0920-1074 SSA Final_12-03-20
0920-1074 SSA Final_12-03-20.docx
Colorectal Cancer Control Program (CRCCP) Monitoring Activities
OMB: 0920-1074
Information Collection Request
REINSTATEMENT WITH CHANGE
Colorectal Cancer Control Program (CRCCP) Monitoring Activities
OMB # 0920-1074
Supporting Statement A
Program Official/Project Officer
Dara Schlueter, MPH
Health Scientist
Division of Cancer Prevention and Control
National Center for Chronic Disease Prevention and Health Promotion
Centers for Disease Control and Prevention
Phone: 770-498-1782
Fax: 770-488-4760
Sax9@cdc.gov
Table of Contents
A. Justification
A1. Circumstances Making the Collection of Information Necessary
A2. Purpose and Use of the Information Collection
A3. Use of Improved Information Technology and Burden Reduction
A4. Efforts to Identify Duplication and Use of Similar Information
A5. Impact on Small Businesses or Other Small Entities
A6. Consequences of Collecting the Information Less Frequently
A7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
A8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A9. Explanation of Any Payment or Gift to Respondents
A10. Protection of the Privacy and Confidentiality of Information Provided by Respondent
A11. Institutional Review Board (IRB) and Justification for Sensitive Questions
A12. Estimates of Annualized Burden Hours and Costs
A13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
A14. Annualized Cost to the Government
A15. Explanation for Program Changes or Adjustments
A16. Plans for Tabulation and Publication and Project Time Schedule
A17. Reason(s) Display of OMB Expiration Date is Inappropriate
A18. Exceptions to Certification for Paperwork Reduction Act Submissions
The goal of this
annual survey is to systematically collect information about the
implementation of program activities from each of the 29 CRCCP
awardees for Component 1 and each of the 6 awardees for Component 2.
We will be using descriptive statistics to produce awardee reports
for use by CDC for program management and technical assistance
planning, as well as for the awardees’ own program
improvement.
Attachment 1: Authorizing Legislation
Attachment 2: CRCCP Logic Model
Attachment 3: CRCCP Evaluation Question Matrix
Attachment 4a: CRCCP Annual Awardee Survey (screenshots)
Attachment 4b: CRCCP Survey Introductory Email
Attachment 4c: CRCCP Survey Reminder Email
Attachment 5a: CRCCP Clinic-Level Data Collection Instrument (screenshots)
Attachment 5b: CRCCP Clinic Data Dictionary
Attachment 5c: CRCCP Clinic-Level Data Collection Introductory Email
Attachment 6a: CRCCP Quarterly Program Update (screenshots)
Attachment 6b: CRCCP Quarterly Program Update Pre-Administration Email
Attachment 6c: CRCCP Quarterly Program Update Administration Email
Attachment 6d: CRCCP Quarterly Program Update Reminder Email
Attachment 7: 60-Day Federal Register Announcement
Attachment 8: Public Comments and Responses
Attachment 9: CRCCP Data Collection Revision Matrix
Attachment 10: Request for Determination of Research Status
Attachment 11: CRCCP DP20-2002 Awardees
The
goal of this information collection is to systematically collect
information about the implementation and outcomes of the CRCCP, a
program including 35 awardees.
CDC
will use resulting information to monitor the implementation of
CRCCP activities and evaluate outcomes achieved across all
awardees.
CDC
will conduct an Annual Awardee Survey, collect clinic-level
information, and collect an awardee-level Quarterly Program Update
representing awardees and their health system partner clinics.
The
subpopulations for both the Annual Awardee Survey and the Quarterly
Program Update are the 35 CRCCP Program Managers/Program Directors.
Clinic-level information, including a CRC screening rate,
represents clinics serving clients ages 50-75 in partner health
systems. The information will be reported to CDC by the CRCCP
Program Managers/Program Directors.
CDC
will use descriptive statistics to produce reports for CDC program
management and CRCCP awardees.
A. Justification
A1. Circumstances Making the Collection of Information Necessary
CDC is requesting a reinstatement with change to OMB No. 0920-1074 for the information collection titled, “Colorectal Cancer Control Program (CRCCP) Monitoring Activities” (expiration date 06/30/2020).
Colorectal cancer (CRC) is the second leading cause of death from cancer in the United States among cancers that affect both men and women. There is substantial evidence that CRC screening reduces the incidence of and death from the disease. Screening for CRC can detect disease early when treatment is more effective and prevent cancer by finding and removing precancerous polyps. Of individuals diagnosed with early stage CRC, more than 90% live five or more years. Despite strong evidence supporting screening, only 68.8% of adults currently report being up to date with CRC screening as recommended by the U.S. Preventive Services Task Force in 2018, with more than 22 million age-eligible adults estimated to be untested. There are significant disparities in screening rates. Lower screening rates are observed among persons with lower incomes, lower education, lack of health insurance, and lack of a regular health provider. Compared to non-Hispanic Whites and non-Hispanic Blacks, persons that are Asian/Pacific Islander, American Indian/Alaskan Native, and Hispanic have lower screening rates. To reduce CRC morbidity, mortality, and associated costs, use of CRC screening tests must be increased among age-eligible adults with the lowest CRC screening rates.
