Spirometry procedures
Understanding Long-term Respiratory Morbidity in Former Styrene-exposed Workers: Medical Survey
Attachment 8 Spirometry SOP.rtf
Spirometry procedures
OMB: 0920-1332
Attachment 8 Spirometry SOP
I
Form
Approved OMB
NO. 0920-xxxx Expiration
Date: xx/xx/20xx
Spirometry may be used to measure lung function volume during forced breathing maneuvers. The important measurements include: forced vital capacity (FVC) or the greatest volume of air exhaled from a maximal inspiration to a complete exhalation, the forced expiratory volume in one second (FEV1) or the volume of air exhaled in the first second of a FVC maneuver, and the ratio between these two values (FEV1/FVC). All procedures will conform to current American Thoracic Society (ATS) and the 2020 American College of Occupational and Environmental Medicine (ACOEM) guidelines.1 Test results will be compared to predicted and lower limit of normal values determined from the third National Health and Nutrition Examination Survey (NHANES III) reference equations.2
II. INDICATIONS
To detect obstructive, restrictive, and mixed lung function patterns.
III. CONTRAINDICATIONS3
Hemoptysis (coughing up blood)
Pneumothorax (collapsed lung)
Chest pain, myocardial infarction (MI), or stroke within the last 3 months
Abdominal (belly) or cerebral (brain) arterial aneurysm
Eye surgery including LASIK within the last 3 months
Acute disorders that might affect subject performance during testing: e.g., chest, back or gastrointestinal (GI) distress/discomfort
Chest or abdominal surgery within last 3 months
Systolic Blood Pressure >180 or Diastolic Blood Pressure >110 mmHg or pulse rate > 110 beats per minute (See Appendix 4 for Blood Pressure Measurement Protocol)
If any of the contraindications are present, the technician will not proceed with the test.
Public
reporting burden of this collection of information is estimated to
average 5 mins per response, including the time for reviewing
instructions, searching existing data sources, gathering and
maintaining the data needed, and completing and reviewing the
collection of information. An agency may not conduct or sponsor, and
a person is not required to respond to a collection of information
unless it displays a currently valid OMB control number. Send
comments regarding this burden estimate or any other aspect of this
collection of information, including suggestions for reducing this
burden to CDC/ATSDR Information Collection Review Office, 1600
Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA
(0920-xxxx).
IV. DESCRIPTION OF EQUIPMENT AND SUPPLIES
S
Public
reporting burden of this collection of information is estimated to
average 5 mins per response, including the time for reviewing
instructions, searching existing data sources, gathering and
maintaining the data needed, and completing and reviewing the
collection of information. An agency may not conduct or sponsor, and
a person is not required to respond to a collection of information
unless it displays a currently valid OMB control number. Send
comments regarding this burden estimate or any other aspect of this
collection of information, including suggestions for reducing this
burden to CDC/ATSDR Information Collection Review Office, 1600
Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA
(0920-xxxx).
Volume Spirometer (See Appendix 2 for manufacturer contact information):
Ohio 822/ 827 or SensorMedics 922 or 1022 dry rolling seal with digital volume encoder to RS232 interface
OMI-HF5 spirometry software program (most recent NIOSH version) installed on a notebook computer
C
Public reporting burden of this collection of information is estimated to average 5 mins per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Information Collection Review Office, 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA (0920-xxxx).
Public reporting burden of this collection of information is estimated to average 5 mins per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Information Collection Review Office, 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA (0920-xxxx).
Public reporting burden of this collection of information is estimated to average 10 mins per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Information Collection Review Office, 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA (0920-xxxx).
alibration syringe (3.00 liters- Hans Rudolph model #5530)
Spirometer hoses (#894787 2” ID x 28” length- SensorMedics)
Spirotube mouthpieces (1/16” OD x 3 3/16 ” length- SensorMedics)
Mouthpiece adaptors – hose reducer (#894788 2” OD, 11/16” ID - SensorMedics)
Nose clips
Miscellaneous supplies: pens, log sheets, barometer, first aid kit (including ammonia capsules), tape measure, scale, germicidal disposable cloths, tissues, memory stick, stopcock grease, room thermometer, waste basket
Flow Spirometer (See Appendix 2 for manufacturer contact information):
EasyOne™ diagnostic spirometer with EasyWare™ software installed on a notebook computer and a USB Connector Spiro Screen or an EasyOnePro™ system.
