PHEP ORR SSAv5-clean
PHEP ORR SSAv5-clean.docx
Operational Readiness Review 2.0
OMB: 0920-1352
Operational Readiness Review 2.0
An Existing Information Collection in Use Without an OMB Control Number
Supporting Statement A
May 17, 2021
Program Official: Chris Reinold, PhD
Title: Lead, Measurement Evaluation and Translation (MET) Team, DSLR
Phone: 404-639-3805
Email: cor4@cdc.gov
Table of Contents
A.1. Circumstances Making the Collection of Information Necessary 3
A.2. Purpose and Use of the Information Collection 4
A.3. Use of Improved Information Technology and Burden Reduction 4
A.4. Efforts to Identify Duplication and Use of Similar Information 5
A.5. Impact on Small Business or Other Small Entities 5
A.6. Consequences of Collecting the Information Less Frequently 5
A.7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5 5
A.8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency 5
A.9. Explanation of Any Payment or Gift to Respondents 6
A.10. Assurance of Confidentiality Provided to Respondents 6
A.11. Justification for Sensitive Questions 6
A.12. Estimates of Annualized Burden Hours and Costs 7
A.13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers 9
A.14. Annualized Cost to the Government 10
A.15. Explanation for Program Changes or Adjustments 10
A.16. Plans for Tabulation and Publication and Project Time Schedule 10
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate 10
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions 11
LIST OF ATTACHMENTS 12
REFERENCE LIST 14
Goal
of the study: To
collect data from all 62 Public Health Emergency Preparedness (PHEP)
cooperative agreement recipients on their preparedness activities
and planning and operational functions related to all 15 PHEP
Capabilities. Intended
use of the resulting data:
The information collection is intended to assess strengths and
challenges facing preparedness programs across the nation and to
identify opportunities for improvement and further technical
support. The
subpopulation to be studied:
All 62 PHEP recipients, including 50 state health departments, 4
major metropolitan areas (Chicago, Los Angeles County, New York
City, and Washington, D.C.), and 8 US territories and freely
associated states (American
Samoa, Guam, Federated States of Micronesia, Northern Mariana
Islands, Puerto Rico, Republic of Palau, Republic of the Marshall
Islands, and U.S. Virgin Islands) Information
Collection Methods:
Jurisdictions will complete a self-assessment using the new
Operational Readiness Review (ORR) 2.0 platform. The ORR system
guides will provide instruction on how to use the ORR application. How
data will be analyzed:
Data will be analyzed with descriptive statistics for the
development of program reports.
Section A – Justification
A.1. Circumstances Making the Collection of Information Necessary
Public health emergency preparedness and response capacity continues to be tested at national, state, local, tribal, and territorial levels. There are ongoing risks related to chemical, biological, radiological, cyber and nuclear incidents, as well as pandemic outbreaks and natural disasters, which emphasize the importance of all-hazards public health preparedness and response at all levels of government. The Centers for Disease Control and Prevention’s (CDC) Division of State and Local Readiness (DSLR) administers the Public Health Emergency Preparedness (PHEP) cooperative agreement to help address these challenges. The PHEP program supports preparedness activities and provides technical assistance to 62 state, local, and territorial jurisdictions including 4 major metropolitan areas: Chicago, Los Angeles County, New York City, and Washington, D.C. (CDC, 2020).
The PHEP program is the largest cooperative agreement at CDC and a critical source of funding for state, local, and territorial health departments to build and strengthen their ability to respond and recover from public health emergencies. The 2019-2024 funding opportunity (CDC-RFA-TP19-1901) (Attachment 4) provides fiscal resources to state, local, and territorial public health agencies to advance their ability to demonstrate response readiness by the end of the period of performance (CDC, 2019). The PHEP cooperative agreement and associated data collection is authorized under Section 319C-1 of the Public Health Service (PHS) Act (47 USC § 247d-3a), as amended (Attachment 1).
This is an existing information collection in use without an OMB Control Number titled, “Operational Readiness Review 2.0” for which DSLR is requesting a three-year Paperwork Reduction Act (PRA) clearance.