To address this problem, the Division of Cancer Prevention and Control issued the Colorectal Cancer Control Program (CRCCP): Organized Approaches to Increase Colorectal Cancer Screening (DP15-1502), a five-year cooperative agreement intended to increase CRC screening rates among an applicant defined target population of persons ages 50-75 years within a partner health system serving a defined geographical area or disparate population. DP15-1502 funded a total of 30 awardees to implement four priority EBIs (provider assessment and feedback, provider reminders, patient reminders, and reducing structural barriers) and supporting strategies (SAs; patient navigation, small media, and activities facilitating community-clinical linkages) to increase clinic-level CRC screening rates within partner health systems. Six of the 30 awardees also received funding to support clinical service delivery.
In 2020, CDC issued a new five-year cooperative agreement, Public Health and Health System Partnerships to Increase Colorectal Cancer Screening in Clinical Settings (DP20-2002), to fund 35 recipients to partner with health systems and individual primary care clinics to implement EBIs to increase CRC screening among defined populations of adults ages 50-75 that have CRC screening rates lower than the national, regional, or local rate. DP20-2002 is the programmatic successor to DP15-1502, with new requirements and an updated logic model. The number of awardees increased from 30 to 35 recipients. In addition to partnering with health systems and their primary care clinics to implement multiple EBIs; partnering with organizations to support implementation of EBIs in those clinics; and collecting high-quality clinic-level data at baseline and annually to assess implementation and identify screening rate changes, DP20-2002 requires recipients to conduct a formal readiness assessment of potential clinics to implement EBIs and use assessment findings to select appropriate EBIs for implementation. DP20-2002 no longer requires implementation of SAs, and eliminated funding to provide direct clinical service delivery; however, recipients can provide clinics with limited resources to support completion of follow-up colonoscopies for persons with inadequate or no insurance after a positive or abnormal CRC screening test.
Based on modified programmatic goals and activities for DP20-2002, the information collection has been revised. Specifically, items within the Annual Awardee Survey and clinic-level data collection were revised, removed, or added to gather the most useful data to inform CDC’s TA and guidance activities during the five-year DP20-2002 funding cycle. We also propose a new awardee-level Quarterly Program Update to collect awardee expenditures; staffing vacancies; program successes and challenges; awardees’ TA needs; and effect of COVID-19 on the CRCCP implementation on a quarterly basis to closely monitor areas for tailored CDC TA. CDC is authorized to collect information by the Public Health Service Act (see Attachment 1 – Authorizing Legislation).
A2. Purpose and Use of the Information Collection
CDC is required by DP20-2002 to monitor and evaluate both process and outcome measures for the CRCCP. Awardees are required to report information to CDC to support these efforts. In redesigning the CRCCP, CDC developed a detailed program logic model to reflect activities/strategies and expected outcomes over time (see Attachment 2 – CRCCP Logic Model). The logic model guided development of CDC’s CRCCP monitoring and evaluation plan, which includes evaluation questions and sub-questions (Attachment 3 – CRCCP Evaluation Question Matrix) to be addressed through systematic monitoring and evaluation of these key strategies and the primary outcome of screening rate changes over time.
Three forms of information collection – two revised and one new – will be implemented to answer our evaluation questions regarding program activities and outcomes. These include an Annual Awardee Survey, clinic-level data collection, and awardee-level Quarterly Program Update.
Annual Awardee Survey
CDC proposes use of an modified CRCCP Annual Awardee Survey (see Attachments 4a – CRCCP Annual Awardee Survey (screenshots)) that eliminates many questions regarding program management (now collected via the proposed awardee-level Quarterly Program Update), clinic service delivery (funding eliminated), and data use (did not yield meaningful results for CDC). Based on experiences during DP15-1502, CDC found that many of the program management elements gathered via the annual survey did not derive high quality, timely data that could be used by CDC staff to provide meaningful TA. We determined that this information would be more useful if monitored on a quarterly basis (see Quarterly Program Update description below). The revised survey continues to assess health IT (now called Data Management) and partnerships, in addition to clinic readiness assessment activities and the effect of COVID-19 on CRC implementation at the awardee level. Survey questions are of various types, including dichotomous, multiple response, and free text. CDC will conduct the survey among all 35 awardees following the end of each program year.
