Supporting Statement B_11082018
Supporting Statement B_11082018.docx
Identification of Behavioral and Clinical Predictors of Early HIV Infection (Project DETECT)
OMB: 0920-1100
Identification of Behavioral and Clinical Predictors of Early HIV Infection
(Project DETECT)
OMB No. 0920-1100
November 8, 2018
Supporting Statement
Part B
Contact:
Kevin Delaney
Epidemiologist, Special Studies and Diagnostics Team
Division of HIV/AIDS Prevention
Centers for Disease Control & Prevention
1600 Clifton Rd, NE, MS E-46
Phone (404) 639-8630
Fax (404) 639-8640
Table of Contents
B. Collection of Information Employing Statistical Methods
1. Respondent Universe and Sampling Methods
2. Procedures for the Collection of Information
3. Methods to Maximize Response Rates and Deal with Nonresponse
4. Tests of Procedures or Methods to be Undertaken
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data
List of Attachments
Attachment number |
Document description |
1 |
Section 301 of the Public Health Service Act |
2 |
60-Day Federal Register Notice |
2a |
Public Comment |
3 |
References |
4 |
Certificate of Confidentiality Approval |
5 |
IRB approval |
6a |
Phase 1 Enrollment Survey A (English) |
6b |
Phase 1 Enrollment Survey A (Spanish) |
7a |
Phase 1 Enrollment Survey B (English) |
7b |
Phase 1 Enrollment Survey B (Spanish) |
8a |
Phase 2 Behavioral Survey (English) |
8b |
Phase 2 Behavioral Survey (Spanish) |
9a |
Phase 2 HIV Symptom and Care Survey (English) |
9b |
Phase 2 HIV Symptom and Care Survey (Spanish) |
10a |
Phase 1 Consent Form (English) |
10b |
Phase 1 Consent Form (Spanish) |
11a |
Phase 2 Consent Form (English) |
11b |
Phase 2 Consent Form (Spanish) |
12a |
Survey Screen Shots (English) |
12b |
Survey Screen Shots (Spanish) |
13 |
Supplemental Study Information |
14 |
Project Determination |
15 |
Privacy Impact Assessment (PIA) |
B. Collection of Information Employing Statistical Methods
1. Respondent Universe and Sampling Method
The respondent population for Project DETECT will be persons who present to Seattle-area clinics who are at high risk for HIV infection or have been diagnosed with established or early HIV infection.
The burden of HIV in Seattle and King County is primarily among men (88%), whites (62%), and men who have sex with men (MSM; 75% including MSM who inject drugs). These percentages are higher than those within the US overall (Table 2.1). While the population of Seattle/King County, and those affected by HIV may not be as diverse as in some other areas, there is value in understanding the behavioral characteristics which are unlikely to be substantially different from those in other areas. Further, the performance of the tests is not anticipated to vary by sociodemographic characteristics.
Variable |
Category |
PHSKC, 2015 % |
National HIV Surveillance System, 2016 % |
Sex |
|
|
|
|
Male |
88% |
76% |
|
Female |
12% |
24% |
Race |
White Non-Hispanic (NH) |
62% |
30% |
|
Black NH |
18% |
41% |
|
Hispanic |
12% |
23% |
|
Asian |
4% |
1% |
|
Native American or Alaskan Native |
1% |
<1% |
|
Pacific Islander |
<1 |
<1% |
|
Two or more races reported |
2% |
4% |
Current age |
0-19 |
1% |
1% |
|
20-29 |
7% |
10% |
|
30-39 |
19% |
17% |
|
40-49 |
29% |
30% |
|
50-59 |
31% |
30% |
|
60+ |
14% |
13% |
Transmission Category |
MSM |
68% |
54% |
|
IDU |
4% |
13% |
|
MSM-IDU |
8% |
5% |
|
Heterosexual |
10% |
26% |
|
Perinatal/Other/Undetermined |
10% |
2% |
Respondent Population
This study is conducted in two phases (see Figure 2.1). A pre-study screen based on risk behavior reported on standard clinic intake forms will comprise a phase 0 which is not part of this information collection request. Approximately 12,500 persons per year present for an HIV test at the PHSKC STD Clinic and complete a standard intake form which will be used in this study to limit the evaluation of new testing technologies in phase 1.