Under the current five-year cooperative agreement, DSLR will collect information related to the 62 recipients’ activities across 15 public health preparedness and response capabilities, which serve as national standards for public health preparedness planning (CDC, 2018). The capabilities cover the following six domains: community resilience, incident management, information management, countermeasures and mitigation, surge management, and biosurveillance (CDC, 2018). These capability standards serve as a framework for state, local, tribal, and territorial preparedness programs to plan, operationalize, and evaluate their ability to respond to and recover from emergencies (CDC, 2018). Collecting data on activities across the 15 capabilities will allow DSLR to monitor recipients’ progress toward program goals and objectives.
A.2. Purpose and Use of the Information Collection
CDC’s Operational Readiness Review (ORR) is a rigorous, evidence-based assessment used to evaluate PHEP recipients’ planning and operational functions (CDC, 2018). The previous version of the ORR was used to measure a jurisdiction’s ability to execute a large emergency response requiring medical countermeasure (MCM) distribution and dispensing. The purpose of this information collection is to expand the ORR to include all 15 preparedness and response capabilities so DSLR can better monitor program impact and support program analysis and improvement across all hazards impacting public health.
The PHEP ORR 2.0 will have three modules: descriptive, planning, and operational, which will allow DSLR to analyze the data for the development of descriptive statistics and to monitor the progress of each recipient toward performance goals. It is intended to promote accountability, track recipient progress toward achieving desired programmatic outcomes, and provide key insights into recipients’ planning and operational strengths, areas of improvement, and technical assistance needs. This expansion is necessary to fully evaluate whether jurisdictions are prepared to respond to and recover from a public health emergency. The new online system will allow DSLR to collect valuable ORR data and facilitate dialog between DSLR reviewers and PHEP recipients.
The Descriptive Module includes the following: Jurisdictional Structure Sheet (Attachment 6), Critical Contact Sheet (Attachment 7), Jurisdictional Data Sheet (Attachment 8), Partner Planning Sheet (Attachment 9), and Workforce Development and Training (Attachment 10).
The Planning Module includes the following: The 15 Public Health Emergency Preparedness and Response Capabilities serve as national standards for public health preparedness planning. They are as follows: 1—Community Preparedness (Attachment 11), 2—Community Recovery (Attachment 12), 3—Emergency Operations Coordination (Attachment 13), 4—Emergency Public Information and Warning (Attachment 14), 5—Fatality Management (Attachment 15), 6—Information Sharing (Attachment 16), 7—Mass Care (Attachment 17), 8—Medical Countermeasure Dispensing and Administration (Attachment 18), 9—Medical Materiel Management and Distribution (Attachment 19), 10—Medical Surge (Attachment 20), 11—Nonpharmaceutical Intervention (Attachment 21), 12—Public Health Laboratory Testing (Attachment 22), 13—Public Health Surveillance and Epidemiological Investigation (Attachment 23), 14—Responder Safety and Health (Attachment 24), and 15—Volunteer Management(Attachment 25).
Capabilities 1 -15, by Domain |
||||||||
Capability / Domain |
1 |
2 |
3 |
4 |
5 |
6 |
||
|
Community Resilience |
Incident Management |
Information Management |
Counter-measures and Mitigation |
Surge Manage-ment |
Bio-surveillance |
||
|
x |
|
|
|
|
|
||
|
x |
|
|
|
|
|
||
|
|
x |
|
|
|
|
||
|
|
|
x |
|
|
|
||
|
|
|
|
|
x |
|
||
|
|
|
x |
|
|
|
||
|
|
|
|
|
x |
|
||
|
|
|
|
x |
|
|
||
|
|
|
|
x |
|
|
||
|
|
|
|
|
x |
|
||
|
|
|
|
x |
|
|
||
|
|
|
|
|
|
x |
||
|
|
|
|
|
|
x |
||
|
|
|
|
x |
|
|
||
|
|
|
|
|
x |
|
||
The Operational Module includes the following: Ops 1 (Attachment 26), Ops 2 (Attachment 27). Facility Setup Drill (Attachment 28), Site Activation Drill (Attachment 29), Staff Notification and Assembly Drill (Attachment 30), Dispensing Throughput Drill (Attachment 31), Tabletop Exercise (TTX) (Attachment 32), Partner role (Par1) (Attachment 33), AFN exercise accommodations or actions (Par2) (Attachment 34)- Joint exercise with emergency management and HCC (Par3) (Attachment 35), Vaccination of Critical Workforce (FE, FSE, or incident) (Attachment 36), Vaccination of Critical Workforce (POD/ DVC setup) (Attachment 37), Vaccination of Critical Workforce (Immunization information system) (Attachment 38), Five-year Distribution Full-Scale Exercise (Attachment 39), Five-year Pan-flu Full-Scale Exercise or Incident (Attachment 40), Five-year Dispensing Full-Scale Exercise or Incident (Attachment 41), and Five-year Dispensing Full-Scale Exercise for each POD exercised (Attachment 42).
