Notification, Documentation, and Recordkeeping
Requirements to Notify FSIS of Adulterated or Misbranded Products, Prepare and Maintain Written Recall Procedures, and Document Certain HACCP Plan Reassessments
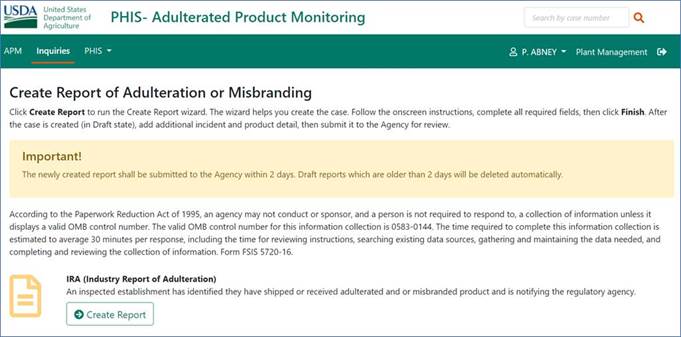
PHIS screenshot for 0583-0144 electronic data collection report of adulteration or misbranding
Notification, Documentation, and Recordkeeping
OMB: 0583-0144
⚠️ Notice: This form may be outdated. More recent filings and information on OMB 0583-0144 can be found here:
© 2026 OMB.report | Privacy Policy