Figure 2.1: Description of Study Phases
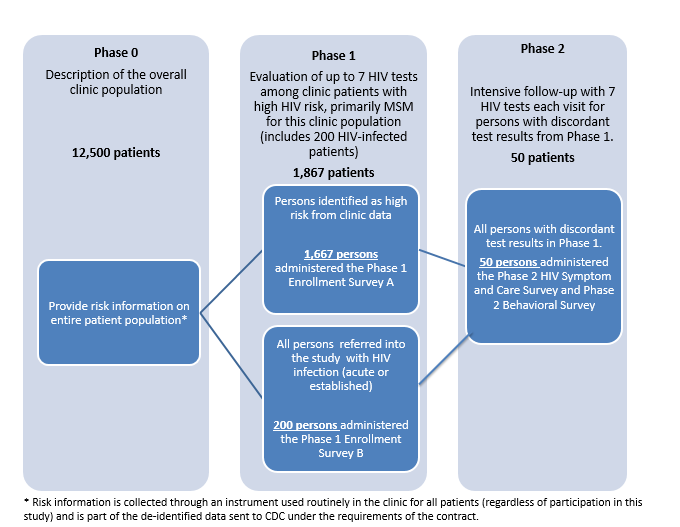
Phase 1 is limited to up to 200 HIV-infected persons per year (identified from the PHSKC STD clinic or other clinic partners who offer HIV testing [see table 2.2] to increase the sample size for the evaluation of test performance) and up to 1,667 MSM at highest risk for HIV infection (identified from the PHSKC STD clinic). In phase 1 of the study we will evaluate test performance by collecting specimens for testing with the HIV testing technologies being evaluated. Phase 1 participants with discordant test results (i.e., those with reactive results on at least one screening test and non-reactive results on another screening test), will be eligible for Phase 2.
Table 2.2: Percentage of HIV-positive participants referred to Project DETECT
New diagnoses of HIV at PHSKC STD Clinic |
7% |
New diagnoses of HIV at Gay City (Clinic) |
6% |
New diagnoses made by private providers in King County |
15% |
Referrals from the UW HIV Information System (UWHIS) |
28% |
PHSKC STD Clinic previously diagnosed |
44% |
In phase 2 we seek to describe the difference in days to detection for the new HIV tests on different specimen types collected. Phase 2 participants will undergo frequent follow-up testing until they are positive on all tests being evaluated, or until they have two consecutive visits with negative test results on all tests (indicating reactive phase 1 tests were false-positive), or completion of 70 days of follow-up.
It is expected that approximately 50 participants per year will enter phase 2 of the study, of which approximately 16 participants will complete the study with false positive results and approximately 32 participants will complete phase 2 follow-up with seroconversion. We expect that approximately 2 participants who begin phase 2 of the study will be lost to follow-up.
Potential participants will be approached for participation through convenience sampling dependent on clinic flow and staff availability if they are 18 years of age or older and able to complete the CASI in English. The following groups will be recruited:
1) persons who present to the PHSKC STD Clinic seeking HIV testing services and are identified as being at high risk of HIV infection† based on their responses to the routinely used clinic intake questionnaire,
2) persons who test antibody-positive through routine HIV testing at the PHSKC STD clinic
3) persons who self-report their HIV-positive status when seeking other services from the PHSKC STD Clinic,
4) persons who tested HIV-antibody positive at a clinical site in Seattle other than the PHSKC STD Clinic and were referred to participate in the study, and
5) persons with test results indicative of early HIV infection§ who tested at a site in Seattle outside of the PHSKC STD clinic and were referred to participate in the study
† Persons are defined as high risk if they are male and have reported any male sex partners in the past 12 months.
§ Early infection is defined according to the CDC recommended HIV testing algorithm (CDC 2014, reference in Attachment 3)
Based on similar HIV studies conducted by UW, it is anticipated that the Phase 1 response rate will be 80% (Stekler, 2013).
For Phase 2, it is estimated that of the 66 participants with discordant results (i.e., at least one positive and one negative Phase 1 test result), 76% (50 persons) will agree to participate in the survey and undergo the consent procedures (Stekler, 2013).