PHEP Logic Model
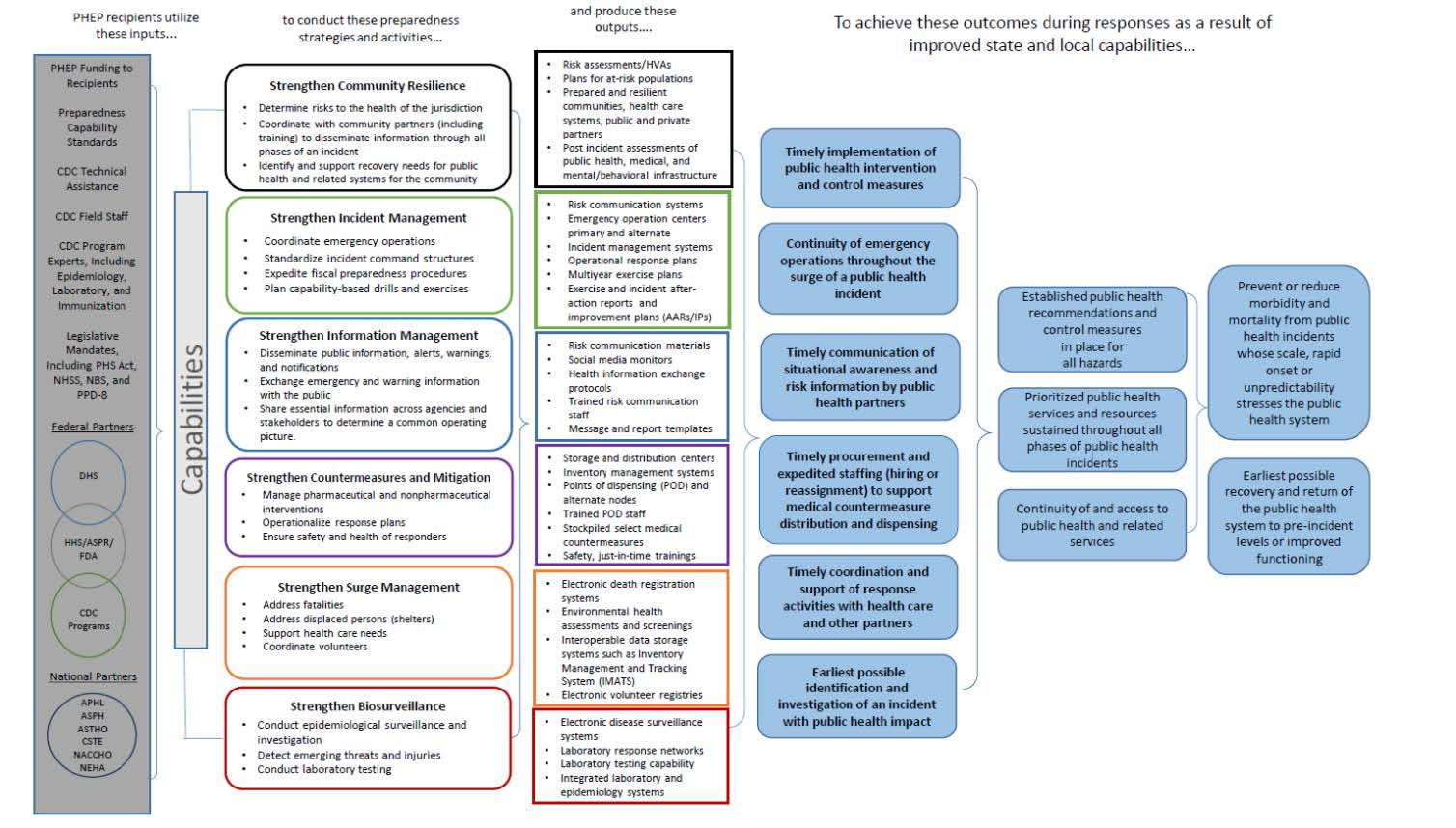
A.3. Use of Improved Information Technology and Burden Reduction
This information collection is intended to be electronic in nature. With the exception of the eight territories and freely associated states, who may have the option to complete fillable PDFs depending on their accessibility, the recipients will be required to submit all responses via the ORR online system. The use of electronic reporting will reduce recipient burden, as the information can be submitted directly into the online system. Direct entry for 54 out of 62 jurisdictions will also reduce the burden associated with transcription and reduce the need for separate data entry at DSLR.
