NLRAD ELR SAHO CX Survey - SLT
Generic Clearance for the Collection of Qualitative Feedback on Agency Service Delivery - APHIS
0377 2021 NLRAD ELR SAHO Survey (20210802)
NLRAD ELR SAHO CX Survey - SLT
OMB: 0579-0377
According to the Paperwork Reduction Act of 1995, an agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a valid OMB control number. The OMB control numbers for this information collection is 0579-0377. The time required to complete this information collection is estimated to average 20 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. |
OMB APPROVED 0579-0377 |
NLRAD Electronic Lab Reporting Project
Survey for State Animal Health Officials
A USDA APHS Veterinary Services (VS) project is underway with the goal to have Laboratories report test findings electronically in order to satisfy NLRAD standards. We would like to gather your input about what you would like from this system. If you are willing to provide input, please return this survey by August ….
The NLRAD Standards will require information to be collected for two categories: Notifiable Diseases and Conditions, and Monitored Diseases (Figure 1). Within the Notifiable Diseases and Conditions category, three sub-categories of diseases have been identified: emergency incidents (such as foreign animal disease investigations), emerging disease incidents, and regulated disease incidents.
Figure 1. NLRAD disease list structure.
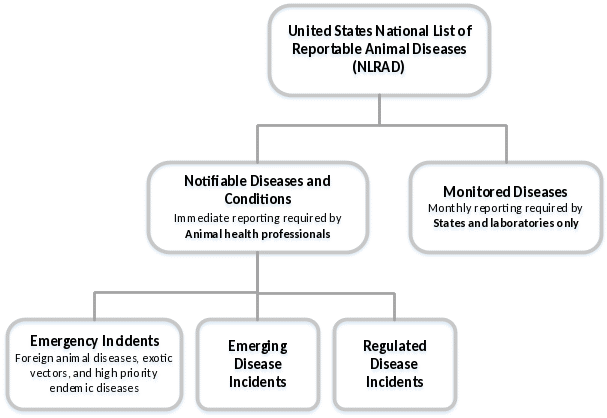
The tables below provide a list of data that will be required for reporting notifiable and monitored diseases as part of the NLRAD standards (emerging disease investigations and outbreaks may require more information depending on the situation). Please indicate your current source for receipt of this information for each data field.
FOR NOTIFIABLE DISEASES:
In the following table, please indicate from what source you most often receive this information, using the following abbreviations for the three types of notifiable diseases: EI – receive for emergency incidents such as foreign animal disease , exotic vectors or high priority endemic disease incidents, ED – receive for emerging disease incidents, RD – receive for regulated disease incidents. If you do not receive the information from any source, enter N/A.
Data field |
Provided by lab? |
Provided by regulatory epidemiologists? |
Provided by another entity? |
(please specify) |
EXAMPLE: Animal species |
EI, ED, RD |
EI, ED, RD |
|
|
EXAMPLE: vaccination history |
|
|
EI |
Herd veterinarian |
Animal species |
|
|
|
|
Animal age |
|
|
|
|
Animal ID |
|
|
|
|
Clinical signs |
|
|
|
|
Case/herd history |
|
|
|
|
Herd type |
|
|
|
|
Vaccination history |
|
|
|
|
State where animal located |
|
|
|
|
Zip code where animal is located |
|
|
|
|
Premises ID where animal is located |
|
|
|
|
Address where animal is located |
|
|
|
|
Owner name |
|
|
|
|
Owner address |
|
|
|
|
Laboratory reason for submission |
|
|
|
|
Sample ID assigned by sample collectors |
|
|
|
|
Date Sample was Collected |
|
|
|
|
Sample ID assigned by laboratory |
|
|
|
|
Lab accession number |
|
|
|
|
Sample type (example: nasal swab, lung, etc.) |
|
|
|
|
Diagnostic test performed |
|
|
|
|
Date Test was Performed |
|
|
|
|
Diagnostic test results |
|
|
|
|
Diagnostic test interpretation |
|
|
|
|
FOR MONITORED DISEASES:
Monitored diseases are generally those that are present in the U.S. and are reported to OIE in 6-month and annual reports. APHIS also uses data gathered to monitor changes in disease over time. For NLRAD monitored diseases, state veterinarians are required to report disease occurrence (e.g. presence/absence) monthly, however additional information may be requested or provided voluntarily to help monitor disease trends and enhance response and control efforts.
In the following table, please indicate what information your office uses to determine presence/absence of a disease in your state, and from what source you most often receive this information. If you do not receive the indicated information from any source, enter N/A.
