2020 BRDPI Supporting Statement Part A
2020 BRDPI Supporting Statement Part A.docx
Biomedical Research and Development Price Index (BRDPI) Survey
OMB: 0608-0069
SUPPORTING STATEMENT
U.S. Department of Commerce Bureau of Economic Analysis
Expenditures Incurred by Recipients of Biomedical Research and Development Awards from the National Institutes of Health
OMB Control No. 0608-0069
General Instructions
Abstract
The Biomedical Research and Development Price Index (BRDPI) is developed and updated annually by the Bureau of Economic Analysis (BEA), Department of Commerce (DOC), under an interagency agreement with the National Institutes of Health (NIH).
The BRDPI measures changes in the weighted average of the prices of all the inputs (e.g. personnel services, supplies, and equipment) purchased with the NIH budget to conduct biomedical research. It is a vital tool for planning the NIH research budget. Annual changes in the BRDPI approximate how much the total NIH budget should be increased to compensate for price increases and to sustain the level of research effort supported during the previous year.
As with any price index, the BRDPI is derived each year based on two variables: the price levels for different types of expenses (wages, supplies, energy costs, etc.) and the shares of those types of expenses, or “weights.” For example, the largest proportion, or greatest weight, among these types of expenses is for the salaries of researchers, where the average wage rate would be the price level. The BRDPI combines price levels with expenditure weights, based on standard microeconomic price theory to derive the overall proportional change in the costs to NIH of funding biomedical R&D. The accuracy of the index thus depends on the accuracy of these two types of data.
Justification
Explain the circumstances that make the collection of information necessary. Identify any legal or administrative requirements that necessitate the collection. Attach a copy of the appropriate section of each statute and regulation mandating or authorizing the collection of information.
Prior to the BRDPI survey, which was first implemented in 2005, the BRDPI estimates for FY 2003 were based on 1993 weights, which weakened the reliability of the information estimated. The survey modernized the BRDPI to account for the changing character of biomedical research, which would be better reflected by up-to-date information on prices and weights. This survey rebases the BRDPI each year because it provides annual updated expenditure weights.
BEA proposes to survey 150 organizations that receive NIH biomedical research awards. Because the top 100 organizations that received NIH awards accounted for about 77 percent of total NIH funding in FY 2019, each of these organizations will be included in the survey sample. An additional 50 organizations will be selected based on a random sample of the remaining organizations that receive NIH grants, in two groups depending on the character of the “Research and Development” that they perform. (See section B.1)
History of the BRDPI:
The BRDPI is developed and updated annually by BEA under an interagency agreement with NIH. Because BEA produces certain price indexes, and because NIH was seeking a reliable price index focused on the inputs used to perform biomedical research and development, NIH requested that BEA develop and update BRDPI estimates annually. These estimates have been, and will continue to be, used extensively by NIH in its budgetary analysis and planning. (Further information on the BRDPI may be found at NIH’s website at
https://officeofbudget.od.nih.gov/pdfs/FY21/gbi/BRDPI_Proj_Jan_2020_Final.pdf
Legal Mandate:
This survey is voluntary. BEA administers the survey and analyzes the survey results on behalf of NIH through an interagency agreement between the two agencies. The authority for the agreement and for the collection of information under the agreement is 15 U.S.C. § 1525 (first paragraph) (DOC’s “special studies authority”), which permits DOC to provide, upon the request of any person, firm or public or private organization (a) special studies on matters within the authority of DOC, including preparing from its records special compilations, lists, bulletins, or reports, and (b) furnishing transcripts or copies of its studies, compilations and other records. BEA has programmatic authority to conduct the survey and analyze survey results under 15 U.S.C. § 1516. NIH’s support for this research is consistent with the Agency’s duties and authority under 42 U.S.C. § 282. Further authority to conduct the survey is found in 45 C.F.R. § 75.302, 75.308, 75.361, and 75.364.
Indicate how, by whom, and for what purpose the information is to be used. Except for a new collection, indicate the actual use the agency has made of the information received from the current collection.