The estimated time for each entry is shown in the Burden Hours Table. It is expected that recipients will spend a total of 3,055 hours in the first year of implementation; in subsequent years this burden is reduced significantly to a total of 1,235 hours annually for completion of the information collection instruments. CDC will submit a Change Request to adjust the burden estimates to reflect the burden of ongoing maintenance instead of the full burden of the initial year. Approximately 90% of the requested burden hours will be spent reporting electronically in the ORR system.
A.4. Efforts to Identify Duplication and Use of Similar Information
There will be no duplication of program performance monitoring data in the ORR system and no duplication of data between systems. This data is intended to help DSLR monitor program improvement and impact. No similar information collection exists.
A.5. Impact on Small Businesses or Other Small Entities
The information collection will require the participation of state and local health departments. Per federal regulations, health departments are not considered small businesses (Attachment 3). Therefore, no small businesses will be involved in this information collection.
A.6. Consequences of Collecting the Information Less Frequently
The frequency of information collection varies by instrument. Recipients are required to complete an initial information submission at the beginning of the information collection period. After initial reporting, information collection instruments may require updates annually, biannually, every three or five years, or as changes occur. This frequency is necessary for DSLR to accurately track program activities and progress. If DSLR collected this data less frequently, the division would not be able to accurately track recipients’ preparedness for public health emergencies, provide necessary technical assistance, identify best practices, or pinpoint areas of improvement. While the initial reporting requires a high time burden, updates will not require as high a burden of time or effort.
A.7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
This request fully complies with the regulation 5 CFR 1320.5.
A.8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A. A 60-day Federal Register Notice was published in Federal Register on June 23, 2020, vol. 85, no. 121, pp. 37659-37661 (Attachment 2). CDC did not receive public comments on this notice.
B. DSLR works with several partner organizations and federal partners outside of CDC. For this information collection, DSLR solicited input and representation from the following sources: prospective state and local users; division subject matter experts (SMEs); external partners, including the Association of Public Health Laboratories (APHL), the Office of the Assistant Secretary for Preparedness and Response (ASPR); the Association of State and Territorial Health Officials (ASTHO), the Council of State and Territorial Epidemiologists (CSTE), and the National Association of County and City Health Officials (NACCHO); and other centers within CDC, including the National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), the National Institute for Occupational Safety and Health (NIOSH), the National Center for Environmental Health (NCEH), and the National Center for Immunization and Respiratory Diseases (NCIRD).
A.9 Explanation of Any Payment or Gift to Respondents
No payment or gift to PHEP recipients will be offered. The recipients are required to submit reports and performance measures as part of the PHEP cooperative agreement.
A.10. Protection of the Privacy and Confidentiality of Information Provided by Respondents
The Center for Preparedness and Response has determined that the Privacy Act does not apply to this information collection. The data collection does not involve collection of sensitive or identifiable personal information. Although contact information is obtained for each funded recipient (state or local jurisdiction), the contact person provides information about the organization, not personal information. No system of records will be created under the Privacy Act.
Information will be kept secure on the HHS Secure Access Management Services (SAMS) system and will only be accessible to project team members. No IIF will be distributed.
Respondents will be notified regarding the provision of IIF through the guidance document that will accompany the PHEP ORR. The guidance will inform recipients that the critical contact sheet will collect IIF and that CDC will keep the information that recipients provide private and secure to the extent permitted by law.
A.11. Institutional Review Board (IRB) and Justification for Sensitive Questions
The information collection is conducted under a nonresearch cooperative agreement (CDC-RFA-TP19-1901) (Attachment 4). The CDC Human Subjects Contact has determined that the information collection is necessary for program management and is not classified as human subjects research. IRB approval is not required (Attachment 5). This information collection does not include sensitive questions.
