App_V2. FNS Response to NASS Comments
App_V2. FNS Response to NASS Comments.docx
WIC Infant and Toddler Feeding Practices Study-2 (WIC ITFPS-2)Year 9 Extension
App_V2. FNS Response to NASS Comments
OMB: 0584-0580
FNS Response to:
Review of
WIC Infant and Toddler Feeding Practices Study-2 (ITFPS-2)
Year 9 Follow Up (Y9FU)
Food and Nutrition Service (FNS)
To:
David Hancock
NASS OMB Clearance Officer
(202) 690 2388
And the Sampling and Frame Development Section of NASS
Peter Quan -peter.quan@usda.gov
Alison Black - alison.black@usda.gov
Beth Schlein - beth.schlein@usda.gov
Linette Lanclos - linette.lanclos@usda.gov
Bayazid Sarkar - bayazid.sarkar@usda.gov
Duan Franklin - franklin.duan@usda.gov
Thank you for your thoughtful review of the ITFPS-2 Year 9 Follow Up. Please see our responses to your comments and questions below as well as indications of where we edited the ICR documents to reflect your comments.
Reviewer Comments:
The first phase sampling frame process to create 40 strata were not clear.
FNS Response: Added language to App_Z.Base study sampling methods.docx further explain the 40 strata.
After the second phase WIC site selection; the selection of the mothers/caregivers within a ‘window’ were not clear.
FNS Response: Added language to App_Z.Base study sampling methods.docx further explain the recruitment “window”.
It’s not clear how the Y9FU Study subsample will be selected.
FNS Response: The subsample obtained from the beginning of the ITFPS-2 sampling will comprise the Y9FU subsample. No new recruitment will take place for this study and no cases will be dropped.
For the Y9FU Study sample, it instructs mothers/caregivers – who were just at a WIC site – to mail measurement results back to WIC. Why not drop it of at the WIC site or have the WIC site mail it back.2.
FNS Response: Mothers/caregivers will be instructed to send their measurement results back to Westat, the contractor conducting data collection. So far in the study, we’ve had good results with participants being responsible for sending their measurement results back – there is low burden and a high return rate as we provide respondents with a postage-paid return envelope. We want to avoid asking the WIC sites to be responsible for mailing back the participants measurement information to reduce burden on the WIC sites.
Incentives to participate in the study seems like they should work.
FNS Response: We agree and have had positive responses in past years of the ITFPS-2.
Will sampled mothers/caregivers receive a copy of the questionnaire?
FNS Response: We avoid giving mothers/caregivers a copy of the questionnaire so as not to prime them for the dietary recall and other questions. They do receive a copy of materials that may help with the dietary recall such as serving size visuals and a school meal journal to track what their child has eaten away from home.
It is not clear if the first or second interview correspondence timeframe overlap with the weight measurement card correspondence timeframe.
FNS Response: (see page 2 of SSB) The SSA describes that the measurement cards will be sent to mothers/caregivers in the advanced letter (Appendix H1/H2) prior to the first and second interviews. The timing of return of the measurement card will be completely at the convenience of the mother/caregiver which could overlap the first or second interview.
It is not clear if Y9FU Study sample interviews will occur periodically as children turn nine years old; or if there will be one designated survey period.
FNS Response: (see pages 1, 2, and 4 of SSB) The interviews will be around the child’s 9th birthday (we will attempt to interview mothers/caregivers within 14 days prior to the child’s birthday to 28 days after the child’s birthday) and since the children’s birthdays are spread out, the interview period will be from April 2022 to about August 2023.
The sample size may not be effective in conducting the hypothesis tests based on the reduced sample from the base study and the content of the sample. The supplemental sample was designed to ensure participants with certain characteristics were represented. Through attrition that may no longer be the case. While it appears that hazard models of ordinal data with repeated measures have been used to impute data; it is unclear what data would be imputed.
FNS Response: (see pages 7-8 of SSB) There are no plans to impute outcomes of interest. Rather, we will limit imputation to a few select sociodemographic characteristics of participants. The imputed characteristics will be used in conjunction with the unimputed responses to define key sociodemographic subgroups. When defining key subgroups, sample size will be considered in order to ensure sufficient size for comparisons on broad outcomes of interest (e.g., mean vitamin D intake). As with weighting, a carefully designed imputation procedure will reduce bias due to item nonresponse (i.e., missing data for particular survey items among those who respond to a given interview).
Authors noted for generic characteristics with certain given percent prevalence, the projected sample sizes at age 9 years are expected to yield confidence interval half-widths ranging from 2.9 to 11.6 percentage points for 95 percent confidence intervals, and from 2.5 to 9.7 percentage points for 90 percent confidence intervals, and are expected to yield CVs ranging from 3.7 percent to 23.7 percent (for the range of estimates considered). Explicit mathematical formula for sample size calculation should be provided in terms of half-width of given confidence limit, precision, sample size and prevalence.
FNS Response: (see page 8 of SSB)
The mathematical formula used to compute the half-widths of the confidence intervals is the following:
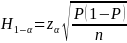
where
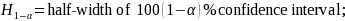

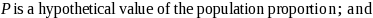
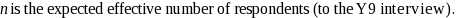
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Fischer, Anthony - NASS |
| File Modified | 0000-00-00 |
| File Created | 2022-04-29 |
© 2026 OMB.report | Privacy Policy