This information collection consists of a web-based questionnaire including seven sections with approximately 17 questions; however, the number of items completed by a respondent will vary due to skip logic. Questions are of various types including dichotomous, multiple response, and open-ended. To reduce burden there are a limited number of questions requiring open-ended or narrative responses. The seven sections of the survey include:
Respondent information
Program Management
Assessment
Data Management (formerly Health IT)
Technical Assistance (formerly Training and Technical Assistance)
Partnerships
COVID-19
The Annual Awardee Survey will be implemented within three months after the end of each awardee program year, which runs from July 1 - June 30. The Program Director or Manager for each cooperative agreement will serve as the survey respondent. Contact information for the awardee is obtained from program administrative systems and used to distribute survey introductory and reminder emails only (See Attachment 4b – CRCCP Survey Introductory Email and Attachment 4c – CRCCP Survey Reminder Email). The CDC contactor will manage primary information collection and send respondents a unique link to an online instrument (not to a website) that will enable awardees’ access to view and enter their survey information. After receiving responses to the survey, the contractor will prepare a validated analysis file and set of reports for CDC to assist in interpreting results. CDC will prepare and distribute awardee-specific and CRCCP summary feedback reports. The online information collection instrument software will be developed using an open-source product called LimeSurvey (limesurvey.org).
Clinic-Level Data Collection
Clinic-level data collection assesses EBI implementation over time and CRCCP’s primary outcome of interest: CRC screening rates within partner health system clinics. Health systems typically include multiple primary care clinic sites. CRCCP awardees collect and report CRCCP clinic-level information for health system partners’ clinic sites that are participating in the CRCCP. Awardees collect baseline information regarding health system and clinic characteristics, EBI/SA implementation, and CRC screening rates. Awardees then collect and report a CRC screening rate and updated information about EBI/SA implementation annually for the duration of the FOA. Each awardee will partner with an estimated 24 primary care clinics to implement the CRCCP. The modified CRCCP DP20-2002 instrument eliminates several variables and includes new and revised variables to better assess health system and clinic characteristics; program reach; CRC screening practices and outcomes; clinics’ quality improvement and monitoring activities; EBI implementation; additional factors that affect EBI implementation over time; and the effect of COVID-19 on CRCCP implementation within clinics (see Attachment 5a– CRCCP Clinic-Level Data Collection Instrument (screenshots) and Attachment 5b – CRCCP Clinic Data Dictionary). Clinic-level information will help CDC to describe program reach; health system and clinic settings; characteristics of the population being served; EBIs and other program activities implemented; screening completion and follow-up care; follow-up colonoscopies and results paid for with CDC funds; and changes in CRC screening rates over time.
The instrument consists of 7 sections with approximately 193 variables; however, the number of items completed by a respondent will vary depending on how many optional, open-ended fields they choose to complete for additional context. In general, the instrument has been reorganized (e.g., sections merged, variables moved to new sections) for increased efficiency and to improve overall data quality. Wording and responses for many variables and their response options have undergone minor revisions to better capture awardees’ partnerships with health systems and clinics, and improve capture of baseline and annual variables. The 7 sections of the clinic-level data collection include:
Record Identification Fields
Health System and Clinic Characteristics, and Clinic Population
Annual CRC Screening Rates and Practices
Monitoring and Quality improvement
Priority EBIs and SAs
Annual Implementation Factors
COVID-19
The clinic data collection will be collected within three months after the end of each awardee program year, which runs from July 1 - June 30. This information collection consists of aggregate data from each clinic site where CRCCP program interventions are implemented. Information will be reported through an online information data entry instrument accessible to awardees on the pre-existing secure CRCCP program website (www.crccp.org) to simplify the reporting process with centralized information collection, validation, access control and technical support (Attachment 5a - CRCCP Clinic-Level Collection Instrument (screenshots)).
Quarterly Program Update
The purpose of the new awardee-level Quarterly Program Update is to collect standardized information from awardees on their (1) federal award spending, (2) current staff vacancies, (3) program successes and challenges, (4) TA needs, and (5) the effect of COVID-19 on CRCCP implementation at the grantee level (Attachments 6a—Quarterly Program Update (screenshots)). These data are collected quarterly to support rapid reporting of programmatic information to support CDC program consultants in providing tailored and meaningful TA. The items included in the Quarterly Program Update are intended to replace many of the program management questions formerly included in the Annual Awardee Survey as the latter were not available until after the end of the program year and were therefore not useful to inform timely TA. The Quarterly Program Update will be administered among all 35 awardees in the month following each program quarter (i.e., October, January, April, July).
