Case Investigation Form
SARS-CoV-2 Epidemiologic Data Collections
3. Case Investigation Form_instrument_OMB_updated_04.23.20
General Public - Case Investigation Form
OMB: 0920-1297
 Form
Approved: OMB: 0920-XXXX Exp. X/XX/XXXX
Form
Approved: OMB: 0920-XXXX Exp. X/XX/XXXX
……………PATIENT IDENTIFIER INFORMATION IS NOT TRANSMITTED TO CDC……………………
Patient first name _______________ Patient last name __________________ Date of birth (MM/DD/YYYY): ____/_____/_______
……………PATIENT IDENTIFIER INFORMATION IS NOT TRANSMITTED TO CDC……………………
C OVID-19
Case Investigation Form
OVID-19
Case Investigation Form
Reporting jurisdiction: ______________ Case state/local ID: ______________
Reporting health department: ______________ CDC 2019-nCoV ID: ______________
Contact ID a: ______________ NNDSS loc. rec. ID/Case ID b: ______________
a. Only complete if case-patient is a known contact of prior source case-patient. Assign Contact ID using CDC 2019-nCoV ID and sequential contact ID, e.g., Confirmed case CA102034567 has contacts CA102034567 -01 and CA102034567 -02. bFor NNDSS reporters, use GenV2 or NETSS patient identifier.
Interviewer informationName of interviewer: Last ______________________________ First______________________________________ Affiliation/Organization: ____________________________________________ Telephone ______________________________ Email _______________________________________________ Date of interview: ________________(MM/DD/YYYY) Date of medical chart abstraction: _________________ (MM/DD/YYYY) Data sources used for this form? Case-patient interview Other interview, specify relationship to case:_______________________ Medical Chart Abstraction Case-patient’s primary language: ____________________ Was this form administered via a translator? □ Yes □ No □ Unknown |
Case-patient demographic information
Report date to CDC (MM/DD/YYYY): ____/_____/_______
Under what process was the case first identified? (check all that apply): PUI/sought care for acute illness Contact tracing of case patient Surveillance system, please specify:___________________________
EpiX notification of travelers; if checked, DGMQID_______________ Unknown Other, specify:_________________
Date of birth (month and year): Month ____ Year _____
Age: ____________ Age units: Years Months Days
Sex: Male Female
Ethnicity: Hispanic/Latino Non-Hispanic/Latino Not specified
Race (check all that apply): White Asian American Indian/Alaska Native Black
Native Hawaiian/Other Pacific Islander Unknown Other, specify: _________________County of Residence:________________________ State of Residence:________
Country of Residence: United States Other, specify_________________
Occupation:__________________________________________
If student, what grade level? __________________________________________
If child, does s/he attend day care? Yes No Unknown
Travel history
In the 14 days prior to illness onset, were you traveling away from your home internationally?
Yes No Unknown
In the 14 days prior to illness onset, were you traveling away from your home within the United States?
Yes No Unknown
Where did you travel 14 days prior to illness onset (list ALL locations, including overnight transits and layovers)?
|
Departure Date (MM/DD/YYYY) |
Departure city, state/province/country |
Arrival Date (MM/DD/YYYY) |
Arrival city, state/province/country |
Trip 1 |
|
|
|
|
Trip 2 |
|
|
|
|
Trip 3 |
|
|
|
|
Trip 4 |
|
|
|
|
Trip 5 |
|
|
|
|
Exposure history
In the 14 DAYS prior to illness, did you have close contact with another lab-confirmed COVID-19 case-patient?