A.12. Estimates of Annualized Burden Hours and Costs
The Descriptive Module [Attachments 6-10] is completed by all PHEP awardees. It includes the following: Jurisdictional Structure Sheet (Attachment 6), Critical Contact Sheet (Attachment 7), Jurisdictional Data Sheet (Attachment 8), Partner Planning Sheet (Attachment 9), and Workforce Development and Training (Attachment 10). The total estimated annualized burden is 1,013 hours.
The Planning Module [Attachments 11-25] is completed by all PHEP awardees. It includes the following: The 15 Public Health Emergency Preparedness and Response Capabilities serve as national standards for public health preparedness planning. They are as follows: 1—Community Preparedness (Attachment 11), 2—Community Recovery (Attachment 12), 3—Emergency Operations Coordination (Attachment 13), 4—Emergency Public Information and Warning (Attachment 14), 5—Fatality Management (Attachment 15), 6— nformation Sharing (Attachment 16), 7—Mass Care (Attachment 17), 8—Medical Countermeasure Dispensing and Administration (Attachment 18), 9—Medical Materiel Management and Distribution (Attachment 19), 10—Medical Surge (Attachment 20), 11— Nonpharmaceutical Intervention (Attachment 21), 12—Public Health Laboratory Testing (Attachment 22), 13—Public Health Surveillance and Epidemiological Investigation (Attachment 23), 14—Responder Safety and Health (Attachment 24), and 15—Volunteer Management (Attachment 25). The total estimated annualized burden is 1,706 hours.
The Operational Module [Attachments 26, 27, 32-40] is completed by all PHEP awardees. It includes the following: Ops 1 (Attachment 26), Ops 2 (Attachment 27). Tabletop Exercise (TTX) (Attachment 32), Partner role (Par1) (Attachment 33), AFN exercise accommodations or actions (Par2) (Attachment 34)- Joint exercise with emergency management and HCC (Par3) (Attachment 35), Vaccination of Critical Workforce (FE, FSE, or incident) (Attachment 36), Vaccination of Critical Workforce (POD/ DVC setup) (Attachment 37), Vaccination of Critical Workforce (Immunization information system) (Attachment 38), Five-year Distribution Full-Scale Exercise (Attachment 39), and Five-year Pan-flu Full-Scale Exercise or Incident (Attachment 40). The total estimated annualized burden is 322 hours.
The Operational Module [Attachments 28, 29, 30, 31, 41, 42] also includes items that are only reported by the 4 major metropolitan areas. These include the Facility Setup Drill (Attachment 28), Site Activation Drill (Attachment 29), Staff Notification and Assembly Drill (Attachment 30), Dispensing Throughput Drill (Attachment 31), Five-year Dispensing Full-Scale Exercise or Incident (Attachment 41), and Five-year Dispensing Full-Scale Exercise for each POD exercised (Attachment 42). The total estimated annualized burden is 14 hours.
Table A12.1 Estimated Annualized Burden Hours
Type of Respondent |
Form Name |
No. of Respondents |
No. of Responses per Respondent |
Avg. Burden per Response (in hrs.) |
Total Burden (in hrs.) |
All PHEP Awardees: State, local, territorial, and metropolitan area jurisdictions |
Jurisdictional structure sheet |
62 |
1 |
3 |
186 |
