Adult smokers ages 25 to 54; Wave 1 Questionnaire
Evaluation of the Food and Drug Administration’s Point-of-Sale Campaign
Attachment_02b_evaluation_questionnaire_Waves_2_3_4
Adult smokers ages 25 to 54; Wave 1 Questionnaire
OMB: 0910-0851
September 2018
Point of Sale Intervention for Tobacco Evaluation (POSITEv)
Waves 2, 3, and 4 Questionnaire
Prepared for
Food and Drug Administration
Janine Delahanty, PhD
Health Scientist
Health Communication and Education
U.S. Food and Drug Administration
Janine.Delahanty@fda.hhs.gov
Prepared by
RTI International
3040 E. Cornwallis Road
Research Triangle Park, NC 27709
RTI Project Number 0213772.003.001.005
_________________________________
RTI International is a registered trademark and a trade name of Research Triangle Institute.
TABLE OF CONTENTS
Section AA: Programming Notes
Section A: Informed Consent and Navigation Instructions
Section C: Tobacco Use Behavior
Section D: Tobacco Use Intentions and Self-Efficacy
Section E: Cessation (Intention, Behavior, Motivation)
Section F: Attitudes, Beliefs, Risk Perceptions and Social Norms
Section G: Media Use and Awareness
Section H: Environment
Section AL: Locator Module
Section J: App-based portion of study
SECTION AA: PROGRAMMING NOTES
PROGRAMMING NOTES
All questions should appear on separate pages.
Participants may refuse to answer any question by clicking “next”. When participants refuse to answer a question, show “Prefer not to answer” as an additional response option and provide a prompt to check that option if they want to skip the question. “Prefer not to answer” will not be displayed unless a question is left unanswered.
If response boxes are used, such as in Hatteras, use radio buttons for questions where only one answer is allowed.
If response boxes are used, use check boxes for questions where more than one answer is allowed.
Prefer Not To Answer/Don’t Know/Refused/None of these are not allowed in combination with other responses.
If the questionnaire interface includes a banner across the top of the page, such as in Hatteras, include the variable name on the banner. In Hatteras, the banner also displays the name of the study, the progress bar, a Help button, and a Log out button. If not, do not include the variable name on the screen.
Except where noted, response options should not be labeled with numbers.
Do not display section headings.
Abbreviations used include ‘R’ for ‘respondent’ and ‘PNTA’ for ‘prefer not to answer’
A back button will be available on every screen for testing ONLY.
A back button will be available on the following screens during production:
Images displayed should be of equal size to one another.
Timestamp Specs
Overall time for respondents – NAVIGATION INSTRUCTIONS through EXIT 1 OR EXIT 3
Overall time by section
SECTION A – INFORMED CONSENT AND NAVIGATION INSTRUCTIONS
SECTION C - C1 through C18
SECTION D – D1 through D4
SECTION E – E1 through E20
SECTION F – F1 through F6
SECTION G – G1 through G12
SECTION H – H1 through ENDCASI2
SECTION AL – FU1 through AL_A2PEM
SECTION J – TBD
All telephone numbers should only accept exactly 10 digits – all numbers and a mask should appear for formatting: XXX-YYY-ZZZZ. Area codes and prefixes should not begin with 0 or 1. The mask will allow the entry of 0 or 1 and the rest of the digits up to 10. Then when Next is pressed, the check happens and a message says that the number is invalid due to the leading 0 or 1, prefix 0 or 1 or less than 10 digits.
CARI Specs:
Consent
AL_INT2 (R willing to provide contact info?)
AL_INT3 (R willing to provide a contact?)
AL_INT4 (R willing to provide a second contact?)
Start recording APP_CONSENT
APP_INSTRUCTIONS1
APP_INSTRUCTIONS2
Stop recording at EXIT 3
SECTION A: INFORMED CONSENT AND NAVIGATION INSTRUCTIONS
[IF CAPI]
Consent Form: Point of Sale Intervention for Tobacco Evaluation (POSITEv)
Form Approved
OMB No. 0910-0851
Exp. Date 04/30/2021
RIHSC No. 17-082CTP
INTERVIEWER, HAND RESPONDENT HARDCOPY CONSENT FORM TO FOLLOW AS YOU READ THE CONSENT TEXT ALOUD.
The Point of Sale Intervention for Tobacco Evaluation (POSITEv) is a research study sponsored by the U.S. Food and Drug Administration. This study is designed to collect information from approximately 4,500 adults across the country about advertisements they have seen and their attitudes towards smoking and programs that help smokers who want to quit. You have completed at least one interview for this study. This study is being conducted again to measure what might have changed over time or what has stayed the same.
Quality Control
We are using a special quality control system on my laptop that will record some of what we say to each other to ensure I am following the correct procedures. The recording will be reviewed by RTI to monitor quality on this project. The recordings will be deleted after my work has been reviewed and will be kept private just like all the other information you provide. The audio files will not be provided to anyone outside of the research team for any purpose. You can still participate in the study even if you do not agree to this recording.
May we use this quality control recording system?
1=YES
2=NO
IF NO, DEACTIVATE COMPUTER AUDIO RECORDED INTERVIEWING FOR THIS CASE
[IF CAPI AND SECA_CONSENTCARI = 1 INITIATE CARI RECORDING]
INTERVIEWER: GIVE RESPONDENT A COPY OF THE YELLOW INFORMED CONSENT
This consent form is for you to keep. It describes the types of questions I will ask, explains that your participation is completely voluntary and that you can stop the interview at any time. The form also reviews risks and benefits, how we keep your information confidential, and how you can get more information about the study. Please let me know if you have any questions about the information on this form.
Do you agree to participate in this study?
1 Yes, I agree to participate in this study
2 No, I do not want to participate in this study
[PROGRAMMING NOTE: IF YES, GO TO SECTION A INSTRUCTIONS; IF NO GO TO END.]
[IF CAWI]
Consent Form: Point of Sale Intervention for Tobacco Evaluation (POSITEv)
Form Approved
OMB No. 0910-0851
Exp. Date 04/30/2021
RIHSC No. 17-082CTP
The Point of Sale Intervention for Tobacco Evaluation (POSITEv) is a research study sponsored by the U.S. Food and Drug Administration. This study is designed to collect information from approximately 4,500 adults across the country about advertisements they have seen and their attitudes towards smoking and programs that help smokers who want to quit. You have completed at least one interview for this study. This study is being conducted again to measure what might have changed over time or what has stayed the same.
Types of Questions
The interview will last about 30-40 minutes and ask questions about your tobacco use, your attitudes towards tobacco, tobacco-related advertising, and personal and household characteristics.
Voluntary Participation
Your participation in this study is completely voluntary. You can refuse to answer any and all questions. Because your contribution is important, we will offer you $30 by check if you complete the survey through the website on or before [FILL EARLY BIRD DATE], or $25 by check after [Early Bird Date], as a token of appreciation for participating. You can stop the interview at any time, however, you will only receive the token of appreciation if you complete the survey. For each follow-up you complete in the future, you will receive a token of appreciation for participating in those additional interviews. Each of these additional interviews will be completely voluntary. We will ask you to provide your consent for each of these interviews if you choose to participate in them.
Risks
There are no physical risks to you from participating in this interview. Some questions are personal in nature and therefore may make you slightly uncomfortable.
Benefits
There are no direct benefits to you from answering our questions. However, you will be contributing to important research.
Confidentiality
You will answer the questions by reading the questions on a computer and entering your answers. Your answers will be labeled with a special number instead of your name. We will only use your name and contact information to stay in touch with you. All of your answers will be kept private to the fullest extent allowable by law and by the technology used. All staff involved in this research are committed to confidentiality. We will not share your specific answers with anyone else outside the research team. Instead, information you provide will be combined with answers of many others and reported in a summary form.
Questions
If you have any questions about the study, you may call the project assistance line toll-free at 1-800-957-6457 between 9 am and 5 pm, Eastern Time, Monday through Friday or email us at fdastudy@rti.org. If you have any questions about your rights as a study participant, you may call RTI's Office of Research Protection at 1-866-214-2043 (a toll-free number). This research study was reviewed and approved by RTI International’s Institutional Review Board (IRB), a committee that evaluates research that involves human participants.
A copy of this consent form is included in the letter we sent you. Or, if you do not have that letter, you can print a copy for your records.
After you select your answer, please press “Next.”
1 Yes, I agree to participate in this study
2 No, I do not want to participate in this study
[PROGRAMMING NOTE: IF YES, GO TO CO INSTRUCTIONS; IF NO GO TO END.]
[IF CAPI, ELSE GO TO C0]
NAVIGATION INSTRUCTIONS
[IF CAPI INCLUDE NAVIGATION INSTRUCTIONS; ELSE GO TO SECTION C INTRODUCTION]
INTERVIEWER, READ: Now I’d like you to read the questions and enter your answers into the laptop yourself. This will allow you to answer the questions in complete privacy. I will not be able to see the answers you type into the computer. You can also skip any question you don’t want to answer by clicking the next button. Let me explain how to use the laptop.
Move laptop so respondent can see the screen and point out the following:
Point to the mouse and say: Please use the mouse to select your answers to the questions.
Point out number keys and say: Please use these keys for questions that ask you to enter a specific number.
Point to “Next” button and say: When you are ready to move to the next question or page, click here. This button will store your answers. Once you have entered your responses and clicked this button, you will not be able to go back and change your answers.
Point to the “Log Out” button and say: The Log Out button will take you out of the survey. Please do not click on it unless you need to stop the survey. This button will save your answers so that you can pick up where you left off when you go back to the survey.
Say: If you have any questions or trouble with the laptop, please ask. If not, click here to begin.”
Point to “Next” button and say “Please answer all of the questions to the best of your abilities.
PROGRAMMER: PROGRAM A NEXT BUTTON AT THE END OF THIS SCREEN
PROGRAMMER: START CASI
SECTION C: TOBACCO USE
INTRODUCTION: The first set of questions are about tobacco products and how often you use them.
C0. Do you now smoke cigarettes . . .
Every day
Some days
Rarely
Not at all
999 Prefer not to answer
ASK: All respondents
C1. Do you consider yourself a smoker?
Yes
No
999 Prefer not to answer
ASK: All respondents
C2. About how long has it been since you last smoked cigarettes? If you smoked a cigarette today please enter 0 days. Please enter days, weeks, months, or years.
PROGRAMMER: ALLOW PARTICIPANTS TO ENTER EITHER DAYS, WEEKS, MONTHS, OR YEARS
C2_1 _____ Days (Range: 0 to 7)
C2_2 _____ Weeks (Range: 0 to 4)
C2_3 _____ Months (Range: 0 to 24)
C2_4 _____ Years (Range: 0 to 2)
PROGRAMMER: NUMERIC RESPONSE. ALLOW A MINIMUM OF 0 AND MAXIMUM OF 7 FOR DAYS. ALLOW A MINIMUM OF 0 AND A MAXIMUM OF 4 FOR WEEKS. ALLOW A MINIMUM OF 0 AND A MAXIMUM OF 24 FOR MONTHS. ALLOW A MINIMUM OF 0 AND A MAXIMUM OF 2 FOR YEARS.
IF ANYTHING ELSE IS TYPED IN, ERROR MESSAGE SHOULD SAY, “YOU HAVE ENTERED A NUMBER OUTSIDE THE ALLOWED RANGE. PLEASE ENTER A NUMBER BETWEEN [0 AND 7/ 0 AND 4/ 0 AND 24/ 0 AND 2].” IN LOWERCASE LETTERS.
ALLOW R TO ENTER DAYS OR WEEKS OR MONTHS OR YEARS.
IF A NUMERIC RESPONSE IS PROVIDED AND THE ‘PREFER NOT TO ANSWER’ BOX IS ALSO CHECKED, ERROR MESSAGE SHOULD SAY “PLEASE DO NOT ENTER A NUMBER OF DAYS OR A NUMBER OF WEEKS WHILE ALSO SELECTING ‘PREFER NOT TO ANSWER’ IN LOWERCASE LETTERS.
ASK: All respondents
C3. Not including today, how many cigarettes did you smoke on the most recent day you smoked? A pack usually has 20 cigarettes in it.
_____ Number of cigarettes (Range: 1–99)
999 Prefer not to answer
PROGRAMMER: NUMERIC RESPONSE. ALLOW A MINIMUM OF 1 AND MAXIMUM OF 99. PROVIDE A CHECKBOX FOR THE ‘PREFER NOT TO ANSWER’ OPTION. ADD VALIDATION CHECK TO PROHIBIT HAVING BOTH A NUMBER RESPONSE AND A CHECKED BOX.
IF ANYTHING ELSE IS TYPED IN, ERROR MESSAGE SHOULD SAY, “YOU HAVE ENTERED A NUMBER OUTSIDE THE ALLOWED RANGE. PLEASE ENTER A NUMBER BETWEEN 1 AND 99.” IN LOWERCASE LETTERS
IF A NUMERIC RESPONSE IS PROVIDED AND THE ‘PREFER NOT TO ANSWER’ BOX IS ALSO CHECKED, ERROR MESSAGE SHOULD SAY “PLEASE DO NOT ENTER A NUMBER OF DAYS OR A NUMBER OF WEEKS WHILE ALSO SELECTING ‘PREFER NOT TO ANSWER’. IN LOWERCASE LETTERS.