Project DETECT Phase 1
For Phase 1 of Project DETECT, each year the contractor will recruit for consent up to 2,334 PHSKC clinic clients (i.e., HIV-negative MSM, HIV-positive persons with early HIV infection or established HIV infection), as well as 250 additional HIV-positive persons (i.e., either with early infection or with established infection) referred to the clinic from other Seattle-area clinics.
All persons seeking services at the PHSKC STD clinic complete an electronic intake questionnaire at a private computer located in the clinic’s waiting area (phase 0). This intake questionnaire is a standard part of clinical care at PHSKC and includes information such as demographics, sexual orientation, and reason for the clinic visit. Full-time research staff will monitor intake data during clinic hours to identify persons eligible to participate in the study (e.g., self-reported HIV-negative MSM at high risk for infection seeking HIV testing or an established HIV positive patient). Study staff will approach eligible persons and escort them to a private room in the clinic to discuss study participation and consent procedures.
Participants who are referred will initiate contact with study staff by phone to make an appointment or present to the clinic, but will not be asked to register as a clinic patient.
Project DETECT Phase 2
To be eligible for Phase 2, Phase 1 participants, whether recruited from among high-risk or HIV-positive persons at the PHSKC clinic, or referred to the study after testing HIV positive at another clinic, must have at least one positive and one negative Phase 1 test (discordant results).
2. Procedures for the Collection of Information
Sample Size
The sample size for HIV test comparison is based on the expected level of precision needed to detect differences between the new HIV tests being evaluated in the project. The sample size selected, a total of 600 participants, including 50 with early HIV infection, is expected to give >80% power to detect differences in sensitivity (proportions of HIV-infected persons who are correctly identified as such) as small as 0.16 in the HIV tests under evaluation, via McNemar’s test for paired proportions adjusted for multiple comparisons.
The investigators anticipate that, during the life of the project, approximately 70 persons will be identified with early HIV infection and complete follow-up, and 4,830 persons will be identified as HIV negative. With this sample size, the study will be powered to detect differences in behavioral survey responses of between 10 and 20% when comparing these two groups (Table 2.3).
The study is powered to detect differences in key behavioral variables. In the 2014 cycle of CDC’s National HIV behavioral surveillance 89% of HIV-negative MSM reported anal sex in the past year, 65% reported condomless anal sex and 12% reported a bacterial sexually transmitted infection in the past year. For these outcomes we would therefore expect to have power to detect differences of 16%-20% between our two study groups of interest.
Table 2.3: Power to detect a difference in proportion of the population reporting an outcome
|
Difference between proportion among those with early HIV infection (N=70) and those who are HIV negative N=4830) |
||||
|
2% |
6% |
10% |
16% |
20% |
Proportion reporting outcome in the HIV- negative population (N=4830) |
|
|
|
|
|
10% |
6.4% |
26.3% |
59.7% |
92.6% |
98.6% |
20% |
5.8% |
18.2% |
43.6% |
81.6% |
94.4% |
30% |
5.6% |
15.5% |
36.8% |
74.9% |
91.0% |
40% |
5.5% |
14.4% |
34.2% |
72.3% |
89.7% |
50% |
5.5% |
14.2% |
34.1% |
73.2% |
90.8% |
60% |
5.5% |
14.8% |
36.5% |
77.9% |
94.1% |
70% |
5.6% |
16.6% |
42.8% |
87.0% |
98.3% |
80% |
5.8% |
21.3% |
57.9% |
98.2% |
100% |
90% |
6.5% |
40.0% |
100% |
100% |
100% |
Collection of Information
Phase 1: Persons who agree to learn more about the project will be escorted into a private room in the STD Clinic. A trained project staff member will discuss the study and read the consent form in its entirety, and will encourage questions about the project. Participation will be completely voluntary. Persons who agree to participate in the project will provide verbal consent, and blood and oral fluid specimens will be taken for the HIV tests. All tests under evaluation (refer to Table 1 in Appendix 13) will be performed on specimens from all participants.
While the tests are processing, the respondent will complete the Phase 1 study survey via an electronic Computer Assisted Self-Interview (CASI) on an encrypted laptop. Clients from the PHSKC STD Clinic, who, in phase 0 have already provided demographic and behavioral information on the standard clinic intake form, will be asked to complete Survey A (Attachment 6) and clients referred from other clinics, who did not complete the standard clinic intake form, will be asked to complete Survey B (Attachment 7).
