5 Audience Feedback Team Sample Online Bulletin Board Prom
Federal COVID Response - Audience Feedback to Inform Ongoing Messaging and Strategies for "Combat COVID" (OD)
Attachment 5 - Audience Feedback Team Sample Online Bulletin Board Prompt 111021
HCP Audience Feedback Activity
OMB: 0925-0769
OMB Control # 0925-0769
Expiration Date 12/31/2021
Public reporting burden for
this collection of information is estimated to average 60 minutes
per response, including the time for reviewing instructions,
searching existing data sources, gathering and maintaining the data
needed, and completing and reviewing the collection of information.
An agency may not conduct or sponsor, and a person is not required
to respond to, a collection of information unless it displays a
current valid OMB control number. Send comments regarding this
burden estimate or any other aspect of this collection of
information, including suggestions for reducing this burden, to NIH,
Project Clearance Branch, 6705 Rockledge Drive, MSC 7974, Bethesda,
MD 20892-7974, ATTN: PRA (0925-0769).
Do not return the completed form to this address.

A 60-Minute Online Bulletin Board Forum
MODERATOR’S GUIDE FOR CONSUMER AND HEALTHCARE PROVIDER (HCP) AUDIENCE FEEBACK TEAMS
Month 01, 202X
BACKGROUND AND INSTRUCTIONS
[MONTH 1 ONLY: Thank you for your participation in this project! This project is sponsored by the Federal COVID Response Team (FCR). The FCR is a cross-agency partnership that includes the U.S. Department of Health and Human Services (HHS), including the Centers for Disease Control and Prevention (CDC), the U.S. Food and Drug Administration (FDA), the National Institutes of Health (NIH), the Biomedical Advanced Research and Development Authority (BARDA), and the U.S. Department of Defense (DOD).
The NIH has set up a partnership among government, industry, and university researchers to identify drugs and other treatments that are most promising. These are called ACTIV trials-- Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV).
The purpose of this Bulletin Board Forum is to discuss your thoughts and feelings about some topics related to COVID-19 and clinical trials. We also want to hear your suggestions on how to best reach you with important information on what’s available for people who get exposed to or get sick with COVID-19. Your feedback will help us share this important information in the most effective way.]
[SUBSEQUENT MONTHS: Thank you for your participation in this project! As a reminder, or if this is your first month joining us, this project is sponsored by the Federal COVID Response Team (FCR). The FCR is a cross-agency partnership that includes the U.S. Department of Health and Human Services (HHS), including the Centers for Disease Control and Prevention (CDC), the U.S. Food and Drug Administration (FDA), the National Institutes of Health (NIH), the Biomedical Advanced Research and Development Authority (BARDA), and the U.S. Department of Defense (DOD).
The NIH has set up a partnership among government, industry, and university researchers to identify drugs and other treatments that are most promising. These are called ACTIV trials-- Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV).
The purpose of this Bulletin Board Forum is to discuss your thoughts and feelings about some topics related to COVID-19 and clinical trials. We also want to hear your suggestions on how to best reach you with important information on what’s available for people who get exposed to or get sick with COVID-19. Your feedback will help us share this important information in the most effective way.]
During the course of this 2-day discussion forum, please keep in mind the following:
Your total time spent on this Bulletin Board Forum will be approximately 60 minutes (across 2 days). It is up to you how you spend your time answering questions, but we encourage to you check in on Day 1 and Day 2 to respond to any follow-up questions from our moderator and to see what others have posted.
A group of participants will take part in this Bulletin Board Forum. You will be asked a set number of questions each day. After you answer each question, you will be able to see how others in the forum answered the same question. You will be able to react to comments from other participants in the Bulletin Board Forum as well as interact with the moderator. We want to hear all opinions and perspectives. Please be respectful of all opinions and perspectives.
There are no right or wrong answers to the questions we ask.
This activity can be completed on a computer (desktop or laptop) or using the smartphone/tablet app, whichever you prefer. At the end of the discussion, we will download everyone’s feedback for the purposes of our report. Please note that there will be NO audio or video recording of this Bulletin Board Forum.
