Form 0920- Final HIV Test Result Survey
Web-based Approaches to Reach Black or African American and Hispanic/Latino MSM for HIV Testing and Prevention Services
Att 3f_ Final HIV Test Results
Final HIV Test Result Survey (completion)
OMB: 0920-1284
Final HIV Test Result Survey
Form Approved
OMB No. 0920-New
Expiration Date: XX/XX/XXXX
Web-based Approaches to reach black or African American and Hispanic/Latino MSM for HIV Testing and Prevention Services
Attachment 3f
Final HIV Test Result Survey
Public reporting burden of this collection of information is estimated to average 5 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Reports Clearance Officer; 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; Attn: OMB-PRA (0920-New)
HIV Test Result Reporting Survey
This survey is only for Know@Home study participants who have used the Know@Home HIV self-test that was sent from the Know@Home study.
Thank you for participating in our study! This survey includes personal questions about the HIV self-test that was sent to you after your 4 month survey. Questions marked with a red asterisk (*) are questions that you must answer to move forward. You may choose not to answer other questions that make you feel uncomfortable.
If you have any questions, problems with using the test, or if you received a positive result, you can call this toll-free study support number 24 hours a day, 7 days a week: 1-800-628-9240.
All
data you give us will be stored on a HIPAA-compliant server and will
only be used for the purposes of this research study.
This survey only asks about the most recent study HIV self-test that you have completed after your 4 month survey.
If you did not test yourself with the Know@Home study HIV self-test that was sent to you after the 4 month survey, stop here and exit.
If
you used the test kit on yourself, and are you ready to take the
survey and upload a photo, click "Next" to continue on to
the survey.
Otherwise,
come back to this link when you are ready!
Are you a participant in the Know@Home Study?*
No, I received the test from a friend
Yes
[HOMETEST_PARTIC]
Source: Created
[ACTION: If HOMETEST_PARTIC == 0, exit survey]
HIV Testing
This survey will ask questions about the most recent study HIV self-test that you have completed. This is the test that was sent to you after you completed the 4 month survey.
We will ask you some questions about the HIV self-test that was sent to you as part of this study.
We need you to verify your test kit box number. Your box number is a total of 7 numbers and/or letters that can be found on the sticker on the top or side of your box. A sample picture of the Know@Home Study HIV self-test is below.
What is your box number? Box Number: .

(Soft-require)
[4MO_BOXNUM]
Source: Created
Please re-enter your box number: Box Number: .
(Soft-require)
[4MO_BOXNUM_CONFIRM]
Source: Created
When did you test yourself using this study HIV self-test?
Day: [4MO_DY_FU]
Month: [4MO_MO_FU]
Year: [4MO_YR_FU]
Source: eSTAMP (edited)
How much time did it take you to conduct the study self-test?
__________ minutes
[4MO_CONDUCTTIME]
Source: Created
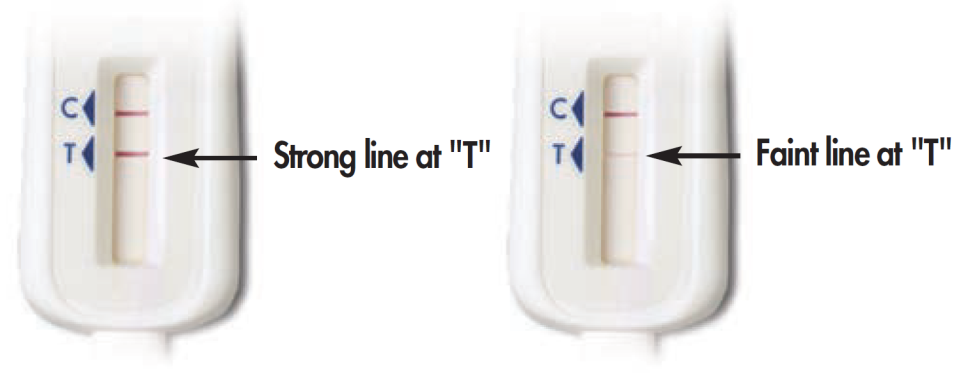
What was the result from the Know@Home Study HIV self-test that you used as part of this study?