Yes No Unknown Date Range: Start Date (MM/DD/YYYY) ____________ End Date (MM/DD/YYYY) ____________
Relationship to COVID-19 source case (select all that apply):
Spouse/Partner
Child
Parent
Other Family
Friend
HCW
Co-worker
Classmate
Roommate
Contact
only – no relationship
Other
(specify):___________________
Exposure setting to the COVID-19 source case (select all that apply):
Household Work Daycare School/University Transit Rideshare Hotel Cruise Ship
Healthcare
Other
(specify): ___________________
In the 14 DAYS prior to illness onset, did you:
Exposure |
Answer |
Date Range |
…have any household members, friends, acquaintances, or co-workers who had fever or respiratory symptoms (e.g. cough, sore throat etc.)? |
Yes No Unknown |
|
…have close contact (e.g. caring for, speaking with, or touching) with any ill persons? |
Yes No Unknown |
|
…attend a mass gathering (e.g., religious event, wedding, party, dance, concert, banquet, festival, sports event, or other event)? |
Yes No Unknown |
|
…use public transportation (bus, train, airplane)? |
Yes No Unknown |
|
…attend or work at a school or daycare? |
Yes No Unknown |
|
…have a household member who attended school or daycare? |
Yes No Unknown |
|
…have close contact (e.g. caring for, speaking with, or touching) with a sick person who had contact with a COVID-19 patient (i.e., secondary contact to confirmed case)? |
Yes No Unknown |
|
…have close contact (e.g. caring for, speaking with, or touching) with a person who had a fever and/or acute respiratory illness and international travel in the past 2 weeks? |
Yes No Unknown If yes where did the person travel:____________________ |
|
In the 14 DAYS prior to illness onset, did you:
Exposure |
Y/N/Unk |
Facility type (Select all that apply) |
Date(s) exposure occurred |
||
Work in healthcare setting:
|
Y N Unk If yes, what was your role: Physician Nurse Administration staff Housekeeping Patient transport Other, specify__________ |
Hospital Urgent Care Doctor’s office/clinic
|
Dialysis unit/center Long Term Care Facility Other (specify)
|
|
|
Volunteer in healthcare setting |
Y N Unk |
Hospital Urgent Care Doctor’s office/clinic
|
Dialysis unit/center Long Term Care Facility Other (specify)
|
|
|
Have direct patient contact |
Y N Unk |
Hospital Urgent Care Doctor’s office/clinic
|
Dialysis unit/center Long Term Care Facility Other (specify)
|
|
|
Visit healthcare setting as a patient (not just for this illness) |
Y N Unk |
Hospital Urgent Care Doctor’s office/clinic |
Dialysis unit/center Long Term Care Facility Other (specify)
|
|
|
Visit healthcare setting for any reason other than as a patient |
Y N Unk |
Hospital Urgent Care Doctor’s office/clinic |
Dialysis unit/center Long Term Care Facility Other (specify)
|
|
|
Contact with a known COVID-19 case-patient in a healthcare setting |
Y N Unk If yes, as a Patient Visitor HCW
|
Hospital Urgent Care Doctor’s office/clinic
|
Dialysis unit/center Long Term Care Facility Other (specify)
|
|
|
Do you reside in an institutional or group setting (e.g. long-term care facility/nursing home, boarding school, college dormitory, etc.)?
Yes No Unknown
How many people in total resided in your household (HH) from the 14 days prior to illness through the date of this interview (excluding you)? ________. A household member is anyone with at least one overnight stay during the 14 days prior to patient’s illness onset to the date of this interview. If patient belongs to multiple HH, group HH members by identifying the 1st HH as A, the 2nd HH as B, etc.
-
HH (if case-patient belongs to >1 HH)
Relation to patient
Sex M/F
Age
(specify unit as years, months, or days)
Did household member have fever or respiratory symptoms (e.g. cough, sore throat, etc.) in the 14 days prior to patient’s illness onset, during the patient’s illness, or 14 days after patient’s illness?
Date of
illness onset of household member
(MM/DD/YYYY)
A B C
Y N Unk
A B C
Y N Unk
A B C
Y N Unk
A B C
Y N Unk
A B C
Y N Unk
A B C
Y N Unk
A B C
Y N Unk
Symptoms
If symptomatic, onset date of first symptom (MM/DD/YYYY): ____/_____/_______ Unknown Asymptomatic
If experienced symptoms, are you Still symptomatic Unknown symptom status Symptoms resolved
If
symptoms resolved, date
of symptom resolution (MM/DD/YYYY): ____/_____/_____
Unknown date
During this illness, did you experience any of the following symptoms?