Data field |
Provided by lab? |
Provided by regulatory epidemiologists? |
Provided by another entity? |
(please specify) |
EXAMPLE: Animal species |
☒ |
☒ |
☐ |
|
Animal species |
☐ |
☐ |
☐ |
|
Animal age |
☐ |
☐ |
☐ |
|
Animal ID |
☐ |
☐ |
☐ |
|
Clinical signs |
☐ |
☐ |
☐ |
|
Case/herd history |
☐ |
☐ |
☐ |
|
Herd type |
☐ |
☐ |
☐ |
|
Vaccination history |
☐ |
☐ |
☐ |
|
State where animal located |
☐ |
☐ |
☐ |
|
Zip code where animal is located |
☐ |
☐ |
☐ |
|
Premises ID where animal is located |
☐ |
☐ |
☐ |
|
Address where animal is located |
☐ |
☐ |
☐ |
|
Owner name |
☐ |
☐ |
☐ |
|
Owner address |
☐ |
☐ |
☐ |
|
Laboratory reason for submission |
☐ |
☐ |
☐ |
|
Sample ID assigned by sample collectors |
☐ |
☐ |
☐ |
|
Date Sample was Collected |
☐ |
☐ |
☐ |
|
Sample ID assigned by laboratory |
☐ |
☐ |
☐ |
|
Lab accession number |
☐ |
☐ |
☐ |
|
Sample type (example: nasal swab, lung, etc.) |
☐ |
☐ |
☐ |
|
Diagnostic test performed |
☐ |
☐ |
☐ |
|
Date Test was Performed |
☐ |
☐ |
☐ |
|
Diagnostic test results |
☐ |
☐ |
☐ |
|
Diagnostic test interpretation |
☐ |
☐ |
☐ |
|
VOLUNTARY INFORMATION FOR MONITORED DISEASES: |
||||
Data field |
Provided by lab? |
Provided by regulatory epidemiologists? |
Provided by another entity? |
(please specify) |
EXAMPLE: Number of confirmed cases |
☐ |
☒ |
☐ |
|
Number of diagnostic tests conducted |
☐ |
☐ |
☐ |
|
Number of confirmed cases |
☐ |
☐ |
☐ |
|
Vaccination status |
☐ |
☐ |
☐ |
|
Number of susceptible animals |
☐ |
☐ |
☐ |
|
Other – please specify* |
☐ |
☐ |
☐ |
|
*For example other epidemiological information needed for investigation of zoonotic diseases
Focusing on results you receive from the laboratory only, how do you receive results now for NOTIFIABLE diseases? (Please check all that apply)
☐ Lab calls SAHO’s office with preliminary results.
☐ Lab calls SAHO’s office with final results.
☐ Lab sends final report to SAHO’s office by regular mail.
☐ Lab emails preliminary report to SAHO’s office (e.g. as an attached pdf document or spreadsheet).
☐ Lab emails final report to SAHO’s office (e.g. as an attached pdf document or spreadsheet).
☐ Lab manually enters results to a state system and the SAHO is able to download or view the report (e.g. as a spreadsheet)
☐ Lab electronically reports results directly to a state database/system as a spreadsheet
☐ Lab electronically messages results directly to a state database/system as an HL7 message
☐ Other (please specify).
Who in your office receives laboratory results now for Notifiable diseases? (check all that apply)
☐ State veterinarian
☐ Assistant state veterinarian
☐ Field epidemiologists
☐ Office staff
☐ Other (please specify)
Are there any differences in laboratory reporting now for the three categories of NOTIFIABLE diseases: emergency incidents (such as foreign animal disease investigations), emerging disease incidents, and regulated disease incidents?
☐ No
☐ Yes (please specify how they are different) [free text field]
Focusing on results you receive from the laboratory only, how do you receive results now for MONITORED diseases? (Please check all that apply)
☐ Lab calls SAHO’s office with preliminary results.
☐ Lab calls SAHO’s office with final results.
☐ Lab sends final report to SAHO’s office by regular mail.
☐ Lab emails preliminary report to SAHO’s office (e.g. as an attached pdf document or spreadsheet).
☐ Lab emails final report to SAHO’s office (e.g. as an attached pdf document or spreadsheet).
☐ Lab manually enters results or uploads the report to a state system which the SAHO reviews
☐ Lab electronically messages results to state database/system. (e.g. results sent directly to an automated storage database/system that is accessed by the SAHO office)
☐ Other (please specify).
Who in your office receives laboratory results now for Monitored diseases? (check all that apply)
☐ State veterinarian
☐ Assistant state veterinarian
☐ Field epidemiologists
☐ Office staff
☐ Other (please specify)
What system(s) do you use to summarize information about diseases that you report to USDA? Check all that apply (We are exploring options for easily feeding data to USDA from the systems you currently use)
☐ USAHERDS
☐ SCS
☐ State owned instance of CoreOne
☐ Other (please specify)
What are the challenges with the way lab information is received and ability to transfer into the NAHRS/NLRAD reporting system? [free text field]
This pilot will be looking at ways to improve NLRAD reporting to both state veterinary offices and to APHIS – for the next set of questions, please provide responses based on what you would like NLRAD reporting to look like for the future.
For FUTURE reporting NLRAD information to APHIS, which scenario is most appealing?
☐ Have lab send results directly to your office, your office reports NLRAD results to APHIS via current reporting process (e.g. no change to current process).
☐ Have lab electronically message results to APHIS which would be routed to your state’s system and your office reports via NAHRS.
☐ Have lab electronically message results to APHIS, but results would only be available to APHIS after your office has reviewed and approved for release (gating process)
☐ Have lab electronically message results to APHIS, with results accessible to your office and to APHIS simultaneously.
☐ Other (please specify)
In the FUTURE, how would you like to be notified that lab results are available for review prior to reporting to APHIS? (check all that apply)
☐ Text
☐ Other (please specify)
What else would assist you with reporting NLRAD information to APHIS? [free text]
If you would like to contact us with any additional questions or input below are contact names and information
Leah Estberg, DVM, MPVM, PhD VMO Veterinary Informaticist Center for Informatics (CFI) Strategy and Policy USDA APHIS Veterinary Services Mobile: 970-631-6517 Email: Leah.Estberg@usda.gov |
Jane A. Rooney, D.V.M. Veterinary Medical Officer (Epidemiology) NLRAD Coordinator Center for Epidemiology and Animal Health Strategy and Policy USDA APHIS Veterinary Services Ph: 970-494-7397 Mobile: 301-789-3064 Email: Jane.A.Rooney@usda.gov |
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | DOCUMENTATION FOR THE GENERIC CLEARANCE |
| Author | 558022 |
| File Modified | 0000-00-00 |
| File Created | 2022-02-09 |
© 2026 OMB.report | Privacy Policy