Calibration syringe
Syringe adapter
Spirette™ mouthpieces (or SDI mouthpiece)
Nose clips
Miscellaneous supplies: pens, log sheets, barometer, first aid kit (including ammonia capsules), tape measure, scale, germicidal disposable cloths, tissues, memory stick, room thermometer, waste basket
V. PREPARATION AND CALIBRATION OF EQUIPMENT
Before Site Visit
Calibrate spirometers and verify them to be in correct operating condition. Verify that software parameters are correctly defined (for volume spirometers see Appendix 1, Part A, for flow spirometers see Appendix 1, Part B).
At Site
Set up equipment and connect cables. Connect power cords to grounded electrical receptacles.
Turn on the spirometer and then the computer.
Record room temperature and barometric pressure.
Perform volume calibration and leak check using 3-Liter syringe (repeat after every 4 hours of testing).
Perform biological control test at beginning and end of each testing session.
VI. PRE-TEST PROCEDURE
Measure and record height and weight of subject without shoes.
Measure and record blood pressure and pulse rate of subject (see Appendix 4 for procedure for obtaining blood pressure). Record blood pressure on wallet card and give to subject for their records. If blood pressure or pulse rate is a contraindication for spirometry (Systolic Blood Pressure >180 or Diastolic Blood Pressure >110 or pulse rate > 110 beats per minute), explain to the subject the reason the test will not be performed and advise the subject to seek immediate guidance from their physician. If blood pressure or pulse rate are elevated (Systolic Blood Pressure between 120 and 180, or Diastolic Blood Pressure between 80 and 110, or pulse rate between 100 and 110 beats per minute) and the test can still be performed, advise the subject to recheck their measurements within a week and if still elevated to seek guidance from their physician.
Administer the pre-test questionnaire and record if contraindications are present (see Appendix 3).
Do not perform test if any contraindications are present.
VII. TEST PROCEDURES
Explain the purpose of the examination and the need for maximal effort from the subject to get accurate results. Tell the subject, “I want to measure how much air you can blow out and how fast you can blow it out.”
Ask the subject to loosen any tight clothing.
Ask the subject to stand during the examination. If unable to perform the test standing, encourage the subject to sit up straight. Record whether subject is sitting or standing.
Demonstrate a deep inspiration and proper placement of the mouthpiece.
Blast out the air in a demonstration of the effort and length of time you expect the subject to blow.
Prepare the spirometry system for collection.
Place nose clip on subject’s nose. Clips may be removed between trials. If the nose clip falls off or is uncomfortable, ask the subject to hold their nose during the FVC maneuvers.
Encourage the subject to stand/sit up straight and not lower chin or bend over. Emphasize not straining with the neck but pushing from the belly.
Give the following instructions (enthusiastic coaching is required, shouting is not):
a. “Open your mouth, take the largest breath of air that you can inhale, while holding the hose near your mouth.”
b. “Quickly, put the mouthpiece into your mouth and seal your lips tightly around it. Blast your air into the tube as hard and fast as you can.”
c. “Keep on blowing out the same breath of air until I tell you to stop.”
d. Coach: “keep blowing, keep going, almost there,” until plateau is observed.
e. “You can stop now.”
f. “Okay, now catch your breath.”
Review the procedure and correct any problems at the end of each trial. Flow-volume curves should have sharp peak flows and volume-time curves must have a plateau and at least 6 seconds of exhalation. Record your impression of the subject’s effort at the end of each trial.
Continue testing until three acceptable trials are completed and the repeatability criteria are met or until a maximum of eight trials have been performed, or until the subject cannot or should not continue (see Appendix 5 for acceptability and repeatability criteria).