ASK: All respondents
C4. [IF C0<4 OR (C2_1 >0 OR C2_2>0 OR C2_3=1)]
On the days that you smoke, how soon after you wake up do you typically smoke your first cigarette of the day? Please enter the number of minutes or hours.
1 _____ Minutes After Waking (Range: 0 to 60)
2 _____ Hours After Waking (Range: 0 to 24)
999 Prefer not to answer
PROGRAMMER: NUMERIC RESPONSE. ALLOW A MINIMUM OF 0 AND MAXIMUM OF 60 FOR MINUTES. ALLOW A MINIMUM OF 0 AND A MAXIMUM OF 24 FOR HOURS.
PROVIDE A CHECKBOX FOR THE ‘PREFER NOT TO ANSWER’ OPTION. ADD VALIDATION CHECK TO PROHIBIT HAVING BOTH A NUMBER RESPONSE AND A CHECKED BOX
IF ANYTHING ELSE IS TYPED IN, ERROR MESSAGE SHOULD SAY, “YOU HAVE ENTERED A NUMBER OUTSIDE THE ALLOWED RANGE. PLEASE ENTER A NUMBER BETWEEN [0 AND 60/ 0 AND 24].” IN LOWERCASE LETTERS
ALLOW R TO ENTER MINUTES OR HOURS. IF MINUTES AND HOURS ARE ENTERED ERROR MESSAGE SHOULD SAY, “PLEASE ENTER A NUMBER OF MINUTES OR A NUMBER OF HOURS, BUT NOT BOTH. IN LOWERCASE LETTERS
ASK: Respondents who report smoking every day, some days, or rarely in C0, or report having smoked within the past 30 days or 4 weeks or 1 month at C2.
TOBACCO PURCHASING BEHAVIOR
C5. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
Do you usually buy your own cigarettes?
Yes
No –> GO TO C15
999 Prefer not to answer
ASK: Respondents who report smoking every day, some days, or rarely in C0, or report having smoked within the past 30 days or 4 weeks or 1 month at C2.
C6. [IF C5=1 OR 999]
Do you usually buy your cigarettes by the carton, pack, or single cigarettes, or do you roll your own?
Carton
Pack
Single cigarettes
Roll your own
999 Prefer not to answer
ASK: Respondents who usually buy their own cigarettes or did not indicate whether they usually buy their own cigarettes.
__________________________________________________________________
C7. [IF C5=1 OR 999]
Now think about cigarettes you purchased for your own personal use in the past 7 days. How many of the past 7 days did you purchase cigarettes?
1 _____ Number of days (Range: 0 to 7)
999 Prefer not to answer
PROGRAMMER: NUMERIC RESPONSE. ALLOW A MINIMUM OF 0 AND MAXIMUM OF 7
PROVIDE A CHECKBOX FOR THE ‘PREFER NOT TO ANSWER’ OPTION. ADD VALIDATION CHECK TO PROHIBIT HAVING BOTH A NUMBER RESPONSE AND A CHECKED BOX
IF ANYTHING ELSE IS TYPED IN, ERROR MESSAGE SHOULD SAY, “YOU HAVE ENTERED A NUMBER OUTSIDE THE ALLOWED RANGE. PLEASE ENTER A NUMBER BETWEEN 0 AND 7” IN LOWERCASE LETTERS
ASK: Respondents who usually buy their own cigarettes or did not indicate whether they usually buy their own cigarettes.
In the past 7 days, how many cartons, packs, single cigarettes, and pouches of roll-your-own tobacco did you buy for your own personal use?
C8_1. ____Cartons (Range: 0 to 99)
C8_2. ____Packs (Range: 0 to 99)
C8_3. ____Single cigarettes (Range: 0 to 99)
C8_4. ____ Pouches of roll your own tobacco (Range: 0 to 99)
999 Prefer not to answer
PROGRAMMER: NUMERIC RESPONSE. ALLOW A MINIMUM OF 0 AND MAXIMUM OF 99 FOR C8_1, C8_2, C8_3, C8_4
PROVIDE A CHECKBOX FOR THE ‘PREFER NOT TO ANSWER’ OPTION. ADD VALIDATION CHECK TO PROHIBIT HAVING BOTH A NUMBER RESPONSE AND A CHECKED BOX
IF ANYTHING ELSE IS TYPED IN, ERROR MESSAGE SHOULD SAY, “YOU HAVE ENTERED A NUMBER OUTSIDE THE ALLOWED RANGE. PLEASE ENTER A NUMBER BETWEEN 0 AND 99.” IN LOWERCASE LETTERS
IF A NUMERIC RESPONSE IS NOT CAPTURED FOR EACH ITEM, ERROR MESSAGE SHOULD SAY “PLEASE ENTER NUMBERS FOR ALL TOBACCO PRODUCTS”. IN LOWERCASE LETTERS
IF A NUMERIC RESPONSE IS PROVIDED AND THE ‘PREFER NOT TO ANSWER’ BOX IS ALSO CHECKED, ERROR MESSAGE SHOULD SAY “PLEASE DO NOT ENTER A PRICE WHILE ALSO SELECTING ‘PREFER NOT TO ANSWER’. IN LOWERCASE LETTERS.
ASK: Respondents who have bought cigarettes for their own personal use in the past 7 days.
C9. [IF C8_1>0]
When you last got a carton of cigarettes for your own personal use, what
price did you pay?
$_ _ _._ _ per carton (Range $0.00 to $150.00)
999 Prefer not to answer
PROGRAMMER: NUMERIC RESPONSE. ALLOW A MINIMUM OF 0 AND MAXIMUM OF 150.00
PROVIDE A CHECKBOX FOR THE ‘PREFER NOT TO ANSWER’ OPTION. ADD VALIDATION CHECK TO PROHIBIT HAVING BOTH A NUMBER RESPONSE AND A CHECKED BOX
IF ANYTHING ELSE IS TYPED IN, ERROR MESSAGE SHOULD SAY, “YOU HAVE ENTERED A NUMBER OUTSIDE THE ALLOWED RANGE. PLEASE ENTER A NUMBER BETWEEN 0 AND 150.00.” IN LOWERCASE LETTERS
IF A NUMERIC RESPONSE IS PROVIDED AND THE ‘PREFER NOT TO ANSWER’ BOX IS ALSO CHECKED, ERROR MESSAGE SHOULD SAY “PLEASE DO NOT ENTER A PRICE WHILE ALSO SELECTING ‘PREFER NOT TO ANSWER’. IN LOWERCASE LETTERS.
ASK: Respondents that purchased at least one carton of cigarettes for their own personal use in the past 7 days.
C10. [IF C8_2>0]
When you last got a pack of cigarettes for your own personal use, what price did you pay?
$_ _ _._ _ per pack (Range $0.00 to $20.00)
999 Prefer not to answer
PROGRAMMER: NUMERIC RESPONSE. ALLOW A MINIMUM OF 0 AND MAXIMUM OF 20.00
PROVIDE A CHECKBOX FOR THE ‘PREFER NOT TO ANSWER’ OPTION. ADD VALIDATION CHECK TO PROHIBIT HAVING BOTH A NUMBER RESPONSE AND A CHECKED BOX
IF ANYTHING ELSE IS TYPED IN, ERROR MESSAGE SHOULD SAY, “YOU HAVE ENTERED A NUMBER OUTSIDE THE ALLOWED RANGE. PLEASE ENTER A NUMBER BETWEEN 0 AND 20.00.” IN LOWERCASE LETTERS
IF A NUMERIC RESPONSE IS PROVIDED AND THE ‘PREFER NOT TO ANSWER’ BOX IS ALSO CHECKED, ERROR MESSAGE SHOULD SAY “PLEASE DO NOT ENTER A PRICE WHILE ALSO SELECTING ‘PREFER NOT TO ANSWER’. IN LOWERCASE LETTERS.
ASK: Respondents that purchased at least one pack of cigarettes for their own personal use in the past 7 days.
C11. [IF C8_1>0 OR C8_2 > 0 OR C8_3>0]
When you last got a single cigarette for your own personal use, what price did you pay?
$_ _ _._ _ per cigarette (Range $0.00 to $15.00)
I have never purchased a single cigarette
999 Prefer not to answer
PROGRAMMER: NUMERIC RESPONSE. ALLOW A MINIMUM OF 0 AND MAXIMUM OF 15.00
PROVIDE A CHECKBOX FOR THE ‘PREFER NOT TO ANSWER’ OPTION. ADD VALIDATION CHECK TO PROHIBIT HAVING BOTH A NUMBER RESPONSE AND A CHECKED BOX
IF ANYTHING ELSE IS TYPED IN, ERROR MESSAGE SHOULD SAY, “YOU HAVE ENTERED A NUMBER OUTSIDE THE ALLOWED RANGE. PLEASE ENTER A NUMBER BETWEEN 0 AND 15.00.” IN LOWERCASE LETTERS
IF A NUMERIC RESPONSE IS PROVIDED AND THE ‘PREFER NOT TO ANSWER’ BOX IS ALSO CHECKED, ERROR MESSAGE SHOULD SAY “PLEASE DO NOT ENTER A PRICE WHILE ALSO SELECTING ‘PREFER NOT TO ANSWER’. IN LOWERCASE LETTERS.
ASK: Respondents that purchased cigarettes for their own personal use in the past 7 days.
C12. [IF C8_4>0]
When you last got a pouch of roll-your-own-tobacco for your own personal use, what price did you pay?
$_ _ _._ _ per pouch (Range $0.00 to $30.00)
999 Prefer not to answer
PROGRAMMER: NUMERIC RESPONSE. ALLOW A MINIMUM OF 0 AND MAXIMUM OF 30.00
PROVIDE A CHECKBOX FOR THE ‘PREFER NOT TO ANSWER’ OPTION. ADD VALIDATION CHECK TO PROHIBIT HAVING BOTH A NUMBER RESPONSE AND A CHECKED BOX
IF ANYTHING ELSE IS TYPED IN, ERROR MESSAGE SHOULD SAY, “YOU HAVE ENTERED A NUMBER OUTSIDE THE ALLOWED RANGE. PLEASE ENTER A NUMBER BETWEEN 0 AND 30.00.” IN LOWERCASE LETTERS
IF A NUMERIC RESPONSE IS PROVIDED AND THE ‘PREFER NOT TO ANSWER’ BOX IS ALSO CHECKED, ERROR MESSAGE SHOULD SAY “PLEASE DO NOT ENTER A PRICE WHILE ALSO SELECTING ‘PREFER NOT TO ANSWER’. IN LOWERCASE LETTERS.
ASK: Respondents that purchased at least one pouch of roll-your-own tobacco for their own personal use in the past 7 days.
C13 INTRO [IF C7 NE 0 OR C8_4 >0]
Next, we would like to ask two questions about where you got cigarettes in the past 7 days. Please think about purchases that you made for your personal use.
C13. [IF C7 NE 0 OR C8_4 >0]
In the past 7 days, have you purchased cigarettes or roll your own tobacco from any of the following locations?
|
|
Yes |
No |
Prefer not to answer |
C13_1. |
At a convenience store or gas station |
1 |
2 |
999 |
C13_2. |
At a grocery store |
1 |
2 |
999 |
C13_3. |
At a drugstore |
1 |
2 |
999 |
C13_4. |
Mass merchandisers such as Wal-Mart, Costco, Sam’s Club |
1 |
2 |
999 |
C13_5. |
At a tobacco shop |
1 |
2 |
999 |
C13_6. |
Other |
1 |
2 |
999 |
ASK: Respondents that purchased cigarettes for their own personal use in the past 7 days.
[IF C13_1=1 OR C13_2=1 OR C13_3=1 OR C13_4=1 OR C13_5=1 OR C13_6=1, ASK C14; ELSE, GO TO C15]
C14. [IF C13_1=1 OR C13_2=1 OR C13_3=1 OR C13_4=1 OR C13_5=1 OR C13_6=1]
Please write the name of the specific store where you usually bought cigarettes or roll-your-own-tobacco in the past 7 days for your own use.
_______________________. (ALLOW 25 ALPHA OR NUMERIC CHARACTERS).
999 Prefer not to answer
PROVIDE A CHECKBOX FOR THE ‘PREFER NOT TO ANSWER’ OPTION. ADD VALIDATION CHECK TO PROHIBIT HAVING BOTH A NUMBER RESPONSE AND A CHECKED BOX
ASK: Respondents who report buying a tobacco product at one of the types of stores listed in C13 in the past 7 days.
CURRENT ELECTRONIC VAPOR PRODUCT USE
This question focuses on electronic vapor products, which include e-cigarettes, e-cigars, e-hookahs, e-pipes, vape pens, tanks, mods, and hookah pens.

C15. Do you now use an electronic vapor product . . .
Every day
Some days
Rarely
Not at all
999 Prefer not to answer
ASK: All respondents
CURRENT SMOKELESS USE
Next, we ask a question about smokeless tobacco which you put in your mouth. You chew, suck or spit some types of smokeless tobacco but not other types. For example, snus is smokeless tobacco that comes in a small pouch that you put inside your lip.
There are many kinds of smokeless tobacco, such as snus pouches, loose snus, moist snuff, dip, spit, and chewing tobacco. Common brands include Redman, Levi Garrett, Beechnut, Skoal, Grizzly, Nordic Ice and Copenhagen.