Symptom |
|
Symptom |
|
Fever ≥100.4F (38C) |
Yes No Unk |
Cough (new onset or worsening of chronic cough) |
Yes No Unk |
Highest temp________ °F |
|
Dry |
Yes No Unk |
Date of onset (MM/DD/YYYY) ___/___/_______ |
|
Productive |
Yes No Unk |
Duration of fever ≥100.4F (38C) (days) ____________ |
|
Bloody sputum (hemoptysis) |
Yes No Unk |
Subjective fever (felt feverish) |
Yes No Unk |
Shortness of breath (dyspnea) |
Yes No Unk |
Chills |
Yes No Unk |
Wheezing |
Yes No Unk |
Fatigue |
Yes No Unk |
Chest Pain |
Yes No Unk |
Muscle aches (myalgia) |
Yes No Unk |
Abdominal pain |
Yes No Unk |
Rash |
Yes No Unk |
Vomiting |
Yes No Unk |
Headache |
Yes No Unk |
Nausea |
Yes No Unk |
Eye redness (conjunctivitis) |
Yes No Unk |
Diarrhea (≥3 loose/looser than normal stools/24hr period) |
Yes No Unk |
Runny nose (rhinorrhea) |
Yes No Unk |
Poor Feeding/Poor appetite |
Yes No Unk |
Sore throat |
Yes No Unk |
Seizures |
Yes No Unk |
Other, specify: |
Yes No Unk |
Other, specify: |
Yes No Unk |
Past medical history
Do you have any pre-existing medical conditions? Yes No Unknown
Chronic Lung Disease |
Yes |
No |
Unknown |
|
Asthma/reactive airway disease |
Yes |
No |
Unknown |
|
Emphysema/COPD |
Yes |
No |
Unknown |
|
Other chronic lung disease |
Yes |
No |
Unknown |
(If YES, specify) |
Active tuberculosis |
Yes |
No |
Unknown |
|
Diabetes Mellitus |
Yes |
No |
Unknown |
|
Cardiovascular disease |
Yes |
No |
Unknown |
|
Hypertension |
Yes |
No |
Unknown |
|
Coronary artery disease |
Yes |
No |
Unknown |
|
Heart failure/Congestive heart failure |
Yes |
No |
Unknown |
|
Cerebrovascular accident/Stroke |
Yes |
No |
Unknown |
|
Congenital heart disease |
Yes |
No |
Unknown |
|
Other |
Yes |
No |
Unknown |
If YES, specify: |
Renal disease |
Yes |
No |
Unknown |
|
Chronic kidney disease/insufficiency |
Yes |
No |
Unknown |
|
End-stage renal disease |
Yes |
No |
Unknown |
|
Dialysis |
Yes |
No |
Unknown |
|
Other |
Yes |
No |
Unknown |
If YES, specify: |
Liver disease |
Yes |
No |
Unknown |
|
Alcoholic hepatitis |
Yes |
No |
Unknown |
|
Chronic liver disease |
Yes |
No |
Unknown |
|
Cirrhosis/End stage liver disease |
Yes |
No |
Unknown |
|
Hepatitis B, chronic |
Yes |
No |
Unknown |
|
Hepatitis C, chronic |
Yes |
No |
Unknown |
|
Non-alcoholic fatty liver disease (NAFLD)/NASH |
Yes |
No |
Unknown |
|
Other |
Yes |
No |
Unknown |
If YES, specify: |
Immunocompromised Condition |
Yes |
No |
Unknown |
|
HIV infection |
Yes |
No |
Unknown |
|
AIDS or CD4 count <200 |
Yes |
No |
Unknown |
|
Solid organ transplant |
Yes |
No |
Unknown |
|
Stem cell transplant (e.g., bone marrow transplant) |
Yes |
No |
Unknown |
|
Cancer: current/in treatment or diagnosed in last 12 months |
Yes |
No |
Unknown |
|
Other |
Yes |
No |
Unknown |
If YES, specify: |
Immunosuppressive therapy |
Yes |
No |
Unknown |
If YES, specify:______________________________ __________________________________________ For what condition: _______________________ _________________________________________ |
Neurologic/neurodevelopmental disorder |
Yes |
No |
Unknown |
If YES, specify: |
Other chronic diseases |
Yes |
No |
Unknown |
If YES, specify: |
Current height: _________ (inches) OR __________ (cm)