The instrument consists of six sections with approximately 11 questions; however, the number of questions completed by each respondent will vary due to skip logic. Question formats include dichotomous, multiple response, and open-ended. The six sections of the Quarterly Program Update include:
Respondent information
Award spending
Staff vacancies
Program successes and challenges
Technical assistance needs
COVID-19
The Program Director or Manager will serve as the respondent for the Quarterly Program Update. The CDC contactor will manage primary information collection and send respondents a unique link to an online instrument (not to a website) that will enable awardees’ access to view and enter their program information. The online instrument will be developed using an open-source product called LimeSurvey (limesurvey.org). Contact information for the awardee is obtained from program administrative systems and used to distribute Quarterly Program Update pre-administration, administration, and reminder emails (Attachments 6b-d). After receiving responses to the Quarterly Program Update, the contractor will prepare an analysis file and set of reports for CDC to assist in interpreting results. CDC will use awardee-specific and aggregate information to inform ongoing CDC TA and guidance.
Together, the proposed information collection activities are expected to contribute to a more effective CRCCP and strengthen CDC’s ability to demonstrate program results. These monitoring activities will also help to identify successful implementation activities that need to be maintained, replicated, or expanded; provide insight into programmatic areas needing improvement; and identify program activities and management efforts requiring immediate CDC TA. Additionally, the information collection supports the national evaluation of the CRCCP, including assessing implementation and program outcomes.
The scope of the information collection is limited to monitoring the public health activities and experiences of CRCCP awardees acting in their official capacity. Personal identifying information will not be collected, and the information collection will not yield information that can be generalized. As such, this information collection will not require IRB review. CDC will use this information to better understand the range of experiences among awardees and as one of many inputs into decision-making and/or program management. In addition, the findings will be reported back to the awardees to help them identify areas for program improvement and successful implementation models and focus networking for shared experiences, lessons learned, and best practices.
A3. Use of Improved Information Technology and Burden Reduction
The Annual Awardee Survey will be administered annually via an online questionnaire allowing respondents to complete and submit their responses electronically (Attachment 4a – CRCCP Annual Awardee Survey (screenshots)). Clinic-level information will be collected annually through an online instrument (Attachment 5a – CRCCP Clinic-Level Data Collection Instrument (screenshots)). The Quarterly Program Update information will be collected following the end of each program quarter (October, January, April, and July) via an online instrument (Attachment 6a – CRCCP Quarterly Program Update (screenshots)). All three information collections use pre-existing web infrastructure and tools easily accessible by CRCCP awardees. These methods were chosen to facilitate the responses for respondents.
A4. Efforts to Identify Duplication and Use of Similar Information
The information to be collected from the CRCCP awardees is unique to the current program and, therefore, not duplicative of other efforts.
A5. Impact on Small Businesses or Other Small Entities
No small businesses will be involved in this information collection.
A6. Consequences of Collecting the Information Less Frequently
The purpose of this request is to ensure collection of information that is not otherwise available in a current, time sensitive, or standardized format to specific or emergent priorities of HHS and CDC. CRCCP information collection was previously approved; however, this request proposes use of a revised CRCCP Annual Awardee Survey (Attachment 4a), revised CRCCP Clinic-Level Data Collection (Attachment 5a), and new Quarterly Program Update (Attachment 6a) to better align with the new cooperative agreement DP20-2002 while only collecting the most essential data elements to support the national evaluation of the CRCCP, as well as CDC’s TA provision. The quarterly program update will be collected following each program quarter (i.e., October, January, April, July) to support rapid reporting of programmatic information to inform ongoing TA provision. Without this information collection, there would be:
No systematic information collection regarding the implementation of program activities and outcomes, as required in the current NOFO.
Less timely and less effective assessment of training and TA needs.
No systematic monitoring and evaluation efforts at the clinic level.
Less effective and less timely assessment of implementation partners and their program activities.
Fewer resources from which to make data-driven decisions that are often required of CDC as well as required of its awardees.
No ability for CDC to conduct a national evaluation of the CRCCP and provide results to Congress and the public.
OMB approval is requested for three years. There are no legal obstacles to reduce the burden.
A7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
There are no special circumstances with this information collection package. This request fully complies with the regulation 5 CFR 1320.5 and will be voluntary.
A8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
PUBLIC NOTICE
Notice of this project was published in the Federal Register on June 5, 2020 in Vol. 85, No 109, pages 34631-34633. (See Attachment 7 – 60-Day Federal Register Announcement). CDC received two non-substantive comments (Attachment 8 – Public Comments and Responses) and provided responses to each.
CONSULTATION
The individuals listed below, including CDC contractors and external partners organizations, provided expert consultation into the redesign of DP20-2002 monitoring activities, including the development of data collection tools and protocols.