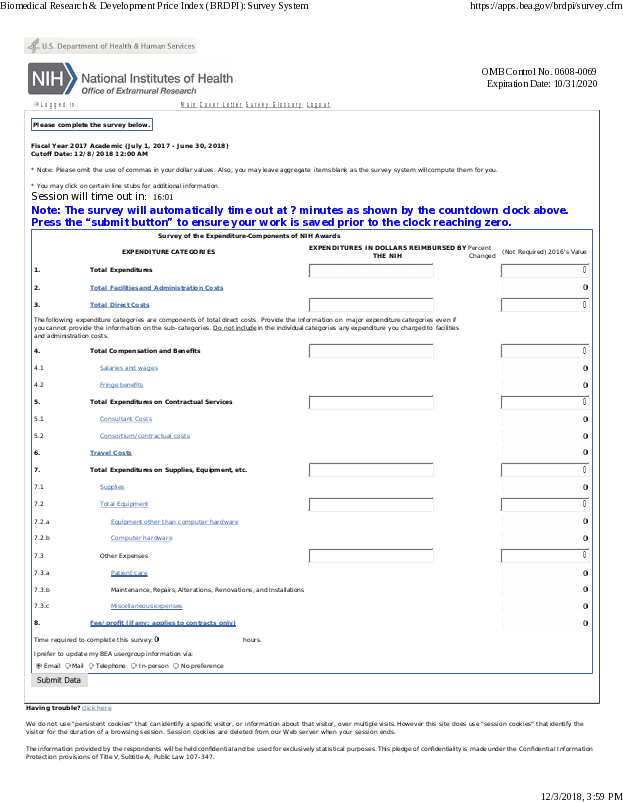
The survey obtains the distribution of expenditures incurred by recipients of biomedical research awards from the National Institutes of Health (NIH) and will provide information on how the NIH award amounts are expended across several major categories.
The information for each of the fiscal years 2020-22, collected each year, will be used by BEA to develop, update, and rebase the weights used to prepare the BRDPI. This survey is currently the only means for updating these expenditure category weights. An electronic survey questionnaire with a cover letter that includes a brief description of, and rationale for, the survey will be sent to potential respondents. Respondents to this proposed survey and future surveys will be the top 100 recipients of NIH biomedical research and development awards, according to the most recent data, plus additional institutions that will be selected randomly with probability of selection proportional to the institution’s share of NIH awards. For each year, a total of 150 institutions (academic and non-academic institutions) will receive the letter and survey.
Section 515 Treasury and General Government Appropriations Act for FY2001 (Public Law 106-554) (the Information Quality Guidelines) applies to the information collected from this survey. The information is collected according to documented procedures in a manner that reflects standard practices accepted by the relevant economic/statistical communities. BEA conducts a thorough review of the survey input data using sound statistical techniques to ensure the quality of the data before the final estimates are released.
The data are collected and reviewed according to documented procedures including the use of checklists, procedures manuals, and on-going review by the appropriate supervisor or team leader. The quality of the data is validated using a battery of computerized edit checks to detect potential errors and to otherwise ensure that the data are accurate, reliable, and relevant for the estimates being made. Data are routinely revised as more complete source data become available. As necessary, information from this survey is shared with NIH through which the survey is developed under an interagency agreement.
Describe whether, and to what extent, the collection of information involves the use of automated, electronic, mechanical, or other technological collection techniques or other forms of information technology, e.g. permitting electronic submission of responses, and the basis for the decision for adopting this means of collection. Also, describe any consideration of using information technology to reduce burden.
The survey will be distributed to the respondents by e-mail. Respondents will be given the option of submitting a completed paper survey by postal (ordinary) mail, attachment to electronic mail, or the Internet to complete and submit the survey. BEA hopes to collect most of the survey data via the Internet and has done so during the previous three years. BEA will place the survey form, reporting instructions, and reporting requirements on its Internet Website (https://www.bea.gov/brdpi/) that is controlled by secure usernames and passwords. The website will provide an alternative, and for some, a more convenient way to transmit, access, and/or retrieve information about the BRDPI survey.