C16. Do you now use smokeless tobacco products . . .
Every day
Some days
Rarely
Not at all
999 Prefer not to answer
ASK: All respondents
CURRENT CIGAR/CIGARILLO USE
The next question is about traditional cigars, cigarillos, little cigars, and filtered cigars. These products go by lots of different names, so please use these descriptions and photos to understand what they are.
Traditional cigars contain tightly rolled tobacco that is wrapped in a tobacco leaf. Some common brands of cigars include Macanudo, Romeo y Julieta, and Arturo Fuente, but there are many others.
Cigarillos, little cigars, and filtered cigars are smaller than traditional cigars. They are usually brown. Some are the same size as cigarettes, and some come with filters or with plastic or wooden tips. Some common brands are Black & Mild, Swisher Sweets, Dutch Masters, Phillies Blunts, Prime Time, and Winchester.

C17. Do you now use traditional cigars, cigarillos, little cigars and/or filtered cigars . . .
Every day
Some days
Rarely
Not at all
999 Prefer not to answer
ASK: All respondents
CURRENT HOOKAH USE
We next ask about smoking tobacco in a hookah, which is a type of water pipe. It is sometimes also called shisha or a “narghile” pipe. From now on, we will use “hookah” to refer to a water pipe, shisha, or narghile pipe that is often used to smoke tobacco.
There are many types of hookahs. People often smoke tobacco in hookahs in groups at cafes or in hookah bars.

C18. Do you now smoke tobacco in a hookah, even one or two puffs . . .
Every day
Some days
Rarely
Not at all
999 Prefer not to answer
ASK: All respondents
SECTION D: TOBACCO USE INTENTIONS AND SELF-EFFICACY
INTRODUCTION: In the next section, we ask you some questions about quitting smoking cigarettes.
SMOKING EXPECTATIONS
D1. Three months from now, how much do you expect to be smoking cigarettes, compared to now?
Not smoking cigarettes at all
A lot less than now
A little less than now
The same amount as now
A little more than now
A lot more than now
999 Prefer not to answer
ASK: All respondents
SELF-EFFICACY FOR QUITTING
D2. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
If you did try to quit smoking cigarettes altogether in the next 3 months, how likely do you think you would be to succeed?
Not at all likely
A little likely
Somewhat likely
Very likely
999 Prefer not to answer
ASK: Respondents who report smoking every day, some days, or rarely in C0, and report having smoked in the past 30 days or 4 weeks or 1 month in C2.
D3. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
How much do you believe that quitting smoking completely is possible for you?
Not at all possible
Somewhat possible
Very possible
999 Prefer not to answer
ASK: Respondents who report smoking every day, some days, or rarely in C0, and report having smoked in the past 30 days or 4 weeks or 1 month in C2.
D4 INTRO: [IF C0<4 AND (C2_1>0 OR C2_2>0 OR C2_3=1)]
How much do you disagree or agree with the following statement?
D4. I feel ready to take a small step toward quitting.
Strongly disagree
Disagree
Neither disagree nor agree
Agree
Strongly agree
999 Prefer not to answer
ASK: Respondents who report smoking every day, some days, or rarely in C0, and report having smoked in the past 30 days or 4 weeks or 1 month in C2.
D5. [IF C0<4 AND (C2_1>0 OR C2_2>0 OR C2_3=1)]
How likely would you be to use each of the following to try to quit smoking cigarettes?
[RANDOMIZE ORDER OF RESPONSE OPTIONS]
|
|
Not at all likely |
A little likely |
Somewhat likely |
Very likely |
Prefer not to answer |
D5_1. |
Nicotine Replacement Therapy (NRT) like nicotine gum, patch, lozenge, nasal spray, or inhaler |
1 |
2 |
3 |
4 |
999 |
D5_2. |
Electronic vapor products, like e-cigarettes, vape pens, or hookah pens |
1 |
2 |
3 |
4 |
999 |
D5_3. |
Prescription medication like Chantix or Zyban (Wellbutrin) |
1 |
2 |
3 |
4 |
999 |
D5_4. |
Call 1-800-QUIT-NOW |
1 |
2 |
3 |
4 |
999 |
D5_5. |
Talk to my doctor about quitting smoking cigarettes |
1 |
2 |
3 |
4 |
999 |
D5_6. |
Visit a website to help me quit like smokefree.gov |
1 |
2 |
3 |
4 |
999 |
ASK: Respondents who report smoking every day, some days, or rarely in C0, and report having smoked in the past 30 days or 4 weeks or 1 month in C2.
SECTION E: CESSATION
QUIT BEHAVIOR
[IF C0<4 AND (C2_1>0 OR C2_2>0 OR C2_3=1)]
E1. Have you ever tried to quit smoking cigarettes?
Yes
No–> GO TO E10
999 Prefer not to answer
ASK: Respondents who report smoking every day, some days, or rarely in C0, and report having smoked in the past 30 days or 4 weeks or 1 month in C2.
E2. [IF E1=1 OR E1=999]
Of all the times you tried to quit smoking cigarettes, what was the longest period you stayed off cigarettes completely? Enter either hours, days, weeks or months or years below.
____ Hours (Range: 0 to 23)
____ Days (Range: 0 to 6)
____ Weeks (Range: 0 to 4)
____ Months (Range: 0 to 11)
____ Years (Range: 0 to 99)
999 Prefer not to answer
PROGRAMMER: ALLOW RESPONDENT TO ENTER EITHER HOURS, DAYS, WEEKS, MONTHS, OR YEARS
IF HOURS SELECTED ALLOW A MINIMUM OF 0 AND MAXIMUM OF 23.
IF DAYS SELECTED ALLOW A MINIMUM OF 0 AND MAXIMUM OF 6.
IF WEEKS SELECTED ALLOW A MINIMUM OF 0 AND MAXIMUM OF 4.
IF MONTHS SELECTED ALLOW A MINIMUM OF 0 AND MAXIMUM OF 11.
IF YEARS SELECTED ALLOW A MINIMUM OF 0 AND MAXIMUM OF 99.
PROVIDE A CHECKBOX FOR THE ‘PREFER NOT TO ANSWER’ OPTION. ADD VALIDATION CHECK TO PROHIBIT HAVING BOTH A NUMBER RESPONSE AND A CHECKED BOX
IF ANYTHING ELSE IS TYPED IN, ERROR MESSAGE SHOULD SAY, “YOU HAVE ENTERED A NUMBER OUTSIDE THE ALLOWED RANGE. PLEASE ENTER A NUMBER BETWEEN 0 AND (HOURS: 23; DAYS: 6; WEEKS: 4; MONTHS: 11; YEARS: 99).” IN LOWERCASE LETTERS
ASK: Respondents who have tried to quit smoking, or do not report whether they have or have not tried.
E3 INTRO:
[IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
For most of the questions in this section, we ask you about the past 3 months. For the next question, please note that we are asking you about the past 6 months.
E3. In the past 6 months, did you intentionally quit smoking cigarettes for at least 24 hours?
Yes
No
999 Prefer not to answer
ASK: Respondents who have last smoked days or weeks ago, but not months
E4 INTRO:
[IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
Now, we are going to ask you about your behavior in the past 3 months again.
E4. In the past 3 months, did you intentionally quit smoking cigarettes for at least 24 hours?
Yes
No–> GO TO E8
999 Prefer not to answer
ASK: Respondents who have tried to quit smoking, or do not report whether they have or have not tried.
E5. [IF E4=1 OR E4=999]
In the past 3 months, how many times have you intentionally quit smoking cigarettes for at least 24 hours?
1 ____ Times [Range: 0 to 99]
999 Prefer not to answer
PROGRAMMER: NUMERIC RESPONSE. ALLOW A MINIMUM OF 0 AND MAXIMUM OF 99
PROVIDE A CHECKBOX FOR THE ‘PREFER NOT TO ANSWER’ OPTION. ADD VALIDATION CHECK TO PROHIBIT HAVING BOTH A NUMBER RESPONSE AND A CHECKED BOX
IF ANYTHING ELSE IS TYPED IN, ERROR MESSAGE SHOULD SAY, “YOU HAVE ENTERED A NUMBER OUTSIDE THE ALLOWED RANGE. PLEASE ENTER A NUMBER BETWEEN 0 AND 99” IN LOWERCASE LETTERS
ASK: Respondents that intentionally quit smoking cigarettes in the past 3 months for at least 24 hours, or respondents that preferred not to indicate whether they had intentionally quit smoking cigarettes in the past 3 months for at least 24 hours.
E6. [IF E4=1 OR E4=999]
When you tried to quit smoking cigarettes in the past 3 months, did you avoid going to places where you used to buy cigarettes in case you might be tempted to buy them?
Never
Once
A few times
Lots of times
999 Prefer not to answer
ASK: Respondents that intentionally quit smoking cigarettes in the past 3 months for at least 24 hours, or respondents that preferred not to indicate whether they had intentionally quit smoking cigarettes in the past 3 months for at least 24 hours.
E7. [IF E4=1 OR E4=999]
When you tried to quit smoking cigarettes in the past 3 months, was there a time when seeing the cigarette pack display in the store gave you an urge to buy cigarettes?
Never
Once
A few times
Lots of times
999 Prefer not to answer
ASK: Respondents that intentionally quit smoking cigarettes in the past 3 months for at least 24 hours, or respondents that preferred not to indicate whether they had intentionally quit smoking cigarettes in the past 3 months for at least 24 hours.
MOTIVATION TO QUIT
E8. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
How much do you want to quit smoking cigarettes?
Not at all
A little
Somewhat
A lot
Prefer not to answer
ASK: Respondents who have last smoked days or weeks ago, but not months
E9. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
In the past 3 months, have you tried to quit smoking cigarettes by reducing or cutting back on the number of cigarettes you smoke?
Yes
No
999 Prefer not to answer
ASK: Respondents who have last smoked days or weeks ago, but not months
E10. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
On a scale of 1-5, where 1 is the lowest and 5 is the highest, how would you rate quitting smoking cigarettes as a priority in your life?
Lowest priority
Highest priority
999 Prefer not to answer
PROGRAMMER: DISPLAY AS A HORIZONTAL SCALE AND LABEL RESPONSE OPTIONS WITH NUMBERS
ASK: Respondents who have last smoked days or weeks ago, but not months
INTENTION TO QUIT
E11. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
Do you plan to quit smoking cigarettes for good . . .
In the next 7 days,
In the next 30 days,
In the next 3 months,
In the next 6 months,
In the next year, or
More than one year from now?
I do not plan to quit smoking cigarettes for good
Not sure/uncertain
999 Prefer not to answer
ASK: Respondents who have last smoked days or weeks ago, but not months
E12. [IF E11=1 OR 2 OR 3 OR 4 OR 5 OR 6]
Have you set a firm date to quit smoking cigarettes?
Yes
No
999 Prefer not to answer
ASK: Respondents who have last smoked days or weeks ago, but not months
CESSATION COGNITION INDEX
E13 INTRO:
[IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
Please tell us how much you disagree or agree with the following statements.
PROGRAMMER: RANDOMIZE ORDER OF E13_1-E13_4]
|
|
Strongly disagree |
Disagree |
Neither agree or disagree |
Agree |
Strongly agree |
Prefer not to answer |
E13_1. |
I have been thinking a lot about quitting smoking cigarettes recently. |
1 |
2 |
3 |
4 |
5 |
999 |
E13_2. |
I am eager for a life without smoking cigarettes. |
1 |
2 |
3 |
4 |
5 |
999 |
E13_3. |
Lately, I have been thinking about which cigarettes during my day would be the hardest to give up. |
1 |
2 |
3 |
4 |
5 |
999 |
E13_4. |
I am not prepared to make changes in my life to quit smoking cigarettes. |
1 |
2 |
3 |
4 |
5 |
999 |
ASK: Respondents who have last smoked days or weeks ago, but not months
E14. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
During the past 3 months, how often would you say you have thought about the changes you will have to make in your life to quit smoking cigarettes?
Never
Once
A few times
Lots of times
999 Prefer not to answer
ASK: Respondents who have last smoked days or weeks ago, but not months
MICROINDICATORS OF QUITTING
E15. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
Compared to three months ago, are you more or less concerned about the price of cigarettes?
Less concerned
Just as concerned
More concerned
999 Prefer not to answer
ASK: Respondents who have last smoked days or weeks ago, but not months
E16. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
In the past 3 months, did you practice not smoking in some situations, or for periods of time?
Yes
No
999 Prefer not to answer
ASK: Respondents who have last smoked days or weeks ago, but not months
E17. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
In the past 3 months, have you stubbed out a cigarette before you finished it because you wanted to quit smoking?
Yes
No
999 Prefer not to answer
ASK: Respondents who have last smoked days or weeks ago, but not months
E18. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
In the past 3 months, how often did you put off purchasing cigarettes because you wanted to quit smoking?
Never
Once
A few times
Lots of times
999 Prefer not to answer
ASK: Respondents who have last smoked days or weeks ago, but not months
E19. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
In the past 3 months, how often have you stopped yourself from having a cigarette when you had the urge to smoke?
Never
Once
A few times
Lots of times
999 Prefer not to answer
ASK: Respondents who have last smoked days or weeks ago, but not months
E20. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
In the past 3 months, how often did you avoid social situations where people were smoking?