Positive / Reactive
Negative / Non-Reactive
Invalid/Test is not working (results do not look like example or there are no lines on the device
[4MO_RESULT]
Source: eSTAMP (edited)
Please select the image that most looks like your test device:
(1)
(2)

(3)
(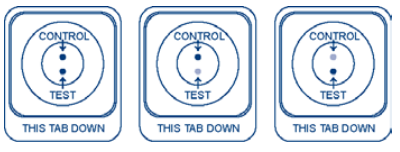
4)
(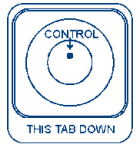
5)
(6)
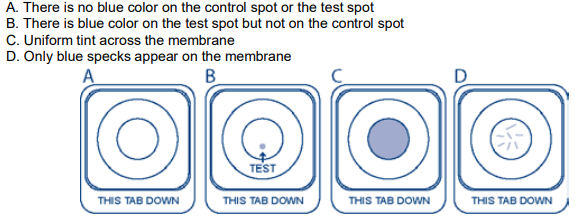
(7) Some lines or dots but my results do not look like the above examples
When you reported a test result, it was different than the image of the test device that you selected. What is your current HIV status?
Positive / Reactive
Negative / Non-reactive
I do not know
[4MO_RECONCILIATION]
Source: Created (edited)
Question 9 or 10 will be displayed based on self-test used by participant.
You mentioned that your OQ test result was invalid or not working. What happened when you tried to run the test? Check all that apply. *
I did not understand the instructions [4MO_INVALID_UNDERSTAND]
I could not see the lines on the test stick clearly 4MO_INVALID_NOCONTROL]
I did not understand what the lines on the test stick meant [4MO_INVALID_LINESISSUE]
I spilled the liquid from the test tube [4MO_INVALID_SPILL]
The test stick fell down and got dirty before I was able to swipe my gums [4MO_INVALID_DIRTY]
There were no lines on the test stick [4MO_INVALID_NOLINES]
I did not follow steps in the order described in the instructions [4MO_INVALID_STEPS]
I did not time the test correctly [4MO_INVALID_TIME]
I did not put the test stick into the test tube [4MO_INVALID_STICK]
I could not swipe the test stick on my gums properly [4MO_INVALID_SAMPLE]
I think a piece of the test kit was missing [4MO_INVALID_MISSING]
Other, please specify______ [4MO_INVALID_OTHER]
(99) I don’t know [4MO_INVALID_DK]
You mentioned that your Insti test result was invalid or not working. What happened when you tried to run the test? Check all that apply. *
I did not understand the instructions [4MO_INVALID_UNDERSTAND]
I could not see the control dots in the results window clearly 4MO_INVALID_NOCONTROL]
I did not understand what the dots in the results window meant [4MO_INVALID_DOTSISSUE]
I spilled the contents of the bottles [4MO_INVALID_SPILL]
The bottles fell down and got dirty before I was able to collect the sample [4MO_INVALID_DIRTY]
There were no lines on the test stick [4MO_INVALID_NOLINES]
I did not follow steps in the order described in the instructions [4MO_INVALID_STEPS]
I did not time the test correctly [4MO_INVALID_TIME]
I did pour the bottles into the test device [4MO_INVALID_POUR]
I could not collect the blood sample properly [4MO_INVALID_SAMPLE]
I think a piece of the test kit was missing [4MO_INVALID_MISSING]
Other, please specify______ [4MO_INVALID_OTHER]
(99) I don’t know [4MO_INVALID_DK]

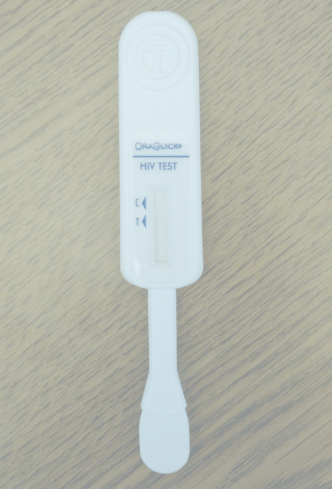
Test Kit Photo
Please
upload a picture of your
used
self-test using the "Browse" button below. If you are using
a smartphone, you have the option of taking a picture now or
uploading a photo already on your phone. When you have chosen
your photo, click "Upload."
An example of a
successful picture is shown below. Please
make sure that the results window is clearly visible.
Note: To receive your token of appreciation, you must take the test, complete the survey within 4 weeks of receiving the test, report the test results, and upload a picture of your test paddle after the test was completed.