All information and opinions you give us will be kept private. Your identity will not be revealed to anyone outside of this discussion group. Please do not share any information which could be used to identify someone, such as someone’s full name. This information will be used for research purposes only and will not be shared with any third parties.
We are not here to influence you or your behaviors in any way. What we seek is your honest feedback. You will not be targeted for any sales or promotional activities as a result of taking part in this discussion.
Your participation is voluntary. What this means is that you are free to decline to answer any questions and you are free to withdraw from the Bulletin Board Forum at any time without penalty.
Feel free to move through the questions at your leisure (at any time during the day). As your moderator, I may contact you on this Bulletin Board Forum to ask a follow-up question about something you wrote.
Project staff may be viewing the Bulletin Board Forum, but the participants and the moderator will be the only ones communicating during the 2-day discussion.
Thank you. Now, let’s get started!
INTRODUCTIONS
[MONTH 1 ONLY: Please introduce yourself using your first name only (no last names) and let us know (a) what state you live in and (b) one thing you like to do for fun.]
[SUBSEQUENT MONTHS: If this is your first time joining, please introduce yourself using your first name only (no last names). If you are returning feedback team member, welcome back! Please share your first name for any new members in this bulletin board activity.
As let us know: (a) what state you live in and (b) [INSERT INTRO QUESTION].]
[Intro questions include:
Favorite seasonal activity
Something you are looking forward to this month
Favorite vacation]
[MONTH 1 ONLY: HEALTHCARE ACCESS AND COVID EXPERIENCE
[CONSUMERS]
POLL: Do you currently have someone you consider a primary care provider? This is someone whom you see for check-ups or common medical problems.
Yes
No
If no, where do you usually go when you are sick and need care or treatment?
POLL. First, we’d like to know more about your personal experience with COVID-19. Please select any of the following that apply to you:
I have not had COVID-19.
I was exposed to COVID-19 at home, at work, or somewhere else, but I did not feel sick.
I was tested for COVID-19 and told my test result was positive.
I had COVID-19 myself.
Someone in my household who I provide care for when they are sick (like a parent, spouse or partner, or child) had COVID-19, and I helped care for them.
Someone who I provide care for but lives outside of my house had COVID-19, and I helped care for them when they were sick.
Someone I knew but do not live with (a friend, other family members) had COVID-19.
I personally stayed overnight in the hospital for COVID-19 treatment.
Someone who I provide care for stayed overnight in the hospital for COVID-19 treatment.
In your own words, briefly walk us through your personal experience being sick with COVID-19. If you did not have COVID-19 personally, tell us about the experience of someone else you know.
If you had COVID-19:
What care or treatments for COVID-19 did you do at home?
If you needed medical care, where did you go?
What care or treatments have you done since your COVID-19 symptoms ended? If you are still having COVID-19 symptoms, what care or treatment have you gotten to help manage those symptoms?
If you provided care for a loved one who had COVID:
What care or treatments for COVID-19 did they do at home?
If they needed medical care, where did you go first?
What care or treatments has your loved one done since their COVID-19 symptoms ended? If they are still having COVID-19 symptoms, what care or treatment have they gotten to help manage those symptoms?
If you have not had COVID-19 personally: What preventive steps or care did you look for to help protect your health?
[HCPs]
Tell me about your experiences treating patients with COVID-19.
What treatments are you aware of for COVID-19, and what is your experience with those treatments in your practice?
How do you learn about new treatments or therapies for COVID-19?
What kinds of questions do your patients have about treatments for COVID-19?
What information would be helpful for conversations with patients?
What information do you feel you are missing, right now, when it comes to treatments for COVID-19?
[MONTH 1 ONLY: CLINICAL TRIAL KNOWLEDGE AND EXPERIENCE
[CONSUMERS]
POLL. Have you or someone you know ever participated in a clinical trial?
Yes