Table A.8.1. Individuals Who Have Provided Consultation on the Project
Consultant |
Title |
Affiliation |
Phone |
Year of Consult |
|
Cam Escoffery
|
Associate Professor |
Emory University |
cescoff@emory.edu |
404-727-4701 |
2015 |
Peggy Hannon |
Director, Associate Professor |
University of Washington School of Public Health |
peggyh@uw.edu |
206-616-7859 |
2015 |
Thuy Vu |
Research Coordinator |
University of Washington School of Public Health |
thuytvu@uw.edu |
206-616-4724 |
2015 |
Annette Maxwell
|
Professor |
University of California – Los Angeles
|
amaxwell@ucla.edu |
310-794-9282 |
2015 |
Kathleen McNamara |
Associate Vice President, Clinical Affairs |
National Association of Community Health Centers (NACHC) |
kmcnamara@nachc.com |
301-347-0400 |
2015 |
Ben Reisler |
Clinical Data Specialist |
National Association of Community Health Centers (NACHC) |
breisler@nachc.com |
301-347-0411 |
A9. Explanation of Any Payment or Gift to Respondents
CDC will not provide payments or gifts to respondents.
A10. Protection of the Privacy and Confidentiality of Information Provided by Respondent
This submission has been reviewed by CDC’s Information System Security Office, who determined that the Privacy Act does not apply. The information to be collected is programmatic in nature and does not involve research with human subjects. IRB approval is not required. No personally identifiable information is collected.
The clinic-level information identifies the partner health system and clinic by name and includes the clinic address – both of which are publicly available. Only aggregate clinic-level data are included in this collection; no patient-level data are collected. The health system and clinic name, in addition to an assigned ID, are used to ensure accurate identification of the clinic when reporting longitudinal (baseline and annual) information, and to compare clinic implementation activities with awardee work plans. Except for in feedback reports to awardees, CDC will not identify the name of the health system or clinic partner. Information is treated in a secure manner and will not be disclosed, unless otherwise compelled by law.
For the Annual Awardee Survey and clinic-level data, each awardee respondent will receive customized feedback reports relating to their program. Awardees will not have access to other awardees’ submissions or individualized reports. Program summary information by awardee and CRCCP aggregate results (e.g., performance ranges) will be shared across programs for awardees to compare performance and identify networking opportunities with others engaging in similar activities. For the Quarterly Program Update, collected information will be shared internally only among CDC staff to inform routine and ad hoc TA provision. CDC team members will utilize secure internal systems, such as SharePoint, to share awardee-specific and aggregate information about awardee expenditures, staffing, successes, challenges, and TA needs.
Information will ultimately be used by CDC to monitor and evaluate the CRCCP; provide feedback and TA to awardees; inform stakeholders on program processes and outcomes; and inform planning for future programs. DCPC evaluators will prepare formal reports periodically. CDC does not plan to create a public use dataset given the programmatic nature of the information and its strict application for monitoring the 35 CRCCP awardees. Program Announcement CDC-RFA-DP20-2002, the CRCCP funding announcement, requires that CDC monitor and evaluate CRCCP processes (i.e., implementation) and outcomes.
Respondents are notified that their information will be maintained in a secure manner and that they will receive individualized feedback reports for their use. There are no advisements that relate to data sharing since CDC has no plans to share information or develop a public-use data set. There is no impact on the respondent’s privacy.
For all information collections, the contractor will host the instrument and archive information on secure network servers. The contractor will aggregate and validate the information for quality and completeness and prepare analysis files and reports for delivery to CDC. All three information collections are secured by technical, physical, and administrative safeguards as outlined below.
Technical
All data reside on a dedicated server on the contractor’s local area network behind the contractor’s firewall and is password protected on its own security domain. Access to the server is limited to the contractor’s authorized project staff. No non-project staff is allowed access to the data. All of the contractor’s project staff are required to sign a confidentiality agreement before passwords and keys are assigned.
Access to the CRCCP program website, which houses the clinic-level data collection instrument (Attachment 5a – CRCCP Clinic-Level Data Collection Instrument (screenshots)), is restricted via a password-protected secure website. Access to awardee-specific reports and clinic-level data entry systems are further restricted within the website. Each awardee has its own directory location, so no awardee has access to another awardee’s information. The CRCCP program website utilizes the Hypertext Transfer Protocol Secure (HTTPS) to ensure secure connections. In addition, the website will enable Strict Transport Security (HSTS), which is in compliance with OMB memorandum M-15-13, Policy to Require Secure Connections across Federal Websites and Web Services.
Once information has been compiled by the contractor and delivered to CDC via electronic transfer, all data are maintained for restricted access on CDC’s secure LAN server with access permission granted by the CDC CRCCP data manager.
Physical
The contractor’s server is housed in a secure facility with restricted access.
Receipt and processing logs are maintained to document data receipt, file processing and report production.