Critical contact sheet (CCS) |
62 |
1 |
80/60 |
83 |
|
Jurisdictional data sheet (JDS) |
62 |
1 |
2.5 |
155 |
|
Partner planning sheet |
62 |
1 |
8 |
496 |
|
Workforce development and training |
62 |
1 |
1.5 |
93 |
|
Subtotal, Module 1 |
62 |
|
|
1,013 |
|
All PHEP Awardees: State, local, territorial, and metropolitan area jurisdictions |
Capability 1 |
62 |
1 |
1 |
62 |
Capability 2 |
62 |
1 |
1 |
62 |
|
Capability 3 |
62 |
1 |
2 |
124 |
|
Capability 4 |
62 |
1 |
1.5 |
93 |
|
Capability 5 |
62 |
1 |
2.5 |
155 |
|
Capability 6 |
62 |
1 |
1 |
62 |
|
Capability 7 |
62 |
1 |
2 |
124 |
|
Capability 8 |
62 |
1 |
3 |
186 |
|
Capability 9 |
62 |
1 |
195/60 |
202 |
|
Capability 10 |
62 |
1 |
2 |
124 |
|
Capability 11 |
62 |
1 |
1.5 |
93 |
|
Capability 12 |
62 |
1 |
1.5 |
93 |
|
Capability 13 |
62 |
1 |
2.5 |
155 |
|
Capability 14 |
62 |
1 |
1.5 |
93 |
|
Capability 15 |
62 |
1 |
75/60 |
78 |
|
Subtotal, Module 2 |
62 |
|
|
1,706 |
|
All PHEP Awardees: State, local, territorial, and metropolitan area jurisdictions |
Ops 1 |
62 |
3 |
20/60 |
62 |
Ops 2 |
62 |
3 |
15/60 |
47 |
|
Tabletop exercise (TTX) |
62 |
1 |
1.5 |
93 |
|
Partner role (Par1) |
62 |
1 |
15/60 |
16 |
|
Access and functional needs exercise accommodations or actions (Par2) |
62 |
1 |
0.5 |
31 |
|
Joint exercise with emergency management and health care coalitions (Par3) |
62 |
1 |
6/60 |
6 |
|
Vaccination of critical workforce (functional exercise, full-scale exercise, or incident) |
62 |
1 |
12/60 |
12 |
|
Vaccination of critical workforce (point of dispensing/ dispensing/vaccination clinic setup) |
62 |
1 |
12/60 |
12 |
|
Vaccination of critical workforce (immunization information system) |
62 |
1 |
12/60 |
12 |
|
Five-year distribution FSE OR five-year pandemic influenza full-scale exercise |
62 |
1 |
0.5 |
31 |
|
Subtotal, Module 3 |
62 |
|
|
322 |
|
PHEP Awardees: Major Metropolitan Area Jurisdictions |
Facility setup drill |
4 |
1 |
45/60 |
3 |
Site activation drill |
4 |
1 |
1 |
4 |
|
Staff notification and assembly drill |
4 |
1 |
1 |
4 |
|
Dispensing throughput drill |
4 |
1 |
12/60 |
1 |
|
Five-year dispensing full-scale exercise or incident |
4 |
1 |
6/60 |
1 |
|
Five-year dispensing full-scale exercise for each point of dispensing site exercised |
4 |
1 |
6/60 |
1 |
|
Subtotal, Module 3, Additional reporting |
4 |
|
|
14 |
|
|
Grand Total |
|
|
|
3,055 |
*Required for four directly funded localities, not state recipients
B. The total annualized cost burden requested is $96,843.50. Estimates of the annualized cost burden to respondents for the collection of information are based on the Department of Labor Bureau of Labor Statistics “May 2018 National Occupational Employment and Wage Estimates, United States” (see www.bls.gov/oes/current/oes_nat.htm#19-0000). The occupation title and hourly wage of employees who will complete the information collection varies by jurisdiction. For the purpose of this cost burden analysis, a proxy occupation was used to represent the average employee involved in the information collection. The mean hourly wage for PHEP cooperative agreement recipients, classified as Emergency Management Directors, is $31.70.