Describe efforts to identify duplication. Show specifically why any similar information already available cannot be used or modified for use for the purposes described in Question 2
BEA is the only Federal agency that collects and develops information on biomedical research and development expenditures for purposes of developing a price index. The only other information comparable to this survey was information acquired by a similar test survey of nine institutions previously administered by Joel Popkin and Associates in 2004, under a contract with NIH. The survey experience demonstrated feasibility, but a survey of only nine institutions does not provide enough reliability to support an annual rebasing of the BRDPI. It is for this reason that BEA has proposed a suitable and dependable survey that will produce statistically reliable data based on a much larger sample of institutions or organizations that receive NIH biomedical research or development grants.
If the collection of information impacts small businesses or other small entities, describe any methods used to minimize burden.
This collection of information does not impose a significant impact on small business or other small entities.
Describe the consequence to Federal program or policy activities if the collection is not conducted or is conducted less frequently, as well as any technical or legal obstacles to reducing burden.
The BRDPI is a vital tool for planning the NIH research budget. Annual changes in the BRDPI approximate how much the total NIH budget should be increased to compensate for price increases and to sustain the level of research effort supported during the previous year. The weights used to construct the index reflect the pattern of NIH expenditures in a designated base year. All price indexes, like the BRDPI, benefit by updating the weights used, which would be achieved in this case by conducting the BRDPI survey.
If the collection were not conducted or conducted less frequently, then the weights applied would be those of the most recent year for which the collection was made. The longer the gap in time between the current year and the survey-data collection year, the more likely it is that the true weights could have changed significantly during that interval. If the true weights change significantly, and the old weights continue to be used, then the BRDPI would be less accurate.
Explain any special circumstances that would cause an information collection to be conducted in a manner:
• requiring respondents to report information to the agency more often than quarterly;
• requiring respondents to prepare a written response to a collection of information in fewer than 30 days after receipt of it;
• requiring respondents to submit more than an original and two copies of any document;
• requiring respondents to retain records, other than health, medical, government contract, grant-in- aid, or tax records for more than three years;
• in connection with a statistical survey, that is not designed to produce valid and reliable results that can be generalized to the universe of study;
• requiring the use of a statistical data classification that has not been reviewed and approved by OMB;
• that includes a pledge of confidentiality that is not supported by authority established in statute or regulation, that is not supported by disclosure and data security policies that are consistent with the pledge, or which unnecessarily impedes sharing of data with other agencies for compatible confidential use; or
• requiring respondents to submit proprietary trade secret, or other confidential information unless the agency can demonstrate that it has instituted procedures to protect the information's confidentiality to the extent permitted by law.
Respondents report information from this collection to BEA only annually.
A survey questionnaire with a cover letter that includes a brief description of, and rationale for, the survey will be sent to potential respondents by August of 2020, 2021, and 2022. A report of the respondent’s expenditures of the NIH award amounts will be requested to be returned no later than December 8, which in most years will be approximately 120 days after mailing.
Respondents will submit responses to the collection only once using the electronic survey questionnaire.
The authority to conduct the BRDPI survey is provided by 15 U.S.C. § 1525, 15 U.S.C. § 1516, and 42 U.S.C. § 282. In addition, NIH is authorized to collect information for the BRDPI by 45 C.F.R. §§75.302 and §75.308, which set forth explicit standards for grantees in establishing and maintaining financial management systems and records, and §75.361 and §75.364, which provide for the retention of such records as well as NIH access to such records.
This collection instrument is designed to produce valid and reliable results that can be generalized to the universe of NIH biomedical research awards recipients.
There are no special circumstances that would require information collection to be conducted in a manner inconsistent with OMB guidelines.
The information provided by the respondents will be held confidential and be used for exclusively statistical purposes. This pledge of confidentiality is made under the Confidential Information Protection and Statistical Efficiency Act of 2018 (CIPSEA) (44 U.S.C. § 3572), which provides that “data or information acquired by an agency under a pledge of confidentiality and for exclusively statistical purposes shall be used by officers, employees, or agents of the agency exclusively for statistical purposes and protected in accordance with such pledge. Data or information acquired by an agency under a pledge of confidentiality for exclusively statistical purposes shall not be disclosed by an agency in identifiable form, for any use other than an exclusively statistical purpose, except with the informed consent of the respondent.”