Once data have been compiled by the contractor and delivered to CDC, all datasets are maintained for restricted access on a secure LAN server, which is housed in a secure facility. All CDC staff are issued identification badges and access to the building is controlled by key cards.
Administrative
CDC and contract staff have developed and implemented an information system security plan to ensure that the information is kept secure. Periodic review and update of the contractor’s security processes is conducted to adjust for needed changes and will be amended as needed to maintain the continued security of the information.
The contractual agreement between CDC and the contractor includes non-disclosure terms. The contractor’s project security team oversees operations to prevent unauthorized disclosure of the CRCCP data.
Once the information has been delivered to CDC, data are housed on CDC’s secure LAN server and restricted access is controlled by the CRCCP data manager.
A11. Institutional Review Board (IRB) and Justification for Sensitive Questions
No information will be collected that are of personal or sensitive nature. IRB approval is not required (Attachment 10 – Request for Determination of Research Status)
A12. Estimates of Annualized Burden Hours and Costs
There are a total of 35 awardees in DP20-2002. The estimated burden hours for the revised CRCCP Annual Awardee Survey (17 survey questions) are based on a pilot test of the information collection instruments by 5 public health professionals. There was a 77% reduction in the number of survey items; however, we anticipate that items requiring financial information (survey items #3 and #12) will require awardees to gather additional program information. Therefore, the estimated burden is 15 minutes per response. The overall burden estimate decreases from 12 to 9 burden hours.
The estimated burden hours for the revised CRCCP clinic-level information collection tool (193 data variables) are based on a pilot test of the information collection instrument by 4 public health professionals. There was a 54% increase in data variables. The estimated burden is 50 minutes per response. CDC estimates an increase in the average of 24 responses per awardee annually to correspond with the number of anticipated health system and clinic partners. The overall burden estimate increases from 192 to 700 burden hours.
The estimated burden hours for the Quarterly Program Update are based on a pilot test of the information collection instrument by 3 public health professionals. In the pilot test, the average time to complete the instrument was approximately 22 minutes. The overall estimate for quarterly submissions is 51 hours burden hours.
Estimates for the average hourly wage for respondents are based on National Occupational Employment and Wage Estimates for “management occupations – medical and health services managers in state government” developed by the Department of Labor, Bureau of Labor Statistics (https://www.bls.gov/oes/current/oes_nat.htm#11-0000) Based on these data, an average hourly wage of $54.68 is estimated for all respondents.
Table A.12.A. Estimated Annualized Burden Hours
Type of Respondent |
Form Name |
Number of Respondents |
Number of Responses per Respondent |
Average Burden per Response (in hr) |
Total Burden (in hr) |
CRCCP Annual Awardee Survey |
35 |
1 |
15/60 |
9 |
|
CRCCP Clinic-level Data Collection Instrument |
35
|
24 |
50/60 |
700 |
|
CRCCP Quarterly Program Update |
35 |
4 |
22/60 |
51 |
|
|
Total |
||||
Table A.12.B. Estimated Annualized Burden Costs
Type of Respondent |
Form Name |
Number of Respondents |
Total Burden Hours |
Hourly Wage Rate |
Total Cost |
CRCCP Awardees |
CRCCP Annual Awardee Survey |
35 |
9 |
$54.68 |
$492 |
CRCCP Clinic-level Data Collection Instrument |
35 |
700 |
$54.68 |
$38,276 |
|
CRCCP Quarterly Program Update |
35 |
51 |
$54.68 |
$2,789 |
|
Total |
|
|
|
|
$41,557 |
A13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
There will be no direct costs to the respondents other than their time to participate in each information collection.
A14. Annualized Cost to the Government
Total operations and maintenance costs includes work performed by both the contractor and CDC personnel. Salary cost of CDC staff include four Health Scientist FTEs (two GS-14 and two GS-13) to lead and coordinate project activities, including data collection, data management, analysis, and report preparation. One hundred and twenty hours of staff time was estimated for each GS-14 FTE and 60 hours of staff time was estimated for each GS-13 FTE annually for this information collection. Cost of the contractor represents an estimated 35% ($154,913) of total annual contract funds ($491,788) allocated for CRCCP data management activities. The estimated cost to the federal government is $781,028. Table A.14 describes how the cost estimate was calculated.
