0.000 |
|
2.500 |
2.500 |
0.000 |
|
2.500 |
2.500 |
1. Production |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
2. Use Information |
|
3.000 |
|
3.000 |
|
3.000 |
|
3.000 |
|
3.000 |
|
3.000 |
|
3.000 |
|
3.000 |
3. Hazard Information |
|
3.500 |
|
3.500 |
|
3.500 |
|
3.500 |
|
3.500 |
|
3.500 |
|
3.500 |
|
3.500 |
II. Human Exposure |
3.000 |
|
6.500 |
9.500 |
0.000 |
|
6.500 |
6.500 |
0.000 |
|
6.500 |
6.500 |
0.000 |
|
6.500 |
6.500 |
A. Ind. Sites Controlled by the Submitter |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1. Operation Description |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
a. Identity |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
b. Type of Operation |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
|
1.000 |
c. Amount and Duration |
|
2.000 |
|
2.000 |
|
2.000 |
|
2.000 |
|
2.000 |
|
2.000 |
|
2.000 |
|
2.000 |
d. Process Description |
|
11.000 |
|
11.000 |
|
11.000 |
|
11.000 |
|
11.000 |
|
11.000 |
|
11.000 |
|
11.000 |
2. Occupational Exposure |
|
13.500 |
|
13.500 |
|
13.500 |
|
13.500 |
|
13.500 |
|
13.500 |
|
13.500 |
|
13.500 |
3. Environmental Release and Disposal |
|
9.500 |
|
9.500 |
|
9.500 |
|
9.500 |
|
9.500 |
|
9.500 |
|
9.500 |
|
9.500 |
B. Industrial Sites Controlled by Others |
2.000 |
11.000 |
2.250 |
15.250 |
0.000 |
11.000 |
2.250 |
13.250 |
0.000 |
11.000 |
2.250 |
13.250 |
0.000 |
11.000 |
2.250 |
13.250 |
1. Operation Description |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2. Worker Exposure and Environmental Release |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
III. List of Attachments |
2.000 |
7.000 |
1.250 |
10.250 |
0.000 |
7.000 |
1.250 |
8.250 |
0.000 |
7.000 |
1.250 |
8.250 |
0.000 |
7.000 |
1.250 |
8.250 |
Physical and Chemical Properties Worksheet |
0.500 |
1.750 |
0.500 |
2.750 |
0.000 |
1.750 |
0.500 |
2.250 |
0.000 |
1.750 |
0.500 |
2.250 |
0.000 |
1.750 |
0.500 |
2.250 |
New Form Fields in 2009 EA |
||||||||||||||||
User Fee Payment Number |
N/A |
N/A |
N/A |
|
0.000 |
0.167 |
0.000 |
0.167 |
0.000 |
0.167 |
0.000 |
0.167 |
0.000 |
0.167 |
0.000 |
0.167 |
Email address |
N/A |
N/A |
N/A |
|
0.000 |
0.017 |
0.000 |
0.017 |
0.000 |
0.017 |
0.000 |
0.017 |
0.000 |
0.017 |
0.000 |
0.017 |
2016 TSCA CBI Substantiation |
||||||||||||||||
6. Chem ID CBI Substantiation Questions |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
0.000 |
9.625 |
4.750 |
14.375 |
0.000 |
9.625 |
4.750 |
14.375 |
III. Non-Chem ID CBI Substantiation Questions |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
0.000 |
1.550 |
0.320 |
1.870 |
0.000 |
1.550 |
0.320 |
1.870 |
Additional Notice Preparation |
||||||||||||||||
Additional Notice Preparation |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
0.000 |
25.670 |
5.000 |
30.670 |
“Points to Consider” Document |
||||||||||||||||
“Points to Consider” Document |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
Pre-Notification Consultation |
||||||||||||||||
Pre-Notification Consultation |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
Post-Notification Communication |
||||||||||||||||
Post-Notification Communication |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
||||||||||||||||
Total |
13.000 |
73.500 |
18.000 |
104.500 |
0.000 |
73.683 |
18.000 |
91.683 |
0.000 |
84.858 |
23.070 |
107.928 |
0.000 |
110.528 |
28.070 |
138.598 |
1 Unless manufacturers (including importers) and processors of chemicals subject to SNURs have either notified recipients of such chemicals and all significant new uses, verified that knowledge of the SNUR has been otherwise acquired by recipients, or verified that recipients are unable to engage in significant new uses, manufacturers (including importers) and processors must file a SNUN.
2 The assumption that there are two manufacturers (including importers) or processors per chemical follows from previous ICRs for these requirements.
3 The assumption that there are two manufacturers (including importers) or processors per chemical follows from previous ICRs for these requirements.
4 These estimates are shown in Table III-3 of the 1994 RIA.
5 As stated in the 1994 Regulatory Impact Analysis of Amendments to Regulations for TSCA section 5 Premanufacture Notifications, the burden estimates for PMN submissions include the time “maintaining a file of the submission” (p. III-13). Because SNUNs, LVE, and LoREX are assumed to have the same burden as PMN submissions, the recordkeeping burden is also included in the reporting burden for these submissions.
6 This factor is derived by dividing the TSCA Section 5 direct and indirect cost estimate provided in the revision ($34,713,428) of 2020, by the original direct and indirect cost estimate of the 2018 TSCA Fees Rule ($28,672,000). The 2020 proposed revision did not have costs broken out on an individual submission basis, so this factor inflates the individual submission estimate by the overall increase between the two documents.
7 This $41,000 review cost is lower than the overall average cost of $55,200 for TSCA section 5 activities that EPA calculated for the 2018 TSCA Fees rule because the $55,200 value includes costs for activities (such as issuing SNURs following a PMN review, and reviewing Notices of Commencement) that are not relevant to SNUNs.
Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | OMB Comments;siu.carolyn@epa.gov |
| File Modified | 0000-00-00 |
| File Created | 2022-07-01 |
© 2026 OMB.report | Privacy Policy