If applicable, provide a copy and identify the date and page number of publications in the Federal Register of the agency's notice, required by 5 CFR 1320.8 (d), soliciting comments on the information collection prior to submission to OMB. Summarize public comments received in response to that notice and describe actions taken by the agency in response to these comments. Specifically address comments received on cost and hour burden.
The Federal Register Notice to solicit public comment was published on April 20, 2020
(Vol. 85, pages 21824-21825, https://www.federalregister.gov/documents/2020/04/20/2020-08239/proposed-information-collection-comment-request-survey-expenditures-incurred-by-recipients-of). No comments were received.
In 2004, seven out of nine potential respondents expressed interest in participating or willingness to support the survey. These seven organizations said that either they will be able to complete the survey form based on available information within their institutions, or they will try to support the survey. There have been no comments since that time, therefore these methods still apply.
Explain any decision to provide any payment or gift to respondents, other than renumeration of contractors or grantees.
No payments or gifts are provided under this program.
Describe any assurance of confidentiality provided to respondents and the basis for the assurance in statute, regulation, or agency policy. If the collection requires a systems of records notice (SORN) or privacy impact assessment (PIA), those should be cited and described here.
The following confidentiality assurance information is provided in the letter sent to the respondents:
The information provided by the respondents will be held confidential and be used for exclusively statistical purposes. This pledge of confidentiality is made under the Confidential Information Protection and Statistical Efficiency Act of 2018 (CIPSEA) (44 U.S.C. § 3572), which provides that “data or information acquired by an agency under a pledge of confidentiality and for exclusively statistical purposes shall be used by officers, employees, or agents of the agency exclusively for statistical purposes and protected in accordance with such pledge. Data or information acquired by an agency under a pledge of confidentiality for exclusively statistical purposes shall not be disclosed by an agency in identifiable form, for any use other than an exclusively statistical purpose, except with the informed consent of the respondent.”
Responses will be kept confidential and will not be disclosed in identifiable form to anyone other than employees or agents of BEA and agents of NIH, without prior written permission from the business or organization filing the data. By law, each employee as well as each agent is subject to a jail term of up to 5 years, a fine of up to $250,000, or both if he or she makes public ANY identifiable information that is reported about a business or institution responding to the survey.
Provide additional justification for any questions of a sensitive nature, such as sexual behavior or attitudes, religious beliefs, and other matters that are commonly considered private. This justification should include the reasons why the agency considers the questions necessary, the specific uses to be made of the information, the explanation to be given to persons from whom the information is requested, and any steps to be taken to obtain their consent.
No questions of a sensitive nature are asked.
Provide estimates of the hour burden of the collection of information.
Indicate the number of respondents, frequency of response, annual hour burden, and an explanation of how the burden was estimated. Unless directed to do so, agencies should not conduct special surveys to obtain information on which to base hour burden estimates. Consultation with a sample (fewer than 10) of potential respondents is desirable. If the hour burden on respondents is expected to vary widely because of differences in activity, size, or complexity, show the range of estimated hour burden, and explain the reasons for the variance. Generally, estimates should not include burden hours for customary and usual business practices.
If this request for approval covers more than one form, provide separate hour burden estimates for each form and aggregate the hour burdens.
Provide estimates of annualized cost to respondents for the hour burdens for collections of information, identifying and using appropriate wage rate categories. The cost of contracting out or paying outside parties for information collection activities should not be included here. Instead, this cost should be included under ‘Annual Cost to Federal Government’.
An estimated 150 institutions are expected to respond to the survey. Based on feedback gathered from respondents, an average burden of 16 hours per respondent was derived, producing an annual reporting burden of 2,400 hours. The burden estimate includes time for reviewing instructions, searching existing data sources, gathering or collecting the information from existing databases or records, completing the survey form, and management review of the completed survey form. The actual burden may vary from institution to institution, depending upon the number and variety of the respondent’s transactions and the ease of assembling the data.