VIII. QUALITY CONTROL PROGRAM
Equipment
The Medical Instrumentation Laboratory (DRDS/FSB) maintains all spirometers, as well as detailed records of calibration results, equipment repairs or modifications, dates of software and hardware changes, and the dates and location of equipment use. Periodically, the accuracy and repeatability of the spirometers is evaluated over a range of mechanically-simulated exhalation maneuvers.1
Test Quality
Every spirometry test is given two overall quality grades, one for the measurement of FVC and one for the measurement of FEV1. The grades are assigned using the following definitions:
FVC Quality Grade:
A = at least three acceptable trials, highest two FVC values match within 100 ml (or within 50 ml if highest FVC from last trial)
B = at least two acceptable trials, highest two FVC values match within 150 ml
C = at least two acceptable trials, highest FVC values match within 250 ml
D = only one acceptable FVC trial
F = no acceptable FVC trials
FEV1 Quality Grade:
A = at least three acceptable trials, highest two FEV1 values match within 100 ml (or within 50 ml if highest FEV1 from last trial)
B = at least two acceptable trials, highest two FEV1 values match within 150 ml
C = at least two acceptable trials, highest FEV1 values match within 250 ml
D = only one acceptable FEV1 trial
F = no acceptable FEV1 trials
The quality of all spirometry tests is reviewed by a quality control specialist. Quality control reports are generated and provided to each spirometry technician. If coaching deficiencies are identified during this review, technicians receive additional training prior to the next testing session.
IX. SAFETY PROCEDURES
To prevent electrical shock, all equipment will be plugged into a grounded electrical outlet.
Infection control measures:
When a volume spirometer is used, a clean hose or mouthpiece filter will be used for each subject.
Only disposable mouthpieces will be used.
Mouthpieces are handled only by the subject.
Spirometers and accessories will be cleaned and disinfected daily.
Technicians will have successfully completed a National Institute for Occupational Safety and Health (NIOSH) spirometry training course and will have been supervised while testing 20 subjects by a trained and experienced technician.
Subjects in wheelchairs will perform the test while seated in their wheelchairs. Wheelchair wheels will be locked prior to testing.
A non-rolling chair will be placed behind subject during the test.
Subjects that become faint or tired may sit for the remainder of the test.
X. EMERGENCY PROCEDURES
An emergency card will be present at the test site. This card will include:
Instructions to call 911.
Arrangement information for transportation to the nearest hospital.
Address and telephone number for the nearest hospital.
A subject who feels faint during testing will be guided onto a stationary chair and encouraged to lower their head towards their knees and to breathe slowly and deeply until recovered.
XI. REFERENCE LIST
Miller MR, Hankinson J, Brusasco V, et al, ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005 Aug; 26(2):319-338.
Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159:179-187.
AARC (American Association for Respiratory Care) clinical practice guideline. Spirometry: 1996 update. Respir Care 1996; 36:629-636.