No
If you or someone you know have personally taken part in a clinical trial, how did you/they find out about it and what made you/them decide to participate?
If you haven’t participated in a clinical trial, tell me more about your thoughts on participating in a clinical trial.
Tell me about any clinical trials you have heard of for COVID-19, and your thoughts about participating or not participating in one.
[HCPs]
Describe your familiarity with clinical trials, and what your professional experience with them has been.
Have you ever referred a patient to a clinical trial? Why or why not?
What would change your mind (positively or negatively) about referring patients to a clinical trial?
How familiar are you with any clinical trials for COVID-19?
Would you be more or less likely to share information with a patient about trials for COVID-19 treatment, compared to other kinds of clinical trials? What considerations would you have?
[MONTH 1 ONLY: TRUSTED SOURCES OF INFORMATION
Next, we are interested in hearing about your preferences looking for information about COVID-19 treatments.
Below is a list of sources people might use for COVID-19 treatment information. Please select the top three you trust the most to give you information about COVID-19 treatments and let us know why you picked these:
PROFESSIONAL ASSOCIATIONS/ORGANIZATIONS/INSTITUTIONS
Association (Specify which one: ______)
Hospital/health clinic
Non-profit organization (Specify which one: ______)
Government agency (Specify which one: ______)
HEALTH PROFESSIONALS
Doctor (primary care doctor or specialist doctor) (Specify which one: _____)
Nurse
Physician assistant
Social worker
COVID-19 testing site staff
COMPLEMENTARY AND INTEGRATIVE MEDICINE PROVIDERS
Acupuncturist
Chiropractor
Herbalist/natural medicine practitioner
ACQUAINTANCES/LOVED ONES
Co-worker/colleague
Family member/spouse/partner
Friend/peer
Support group
Someone else in your local community, school, or faith organization
MEDIA
Magazine (Specify which one: ______)
Newspaper (Specify which one: ______)
Radio station (Specify which one: ______)
Television station (Specify which one: ______)
Podcast (Specify which one: ______)
Scientific journal (Specify which one: ______)
INTERNET/SOCIAL MEDIA
Online search (Specify which ones: Google, Bing, etc.:_____)
Online discussion group (Specify which one: ______)
Social media (Specify which ones: Facebook, Instagram, Snapchat, Twitter, LinkedIn, etc.: _____)
Websites (Specify which ones: ______)
Website of medication manufacturer (Specify which ones: ______)
OTHER, please specify: ______: (1)__________________________________ , (2)__________________ and (3)________________________________.
Of the following, how do you prefer to receive information about COVID-19 treatments? Please select your top two preferred formats and let us know why you picked them:
Brochure
Email
Infographic
In-person conversation
Fact sheet
Podcast
Poster
Social media post (e.g., Facebook, Instagram, Snapchat, Twitter, LinkedIn)
Regular mail
Video
Webinar
Website
Other, please specify: ______: (1)__________________________________ and (2)_____________________________________________________________.
If you only had the following two options, do you prefer reviewing health information in print form or in digital/electronic form? PICK ONLY ONE.
_____ Print form
_____ Digital/electronic form
MESSAGE TESTING
MESSAGE OR AD CONCEPT QUESTIONS TO REMAIN THE SAME ACROSS MONTHS; HOWEVER NEW AD MESSAGES/CONCEPTS WILL BE PRESENTED TO PARTICIPANTS BASED ON EACH UNIQUE TOPIC]
Please review each of the following communication materials and respond to the review prompts. Thank you in advance for responding to these prompts about the test communication materials!
[REVIEW PROMPTS]
One thing in this [test communication material] that grabbed your attention and why (for example, what was something surprising or new to you?).
How clear and easy to understand is this [test communication material]? If there was something confusing to you, let us know what it was, why, and any suggestions you might have for wording it more clearly.
POLL. To what degree do you feel like this [test communication material] is speaking directly to you, like the [test communication material] understands you and your situation?
1 to 7 scale, 1 is “Not at all relatable” and 7 is “Extremely relatable.”
How did reading [test communication material] change your thoughts about clinical trials for COVID-19 treatments? What, if anything, might you do differently after reading this [test communication material] (this includes learning more about this topic)?
[CONSUMERS]
Available COVID-19 Treatment Options [CLICK HERE TO REVIEW]
Page 1 of 2