Current weight: _________ (pounds) OR __________ (kg)
If female, are you currently pregnant? Yes Weeks pregnant at illness onset_________ No Unknown
If female, are you postpartum (
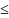 6
weeks postpartum)? Yes
No Unknown
6
weeks postpartum)? Yes
No UnknownIf female, are you breastfeeding? Yes No Unknown
If child, is he/she being breastfed? Yes No Unknown
Social history
Do you currently smoke cigarettes? Yes No Unknown
If yes, how many packs of cigarettes per day? ______ For how many years? _____Have you ever smoked cigarettes? Yes No Unknown
If yes, how many packs of cigarettes per day? ______ For how many years? _____ How long since you last smoked a cigarette? ___(m) ___(y)Do you currently use e-cigarettes/vape-pen? Yes No Unknown
In the past year, how often did you have a drink containing alcohol?
Never Monthly or less 2-4 times a month 2-3 times per week 4 or more times per week
Course of Illness
Do you feel back to normal? Yes No Not applicable (patient deceased) Not applicable (patient asymptomatic) Unknown
If yes, when did you feel back to normal? _____/_____/_____ (MM/DD/YYYY)
Did you miss work or school for this illness? Yes No Unknown
If yes, how many days during illness? __________
Did you receive any medical care for the illness? Yes No Unknown
If yes, where and which dates did you seek care after this illness started (check all that apply)? [Please add extra visit dates in ‘notes’ box]
Doctor’s office Date 1: ______/_____/_______ (MM/DD/YYYY) Date 2: ______/_____/_______ (MM/DD/YYYY)
Emergency room Date 1: ______/_____/_______ (MM/DD/YYYY) Date 2: ______/_____/_______ (MM/DD/YYYY)
Retail store/pharmacy Date 1: ______/_____/_______ (MM/DD/YYYY) Date 2: ______/_____/_______ (MM/DD/YYYY)
Health department Date 1: ______/_____/_______ (MM/DD/YYYY) Date 2: ______/_____/_______ (MM/DD/YYYY)
Urgent care Date 1: ______/_____/_______ (MM/DD/YYYY) Date 2: ______/_____/_______ (MM/DD/YYYY)
Telephone triage line Date 1: ______/_____/_______ (MM/DD/YYYY) Date 2: ______/_____/_______ (MM/DD/YYYY)
Other __________ Date 1: ______/_____/_______ (MM/DD/YYYY) Date 2: ______/_____/_______ (MM/DD/YYYY)
Unknown
Was the patient hospitalized? Yes No Unknown If YES, please fill out hospitalization section below If no, skip to Question #53
Purpose:
Clinical indication No clinical indication
(e.g., isolation for public health)
Hospitalization
Hospital name: ____________________________________________________ Hospital phone: _____________________________
If yes, Admission date 1 ___/___/___ (MM/DD/YYYY) , discharge date 1 ___/___/____ (MM/DD/YYYY) Patient still hospitalized
To where was the patient discharged?
Home Transferred to another hospital Nursing facility/rehab Hospice Other ______________ Unknown
If hospitalized more than once, please enter the second hospitalization’s admission and discharge dates:
Hospital name: ____________________________________________________ Hospital phone: _____________________________
Admission date 2 ______/_____/_______ (MM/DD/YYYY) Discharge date 2______/_____/_______ (MM/DD/YYYY)
Patient still hospitalized
To where was the patient discharged?