APPENDIX 1
Part A: Volume Spirometer Setup Parameters
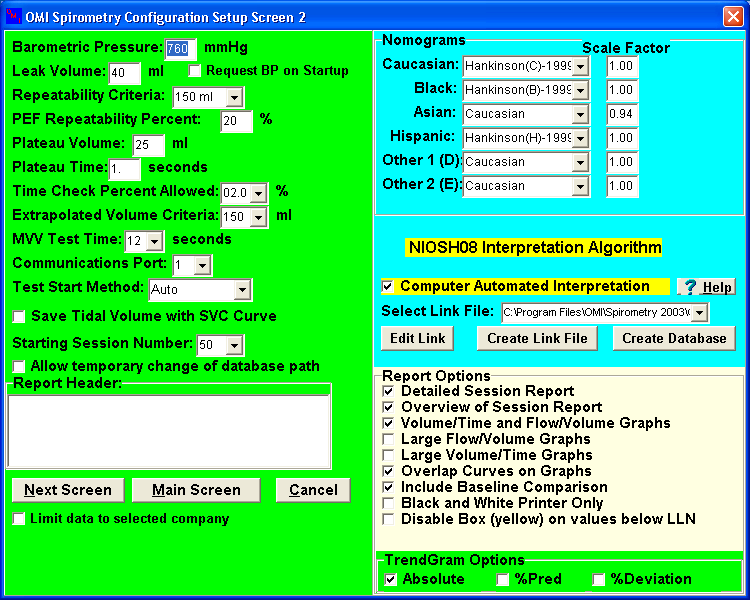
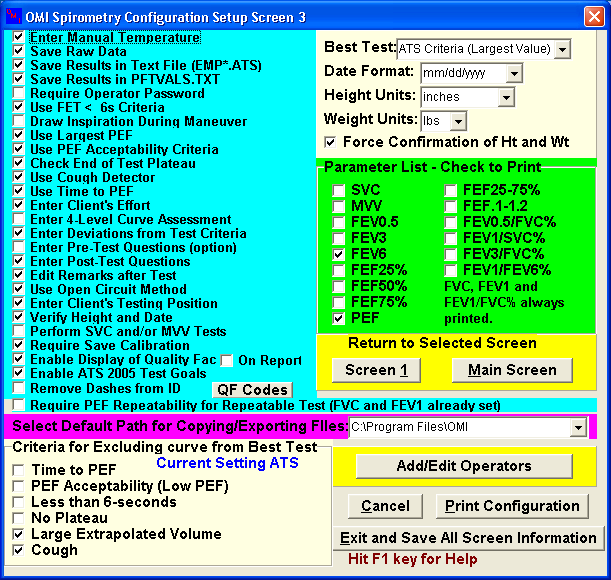
Part B: Flow Spirometer Setup Parameters
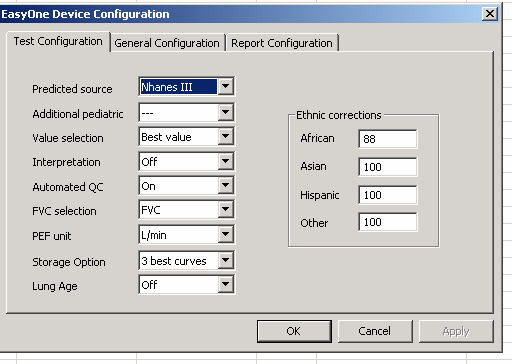
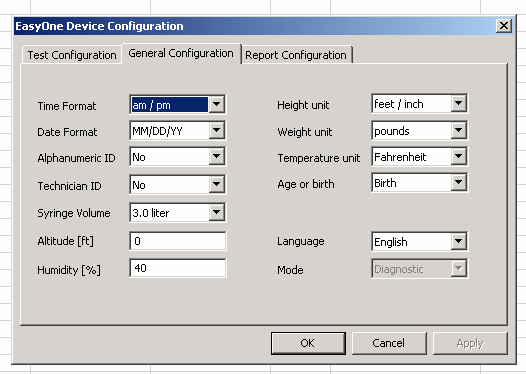
APPENDIX 2
Manufacturer Contact Information
SensorMedics Spirometer
22705 Savi Ranch Parkway
Yorba Linda, CA 92887-4645
Phone: (714) 283-1830
(800) 520-4368
OMI –HF5 spirometry software program
Occupational Marketing, Inc.
11211 Kathy Freeway Ste 420
Houston, TX 77079
Phone: (713) 468-3201
EasyOne™ Spirometer (including EasyWare™ software)
ndd Medical Technologies
17 Progress Ave
Chelmsford, MA 01824
Phone: (978) 244-0620
Spirotube mouthpieces
Occupational Marketing, Inc.
11211 Kathy Freeway Ste 420
Houston, TX 77079
Phone: (713) 468-3201
Calibration syringes
Hans Rudolph, Inc.