Never
Once
A few times
Lots of times
999 Prefer not to answer
ASK: Respondents who have last smoked days or weeks ago, but not months
SECTION F: ATTITUDES, BELIEFS & RISK PERCEPTIONS, SOCIAL NORMS
INTRODUCTION: Now we would like to ask you some questions about your attitudes and beliefs.
SMOKING BELIEFS
F1. How harmful do you think smoking cigarettes is to people’s health in general?
Not at all harmful
Slightly harmful
Somewhat harmful
Very harmful
Extremely harmful
999 Prefer not to answer
ASK: All Respondents
__________________________________________________________________
F2. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
Please tell us how much do you disagree or agree with the following statements about smoking cigarettes.
PROGRAMMER: RANDOMIZE ORDER OF F2_1 to F2_7]
|
|
Strongly disagree |
Disagree |
Neither agree or disagree |
Agree |
Strongly agree |
Prefer not to answer |
F2_1. |
I would be more energetic right now if I didn’t smoke cigarettes. |
1 |
2 |
3 |
4 |
5 |
999 |
F2_2. |
I’m embarrassed that I smoke cigarettes. |
1 |
2 |
3 |
4 |
5 |
999 |
F2_3. |
Smoking cigarettes is hazardous to my health. |
1 |
2 |
3 |
4 |
5 |
999 |
F2_4. |
Smoking cigarettes is pleasurable. |
1 |
2 |
3 |
4 |
5 |
999 |
F2_5. |
Smoking cigarettes reduces stress. |
1 |
2 |
3 |
4 |
5 |
999 |
F2_6. |
Smoking cigarettes helps me concentrate. |
1 |
2 |
3 |
4 |
5 |
999 |
F2_7. |
Smoking cigarettes helps keep my weight down. |
1 |
2 |
3 |
4 |
5 |
999 |
ASK: Respondents who have last smoked days or weeks ago, but not months
__________________________________________________________________
F3. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
In the past 3 months, how often did you think about the harm your cigarette smoking might be doing to you?
Never
Once
A few times
Lots of times
999 Prefer not to answer
ASK: Respondents who have last smoked days or weeks ago, but not months
F4. How likely do you think you are to develop a smoking-related disease as a result of smoking cigarettes?
Extremely unlikely
Very unlikely
Very likely
Extremely likely
999 Prefer not to answer
ASK: All Respondents
F5. Please tell us how much you disagree or agree that smoking cigarettes increases your risk of . . .
PROGRAMMER: RANDOMIZE ORDER OF F5_1 to F5_4
|
|
Strongly Disagree |
Disagree |
Neither agree or disagree |
Agree |
Strongly Agree |
Prefer not to answer |
F5_1. |
Lung cancer? |
1 |
2 |
3 |
4 |
5 |
999 |
F5_2. |
Heart disease? |
1 |
2 |
3 |
4 |
5 |
999 |
F5_3. |
Emphysema? |
1 |
2 |
3 |
4 |
5 |
999 |
F5_4. |
Chronic obstructive pulmonary disorder (COPD) or chronic bronchitis? |
1 |
2 |
3 |
4 |
5 |
999 |
ASK: All Respondents
CESSATION BELIEFS
F6. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
How much do you think your health would improve if you were to stop smoking cigarettes for good?
Not at all
A little
Somewhat
A lot
999 Prefer not to answer
ASK: Respondents who have last smoked days or weeks ago, but not months
SECTION G: MEDIA USE AND AWARENESS
INTRODUCTION: Next, we’d like to ask you about your use of TV and other media.
GENERAL MEDIA EXPOSURE
G1. How often do you…
PROGRAMMER: RANDOMIZE ORDER OF G1_1 to G1_4
|
|
Never
|
Once a month or less
|
Every few weeks
|
1-3 days a week |
4-6 days a week |
About once a day |
Several times a day |
Prefer not to answer |
G1_1. |
Watch television, including streaming TV (Hulu, Netflix, or Amazon Prime)? |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
999 |
G1_2. |
Watch videos on YouTube? |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
999 |
G1_3. |
Listen to streaming radio? |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
999 |
G1_4. |
Listen to radio over the air? |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
999 |
ASK: All respondents
G2. Thinking about the social networking sites you use, about how often do you visit or use the following…
PROGRAMMER: RANDOMIZE ORDER OF G2_1 to G2_6
|
|
Never
|
Once a month or less
|
Every few weeks
|
1-3 days a week |
4-6 days a week |
About once a day |
Several times a day |
Prefer not to answer |
G2_1. |
Facebook? |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
999 |
G2_2. |
Instagram? |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
999 |
G2_3. |
Twitter? |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
999 |
G2_4. |
Tumblr? |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
999 |
G2_5. |
Snapchat? |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
999 |
G2_6. |
Pinterest? |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
999 |
ASK: All respondents
ANTI-SMOKING MEDIA EXPOSURE
G3. In the past 3 months, how frequently have you seen or heard the following slogan or theme on the TV, radio, or Internet?
Tips from Former Smokers (Tips) PROGRAMMER: INSERT EXAMPLE AD
Never
Once
A few times
Lots of times
999 Prefer not to answer
ASK: All respondents
AD
FOR G3:
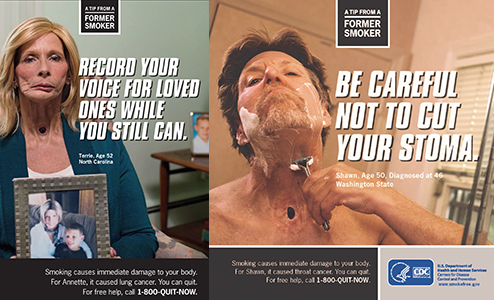
FALSE AD IDENTIFICATION
G3_1. In the past 3 months, how frequently have you seen or heard the following slogan or theme on the TV, radio, or Internet?
Stop Smoking Start Repairing

Never
Once
A few times
Lots of times
999 Prefer not to answer
ASK: All respondents
EVERY TRY COUNTS EXPOSURE
G4. In the past 3 months, how frequently have you seen or heard the following slogan or theme?
Every Try Counts
PROGRAMMER: RANDOMLY INSERT 1 OF THE FOLLOWING SIX EXAMPLE ADS
Ad 1: “You didn’t fail at quitting”

Ad 2: “If at first you don’t succeed”

Ad 3: “Every time you put out a cigarette”

Ad 4: “Keep your head up”

Ad 5: [INSERT NAME OF AD]
[INSERT AD 5 GRAPHIC]
Ad 6: [INSERT NAME OF AD]
[INSERT AD 6 GRAPHIC]
Never
Once
A few times
Lots of times
999 Prefer not to answer
ASK: All respondents
VERIFICATION. To show us that you are paying attention, please select Never as your response to this item.
1 Never
2 Rarely
3 Sometimes
4 Often
5 Very Often
999 Prefer not to answer
GO TO: IF G4 = 1 OR 999 GO TO G6, ELSE GO TO G5
ASK: All respondents
G5. [IF G4>=2 AND G4 NE 999]
You said you have seen or heard Every Try Counts. Where have you seen or
heard it? Please check “yes” or “no” for each item.
PROGRAMMER: INSERT AD SELECTED IN G4
PROGRAMMER: RANDOMIZE ORDER OF G5_1 to G5_7
|
|
Yes |
No |
Prefer not to answer |
G5_1. |
Inside of a store |
1 |
2 |
999 |
G5_2. |
Outside of a store |
1 |
2 |
999 |
G5_3. |
At the gas pump |
1 |
2 |
999 |
G5_4. |
On a billboard |
1 |
2 |
999 |
G5_5. |
On television |
1 |
2 |
999 |
G5_6. |
On the internet and/or on social media |
1 |
2 |
999 |
G5_7. |
On the radio |
1 |
2 |
999 |
ASK: Respondents that indicated they saw or hear the ‘Every Try Counts’ slogan or theme once, a few times, or a lot of times in the past 3 months.
IDENTIFICATION WITH CAMPAIGN MESSAGE
G6. [IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
Please tell us how much you disagree or agree with the following statements about smoking cigarettes.
PROGRAMMER: RANDOMIZE ORDER OF G6_1 to G6_7 BUT KEEP PLACEMENT OF G6_C CONSTANT
|
|
Strongly Disagree |
Disagree |
Neither agree or disagree |
Agree |
Strongly Agree |
Prefer not to answer |
G6_1. |
I feel like a failure when I start smoking again after quitting. |
1 |
2 |
3 |
4 |
5 |
999 |
G6_2. |
Every quit attempt I make is a step towards quitting smoking cigarettes for good. |
1 |
2 |
3 |
4 |
5 |
999 |
G6_3. |
The more times I try to quit smoking cigarettes, the more likely I am to quit for good. |
1 |
2 |
3 |
4 |
5 |
999 |
G6_4. |
It may take me several quit attempts to quit smoking cigarettes for good. |
1 |
2 |
3 |
4 |
5 |
999 |
G6_5. |
I have a chance to learn something new with every quit attempt. |
1 |
2 |
3 |
4 |
5 |
999 |
G6_C. |
Please select the option labeled ‘Disagree’ as your answer. |
1 |
2 |
3 |
4 |
5 |
999 |
G6_6. |
It’s important for me to learn not to smoke cigarettes in situations where I typically smoke. |
1 |
2 |
3 |
4 |
5 |
999 |
G6_7. |
With each quit attempt, I become better at quitting. |
1 |
2 |
3 |
4 |
5 |
999 |
ASK: Respondents who have last smoked days or weeks ago, but not months
G7 INTRO:
[IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
Please tell us how much you disagree or agree with the following statements.
PROGRAMMER: RANDOMIZE ORDER OF G7_1-G7_4
G7. When I think about quitting smoking cigarettes, I feel…
|
|
Strongly Disagree |
Disagree |
Neither agree or disagree |
Agree |
Strongly Agree |
Prefer not to answer |
G7_1. |
Confident |
1 |
2 |
3 |
4 |
5 |
999 |
G7_2. |
Hopeful |
1 |
2 |
3 |
4 |
5 |
999 |
G7_3. |
Discouraged |
1 |
2 |
3 |
4 |
5 |
999 |
G7_4. |
Stressed |
1 |
2 |
3 |
4 |
5 |
999 |
ASK: Respondents who have last smoked days or weeks ago, but not months
G8 INTRO:
[IF C0<4 OR (C2_1>0 OR C2_2>0 OR C2_3=1)]
Please tell us how much you disagree or agree with the following statements.
PROGRAMMER: RANDOMIZE ORDER OF G8_1-G8_6
G8. I continue to smoke cigarettes because…
|
|
Strongly Disagree |
Disagree |
Neither agree or disagree |
Agree |
Strongly Agree |
Prefer not to answer |
G8_1. |
I’m addicted to smoking. |
1 |
2 |
3 |
4 |
5 |
999 |
G8_2. |
I enjoy smoking. |
1 |
2 |
3 |
4 |
5 |
999 |
G8_3. |
I don’t have enough willpower. |
1 |
2 |
3 |
4 |
5 |
999 |
G8_4. |
I’m stressed out. |
1 |
2 |
3 |
4 |
5 |
999 |
G8_5. |
I don’t have the support I need from friends and family. |
1 |
2 |
3 |
4 |
5 |
999 |
G8_6. |
I haven’t tried to quit enough times. |
1 |
2 |
3 |
4 |
5 |
999 |
ASK: Respondents who have last smoked days or weeks ago, but not months
G9 INTRO:
Now we would like to show you some ads that you may have seen close to places that sell tobacco or online.
PROGRAMMER: RANDOMIZE PRESENTATION OF G9_1 AND G9_2
G9_1. Apart from this survey, how frequently have you seen this ad in the past 3
months?
Never
Once
A few times
Lots of times
999 Prefer not to answer
PROGRAMMER: DISPLAY NEW PRINT CAMPAIGN AD 1
[INSERT NEW PRINT AD]
ASK: All respondents
G9_2. Apart from this survey, how frequently have you seen this ad in the past 3 months?
Never
Once
A few times
Lots of times
999 Prefer not to answer
PROGRAMMER: DISPLAY DIGITAL AD
[INSERT DIGITAL AD]
ASK: All respondents
PROGRAMMER: RANDOMIZE PRESENTATION OF G9_3 AND G9_4
G9_3. Apart from this survey, how frequently have you seen this ad in the past 3 months?
Never
Once
A few times
Lots of times
999 Prefer not to answer
PROGRAMMER: RANDOMLY INSERT 1 OF THE FOLLOWING FOUR EXAMPLE ADS
Ad 1: “You didn’t fail at quitting”

Ad 2: “If at first you don’t succeed”

Ad 3: “Every time you put out a cigarette”

Ad 4: “Keep your head up”

ASK: All respondents
G9_4. Apart from this survey, how frequently have you seen this ad in the past 3 months?
Never
Once
A few times
Lots of times
999 Prefer not to answer
PROGRAMMER: RANDOMLY INSERT 1 OF THE REMAINING THREE EXAMPLE ADS NOT SHOWN IN G9_3
ASK: All respondents
Y_video INTRO:
Now we would like to show you some videos. [IF WEB: Please make sure your computer’s volume is set to an appropriate level. You may be prompted by your computer to download a program enabling video playback to view the videos.]
ASK: All respondents.
Y_video1 [IF WEB]
Please try to view this test video to make sure you can see it.