Home Transferred to another hospital Nursing facility/rehab Hospice Other ______________ Unknown
First recorded vital signs: Temp_________ (Unit: °F / oC) Blood pressure: ________ (systolic) / ________ (diastolic)
Heart rate: _________ Resp rate:___________
O2 Sat: _______________ (Type of support required when O2 saturation was measured:
Room Air Nasal Cannula Face Mask CPAP or BIPAP High Flow Nasal Cannula Invasive mechanical ventilation
Other, specify: Unknown
Fraction of Inspired Oxygen/Flow __________ % Liters/minute (LPM) Unknown NA
First recorded laboratory values for:
Date
(MM/DD/YYYY)
Value
Unit
White blood cell (WBC) count
Cells x 109/L x 1 000/μL Other: ______
Absolute neutrophil count
Cells x 109/L x 1 000/μL Other: ______
Absolute lymphocyte count
Cells x 109/L x 1 000/μL Other: ______
Platelets (Plt)
Cells x 109/L x 1 000/μL Other: ______
Aspartate transaminase (AST)
U/L IU/L Other: ______
Alanine aminotransferase (ALT)
U/L IU/L Other: ______
Lactate dehydrogenase (LDH)
U/L IU/L Other: ______
Was the patient admitted to an intensive care unit (ICU)? Yes No Unknown
ICU admission date 1 ______/_____/_______ (MM/DD/YYYY) ICU discharge date 1 ______/_____/_______ (MM/DD/YYYY)
ICU admission date 2 ______/_____/_______ (MM/DD/YYYY) ICU discharge date 2 ______/_____/_______ (MM/DD/YYYY)
During hospitalization, did the patient receive...
-
Start Date (MM/DD/YYYY)
Last Date (MM/DD/YYYY)
Total Days
Supplemental Oxygen?
Y N Unk
BiPaP or CPAP use?
Y N Unk
High flow nasal cannula?
Y N Unk
Invasive mechanical ventilation?
Y N Unk
ECMO?
Y N Unk
Did the patient receive a discharge diagnosis of pneumonia (refer to clinical discharge summary)?
Yes No Unknown
Did the patient receive a discharge diagnosis of acute respiratory distress syndrome (ARDS) (refer to clinical discharge summary)?
Yes No Unknown
Clinical Discharge Diagnoses and ICD10 Discharge Codes
Clinical Discharge Diagnoses
ICD-10-CM Code
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
Did the patient receive any antiviral medications during hospitalization for this illness:
Medication |
|
Dose |
Frequency |
Start Date (MM/DD/YYYY) |
Last Date (MM/DD/YYYY) |
Total Days |
Remdesivir |
PO IV IM |
|
|
|
|
|
Other:____________________ |
PO IV IM |
|
|
|
|
|
Other:____________________ |
PO IV IM |
|
|
|
|
|
Imaging
Was a chest x-ray taken? Yes No Unknown
Were any of these chest x-rays abnormal? Yes No Unknown
Date of first abnormal chest x-ray: ______/_____/_______ (MM/DD/YYYY
For first abnormal chest x-ray, please check all that apply: Report not available:
Air space density
|
|
Cannot rule out pneumonia |
|
ARDS (acute respiratory distress syndrome) |
|
Other
|
|
Air space opacity |
|
Consolidation |
|
Lung infiltrate |
|
Pleural Effusion |
|
Bronchopneumonia/pneumonia |
|
Cavitation |
|
Interstitial infiltrate |
|
Empyema |
|
Was a chest CT/MRI taken? Yes No Unknown
Were any of these chest CT/MRIs abnormal? Yes No Unknown
Date of first abnormal CT/MRI: ______/_____/_______ (MM/DD/YYYY)
For first abnormal chest CT/MRI, please check all that apply: Report not available:
Air space density |
|
ARDS (acute respiratory distress syndrome) |
|
Empyema |
|
Englarge epiglottis
|
|
Air space opacity/opacification |
|
Lung infiltrate |
|
Pneumothorax |
|
Tracheal narrowing |
|
Bronchopneumonia/pneumonia |
|
Interstitial infiltrate |
|
Pneumomediastinum |
|
Ground glass opacities |
|
Consolidation |
|
Lobar infiltrate |
|
Widened mediastinum |
|
Other |
|
Cavitation |
|