The burden of the survey will decrease as the respondents become familiar with the survey and develop routine database reports to respond. This is especially the case for the top 100 respondents that are surveyed each year.
Cost to the Respondents for their time:
The total estimated annual cost of the survey’s burden to the public is $106,080 (see table below). This estimate assumes an hourly burden and corresponding direct monetary costs (average wage or salary compensation) to the respondent. Based on the feedback gathered from potential NIH award recipients, this survey will be prepared by a professional employee with an average hourly wage of $34.00. Assuming the employee completing the survey is entitled to fringe benefits, the total cost (wages plus fringe benefits) to the respondent would be $44.20 per hour. (Benefits were assumed to be 30 percent of wages, based on the latest available Bureau of Labor Statistics report on Employer Costs for Employee Compensation.) Given the estimated average burden of 16 hours per respondent, for the 150 respondents who complete the survey, and the $44.20 average hourly compensation rate for these individuals, the total average cost per response per institution would be approximately $708.

Provide an estimate for the total annual cost burden to respondents or record keepers resulting from the collection of information. (Do not include the cost of any hour burden already reflected on the burden worksheet).
The cost estimate should be split into two components: (a) a total capital and start-up cost component (annualized over its expected useful life) and (b) a total operation and maintenance and purchase of services component. The estimates should consider costs associated with generating, maintaining, and disclosing or providing the information. Include descriptions of methods used to estimate major cost factors including system and technology acquisition, expected useful life of capital equipment, the discount rate(s), and the time period over which costs will be incurred. Capital and start-up costs include, among other items, preparations for collecting information such as purchasing computers and software; monitoring, sampling, drilling and testing equipment; and record storage facilities.
If cost estimates are expected to vary widely, agencies should present ranges of cost burdens and explain the reasons for the variance. The cost of purchasing or contracting out information collections services should be a part of this cost burden estimate. In developing cost burden estimates, agencies may consult with a sample of respondents (fewer than 10), utilize the 60-day pre-OMB submission public comment process and use existing economic or regulatory impact analysis associated with the rulemaking containing the information collection, as appropriate.
Generally, estimates should not include purchases of equipment or services, or portions thereof, made: (1) prior to October 1, 1995, (2) to achieve regulatory compliance with requirements not associated with the information collection, (3) for reasons other than to provide information or keep records for the government, or (4) as part of customary and usual business or private practices.
There are no capital/start-up or ongoing operation/maintenance costs associated with this information collection.
Provide estimates of annualized cost to the Federal government. Also, provide a description of the method used to estimate cost, which should include quantification of hours, operational expenses (such as equipment, overhead, printing, and support staff), and any other expense that would not have been incurred without this collection of information.
Agencies may also aggregate cost estimates from Question 12, 13, and 14 in a single table.
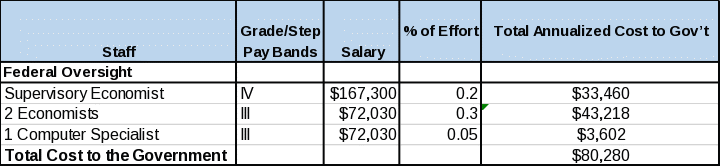
The estimated cost to the Federal Government to administer this survey is approximately $80,000 per year—about 50 percent of the payment for the existing interagency agreement between NIH and BEA to prepare the BRDPI estimates.
Explain the reasons for any program changes or adjustments reported in ROCIS.
There are no changes to the information collection since the last OMB approval.
For collections of information whose results will be published, outline plans for tabulation and publication. Address any complex analytical techniques that will be used. Provide the time schedule for the entire project, including beginning and ending dates of the collection of information, completion of report, publication dates, and other actions.
Survey distribution is expected to be August of 2020, 2021 and 2022. Data collection, estimation and tabulation will be done from October through early December of the same year. The results, analysis and report will be submitted to NIH in December. The BRDPI and some supporting details and analysis are expected to be published by January of the same fiscal year.