Table A.12.2. Hourly Wage Estimates for PHEP Cooperative Agreement Recipients
Occupation Code |
Occupation Title |
Mean Hourly Wage |
11-9161 |
Emergency Management Directors |
$31.72 |
19-1041 |
Epidemiologists |
$31.69 |
Table A.12.3. Estimated Annualized Burden Costs
Type of Respondent |
Form Name |
Total Burden (in hrs.) |
Hourly Wage Rate |
Total Respondent Costs |
All PHEP Awardees: State, local, territorial, and metropolitan area jurisdictions |
Jurisdictional structure sheet |
186 |
$31.70 |
$5,896.20 |
Critical contact sheet (CCS) |
83 |
$31.70 |
$2,631.10 |
|
Jurisdictional data sheet (JDS) |
155 |
$31.70 |
$4,913.50 |
|
Partner planning sheet |
496 |
$31.70 |
$15,723.20 |
|
Workforce development and training |
93 |
$31.70 |
$2,948.10 |
|
Subtotal, Module 1 |
1,013 |
|
$32,112.10 |
|
All PHEP Awardees: State, local, territorial, and metropolitan area jurisdictions |
Capability 1 |
62 |
$31.70 |
$1,965.40 |
Capability 2 |
62 |
$31.70 |
$1,965.40 |
|
Capability 3 |
124 |
$31.70 |
$3,930.80 |
|
Capability 4 |
93 |
$31.70 |
$2,948.10 |
|
Capability 5 |
155 |
$31.70 |
$4,913.50 |
|
Capability 6 |
62 |
$31.70 |
$1,965.40 |
|
Capability 7 |
124 |
$31.70 |
$3,930.80 |
|
Capability 8 |
186 |
$31.70 |
$5,896.20 |
|
Capability 9 |
202 |
$31.70 |
$6,403.40 |
|
Capability 10 |
124 |
$31.70 |
$3,930.80 |
|
Capability 11 |
93 |
$31.70 |
$2,948.10 |
|
Capability 12 |
93 |
$31.70 |
$2,948.10 |
|
Capability 13 |
155 |
$31.70 |
$4,913.50 |
|
Capability 14 |
93 |
$31.70 |
$2,948.10 |
|
Capability 15 |
78 |
$31.70 |
$2,472.60 |
|
Subtotal, Module 2 |
1,706 |
|
$54,080.20 |
|
All PHEP Awardees: State, local, territorial, and metropolitan area jurisdictions |
Ops 1 |
62 |
$31.70 |
$1,965.40 |
Ops 2 |
47 |
$31.70 |
$1,489.90 |
|
Tabletop exercise (TTX) |
93 |
$31.70 |
$2,948.10 |
|
Partner role (Par1) |
16 |
$31.70 |
$507.20 |
|
Access and functional needs exercise accommodations or actions (Par2) |
31 |
$31.70 |
$982.70 |
|
Joint exercise with emergency management and health care coalitions (Par3) |
6 |
$31.70 |
$190.20 |
|
Vaccination of critical workforce (functional exercise, full-scale exercise, or incident) |
12 |
$31.70 |
$380.40 |
|
Vaccination of critical workforce (point of dispensing/ dispensing/vaccination clinic setup) |
12 |
$31.70 |
$380.40 |
|
Vaccination of critical workforce (immunization information system) |
12 |
$31.70 |
$380.40 |
|
Five-year distribution FSE OR five-year pandemic influenza full-scale exercise |
31 |
$31.70 |
$982.70 |
|
Subtotal, Module 3 |
322 |
|
$10,207.40 |
|
PHEP Awardees: Major Metropolitan Area Jurisdictions |
Facility setup drill |
3 |
$31.70 |
$95.10 |
Site activation drill |
4 |
$31.70 |
$126.80 |
|
Staff notification and assembly drill |
4 |
$31.70 |
$126.80 |
|
Dispensing throughput drill |
1 |
$31.70 |
$31.70 |
|
Five-year dispensing full-scale exercise or incident |
1 |
$31.70 |
$31.70 |
|
Five-year dispensing full-scale exercise for each point of dispensing site exercised |
1 |
$31.70 |
$31.70 |
|
Subtotal, Module 3, Additional reporting |
14 |
|
$443.80 |
|
|
Grand Total |
3,055 |
|
$96,843.50 |
A.13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
There will be no direct costs to the respondents other than their time to participate in each information collection. Capital and start-up costs will not be required for this information collection.
A.14. Annualized Cost to the Government
The total annualized cost to the federal government is $612,340,378 based on the costs itemized below.
The project funding for the five-year cooperative agreement is $3,061,250,000. This is an annualized cost of $612,250,000.
The total cost of development of the PHEP ORR information technology system is $451,890. This is an annualized cost of $90,378 over the five-year budget period.
Table A.14.1. Cost of Development of the Online ORR System
Aspect of Project |
Employment Level |
Hours Worked |
Hourly Wage Rate |
Total Cost |
Development of content |
GS-14 |
1,200 hours |
$65.56 |
$78,672 |
GS-13 |
2,300 hours |
$50.83 |
$116,909 |
|
Development of IT system to collect data |
GS-14 |
1,100 hours |
$65.56 |
$72,116 |
GS-13 |
2,500 hours |
$50.83 |
$127,075 |
|
GS-12 |
415 hours |
$44.21 |
$18,347 |
|
Contract hours |
Deloitte |
1,132 hours |
$34.25 |
$38,771 |
Total |
|
$451,890 |
||
A.15. Explanation for Program Changes or Adjustments
This is a new information collection.