PROGRAMMER: DISPLAY OCEAN VIDEO
Are you able to view and hear this video?
1 Yes
2 No
PROGRAMMER: IF Y_video1 IS NO (=2), DISPLAY THIS MESSAGE:
Viewing and hearing the videos in this survey is important. Please turn up the volume on your device. If you cannot view the video, try logging into the survey using a different computer or browser. If that doesn’t work, we will show you some images of the advertisements.
PROGRAMMER: IF NO, NEED TO BEGIN WITH THE VIEWING OF THE VIDEO WHEN R COMES BACK TO THE SURVEY FROM A DIFFERENT DEVICE.
ASK: Web respondents.
Y_video2 [IF WEB AND Y_video1=2]
PROGRAMMER: DISPLAY OCEAN VIDEO
Now are you able to view and hear this video?
1 Yes
2 No
PROGRAMMER: IF Y_video2 IS NO (=2), DISPLAY THIS MESSAGE:
Okay, since you are not able to see or hear the videos, we will show you some images of the advertisements instead of showing you the videos.
ASK: Web respondents who are not able to view the video the first time.
G9_5_1 INTRO [IF IN PERSON OR (WEB AND (Y_video1=1 OR Y_video2=1])):
Now we would like to show you some advertisements that have been shown in the U.S. Once you have viewed the video, please click on the forward arrow below to continue with the survey.
PROGRAMMER: DISPLAY VIDEOS IN RANDOM ORDER
G9_5_1: “QUIT BUDDY” AD VIDEO
G9_5_2: “NEW” AD (TBD) VIDEO
PROGRAMMER: VIDEO AND QUESTION TEXT SHOULD BE DISPLAYED ON SAME SCREEN. GREY OUT THE ‘Next’ BUTTON FOR 15 SECONDS]
Apart from this survey, how frequently have you seen this ad in the past 3 months?
Never
Once
A few times
Lots of times
999 Prefer not to answer
ASK: In-person respondents OR web respondents who are able to view the test video for the first or second time.
G9_6_1 INTRO [IF WEB AND Y_video2=2]:
Now we would like to show you some advertisements that have been shown in the U.S. Once you have viewed the screenshot, please click on the forward arrow below to continue with the survey.
PROGRAMMER: RANDOMIZE ORDER OF SCREENSHOTS
G9_6_1: “QUIT BUDDY” AD SCREENSHOT
G9_6_2: “NEW” AD (TBD) SCREENSHOT
Apart from this survey, how frequently have you seen this ad in the past 3 months?
Never
Once
A few times
Lots of times
999 Prefer not to answer
ASK: Web respondents who are unable to view the test video even after trying to view it a second time.
PERCEIVED EFFICACY OF CAMPAIGNS
G10_1. Please tell us how much you disagree or agree with the following statements about this ad.
PROGRAMMER: RANDOMIZE PRESENTATION, DISPLAY CAMPAIGN AD FOR AD SHOWN IN G9_1.
|
|
Strongly Disagree |
Disagree |
Neither Agree or Disagree |
Agree |
Strongly Agree |
Prefer not to answer |
G10_1_1. |
This ad is worth remembering. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_1_2 |
This ad grabbed my attention. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_1_3. |
This ad is powerful. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_1_4. |
This ad is informative. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_1_5. |
This ad is meaningful to me. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_1_6. |
This ad is convincing. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_1_7. |
This ad made me want to quit smoking cigarettes. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_1_8. |
This ad made me feel motivated to try to quit smoking cigarettes. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_1_9. |
This ad made me feel hopeful about quitting smoking cigarettes. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_1_10. |
This ad made me feel understood. |
1 |
2 |
3 |
4 |
5 |
999 |
ASK: All respondents
G10_2. Please tell us how much you disagree or agree with the following statements about this ad.
PROGRAMMER: RANDOMIZE PRESENTATION. DISPLAY CAMPAIGN AD FOR G9_2.
|
|
Strongly Disagree |
Disagree |
Neither Agree or Disagree |
Agree |
Strongly Agree |
Prefer Not to Answer |
G10_2_1. |
This ad is worth remembering. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_2_2. |
This ad grabbed my attention. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_2_3. |
This ad is powerful. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_2_4. |
This ad is informative. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_2_5. |
This ad is meaningful to me. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_2_6. |
This ad is convincing. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_2_7. |
This ad made me want to quit smoking cigarettes. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_2_8. |
This ad made me feel motivated to try to quit smoking cigarettes. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_2_9. |
This ad made me feel hopeful about quitting smoking cigarettes. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_2_10. |
This ad made me feel understood. |
1 |
2 |
3 |
4 |
5 |
999 |
ASK: All respondents
G10_5. Please tell us how much you disagree or agree with the following statements about this ad.
PROGRAMMER: RANDOMIZE PRESENTATION. DISPLAY SCREENSHOT OF VIDEO CAMPAIGN SHOWN IN G9_5.
|
|
Strongly Disagree |
Disagree |
Neither Agree or Disagree |
Agree |
Strongly Agree |
Prefer Not to Answer |
G10_5_1. |
This ad is worth remembering. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_5_2. |
This ad grabbed my attention. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_5_3. |
This ad is powerful. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_5_4. |
This ad is informative. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_5_5. |
This ad is meaningful to me. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_5_6. |
This ad is convincing. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_5_7. |
This ad made me want to quit smoking cigarettes. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_5_8. |
This ad made me feel motivated to try to quit smoking cigarettes. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_5_9. |
This ad made me feel hopeful about quitting smoking cigarettes. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_5_10. |
This ad made me feel understood. |
1 |
2 |
3 |
4 |
5 |
999 |
ASK: All respondents
G10_6. Please tell us how much you disagree or agree with the following statements about this ad.
PROGRAMMER: RANDOMIZE PRESENTATION. DISPLAY SCREEN SHOT OF VIDEO AD SHOWN IN G9_6.
|
|
Strongly Disagree |
Disagree |
Neither Agree or Disagree |
Agree |
Strongly Agree |
Prefer Not to Answer |
G10_6_1. |
This ad is worth remembering. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_6_2. |
This ad grabbed my attention. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_6_3. |
This ad is powerful. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_6_4. |
This ad is informative. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_6_5. |
This ad is meaningful to me. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_6_6. |
This ad is convincing. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_6_7. |
This ad made me want to quit smoking cigarettes. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_6_8. |
This ad made me feel motivated to try to quit smoking cigarettes. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_6_9. |
This ad made me feel hopeful about quitting smoking cigarettes. |
1 |
2 |
3 |
4 |
5 |
999 |
G10_6_10. |
This ad made me feel understood. |
1 |
2 |
3 |
4 |
5 |
999 |
ASK: All respondents
COMPREHENSION OF CAMPAIGN MESSAGE
G11. Please pick the answer below that you think best fits the main message of all of the ads we showed you. There may be more than one right answer.
Quitting smoking takes practice.
It may take several attempts to quit smoking for good.
You learn something every time you try to quit smoking.
It is important to talk to your doctor before quitting smoking.
Quitting smoking reduces your chances of getting lung cancer.
999 Prefer not to answer
PROGRAMMER: RANDOMIZE ORDER OF RESPONSE OPTIONS
ASK: All respondents
BRAND IDENTIFICATION
G12. In the past 3 months, did you talk to anyone, either in person or online, about the “Every Try Counts” ads?
Yes
No
999 Prefer not to answer
ASK: All respondents
SECTION H: ENVIRONMENT
INTRODUCTION: This section asks some additional questions about you and your environment.
BLUNT USE
H1. Do you now use a “blunt” (a cigar, cigarillo, little cigar, or filtered cigar
with marijuana in it) . . .
Every day
Some days
Rarely
Not at all
999 Prefer not to answer
ASK: All respondents
TOBACCO ENVIRONMENT IN HOME
H2. The next question asks about rules of using tobacco inside your home. Please
think about everyone who might be in your home including children, adults, visitors, guests, or workers. For tobacco products that are burned, such as cigarettes, cigars, pipes or hookah, which statement best describes the rules about smoking a tobacco product inside your home?
It is not allowed anywhere or at any time inside my home
It is allowed in some places or at some times inside my home
It is allowed anywhere and at any time inside my home
999 Prefer not to answer
ASK: All respondents
H3. Other than you, has anyone who lives with you used any of the following during the past 30 days? Select all that apply.
[PROGRAM SO THAT RESPONDENTS CAN SELECT MORE THAN ONE RESPONSE ON 2–5]
Cigarettes
Traditional cigars, cigarillos, little cigars, or filtered cigars, such as Macanudo, Romeo y Julieta, Arturo Fuente, Black & Mild, Swisher Sweets, Dutch Masters, Phillies Blunts, Prime Time, or Winchester
Tobacco out of a water pipe (also called “hookah”)
Electronic vapor products, also called e-cigarettes, e-cigars, e-hookahs, e-pipes, vape pens, tanks, mods, and hookah pens
Any other form of tobacco
No, no one who lives with me has used any form of tobacco during the past 30 days
999 Prefer not to answer
IF ANY RESPONSE 1- 6 IS SELECTED AND THE ‘PREFER NOT TO ANSWER’ BOX IS ALSO CHECKED, ERROR MESSAGE SHOULD SAY “YOU CANNOT SELECT THE OPTION ‘PREFER NOT TO ANSWER’ AND ANY OTHER OPTION’ IN LOWERCASE LETTERS.
IF RESPONSE 6 IS SELECTED WITH ANY OF THE RESPONSES 1 – 5, ERROR MESSAGE SHOULD SAY “YOU CANNOT SELECT THE OPTION “NO, NO ONE WHO LIVES WITH ME HAS USED ANY FORM OF TOBACCO DURING THE PAST 30 DAYS” AND ANY OTHER OPTION” IN LOWERCASE LETTERS.
ASK: All respondents
PHYSICAL HEALTH
H5. In general, how would you rate your physical health?
Poor
Fair
Good
Very good
Excellent
999 Prefer not to answer
ASK: All respondents
MARITAL STATUS
H6. What is your marital status?
Now married –> GO TO H11
Widowed
Divorced
Separated
Never married
999 Prefer not to answer
ASK: All respondents
H7. [IF H6 >1 OR H6 = 999] Do you share a household with a boyfriend, girlfriend, or partner?
Yes
No
999 Prefer not to answer
ASK: All respondents that are not married
MENTAL HEALTH
H11. Now thinking about your mental health, which includes stress, depression, and emotional problems, for how many days during the past 30 days was your mental health not good?
1 ____ Number of days (Range: 0 to 30)
999 Prefer not to answer
PROGRAMMER: NUMERIC RESPONSE. ALLOW A MINIMUM OF 0 AND MAXIMUM OF 30
PROVIDE A CHECKBOX FOR THE ‘PREFER NOT TO ANSWER’ OPTION. ADD VALIDATION CHECK TO PROHIBIT HAVING BOTH A NUMBER RESPONSE AND A CHECKED BOX
IF ANYTHING ELSE IS TYPED IN, ERROR MESSAGE SHOULD SAY, “YOU HAVE ENTERED A NUMBER OUTSIDE THE ALLOWED RANGE. PLEASE ENTER A NUMBER BETWEEN 0 AND 30” IN LOWERCASE LETTERS
IF A NUMERIC RESPONSE IS PROVIDED AND THE ‘PREFER NOT TO ANSWER’ BOX IS ALSO CHECKED, ERROR MESSAGE SHOULD SAY “PLEASE DO NOT ENTER A NUMBER OF DAYS WHILE ALSO SELECTING ‘PREFER NOT TO ANSWER’. IN LOWERCASE LETTERS.
ASK: All respondents
__________________________________________________________________
H12. During the past 30 days, for about how many days did poor physical or mental health keep you from doing your usual activities, such as self-care, work, or recreation?
1 ____ Number of days (Range: 0 to 30)
999 Prefer not to answer
PROGRAMMER: NUMERIC RESPONSE. ALLOW A MINIMUM OF 0 AND MAXIMUM OF 30
PROVIDE A CHECKBOX FOR THE ‘PREFER NOT TO ANSWER’ OPTION. ADD VALIDATION CHECK TO PROHIBIT HAVING BOTH A NUMBER RESPONSE AND A CHECKED BOX
IF ANYTHING ELSE IS TYPED IN, ERROR MESSAGE SHOULD SAY, “YOU HAVE ENTERED A NUMBER OUTSIDE THE ALLOWED RANGE. PLEASE ENTER A NUMBER BETWEEN 0 AND 30” IN LOWERCASE LETTERS
IF A NUMERIC RESPONSE IS PROVIDED AND THE ‘PREFER NOT TO ANSWER’ BOX IS ALSO CHECKED, ERROR MESSAGE SHOULD SAY “PLEASE DO NOT ENTER A NUMBER OF DAYS WHILE ALSO SELECTING ‘PREFER NOT TO ANSWER’. IN LOWERCASE LETTERS.
ASK: Respondents who report 1 or more days of poor mental health in the past 30 days.
__________________________________________________________________
SEX/GENDER
H13. What is your current gender identity?
Male
Female
Trans male/Trans man
Trans female/Trans woman
Genderqueer/Gender non-conforming/Intersex
Different identity
999 Prefer not to answer
ASK: All respondents
H13OT. [IF H13=6]
Please specify your current gender identity.