Make
sure your photo has finished uploading before clicking "Next"
or you will not get your Amazon gift card!
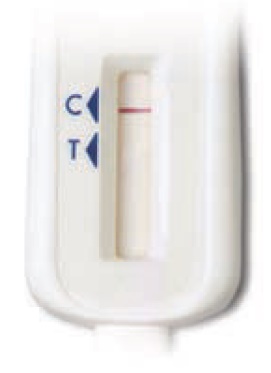
When
your photo has finished uploading, it will look like this:

Please upload a picture of your used HIV self-test kit here:________
* Soft-required
[4MO_PHOTO_SUBMIT]
Source:
AMIS Test Kit Upload 2017
Remember... don't click "Next" until you see the grey box with your file name and a red X!
If INSTI is used by participant
Self-Test Usability
Did you use the instructions sheet?
No
Yes
[INSTRUCTION_TEST]
Source: INSTI HSTAR Study 2018
Were the instructions easy to follow?
No
Yes
[INSTRUCTION_EASY_TEST]
Source: INSTI HSTAR Study 2018
Were the pictures helpful?
No
Yes
[INSTRUCTION_PICTURES_HELPFUL]
Source: INSTI HSTAR Study 2018
Was the device easy to use?
No
Yes
[DEVICE_EASY_TEST]
Source: INSTI HSTAR Study 2018
Would you use this test again?
No
Yes
[REPEAT_USE_TEST]
Source: INSTI HSTAR Study 2018
Would you recommend this test to a sexual partner/friend?
No
Yes
[RECOMMEND_TEST]
Source: INSTI HSTAR Study 2018
Some participants may be selected to provide another finger-stick blood sample and you would receive a separate token of appreciation. If selected, may we contact you?
No
Yes
End
ACTION: Webhook pushes survey completion/results to SMART
Thank you for reporting your rapid HIV home test results! A staff member may be in contact with you soon regarding the results that you reported.
If you have any questions, problems with using the tests, or if you test positive, you can call this toll-free support number 24 hours a day, 7 days a week: 1-800-628-9240.
Study staff may be in contact with you soon regarding the results that you reported. If you think you are at risk or that you may have been exposed to HIV, it will be important for you to test again in three months. If you want to learn more about HIV, where to get more information, or where to get tested or receive care in your area, please click on the following links.
If you are currently HIV-negative, pre-exposure prophylaxis (PrEP) may be a potential option for you. PrEP is a way for people who do not have HIV to lower their risk of getting HIV by taking a pill every day. To learn more, please visit some of the links below.
If you want to learn more about HIV, where to get more information, or where to get tested in your area, please click on the following links:
Information about HIV
HIV Testing Resources
CDC HIV Testing Locator (https://gettested.cdc.gov/)
CDC HIV Testing Information Page (https://www.cdc.gov/hiv/testing/)
HIV.gov HIV Testing Locator (https://www.hiv.gov/locator)
AIDSvu HIV Testing Locator (https://aidsvu.org/services)
PrEP Resources
Centers for Disease Control PrEP Resources (https://www.cdc.gov/actagainstaids/campaigns/starttalking/materials/prepresources.html)
Centers for Disease Control PrEP Information (https://www.cdc.gov/hiv/risk/prep/index.html)
The Fenway Institute: What is PrEP? (http://thefenwayinstitute.org/prepinfo/)
PrEP Locator (https://preplocator.org/)
HIV.gov PrEP Information Page (https://www.hiv.gov/hiv-basics/hiv-prevention/using-hiv-medication-to-reduce-risk/pre-exposure-prophylaxis)
If you have any questions or comments, you may contact study staff at iSTAMP@emory.edu or (404) 727-4340, or the Principal Investigator, Dr. Patrick Sullivan of Emory University, at (404) 727-2038 or pssulli@emory.edu. To get more information about HIV, please visit: www.cdc.gov/hiv.
You can use your study mobile app to set up an appointment to talk to an experienced HIV counselor. If you wish to do that, please go back to the study app now. Study staff will also be in contact with you in 3-5 business days regarding your token of appreciation.
You
may now safely close your browser tab or window.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Weiss, Kevin |
| File Modified | 0000-00-00 |
| File Created | 2021-01-14 |
© 2026 OMB.report | Privacy Policy