Table A.14. Estimated Annualized Cost to the Federal Government
Staff (FTE) |
Average Hours per Collection |
Average Hourly Rate |
Average Cost |
Lead Health Scientist (GS-14) Lead overall coordination of all aspects of CRCCP information collection, including administration, analysis, and reporting for the awardee survey, clinic-level data collection, and quarterly program update. |
120 |
$59.86 |
$7,183 |
Health Scientist (GS-14) Lead coordination of clinic-data collection and quarterly program update, data management, analysis, and report preparation. |
120 |
$59.86 |
$7,183 |
Health Scientist (GS-13) Lead coordination of awardee survey administration, analysis, and report preparation |
60 |
$50.66 |
$3,040 |
Health Scientist (GS-13) Lead preparation and submission of OMB package, and planning and coordination of data collection tools and protocols |
60 |
$50.66 |
$3,040 |
Contractor Costs |
|
|
|
Annualized Cost of Contract with Information Management Services Responsible for building online instrument platform, information collection, coding and entry, quality control, analysis, report preparation |
|
|
$680,582 |
CDC Data Manager Responsible for supporting data management, analysis, and report preparation |
|
|
$80,000 |
Estimated Total Cost of Information Collection |
$781,028 |
||
A15. Explanation for Program Changes or Adjustments
This is a request to reinstate OMB No. 0920-1074 to reflect modified goals for the new cooperative agreement and a modified monitoring plan. During the previous CRCCP cooperative agreement DP15-1502 (July 1, 2015 – June 30, 2020), the information collection consisted of an annual grantee survey and clinic-level information collection. The proposed changes include modifying the name of the Annual Grantee Survey to “Annual Awardee Survey” and removing data collection related to clinical service delivery (Component 2 eliminated) and program management (more appropriately assessed on a quarterly basis via the Quarterly Program Update; Attachment 4a – CRCCP Annual Awardee Survey). The modified survey decreased from 73 to 17 questions and reduced burden from 12 to 9 hours. The revised clinic-level data collection instrument (see Attachment 5a – CRCCP Clinic-Level Data Collection Instrument) includes variables to assess health system and clinic characteristics; program reach; CRC screening practices and outcomes; clinics’ quality improvement and monitoring activities; EBI implementation; additional factors that affect EBI implementation over time; and the effect of COVID-19 on CRCCP implementation within clinics. The instrument was also reorganized (e.g., sections merged, variables moved to new sections) for increased efficiency and overall data quality improvement. CDC estimates an average of 24 responses per awardee annually (compared to 12 responses previously) to correspond with the number of anticipated health system and clinic partners. These changes increased the number of variables from 125 to 193, and increased burden from 192 hours to 700 hours. In addition, we propose the addition of an awardee-level Quarterly Program Update to monitor awardees’ federal award spending; staffing vacancies; program successes and challenges; technical assistance needs; and the effect of COVID-19. The new Quarterly Program Update consists of 11 questions and adds 51 burden hours. The overall burden increases from 204 to 760 burden hours. Exhibit 1 illustrates key changes proposed for CRCCP information collection. See Attachment 9 – CRCCP Data Collection Revision Matrix for a list of specific changes by section for each instrument, and rationale for each change within the revised instruments.
Exhibit 1: Key Changes to CRCCP Information Collection Plan
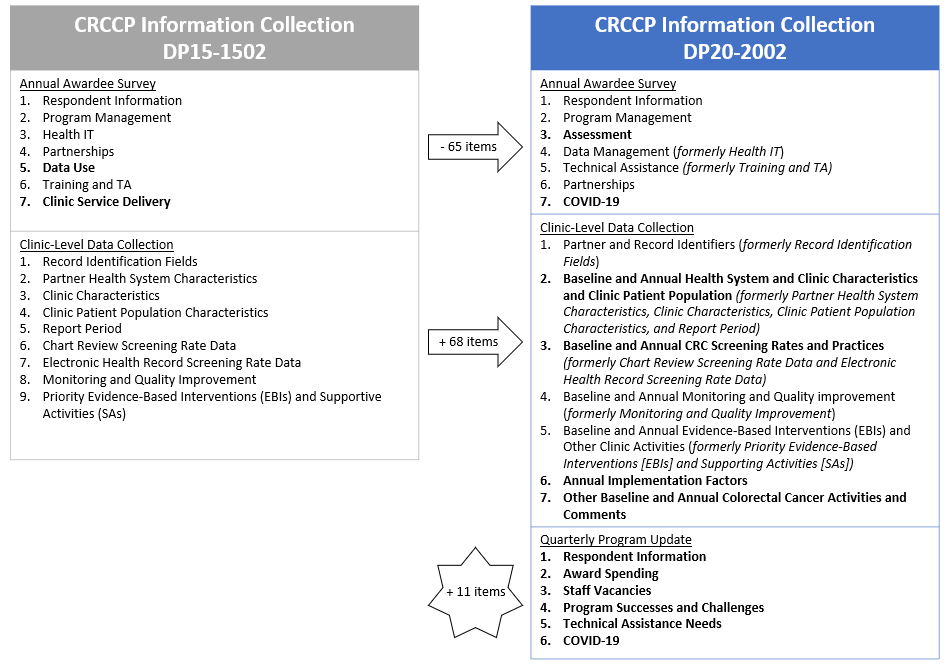
Table A.15. Changes in Information Collection
Type of respondents |
Form |
|
Number of respondents
|
Frequency of Response |
Average Burden per Response (in hours) |
Total Response Burden (in hours) |
CRCCP Awardees |
CRCCP Annual Awardee Survey
|
Prior Approval |
30 |
1 |
.48 |
12 |
Reinstatement Request |
35 |
1 |
.25 |
9 |
||
Net Change |
+5 |
- |
-.23 |
-3 |
||
CRCCP Clinic-Level Data Collection |
Prior Approval |
30 |
12 |
.53 |
192 |
|
Reinstatement Request |
35 |
24 |
.83 |
700 |
||
Net Change |
+5 |
+12 |
-.30 |
+508 |
||
CRCCP Program Update Tool |
Prior Approval |
- |
- |
- |
- |
|
Reinstatement Request |
35 |
4 |
.37 |
51 |
||
Net Change |
+35 |
+4 |
+.37 |
+51 |
||
Total: |
+556 |
|||||