________ [ALLOW 20 ALPHA CHARACTERS]
999 Prefer not to answer
PROVIDE A CHECKBOX FOR THE ‘PREFER NOT TO ANSWER’ OPTION. ADD VALIDATION CHECK TO PROHIBIT HAVING BOTH A NUMBER RESPONSE AND A CHECKED BOX
ASK: Respondents who indicate a different gender identity than those listed in H13.
[IF CAPI]
ENDCASI Thank you for your answering these questions. When you leave this screen, the responses you entered into the laptop can no longer be seen by you, the interviewer, or anyone else who uses this computer. When you are ready, please press NEXT to complete this part of the interview.
PROGRAMMER: ONCE NEXT IS ENTERED FOR ENDCASI, NO ONE CAN RE-ENTER THE CASI PORTION OF THE INTERVIEW.
ASK: All respondents answering by ACASI
[IF CAPI]
ENDCASI2 Please tell your interviewer that you are finished.
Interviewer: Enter the code to move to the next section.
ASK: All respondents answering by ACASI
SECTION AL. LOCATOR MODULE
CHECK BOX 1:
ELSE CONTINUE |
[IF CAPI FILL: Instructions to interviewer: read all text and questions in regular type.]
[IF CAPI FILL: Before we finish the interview, I would just like to confirm that we have your correct contact information.]
[IF CAWI FILL: Please confirm that we have your correct contact information.]
This information is held securely and privately by RTI and will only be used to help contact you in the future.
AL-FU1. Is this correct?
[IF CAWI FILL: Please update any information that is not correct]
[IF CAPI FILL: INTERVIEWER, IF ANY INFORMATION IS NOT CORRECT, PROMPT RESPONDENT FOR UPDATED INFORMATION.]
PROGRAMMER: DISPLAY CONTACT INFORMATION FROM WAVE 1 QUESTIONNAIRE; ALLOW EDITS
INTERVIEWER: ATTEMPT TO COLLECT ANY CONTACT INFORMATION THAT IS MISSING
FI: ATTEMPT TO ADDRESS ANY CONCERNS BEFORE SELECTING MOVING FORWARD WITHOUT CONTACT INFORMATION. REMIND THE RESPONDENT OF THE FOLLOWING:
THEY HAVE THE OPPORTUNITY TO RECEIVE [IF WAVE = 2 FILL: UP TO $60 IF THEY PARTICIPATE IN FUTURE INTERVIEWS; IF WAVE = 3 FILL: $30 IF THEY PARTICIPATE IN A FUTURE INTERVIEW], BUT WE NEED TO BE ABLE TO FIND THEM TO INVITE THEM TO PARTICIPATE.
[IF WAVE = 2 FILL: FUTURE INTERVIEWS; IF WAVE = 3 FILL: THE FUTURE INTERVIEW] CAN BE COMPLETED ONLINE.
TO INVITE THEM TO PARTICIPATE IN AN ADDITIONAL PORTION OF THE STUDY WE NEED THEIR CONTACT INFORMATION. THEY CAN RECEIVE UP TO AN ADDITIONAL [IF WAVE = 2 FILL: $10; IF WAVE = 3 FILL: $5] IF THEY PARTICIPATE IN THIS ADDITIONAL PORTION OF THE STUDY.
CONTACT INFORMATION IS HELD SECURELY AND PRIVATELY AND WILL ONLY BE USED TO CONTACT THEM IN THE FUTURE.
AL-FU1_FNAME FIRST NAME ________ (ALLOW 50 ALPHA CHARACTERS)
AL-FU1_LNAME LAST NAME ________ (ALLOW 50 ALPHA OR NUMERIC CHARACTERS)
AL-FU1_ADD1 street NUMBER ________ (ALLOW 50 ALPHA OR NUMERIC CHARACTERS)
AL-FU1_ADD2 STREET NAME ________________ (ALLOW 50 ALPHA OR NUMERIC CHARACTERS)
AL-FU1_APT Apartment Number ______ (ALLOW 10 ALPHA OR NUMERIC CHARACTERS)
AL-FU1_CITY cITY ________ (ALLOW 50 ALPHA OR NUMERIC CHARACTERS)
AL-FU1_STATE STATE ____ (PROGRAMMER: DROPDOWN FIELD OF 50 STATES)
AL-FU1_ZIP ZIP ____ (ALLOW 5 NUMERIC CHARACTERS)
AL-FU1_HPHONE home phone:_______________ [ALLOW 10 NUMERIC CHARACTERS]
AL-FU1_CELLPHONE cell phone:_____________ [ALLOW 10 NUMERIC CHARACTERS]
AL-FU1_EMAIL e-mail address: ___________@____ [ALLOW 40 CHARACTERS]
Continue
999 Prefer not to answer
ASK: All respondents Wave 2 or 3
CAWI_INCENTIVE [IF CAWI]
We will send you a check for $[INCENTIVE] as a token of appreciation for participating to the address you provided. Please allow 3 – 4 weeks for the check to arrive.
If you do not wish to receive a check, please check the box, “I DECLINE TO RECEIVE A CHECK”
I DECLINE TO RECEIVE A CHECK
INCENTIVE: IF DATE BEFORE ‘EARLY BIRD’ DATE FILL 30; OTHERWISE FILL 25.
ASK: Web respondents
BLINE_CONTACT1 [IF CAPI AND R PROVIDED CONTACT PERSON IN BASELINE FILL: During the last interview you provided contact information for another person who would always know your whereabouts. I would also like to confirm that person’s contact information.]
[IF CAWI AND R PROVIDED A CONTACT PERSON IN BASELINE FILL: During the last interview you provided contact information for another person who would always know your whereabouts. Please confirm that we have that person’s correct contact information.]
PROGRAMMER: DISPLAY CONTACT INFORMATION FROM WAVE 1 QUESTIONNAIRE; ALLOW EDITS
BLINE_CONTACT1_FNAME contact 1 FIRST NAME ________ (ALLOW 50 ALPHA CHARACTERS)
BLINE_CONTACT1_LNAME contact 1 LAST NAME ________ (ALLOW 50 ALPHA OR NUMERIC CHARACTERS)
BLINE_CONTACT1_RELAT contact 1 RELATIONSHIP ________
BLINE_CONTACT1_HPHONE CONTACT 1 home phone:_______________ [ALLOW 10 NUMERIC CHARACTERS]
BLINE_CONTACT1_CELLPHONE CONTACT 1 cell phone:_________________ [ALLOW 10 NUMERIC CHARACTERS]
BLINE_CONTACT1_EMAIL CONTACT 1 e-mail address: ___________@______ [ALLOW 40 CHARACTERS]
Continue
999 Prefer not to answer
ASK: All respondents Wave 2 or 3
BLINE_CONTACT2 [IF CAPI AND R PROVIDED A SECOND CONTACT PERSON IN BASELINE FILL: During the last interview you provided contact information for another person who would always know your whereabouts. I would also like to confirm that person’s contact information]
[IF CAWI AND R PROVIDED A SECOND CONTACT PERSON IN BASELINE FILL: During the last interview you provided contact information for another person who would always know your whereabouts. Please confirm that we have that person’s correct contact information.]
As a reminder, this information is kept private and not shared with anyone outside the study. We will only use this information if we need to contact you and are unable to do so using the information you have already provided to us.
PROGRAMMER: DISPLAY CONTACT INFORMATION FROM WAVE 1 QUESTIONNAIRE; ALLOW EDITS
BLINE_CONTACT2_FNAME contact 2 FIRST NAME ________ (ALLOW 50 ALPHA CHARACTERS)
BLINE_CONTACT2_LNAME contact 2 LAST NAME ________ (ALLOW 50 ALPHA OR NUMERIC CHARACTERS)
BLINE_CONTACT2_RELAT contact 2 RELATIONSHIP ________
BLINE_CONTACT2_HPHONE CONTACT 2 home phone:_______________ [ALLOW 10 NUMERIC CHARACTERS]
BLINE_CONTACT2_CELLPHONE CONTACT 2 cell phone:_________________ [ALLOW 10 NUMERIC CHARACTERS]
BLINE_CONTACT2_EMAIL CONTACT 2 e-mail address: ___________@______ [ALLOW 40 CHARACTERS]
Continue
999 Prefer not to answer
ASK: All respondents Wave 2 or 3
[FOR BOTH CAPI AND CAWI. IF NO CONTACTS PROVIDED AT BASELINE OR ONLY 1 CONTACT PROVIDED AT BASELINE]
AL_INT3 It is often difficult to get in touch with people if their contact information changes. Should this occur, we would still like you to participate in the follow-up interviews. In case we have difficulty getting in touch with you in the future, could you please give me the contact information for another person, who is not currently living with you, who will always know your whereabouts? This information will be kept private and not shared with anyone outside the study. We will only use this information if we need to contact you and are unable to do so using the information you have already provided to us.
IF NECESSARY: This might be a family member, a close friend, or someone else who knows where you are. We would only contact this person if we could not reach you.
YES
NO
999 PREFER NOT TO ANSWER
ASK: Respondents willing to provide additional personal contact information.
AL_A1NAF [IF AL_INT3 = 1]
What is this person's name?
FIRST NAME: ________ [ALLOW 20 ALPHA CHARACTERS]
999 PREFER NOT TO ANSWER
PROGRAMMER: THIS PART OF THE SCREEN CAPTURES THE ALTERNATE PERSON'S FIRST NAME AND SHOULD BE DISPLAYED ON THE SAME SCREEN AS AL_A1NAL
IF A NUMERIC RESPONSE IS PROVIDED AND THE ‘PREFER NOT TO ANSWER’ BOX IS ALSO CHECKED, ERROR MESSAGE SHOULD SAY “PLEASE DO NOT ENTER A NAME WHILE ALSO SELECTING ‘PREFER NOT TO ANSWER’ IN LOWERCASE LETTERS.
ASK: Respondents willing to provide additional personal contact information and agreed to give contact information for another person who is not currently living with them and who will always know their whereabouts.
AL_A1NAL [IF AL_INT3 = 1]
What is this person's name?
LAST NAME: ________ [ALLOW 20 ALPHA CHARACTERS]
999 PREFER NOT TO ANSWER
PROGRAMMER: THIS PART OF THE SCREEN CAPTURES THE ALTERNATE PERSON’S LAST NAME AND SHOULD BE DISPLAYED ON THE SAME SCREEN AS AL_A1NAF
IF A NUMERIC RESPONSE IS PROVIDED AND THE ‘PREFER NOT TO ANSWER’ BOX IS ALSO CHECKED, ERROR MESSAGE SHOULD SAY “PLEASE DO NOT ENTER A NAME WHILE ALSO SELECTING ‘PREFER NOT TO ANSWER’. IN LOWERCASE LETTERS.
ASK: Respondents willing to provide additional personal contact information and agreed to give contact information for another person who is not currently living with them and who will always know their whereabouts.
AL_A1REL [IF AL_INT3 = 1]
How is [IF AL_A1NAF NE 999 FILL AL_A1NAF, ELSE FILL: this person] related to you?
MOTHER
FATHER
STEPMOTHER (INCLUDING FOSTER OR ADOPTED MOTHER)
STEPFATHER (INCLUDING FOSTER OR ADOPTED FATHER)
GRANDMOTHER
GRANDFATHER
DAUGHTER
SON
AUNT
UNCLE
SISTER (INCLUDING HALF, STEP, FOSTER OR ADOPTED SISTER)
BROTHER (INCLUDING HALF, STEP, FOSTER OR ADOPTED BROTHER)
OTHER RELATIVE
FRIEND
GIRLFRIEND (NOT LIVING WITH RESPONDENT)
BOYFRIEND (NOT LIVING WITH RESPONDENT)
COWORKER
HUSBAND/WIFE (NOT LIVING WITH RESPONDENT)
EX-HUSBAND OR WIFE
OTHER, SPECIFY
999 PREFER NOT TO ANSWER
PROGRAMMER: IF AL_A1NAF NE 999 FILL AL_A1NAF, ELSE FILL: this person
DISPLAY: AL_A1NAF is the name of the person not currently living with the R who will always know their whereabouts, collected at AL_A1NAF.
ASK: Respondents willing to provide additional personal contact information and agreed to give contact information for another person who is not currently living with them and who will always know their whereabouts.
AL_A1RELs [IF AL_A1REL = 20]
Please specify how [IF AL_A1NAF NE 999 FILL AL_A1NAF, ELSE FILL: this person] is related to you?
SPECIFY:_____________[ALLOW 25 ALPHA CHARACTERS]
999 PREFER NOT TO ANSWER
PROGRAMMER: IF AL_A1NAF NE 999 FILL AL_A1NAF, ELSE FILL: this person
DISPLAY: AL_A1NAF is the name of the person not currently living with the R who will always know their whereabouts, collected at AL_A1NAF.
ASK: Respondents willing to provide additional personal contact information and agreed to give contact information for another person who is not currently living with them and who will always know their whereabouts.
AL_A1HPH [IF AL_INT3 = 1]
What is [IF AL_A1NAF NE 999 FILL AL_A1NAF’s, ELSE FILL: this person’s] home telephone number, including area code?