Pleural effusion |
|
|
|
|
|
Lab Results
SARS-CoV-2 Testing (Please report further test results in comments)
-
Date of sample collection (MM/DD/YYYY)
Sample Type
Result
NP OP Sputum Other, specify:
___________________
Pos Neg Inconclusive
NP OP Sputum Other, specify:
___________________
Pos Neg Inconclusive
NP OP Sputum Other, specify:
___________________
Pos Neg Inconclusive
NP OP Sputum Other, specify:
___________________
Pos Neg Inconclusive
NP OP Sputum Other, specify:
___________________
Pos Neg Inconclusive
Was patient tested for other viral respiratory pathogens during their illness? Yes (report results below) No Unknown
|
Positive |
Negative |
Not Tested/ Unknown |
Collection Date (MM/DD/YYY) |
Specimen Type |
Flu A/H1 |
|
|
|
____/____/________ |
|
Flu A/H3 |
|
|
|
____/____/________ |
|
Flu B |
|
|
|
____/____/________ |
|
Flu (no type) |
|
|
|
|
|
Respiratory syncytial virus/RSV |
|
|
|
____/____/________ |
|
Adenovirus |
|
|
|
____/____/________ |
|
Parainfluenza virus 1 |
|
|
|
____/____/________ |
|
Parainfluenza virus 2 |
|
|
|
____/____/________ |
|
Parainfluenza virus 3 |
|
|
|
____/____/________ |
|
Parainfluenza virus 4 |
|
|
|
____/____/________ |
|
Respiratory syncytial virus/RSV |
|
|
|
____/____/________ |
|
Human metapneumovirus |
|
|
|
____/____/________ |
|
Rhinovirus/enterovirus |
|
|
|
____/____/________ |
|
Human coronavirus 229E |
|
|
|
____/____/________ |
|
Human coronavirus HKU1 |
|
|
|
____/____/________ |
|
Human coronavirus NL63 |
|
|
|
____/____/________ |
|
Human coronavirus OC43 |
|
|
|
____/____/________ |
|
Other, specify: ______________________ |
|
|
|
____/____/________ |
|
Were any bacterial culture tests performed during their illness? Yes No Unknown
If yes, was there a positive culture for a bacterial pathogen? Yes No Unknown
If yes, specify pathogen: __________________________________________________
If yes, specify date of culture (MM/DD/YYYY): ____________
If yes, site where pathogen identified: Blood Sputum Bronchoalveolar lavage (BAL) Endotracheal aspirate Pleural fluid
Cerebrospinal fluid (CSF) Other, specify: ______________
If more than one bacterial culture test was performed, please record in additional comments.
Outcome
Did the patient die as a result of this illness?
Yes, Date: _____/_____/_____ (MM/DD/YYYY) No Unknown
Where did the death occur: Home Hospital ER Hospice Other, specify______________________________
(If the following information is not currently available, please send an update later using death certificate or death note in hospital record.)
Contribution of COVID-19 to death Underlying/primary Contributing/secondary No contribution to death Unknown
Was autopsy performed? Yes No Unknown
Primary Cause of death (death certificate/coroner) _______________________________________________________________

Any
additional comments or notes?
This is the end of the case investigation form. Thank you very much for your time. If you have any questions please feel free to contact the CDC at 770-488-7100 or eocevent330@cdc.gov
Public reporting burden of this collection of information is estimated to average 60 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information including suggestions for reducing this burden to CDC/ATSDR Reports Clearance Officer; 1600 Clifton Road NE, MS D-74 Atlanta, Georgia 30333; ATTN: PRA (0920-1011).
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Modified | 0000-00-00 |
| File Created | 0000-00-00 |
© 2026 OMB.report | Privacy Policy