Page 2 of 2

I’ve Never Had COVID-19 [CLICK HERE TO REVIEW]
Page 1 of 2

Page 2 of 2

[HCPs]
High-Risk COVID-19 Patients May Avoid Hospitalization with Monoclonal Antibody Treatment [CLICK HERE TO REVIEW]
Page 1 of 2

Page 2 of 2
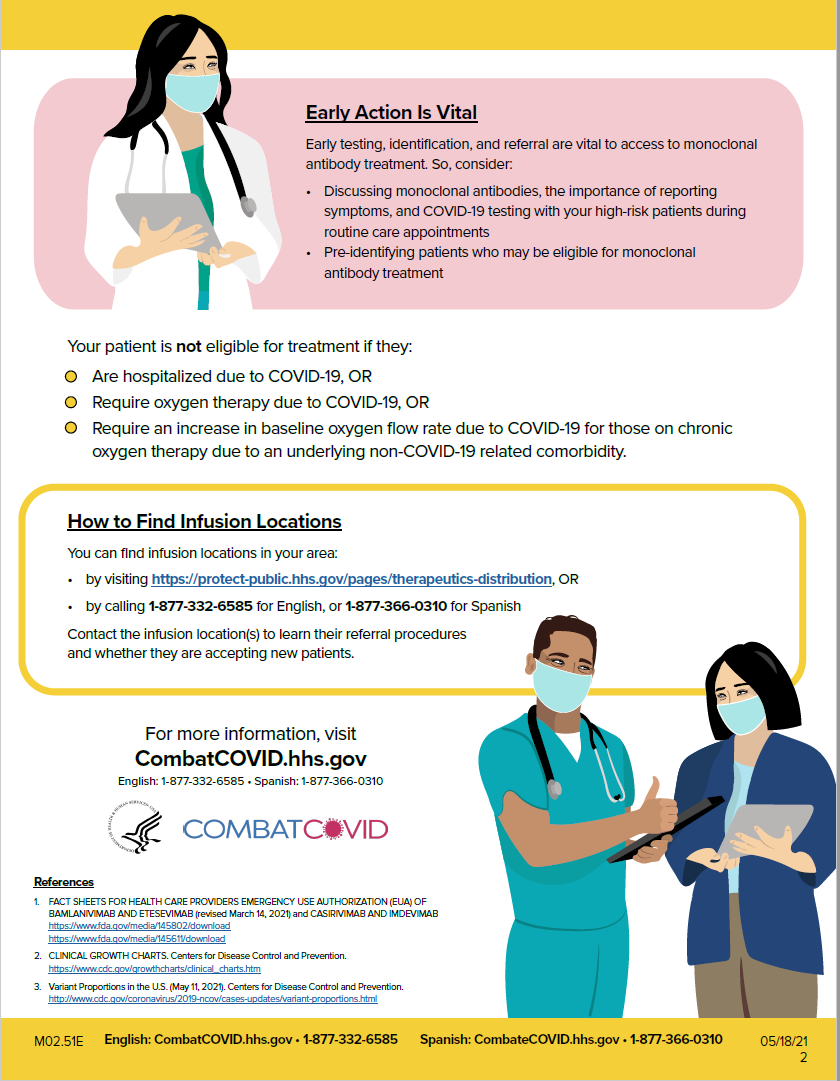
Talking with Patients about Monoclonal Antibodies for COVID-19: Tips and Frequently Asked Questions [CLICK HERE TO REVIEW]
Page 1 of 3

Page 2 of 3

Page 3 of 3

INFORMATION RECALL
Which ONE piece of information do you remember MOST clearly?
What was it about that information that made it stand out to you?
What do you remember about the wording/text/visuals/images of that information?
What do you feel the main idea of that information was trying to communicate?
What is one new thing you learned from that information?
CLOSING POLL :
[CONSUMERS]
[MONTH 1:]
How likely are you to find out more about clinical trials for COVID-19 treatments?
If you or a loved one had COVID-19, how likely are you to participate in a trial for COVID-19 treatments?
1 to 7 scale, 1 is “Not at all likely” and 7 is “Extremely likely.”
Why did you rate this question in this manner?
Where would you go for more information?
[SUBSEQUENT MONTHS:]
Since our last discussion, have you taken any action to find out more about clinical trials for COVID-19 treatments?
Yes/No
If yes, what action did you take?
If no, why did you choose not to take action?
Compared to a few months ago, how likely are to consider a clinical trial for COVID-19 treatment for yourself?
More likely, no change, less likely
Why did you choose your answer?
Compared to a few months ago, how likely are to consider a clinical trial for COVID-19 treatment for a loved one?
More likely, no change, less likely
Why did you choose your answer?
[HCPs]
[MONTH 1:
How likely are you to find out more about clinical trials for COVID-19 treatments?
1 to 7 scale, 1 is “Not at all likely” and 7 is “Extremely likely.”
Why did you rate this question in this manner?
Where would you go for more information?
How well-prepared do you feel to answer patients’ questions about clinical trials for COVID-19 treatments?
1 to 7 scale, 1 is “Not at all prepared” and 7 is “Extremely prepared.”
Why did you rate this question in this manner?
Where would you go for more information?]
[SUBSEQUENT MONTHS:
Compared to a few months ago, how likely are you to share information about clinical trials for COVID-19 treatments with patients?
More likely, no change, less likely
Why did you choose your answer?
Compared to a few months ago, how likely are you to share information about clinical trials for COVID-19 treatments with other providers?
More likely, no change, less likely
Why did you choose your answer?]
Finally, please share any additional questions you have about clinical trials to find new, better treatments for COVID-19.
CLOSE
Those are all the questions we have. Thank you very much, again, for participating and be well!
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Rosita Thomas |
| File Modified | 0000-00-00 |
| File Created | 2021-11-24 |
© 2026 OMB.report | Privacy Policy