HOME TELEPHONE NUMBER: _______[ALLOW 10 NUMERIC CHARACTERS]
999 PREFER NOT TO ANSWER
OPEN END NUM
VALIDATION: MIN 0 MAX 9999999999
PROGRAMMER: VALIDATE FORMAT FOR PHONE NUMBER. IF FORMAT IS INCORRECT, PLEASE DISPLAY:
“PLEASE VERIFY THIS AREA CODE WITH RESPONDENT. PRESS “CANCEL” TO CORRECT AREA CODE, OR “OK” TO CONTINUE.
IF A NUMERIC RESPONSE IS PROVIDED AND THE ‘PREFER NOT TO ANSWER’ BOX IS ALSO CHECKED, ERROR MESSAGE SHOULD SAY “PLEASE DO NOT ENTER A PHONE NUMBER WHILE ALSO SELECTING ‘PREFER NOT TO ANSWER’ IN LOWERCASE LETTERS.
IF AL_A1NAF NE 999 FILL AL_A1NAF’s, ELSE FILL: this person’s
DISPLAY: AL_A1NAF is the name of the person not currently living with the R who will always know their whereabouts, collected at AL_A1NAF.
ASK: Respondents willing to provide additional personal contact information and agreed to give contact information for another person who is not currently living with them and who will always know their whereabouts.
AL_A1CPH [IF AL_INT3 = 1]
What is [IF AL_A1NAF NE 999 FILL AL_A1NAF’s, ELSE FILL: this person’s] cell phone number, including area code?
CELL PHONE NUMBER:____________ [ALLOW 10 NUMERIC CHARACTERS]
999 PREFER NOT TO ANSWER
OPEN END NUM
VALIDATION: MIN 0 MAX 9999999999
PROGRAMMER: VALIDATE FORMAT FOR PHONE NUMBER. IF FORMAT IS INCORRECT, PLEASE DISPLAY:
“PLEASE VERIFY THIS AREA CODE WITH RESPONDENT. PRESS “CANCEL” TO CORRECT AREA CODE, OR “OK” TO CONTINUE.
IF A NUMERIC RESPONSE IS PROVIDED AND THE ‘PREFER NOT TO ANSWER’ BOX IS ALSO CHECKED, ERROR MESSAGE SHOULD SAY “PLEASE DO NOT ENTER A PHONE NUMBER WHILE ALSO SELECTING ‘PREFER NOT TO ANSWER’ IN LOWERCASE LETTERS.
IF AL_A1NAF NE 999 FILL AL_A1NAF’s, ELSE FILL: this person’s
DISPLAY: AL_A1NAF is the name of the person not currently living with the R who will always know their whereabouts, collected at AL_A1NAF.
ASK: Respondents willing to provide additional personal contact information and agreed to give contact information for another person who is not currently living with them and who will always know their whereabouts.
AL_A1PEM [IF AL_INT3 = 1]
What is [IF AL_A1NAF NE 999 FILL AL_A1NAF’s, ELSE FILL: this person’s] personal e-mail address?
Interviewer: Enter the address as __________@@___[ALLOW 40 CHARACTERS]
999 PREFER NOT TO ANSWER
PROGRAMMER: IF AL_A1NAF NE 999 FILL AL_A1NAF’s, ELSE FILL: this person’s
IF A NUMERIC RESPONSE IS PROVIDED AND THE ‘PREFER NOT TO ANSWER’ BOX IS ALSO CHECKED, ERROR MESSAGE SHOULD SAY “PLEASE DO NOT ENTER AN EMAIL ADDRESS WHILE ALSO SELECTING ‘PREFER NOT TO ANSWER’ IN LOWERCASE LETTERS.
DISPLAY: AL_A1NAF is the name of the person not currently living with the R who will always know their whereabouts, collected at AL_A1NAF.
ASK: Respondents willing to provide additional personal contact information and agreed to give contact information for another person who is not currently living with them and who will always know their whereabouts.
AL_INT4 [IF NO CONTACTS PROVIDED AT BASELINE AND AL_INT3 = 1]
To be certain that we are able to contact you for a future interview, we would also like the contact information for one additional person, who is not currently living with you, who would always know your whereabouts. This information will be kept private and will only be used by the study to help contact you for a future interview. Could you share this information with us?
If necessary, read: This might be a family member, a close friend, or someone else who knows where you are. We would only contact this person if we could not reach you.
YES
NO
999 PREFER NOT TO ANSWER
ASK: Respondents willing to provide additional personal contact information and agreed to give contact information for another person who is not currently living with them and who will always know their whereabouts.
AL_A2NAF [IF AL_INT4 = 1]
What is this person's name?
FIRST NAME: ______________ [ALLOW 20 ALPHA CHARACTERS]
999 PREFER NOT TO ANSWER
PROGRAMMER: THIS PART OF THE SCREEN CAPTURES THE ALTERNATE PERSON'S FIRST NAME AND SHOULD BE DISPLAYED ON THE SAME SCREEN AS AL_A2NAL
ASK: Respondents willing to provide additional personal contact information and agreed to give contact information for another person who is not currently living with them and who will always know their whereabouts.
AL_A2NAL [IF AL_INT4 = 1]
What is this person's name?
LAST NAME: ____________ [ALLOW 20 ALPHA CHARACTERS]
999 PREFER NOT TO ANSWER
PROGRAMMER: THIS PART OF THE SCREEN CAPTURES THE ALTERNATE PERSON’S LAST NAME AND SHOULD BE DISPLAYED ON THE SAME SCREEN AS AL_A2NAF
ASK: Respondents willing to provide additional personal contact information and agreed to give contact information for another person who is not currently living with them and who will always know their whereabouts.
AL_A2REL [IF AL_INT4 = 1]
How is [IF AL_A2NAF NE 999 FILL AL_A2NAF, ELSE FILL: this person] related to you?
MOTHER
FATHER
STEPMOTHER (INCLUDING FOSTER OR ADOPTED MOTHER)
STEPFATHER (INCLUDING FOSTER OR ADOPTED FATHER)
GRANDMOTHER
GRANDFATHER
DAUGHTER
SON
AUNT
UNCLE
SISTER (INCLUDING HALF, STEP, FOSTER OR ADOPTED SISTER)
BROTHER (INCLUDING HALF, STEP, FOSTER OR ADOPTED BROTHER)
OTHER RELATIVE
FRIEND
GIRLFRIEND (NOT LIVING WITH RESPONDENT)
BOYFRIEND (NOT LIVING WITH RESPONDENT)
COWORKER
HUSBAND/WIFE (NOT LIVING WITH RESPONDENT)
EX-HUSBAND OR WIFE
OTHER, SPECIFY
999 PREFER NOT TO ANSWER
PROGRAMMER: IF AL_A2NAF NE 999 FILL AL_A2NAF, ELSE FILL: this person
DISPLAY: AL_A2NAF is the name of the person not currently living with the R who will always know their whereabouts, collected at AL_A2NAF.
ASK: Respondents willing to provide additional personal contact information and agreed to give contact information for another person who is not currently living with them and who will always know their whereabouts.
AL_A2RELs [IF AL_A2REL=20]
Please specify how [IF AL_A2NAF NE 999 FILL AL_A2NAF, ELSE FILL: this person] is related to you?
SPECIFY:_____________[ALLOW 25 ALPHA CHARACTERS]
999 PREFER NOT TO ANSWER
PROGRAMMER: IF AL_A2NAF NE 999 FILL AL_A2NAF, ELSE FILL: this person
DISPLAY: AL_A2NAF is the name of the person not currently living with the R who will always know their whereabouts, collected at AL_A2NAF.
ASK: Respondents willing to provide additional personal contact information and agreed to give contact information for another person who is not currently living with them and who will always know their whereabouts.
AL_A2HPH [IF AL_INT4 = 1]
What is [IF AL_A2NAF NE 999 FILL AL_A2NAF’s, ELSE FILL: this person’s] home telephone number, including area code?
HOME TELEPHONE NUMBER: _______[ALLOW 10 NUMERIC CHARACTERS]
999 PREFER NOT TO ANSWER
OPEN END NUM
VALIDATION: MIN 0 MAX 9999999999
PROGRAMMER: VALIDATE FORMAT FOR PHONE NUMBER. IF FORMAT IS INCORRECT, PLEASE DISPLAY:
“PLEASE VERIFY THIS AREA CODE WITH RESPONDENT. PRESS “CANCEL” TO CORRECT AREA CODE, OR “OK” TO CONTINUE.
IF AL_A2NAF NE 999 FILL AL_A2NAF’s, ELSE FILL: this person’s
DISPLAY: AL_A2NAF is the name of the person not currently living with the R who will always know their whereabouts, collected at AL_A2NAF.
ASK: Respondents willing to provide additional personal contact information and agreed to give contact information for another person who is not currently living with them and who will always know their whereabouts.
AL_A2CPH [IF AL_INT4 = 1]
What is [IF AL_A2NAF NE 999 FILL AL_A2NAF’s, ELSE FILL: this person’s] cell phone number, including area code?
CELL PHONE NUMBER:____________ [ALLOW 10 NUMERIC CHARACTERS]
999 PREFER NOT TO ANSWER
OPEN END NUM
VALIDATION: MIN 0 MAX 9999999999
PROGRAMMER: VALIDATE FORMAT FOR PHONE NUMBER. IF FORMAT IS INCORRECT, PLEASE DISPLAY:
“PLEASE VERIFY THIS AREA CODE WITH RESPONDENT. PRESS “CANCEL” TO CORRECT AREA CODE, OR “OK” TO CONTINUE.
IF AL_A2NAF NE 999 FILL AL_A2NAF’s, ELSE FILL: this person’s
DISPLAY: AL_A2NAF is the name of the person not currently living with the R who will always know their whereabouts, collected at AL_A2NAF.
ASK: Respondents willing to provide additional personal contact information and agreed to give contact information for another person who is not currently living with them and who will always know their whereabouts.
AL_A2PEM [IF AL_INT4 = 1]
What is [IF AL_A2NAF NE 999 FILL AL_A2NAF’s, ELSE FILL: this person’s] personal e-mail address?
Interviewer: Enter the address as _________@@____[ALLOW 40 CHARACTERS]
999 PREFER NOT TO ANSWER
PROGRAMMER: IF AL_A2NAF NE 999 FILL AL_A2NAF’s, ELSE FILL: this person’s
DISPLAY: AL_A2NAF is the name of the person not currently living with the R who will always know their whereabouts, collected at AL_A2NAF.
ASK: Respondents willing to provide additional personal contact information and agreed to give contact information for another person who is not currently living with them and who will always know their whereabouts.
INTERVIEWER READ: Thank you for providing this information.
SECTION J: APP-BASED PORTION OF STUDY
J0. [IF (WAVE = 2 AND APP_CONSENT (Wave 1) = 2 OR MISSING) OR (WAVE = 3 AND FU_APP_CONSENT (Wave 2) = 2 OR MISSING)] Do you have a smartphone?
YES GO TO FU_APP_CONSENT
NO EXIT 1
999 Prefer not to answer
ASK: All respondents who did not consent to app-based portion of study at Wave 1 or 2.
FU_APP_CONSENT [J0 = 1]
[IF CAPI: INTERVIEWER: GIVE RESPONDENT A COPY OF THE GREEN INFORMED CONSENT]
Thank you for completing the Point of Sale Intervention for Tobacco Evaluation (POSITEv) questionnaire. When you completed the questionnaire, you said that you own a smartphone. We are inviting you to participate in a smartphone application-based component of our study. This new approach to research uses your phone’s technology to determine how often you go to stores that sell tobacco products. When analyzed together with the survey responses that you already provided, information from this app will help the FDA to collect information about advertisements adults have seen and their attitudes towards smoking and programs that help smokers who want to quit.
If you choose to download the smartphone application (or app), it will use your phone’s location services to record the time, date, and your location whenever you go into and exit these kinds of stores. This technology only works when your location services are activated. As a result, we hope that you will keep location services activated as much as possible.
The app will also ask you to complete a short questionnaire (5 minutes or less) [IF WAVE = 2 FILL: two times; IF WAVE = 3 FILL: once] over the next several months. After you complete [IF WAVE = 2 FILL: each questionnaire; IF WAVE = 3 FILL: the questionnaire] with the app, you will receive a $5 electronic gift card that can be redeemed from an online vendor. You will receive an e-mail containing instructions on how to redeem your gift card. The app will not interact with, obtain information from, or transmit information to any of the other apps installed on the phone. Although the app will access location data from the phone, no location data will be logged except data for the mapped convenience stores. The data obtained from the app will not be used for any purpose except for analysis.
The app was developed by a company that develops apps to research health and behavior. If you choose to participate, [IF CAPI FILL: I / IF WEB FILL: we] will give you the instructions to download the app onto your phone now. Afterwards, you will not need to do anything else with the app except answer [IF WAVE = 2 FILL: two brief questionnaires; IF WAVE = 3 FILL: one brief questionnaire] over the next several months when prompted by the app to do so. However, you may be prompted to approve and/or download any updates that we create to make the app work better.
Types of Questions
The app-based [IF WAVE = 2 FILL: questionnaires ask; IF WAVE = 3 FILL: questionnaire asks] about the types of ads you’ve seen, your tobacco use, intention to quit smoking, and tobacco purchasing behaviors.
Voluntary Participation
Your participation in this study is completely voluntary. If you refuse to participate in this portion of the evaluation, you can still participate in the other parts of the study (a total of 4 online or in-person questionnaires over 24 months). You can decide not to answer any or all questions asked by the app or delete the app from your phone at any time. We simply ask that you contact us when you do so. You can stop the app-based questionnaire at any time, however, you will only receive the $5 token of appreciation if you complete the questionnaire.