A16. Plans for Tabulation and Publication and Project Time Schedule
The CRCCP Annual Awardee Survey and CRCCP Clinic-Level Data Collection Instrument will be completed annually within 3 months after the end of each program year (July – September). The Quarterly Program Update will be completed the month following the end of each quarter (October, January, April, and July). Data validation, analysis, and report preparation and dissemination will follow. A summary timeline is provided below:
Estimated Project Time Schedule
Activity |
Time Schedule |
CRCCP Clinic-Level Data Collection Introductory Email sent to respondents |
July One week prior to information collection |
CRCCP Clinic-Level Data Collection Instrument available for reporting, information collection conducted |
July-September Awardees report annual data once each year for each clinic |
Introductory emails for Annual Awardee Survey sent to respondents with link to survey; information collection begins. |
July Information collection continued for 30 business days |
Annual Awardee Survey reminder emails sent to non-responders |
July 10 business days after introductory emails sent |
Annual Awardee Survey data validation |
August |
Annual Awardee Survey analysis and dissemination |
September - October Completed 2 months after end of information collection |
Pre-administration emails for 1st quarter Quarterly Program Update sent to respondents |
September One week prior to information collection |
Administration emails for 1st Quarterly Program Update sent to respondents with link to instrument; information collection begins |
October Following end of first quarter, July – September Information collection continues for 10 business days |
Reminder emails for 1st Quarter Program Update sent (non-responders only) |
October 10 business days after introduction email |
CRCCP Clinic-Level Data validation |
October - November |
Data analysis and dissemination for 1st Quarter Program Update (awardees and CDC-only) |
November Completed 1 month after end of information collection |
CRCCP Baseline and Annual Data analysis and dissemination |
December – January Completed 4 months after end of information collection |
Pre-administration emails for 2nd Quarterly Program Update sent to respondents |
December One week prior to information collection |
Administration emails for 2nd Quarter Program Update sent to respondents with link to instrument; information collection begins |
January Following end of second quarter, October – December Information collection continues for 10 business days |
Reminder emails for 2nd Quarter Program Update sent (non-responders only) |
January 10 business days after introduction email |
Data analysis and dissemination for 2nd Quarter Program Update (awardees and CDC-only) |
February Completed 1 month after end of information collection |
Pre-administration emails for 3rd Quarter Program Update sent to respondents |
March One week prior to information collection |
Administration emails for 3rd Quarter Program Update sent to respondents with link to instrument; information collection begins |
April Following end of 3rd quarter, January – March Information collection continues for 10 business days |
Reminder emails for 3rd Quarter Program Update sent (non-responders only) |
April 10 business days after introduction email |
Data analysis and dissemination for 3rd Quarter Program Update (awardees and CDC-only) |
May Completed 1 month after end of information collection |
Pre-administration emails for 4th quarter Quarterly Program Update sent to respondents |
June One week prior to information collection |
Administration emails for 4th Quarter Program Update sent to respondents with link to instrument; information collection begins |
July Following end of 4th quarter, April – June Information collection continues for 10 business days |
Reminder emails for 4th Quarter Program Update sent (non-responders only) |
July 10 business days after introduction email |
Data analysis and dissemination for 4th Quarter Program Update (awardees and CDC-only) |
August Completed 1 month after end of information collection |
A17. Reason(s) Display of OMB Expiration Date is Inappropriate
We are requesting no exemption.
A18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification. These activities comply with the requirements in 5 CFR 1320.9.
Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | gel2 |
| File Modified | 0000-00-00 |
| File Created | 2021-01-13 |
© 2026 OMB.report | Privacy Policy