A.16. Plans for Tabulation and Publication and Project Time Schedule
Table 16.A.1. Project Time Schedule
Activity |
Schedule |
Date of Awards |
July 2019, July 2020, July 2021, July 2022, July 2023 |
PHEP ORR Guidance Completed |
March 2021 |
PRA Clearance Obtained |
May 2021 |
ORR Online System Completed |
July 2021 |
Information Collection |
2021, 2022, 2023, 2024 |
Reporting |
2022, 2023, 2024 |
A national ORR report will be developed based on the data collected through the system. This will present aggregated national data and specific recipient data.
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate
The display of OMB expiration date is appropriate.
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification.
LIST OF ATTACHMENTS AND APPENDICES
Attachment 1 – Authorizing Legislation
Attachment 2 – 60-day Federal Register Notice
Attachment 3 – Code of Federal Regulations Title 13 Business Credit and Assistance
Attachment 4 – PHEP Cooperative Agreement Funding Opportunity Announcement (CDC-RFA-TP19-1901)
Attachment 5 – Non-Research Determination
Attachment 6 - Jurisdictional Structure Sheet
Attachment 7 – Critical Contact Sheet
Attachment 8 – Jurisdictional Data Sheet
Attachment 9 - Partner Planning Sheet
Attachment 10- Workforce Development and Training
Attachment 11 – Capability 1
Attachment 12 – Capability 2
Attachment 13 – Capability 3
Attachment 14 – Capability 4
Attachment 15 – Capability 5
Attachment 16 – Capability 6
Attachment 17 – Capability 7
Attachment 18 – Capability 8
Attachment 19 – Capability 9
Attachment 20 – Capability 10
Attachment 21 – Capability 11
Attachment 22 - Capability 12
Attachment 23 – Capability 13
Attachment 24 – Capability 14
Attachment 25 – Capability 15
Attachment 26 – Ops 1
Attachment 27- Ops 2
Attachment 28- Facility Setup Drill
Attachment 29- Site Activation Drill
Attachment 30- Staff Notification and Assembly Drill
Attachment 31- Dispensing Throughput Drill
Attachment 32- Tabletop Exercise (TTX)
Attachment 33- Partner role (Par1)
Attachment 34- AFN exercise accommodations or actions (Par2)
Attachment 35- Joint exercise with emergency management and HCC (Par3)
Attachment 36- Vaccination of Critical Workforce (FE, FSE, or incident)
Attachment 37- Vaccination of Critical Workforce (POD/ DVC setup)
Attachment 38 - Vaccination of Critical Workforce (Immunization information system)
Attachment 39 – Five-year Distribution Full-Scale Exercise
Attachment 40 - Five-year Pan-flu Full-Scale Exercise or Incident
Attachment 41 - Five-year Dispensing Full-Scale Exercise or Incident
Attachment 42 - Five-year Dispensing Full-Scale Exercise for each POD exercised
Appendix 1 – PHEP ORR 2.0 Guidance
REFERENCE LIST
Centers for Disease Control and Prevention. (March 27, 2020). Public Health Emergency Preparedness (PHEP) Cooperative Agreement. https://www.cdc.gov/cpr/readiness/phep.htm
Centers for Disease Control and Prevention. (October 15, 2018). Operational Readiness Review. https://www.cdc.gov/cpr/readiness/orr.html
Centers for Disease Control and Prevention, Center for Preparedness and Response. (October 2018). Public Health Emergency Preparedness and Response Capabilities: National Standards for State, Local, Tribal, and Territorial Public Health. https://www.cdc.gov/cpr/readiness/00_docs/CDC_PreparednesResponseCapabilities_October2018_Final_508.pdf
Centers for Disease Control and Prevention, Office of Public Health Preparedness and Response. (2019). Public Health Emergency Preparedness Cooperative Agreement, CDC-RFA-TP19-1901. https://esp.cdc.gov/sites/ophpr/DSLR/PSB/PODM/Resources/CDC-RFA-TP19-1901%20NOFO.pdf
Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Sanborn, Molly (CDC/DDPHSIS/CPR/DSLR) |
| File Modified | 0000-00-00 |
| File Created | 2022-03-03 |
© 2026 OMB.report | Privacy Policy