Phase 2: Participants whose results from Phase 1 include at least one positive and at least one negative test (i.e., are “discordant”) will immediately be invited to participate in Phase 2, which will include up to 9 serial follow-up visits(with the first follow-up visit within 3 days). Participants who consent to enroll in Phase 2 will be asked to come to the clinic at regular intervals – up to 9 visits over a 70-day period. The timing of the follow up visits is designed to identify differences, expected to be in a matter of days (not weeks), in the time from HIV exposure to detection by a given test (the window period) for each test. At each visit, the participant will have blood and oral fluid specimens collected for the performance of all HIV tests under evaluation to determine the window period for the detection of HIV infection in whole blood and oral fluid specimens for the respective tests. At each visit, the participant will also complete the Phase 2 HIV Symptom and Care Survey via the CASI system on an encrypted laptop. The CASI software will pre-populate some information from the participant’s last clinic visit (e.g., race/ethnicity, age) to reduce the burden for these study participants. Phase 2 participants will be followed up until the time their HIV tests all show the same result (i.e., all positive or all negative), or until 70-days have elapsed, whichever is earliest. At the last Phase 2 follow-up visit, participants will be asked to complete the Phase 2 Behavioral Survey using the CASI software on an encrypted laptop.
Quality Control Measures
The use of CASI for electronic data capture allows for real-time quality control. Pre-programmed skip patterns will ensure that respondents are not asked irrelevant questions, which means respondents can move through the survey smoothly and efficiently. Automated validation checks incorporated into the survey will assist the participant when incomplete or implausible responses are provided.
The Contractor, UW, will upload de-identified data from the surveys and HIV test results to CDC using a secure, encrypted File Transfer Protocol (FTP) site operated by CDC at regular intervals. CDC will run quality assurance checks as another way to ensure that data quality is maintained throughout the life of the project.
3. Methods to Maximize Response Rates and Minimize Nonresponse
The investigators involved in this project have extensive experience recruiting and retaining clinic clients for projects involving the collection of biologic specimens, survey data, and serial follow-up. Project staff will approach clinic clients identified through the routinely collected clinic intake questionnaire as eligible for the study, and will escort them to a private room in the clinic to discuss study participation and consent.
Based on similar HIV studies conducted by UW, it is anticipated that the Phase 1 response rate among persons approached in the PHSKC Clinic will be 78%. The response rate among persons referred to the study from other clinics is estimated to be 100% (since these persons are coming to the clinic specifically to participate in the study). Therefore, the overall response rate for Phase 1 will be 80%.
For Phase 2, the Investigators estimate that of the 66 participants with discordant results, 76% (50 persons) will agree to participate in the survey and undergo the consent procedures.
Because response rate is critical to the success of the proposed project, and recognizing the time burden associated with the survey and the discomfort of specimen collection, tokens of appreciation will be offered to all participants in Phase 1 and at every visit in Phase 2. Two meta-analyses (Church 1993; Edwards et al, 2002) full references in Attachment 3) found that cross-sectional studies using prepaid monetary incentives yielded an average increase in response rates. In addition, persons at risk for HIV infection have frequently been the focus of health-related data collections, in which tokens of appreciation are the norm (Mackellar et al., 2009; Thiede et al., 2005; full references in Attachment 3). Jackle and Lynn (2008, reference in Attachment 3) found that incentives at multiple visits in a longitudinal study decreased attrition at all visits. Tokens have been used at each visit in other Department of Health and Human Services studies with longitudinal designs involving both survey responses and collection of biological specimens, e.g. the National Health and Nutrition Examination Survey (NHANES, OMB No. 0920-0950, exp. 11/30/2015). For the proposed data collection, the Contractor will provide $40 to participants for the Phase 1 study visit and $50 per study visit for participants followed longitudinally in Phase 2. The token of appreciation amounts in this study are consistent with a recent HIV testing study conducted by UW among MSM in the Seattle metropolitan area, which had an 80% response rate, and are less than those provided during the 2013-2014 NHANES data collection cycle (NHANES Interviewer Procedures Manual, 2013, reference in Attachment 3).