Publication of survey results or data will be governed by CIPSEA. (See Section A.10 on Assurance of Confidentiality.)
If seeking approval to not display the expiration date for OMB approval of the information collection, explain the reasons that display would be inappropriate.
The agency plans to display the expiration date for OMB approval of the information collection on all instruments.
Explain each exception to the certification statement identified in “Certification for Paperwork Reduction Act Submissions."
Certification Statement for Paperwork Reduction Act Submissions
On behalf of this Federal agency, I certify that the collection of information encompassed by this request complies with 5 CFR 1320.9 and the related provisions of 5 CFR 1320.8(b)(3).
The following is a summary of the topics, regarding the proposed collection of information, that the certification covers:
It is necessary for the proper performance of agency functions;
It avoids unnecessary duplication;
It reduces burden on small entities;
It uses plain, coherent, and unambiguous terminology that is understandable to respondents;
Its implementation will be consistent and compatible with current reporting and recordkeeping practices;
It indicates the retention period for recordkeeping requirements;
It informs respondents of the information called for under 5 CFR 1320.8(b)(3):
-
i. Why the information is being collected;
ii. Use of information;
iii. Burden estimate;
iv. Nature of response (voluntary, required for a benefit, or mandatory);
v. Nature and extent of confidentiality; and
vi. Need to display currently valid OMB control number;
It was developed by an office that has planned and allocated resources for the efficient and effective management and use of the information to be collected (see note in Item 19 of the instructions);
It uses effective and efficient statistical survey methodology; and
It makes appropriate use of information technology.
If you are unable to certify compliance with any of these provisions, identify the item and explain the reason in Question 18 of the Supporting Statement.
If there are not exceptions to the certification statement, the following response would apply:
The agency certifies compliance with 5 CFR 1320.9 and the related provisions of 5 CFR 1320.8(b)(3).
Attachments
Attachment I: Statement A-1 Statutes and Regulations
The authority for NIH to collect information for the BRDPI
45 C.F.R Subpart C Section 74.21
Title 45 - Public Welfare
Subtitle A - DEPARTMENT OF HEALTHAND HUMAN SERVICES
Subchapter A - GENERAL ADMINISTRATION
Part 75 – Uniform Administrative Requirements, Cost Principles, and Audit Requirements for HHS Awards
Subpart D – Post Federal Award Requirements
Section 75.302 – Financial management and standards for financial management systems
Section 75.308 – Revision of budget and program plans
Section 75.361 – Retention requirements for records
Section 75.364 – Access to records
The authority for the NIH and BEA to make this collection
“Special Studies Authority”:
15 U.S. Code § 1525. (first paragraph)
The Secretary of Commerce is authorized, upon the request of any person, firm, organization, or others, public or private, to make special studies on matters within the authority of the Department of Commerce; to prepare from its records special compilations, lists, bulletins, or reports; to perform the functions authorized by section 1152 of this title; and to furnish transcripts or copies of its studies, compilations, and other records; upon the payment of the actual or estimated cost of such special work.
NIH’s support for this research is consistent with the Agency’s duties and authority
42 U.S.C. § 282
(b)Duties and authority In carrying out the purposes of section 241 of this title, the Secretary, acting through the Director of NIH—
(7)
(A)shall, through the Division of Program Coordination, Planning, and Strategic Initiatives—
(i)identify research that represents important areas of emerging scientific opportunities, rising public health challenges, or knowledge gaps that deserve special emphasis and would benefit from conducting or supporting additional research that involves collaboration between 2 or more national research institutes or national centers, or would otherwise benefit from strategic coordination and planning;
Attachment II: Statement A-3 Collection Form and Instructions/Glossary
Page
|
DOC
PRA ICR TOOLS MAY 2020

| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | DOC PRA TOOLS 2020 |
| Subject | 2020 |
| Author | Dumas, Sheleen (Federal) |
| File Modified | 0000-00-00 |
| File Created | 2021-01-13 |
© 2026 OMB.report | Privacy Policy