7200 Wyandotte
Kansas City, MO 64114
Phone: (816) 363-5522
Nose clips
Medical Graphics Corporation
350 Oak Grove Parkway
St. Paul, MN 55127
Phone: (651) 484-4874
Spirette mouthpieces
ndd Medical Technologies
2 Dundee Park
Andover, MA 01810
Phone: (877) 904-0090
APPENDIX 3
Control Card
Project Name
Subject ID
|
Name |
|||||
Birthdate
|
Age |
Gender
|
||||
Height (Nearest ½ inch)
|
Weight (pounds) |
Blood Pressure |
Pulse |
|||
Current Medications/Eye Drops/Inhalers
|
||||||
Initial when complete: |
Questionnaire
|
Methacholine
|
||||
Spirometry
|
|
|||||
Bronchodilator
|
|
|||||
Spirometry Contraindications: YES / NO Within the last 3 months: chest pain (angina), heart attack, stroke, eye surgery (including LASIK surgery), chest/abdominal surgery Ever: coughing up blood, collapsed lung, arterial aneurysm of the belly or brain Current: gastrointestinal distress, chest discomfort, back discomfort Systolic BP >180, diastolic BP >110, pulse >110 bpm
|
|
Bronchodilator Contraindications: YES / NO History of irregular heart beat (arrhythmia) Systolic BP >160, diastolic BP >100, pulse >100 bpm |
|
Methacholine Challenge Contraindications: YES / NO History of irregular heart beat (arrhythmia), known pregnancy, breastfeeding, on oral/eye drop beta-blocker medication Systolic BP >160, diastolic BP >100, pulse >100 bpm, FEV1 <70% predicted, FEV1 <1.5 liters, poor reproducibility of FEV1 |
|
|
Use of short-acting bronchodilator within last 4 hours? YES/NO Use of intermediate or long-acting bronchodilator on morning of test? YES/NO Smoked within last 2 hours? YES/NO Respiratory infection within last 4 weeks? YES/NO
|
Predicted FEV1 ________ Best FEV1 _________ Best %predicted FEV1 ________ FEV1 after MCT ________ % decline FEV1 from personal best after MCT __________ Treatment after MCT ____________________________________________________________ Best FEV1 after treatment following MCT _________
|
|
APPENDIX 4
Blood Pressure Measurement Protocol
Ask the subject to refrain from smoking or ingesting caffeine for 30 minutes prior to blood pressure measurement.
Have the subject sit quietly for at least 5 minutes before taking blood pressure.
Seat the subject with feet flat on the floor. Ask the subject to bare their upper arm. Support the subject’s arm at heart level.
Select appropriate cuff size (bladder should encircle at least 80% of the upper arm).
Measure and record blood pressure.
If Systolic Blood Pressure >180 or Diastolic Blood Pressure >110, then wait 2 minutes and take and record a second blood pressure measurement. If Systolic Blood Pressure <180 and Diastolic Blood Pressure <110 on the second measurement, use the second measurement when determining contraindications.
APPENDIX 5
Quality Assessment
Acceptability Criteria
The following criteria will be used to judge whether a trial is acceptable:
No hesitation or false starts
The volume of back-extrapolation (Vext) must be less than 5% of the FVC or 150 ml, whichever is greater
No coughing during the first second
No glottis closure
No mouthpiece obstruction by tongue or dentures
No leaks
A plateau in the volume-time curve is achieved with at least 6 seconds of exhalation
No extra breaths
Repeatability Criteria
After collecting three acceptable trials, the following criteria will be used to judge whether a test is repeatable:
The two largest FVC values from acceptable trials should agree within 150 ml
The two largest FEV1 values from acceptable trials should agree within 150 ml
| File Type | text/rtf |
| File Title | NIOSH/DRDS/FSB |
| Author | kbk1 |
| Last Modified By | Sawyer, Tamela (CDC/NIOSH/OD/ODDM) |
| File Modified | 2020-09-15 |
| File Created | 2020-05-22 |
© 2026 OMB.report | Privacy Policy