Research Study Assistants will each be provided with a study cell phone to use to maintain contact with all Part 2 study participants. Research Staff will also serve as a source of support to participants recently diagnosed with HIV to help them identify HIV care resources in the area. The following will be used to ensure that follow-up and retention are as complete as possible:
At the first visit, the participant will be asked for multiple methods of contact. The participant will inform study staff of their preference regarding the order of contact.
The day prior to a scheduled appointment, participants will receive reminders through their preferred method of contact with a request for confirmation (e.g., SMS text). Participants who miss a scheduled appointment will be contacted and rescheduled, ideally for the same or following day. Contact will be pursued through all channels unless the participant indicates that s/he would like to withdraw from the study.
As part of the consent process prior to enrollment, study staff will ensure that participants are well informed about the purpose of the study and the importance of follow-up, and will continue to provide test results and education to the participant throughout the follow-up period. Investigators will maintain a safe and friendly environment with diverse and well-trained staff. Clinic hours will allow for flexible appointment times for participants who would like to be seen before, during, or after the typical workday. The Research Staff will see participants after clinic closure, as necessary.
Recruitment and retention will be monitored through ongoing data reports generated weekly and monthly from the data submitted to CDC. The project area staff and CDC will use the data in these reports to identify problems with recruitment or retention. When a problem with recruitment or retention arises during data collection, research staff will be instructed to consult with local stakeholders and facility staff to identify solutions to the problem.
Non-response bias will be assessed using data from the standard clinic intake form administered as part of phase 0. Study participants will be compared to those who attended the clinic but did not participate. For any potential participants referred from other clinics data will not be available to assess non-response bias, however, the non-response is not expected to be large or different.
4. Tests of Procedures or Methods to be Undertaken
The data collection instruments were developed using questions from previous CDC projects and with input from UW, based on questions used in their previous research. CDC and contract staff will test the skip patterns and responses in the surveys that will be used for the proposed information collection.
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data
Consultants on Statistical Aspects
Chris Johnson, MS
Biostatistician
Division of HIV/AIDS Prevention
U.S. Centers for Disease Control and Prevention
(404) 639-2989
Contractor
CDC awarded a contract to UW in 2014 to conduct Project DETECT. The contract staff is involved with all aspects of designing and implementing the study. The survey data and HIV test results will be collected by contract staff and merged using a unique study ID. No identifiers will be included in datasets prepared for CDC. The contractor will deliver the de-identified but sensitive information from the study using an encrypted File Transfer Protocol. The contractor will participate with CDC in analysis of the data.
Joanne Stekler, MD
Principal Investigator (Contractor)
University of Washington, Department of Medicine
325 Ninth Avenue
Seattle, WA 98104
Phone: (206) 744-8312
David Katz, PhD, MPH
Research Scientist (Contractor)
University of Washington, Department of Medicine
325 Ninth Avenue
Seattle, WA 98104
Phone: 206-744-5877
CDC Project Staff
The CDC staff members who are involved with the various aspects of designing and implementing the study are listed below. CDC staff will not be directly involved in the data collection, and will not be in contact with study participants. CDC will receive only study data with no information in identifiable form. The data collected will be analyzed by CDC staff. All CDC project staff can be reached at the following address and phone number:
Behavioral and Clinical Surveillance Branch
Division of HIV/AIDS Prevention
Centers for Disease Control and Prevention
1600 Clifton Rd, NE MS E-46
Atlanta, GA 30333
Phone: (404) 639-2090
Kevin Delaney, PhD Epidemiologist Email: khd8@cdc.gov Phone: 404-639-8630
|
Laura Wesolowski, PhD Diagnostics Activity Lead Email: lig7@cdc.gov Phone: 404-639-6007
|
Pollyanna Chavez, PhD Epidemiologist Email: geo5@cdc.gov Phone: 404-639-1742 |
Steve Ethridge Epidemiologist Email: sfe1@cdc.gov Phone: 404-639-6059
|
Joseph Prejean, PhD Chief, Behavioral and Clinical Surveillance Branch Email: nzp1@cdc.gov Phone: 404-639-5273
|
|
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | ziy6 |
| File Modified | 0000-00-00 |
| File Created | 2021-03-29 |
© 2026 OMB.report | Privacy Policy