Risks
There are no physical risks to you from participating in this component of the study. Some questions in the app-based questionnaire may be personal and might make you mildly uncomfortable. Wait until you are in a safe place to complete the questionnaires when prompted to do so. Please do not complete the questionnaires when driving, operating machinery, or performing other tasks that could put you or other people in danger. All of the information we collect from the app will be kept private and only used for research. We cannot guarantee the privacy of all of your information when it is communicated electronically, but we are making every possible effort to keep your information private.
Benefits
There are no direct benefits to you from answering our questions. However, you will be contributing to important research.
Costs
There is no cost to download the app. However, data collected by the app will count towards any data usage limits included in your phone plan. Before downloading the app, we recommend that you make sure that you have a data plan on your phone. You will be responsible for the costs of your phone bill including any data usage for the app. We estimate that the app will use 50MB (megabytes) of data per month. In 2017, most data plans ranged from 500MB of data per month to unlimited data. Charges for going over your data limit vary greatly by phone carrier but may range from $0 to $30 each time you go over. You may be charged this amount more than once. The app may also affect the battery life of your phone.
Confidentiality
We ask you for your e-mail address and phone number so that we can remind you to complete the [IF WAVE = 2 FILL: brief questionnaires; IF WAVE = 3 FILL: brief questionnaire]. You will also use a username that we provide you to sign into the app. We also ask for your e-mail address to provide you with a $5 electronic gift card in appreciation for your time. The application will not collect information from other apps in your phone. The app will not record any information about what you do in stores. Your survey answers and your location data will be recorded by the app and stored on the app vendor’s secure server. Your name will be kept private. Your answers will be labeled with a special number instead of your name. This makes it so only research staff will know these are your answers. Your specific answers to the survey questions will not be shared outside of the app vendor’s or RTI’s research teams. Instead, the information you provide will be combined with answers of many others and reported in a summary form. All of your answers will be kept private. We may send your data over the internet. It is not completely safe to send data through the Internet but we are doing everything we can to protect your data. All staff involved in this research are committed to keeping your information private. At the end of the study, we will ask you to delete the app so that it no longer collects information from you. Once RTI receives your information from the app, the app vendor will delete all information about you on their servers. If you withdraw from the study, we will ask you to delete the app, but some copies of your visits to convenience stores that sell tobacco may not be able to be destroyed or deleted.
Questions
If you have any questions about the study, you may call the project assistance line toll-free at 1-800-957-6457 between 9 am and 5 pm, Eastern Time, Monday through Friday or email us at fdastudy@rti.org. If you have any questions about your rights as a study participant, you may call RTI's Office of Research Protection at 1-866-214-2043 (a toll-free number). This research study was reviewed and approved by RTI International’s Institutional Review Board (IRB), a committee that evaluates research that involves human participants.
[IF CAWI FILL: You can print a copy of this consent form for your records.]
Do you agree to participate in the app-based portion of the study?
 Yes
GO TO J2
Yes
GO TO J2 No
GO TO FU_APP_REFUSAL
No
GO TO FU_APP_REFUSAL
ASK: Respondents who did not consent to app-based data collection at Wave 1 and report having a smartphone at Wave 2 or respondents who did not consent to app-based data collection at Wave 2 and report having a smartphone at Wave 3.
J1. [(IF WAVE = 2 AND APP_CONSENT (Wave 1)= 1) OR (WAVE = 3 AND FU_APP_CONSENT (Wave 2) = 1) OR (WAVE = 4 AND FU_APP_CONSENT (Wave 3) = 1)]Have you changed phones since [LASTINT]?
YES
NO
999 PREFER NOT TO ANSWER
PROGRAMMER: FILL LASTINT
DEFINE LASTINT: DATE OF LAST INTERVIEW
ASK: Respondents who agreed to participate in app-based data collection in the previous wave of data collection (Wave 1 or 2 or 3) .
J2. [IF (WAVE = 2 AND APP_CONSENT (Wave 1) = 1) OR (WAVE = 3 AND FU_APP_CONSENT (Wave 2) = 1) OR (WAVE = 4 AND FU_APP_CONSENT (Wave 3) = 1)] Do you have an iPhone or Android?
1 iPhone
2 Android
3 DON’T KNOW
999 Prefer not to answer
ASK: Respondents who agreed to participate in app-based data collection in the previous wave of data collection (Wave 1 or 2 or 3).
J2a [IF J2 = 1] Please select your iPhone from the list below.
PROGRAMMER: USE DROP DOWN LIST FOR RESPONSE CATEGORIES
iPhone 5
iPhone 5c
iPhone 5s
iPhone 6
iPhone 6 Plus
iPhone 6s
iPhone 6s Plus
iPhone SE
iPhone 7
iPhone 7 Plus
iPhone 8
iPhone 8 Plus
iPhone X
OTHER
DON’T KNOW
ASK: Respondents who agreed to participate in app-based data collection in the previous wave of data collection (Wave 1 or 2 or 3).
J2a1 [IF J2 = 1] We want to know what operating system your iPhone is using. To check, please go to “Settings”, then “General”, then “About”, then “Version”. [IF CAPI: What is the version number of your operating system / [IF WEB: Please enter the version number of your operating system here]:
_________ VERSION NUMBER OF OPERATING SYSTEM
DON’T KNOW
PREFER NOT TO ANSWER
ASK: Respondents who agreed to participate in app-based data collection in the previous wave of data collection (Wave 1 or 2 or 3) and have an iPhone.
IF FU_APP_CONSENT = 1 GO TO APP_INSTRUCTIONS1
J2b [IF J2 = 2] Please select your phone from the list below.
PROGRAMMER: USE DROP DOWN LIST FOR RESPONSE CATEGORIES
Samsung Galaxy S7
Alpha4
Alpha1
LG K8(2018)
OTHER
DON’T KNOW
ASK: Respondents who agreed to participate in app-based data collection in the previous wave of data collection (Wave 1 or 2 or 3) and have an android.
J2b2 [IF J2 = 2] We want to know what operating system your android is using to help us know more about how the POSITEv app is working. To check, please go to “Settings”, tap “About Phone” or “About Device”, and tap “Android Version” to display your version information. [IF CAPI: What is the version number of your operating system / [IF WEB: Please enter the version number of your operating system here]:
_________ VERSION NUMBER OF OPERATING SYSTEM
DON’T KNOW
PREFER NOT TO ANSWER
ASK: Respondents who agreed to participate in app-based data collection in the previous wave of data collection (Wave 1 or 2 or 3) and have an android
IF FU_APP_CONSENT = 1 GO TO APP_INSTRUCTIONS1
J3. [IF J1 = 2 OR 999] We are interested in knowing if you have turned off all location services on your phone. Since installing the app, have you ever turned off your phone’s location services, meaning the ability for your phone to detect its geographic location?
1 YES
2 NO
999 PREFER NOT TO ANSWER
ASK: Respondents who participated in app-based data collection in the previous wave and have not changed phones or indicated they preferred not to answer whether they changed phones.
J3a. [IF J3 = 1] Why have you turned off your phone’s location services? Please select all that apply.
1 Privacy reasons
2 Data usage
3 Battery usage
4 Concern from family member or friend
5 Memory issue
6 Something else (specify)______________
999 PREFER NOT TO ANSWER
ASK: Respondents who participated in app-based data collection in the previous wave, have not changed phones or indicated they preferred not to answer whether they changed phones, and indicated they had turned off their phone’s location services.
J3b. [IF J3 = 1] During a typical week, how often are the location services for your phone off?
More than 75% of the time
Between 50% to 75% of the time
Between 25% to 50% of the time
Less than 25% of the time
999 PREFER NOT TO ANSWER
ASK: Respondents who participated in app-based data collection in the previous wave, have not changed phones or indicated they preferred not to answer whether they changed phones, and indicated they had turned off their phone’s location services.
J4. [IF J1 = 2 OR 999] Have you changed the POSITEv app settings to turn off access to location services?
YES
NO
999 PREFER NOT TO ANSWER
ASK: Respondents who participated in app-based data collection in the previous wave and have not changed phones or indicated they preferred not to answer whether they changed phones.
J4a. [IF J4 = 1] Why have you changed the POSITEv app settings to turn off access to location services? Please select all that apply.
1 Privacy reasons
2 Data usage
3 Battery usage
4 Concern from family member or friend
5 Memory issue
6 Something else (specify)______________
999 PREFER NOT TO ANSWER
ASK: Respondents who participated in app-based data collection in the previous wave, have not changed phones or indicated they preferred not to answer whether they changed phones, and indicated they had changed the POSITEv app settings to disallow access to location services.
J4b [IF J4 = 1] How long ago did you change the POSITEv app settings to turn off access to location services? Please answer in days, weeks, or months.
____ Days (Range: 0 to 7)
____ Weeks (Range: 0 to 4)
____ Months (Range: 0 to 12)
999 Prefer not to answer
ASK: Respondents who participated in app-based data collection in the previous wave, have not changed phones or indicated they preferred not to answer whether they changed phones, and indicated they had changed the POSITEv app settings to disallow access to location services.
J4c [IF J4 = 1] Since then, have you changed the POSITEv app settings back?
YES
NO
999 Prefer not to answer
ASK: Respondents who participated in app-based data collection in the previous wave, have not changed phones or indicated they preferred not to answer whether they changed phones, and indicated they had changed the POSITEv app settings to disallow access to location services.
J4d [IF J4c = 1] About how long ago did you change the POSITEv app settings back to allow access to location services? Please tell me in days, weeks, or months.
____ Days (Range: 0 to 7)
____ Weeks (Range: 0 to 4)
____ Months (Range: 0 to 12)
999 Prefer not to answer
ASK: Respondents who participated in app-based data collection in the previous wave, have not changed phones or indicated they preferred not to answer whether they changed phones, and indicated they had changed the POSITEv app settings to disallow access to location services but have changed them back to allow access to location services
J5. [IF J1 = 2 OR 999] Have you changed the POSITEv app settings to turn off notifications?
YES
NO
999 Prefer not to answer
ASK: Respondents who participated in app-based data collection in the previous wave and have not changed phones or indicated they preferred not to answer whether they changed phones.
J5a. [IF J5 = 1]: Why have you changed the POSITEv app settings to turn off notifications?
1 Privacy reasons
2 Data usage
3 Battery usage
4 Concern from family member or friend
5 Memory issue
6 Something else (specify)______________
999 PREFER NOT TO ANSWER
ASK: Respondents who participated in app-based data collection in the previous wave and have not changed phones or indicated they preferred not to answer whether they changed phones, and have changed the POSITEv app settings to disallow notifications.
J5b. [IF J5 = 1] How long ago did you change the POSITEv app settings to turn off notifications? Please answer in days, weeks, or months.
____ Days (Range: 0 to 7)
____ Weeks (Range: 0 to 4)
____ Months (Range: 0 to 12)
999 Prefer not to answer
ASK: Respondents who participated in app-based data collection in the previous wave and have not changed phones or indicated they preferred not to answer whether they changed phones, and have changed the POSITEv app settings to disallow notifications.
J5c. [IF J5 = 1] Have you changed the POSITEv app settings back to allow notifications since then?
YES
NO
999 Prefer not to answer
ASK: Respondents who participated in app-based data collection in the previous wave and have not changed phones or indicated they preferred not to answer whether they changed phones, and have changed the POSITEv app settings to disallow notifications.
J5d. [IF J5c = 1] About how long ago did you change the POSITEv app settings back to allow notifications? Please answer in days, weeks, or months.
____ Days (Range: 0 to 7)
____ Weeks (Range: 0 to 4)
____ Months (Range: 0 to 12)
999 Prefer not to answer
ASK: Respondents who participated in app-based data collection in the previous wave and have not changed phones or indicated they preferred not to answer whether they changed phones, and have changed the POSITEv app settings to disallow notifications and have changed the settings back to allow notifications.
J6. [IF J1 = 2 OR 999] Have you logged out of the POSITEv app since you downloaded it?
YES
NO
999 Prefer not to answer
ASK: Respondents who participated in app-based data collection in the previous wave and have not changed phones or indicated they preferred not to answer whether they changed phones.
J6a. [IF J6 = 1] Why have you logged out of the POSITEv app? Please select all that apply.
1 Privacy reasons
2 Data usage
3 Battery usage
4 Concern from family or friend
5 Memory issue
6 Something else (specify)______________
999 PREFER NOT TO ANSWER
ASK: Respondents who participated in app-based data collection in the previous wave and have not changed phones or indicated they preferred not to answer whether they changed phones, and have logged out of the POSITEv app since they downloaded it.
J6b. [IF J6 = 1] How long ago did you log out of the POSITEv app? Please answer in days, weeks, or months.
____ Days (Range: 0 to 7)
____ Weeks (Range: 0 to 4)
____ Months (Range: 0 to 12)
999 Prefer not to answer
ASK: Respondents who participated in app-based data collection in the previous wave and have not changed phones or indicated they preferred not to answer whether they changed phones, and have logged out of the POSITEv app since thy downloaded it.
J6c. [IF J6 = 1] Have you logged back into the POSITEv app?
YES
NO
999 Prefer not to answer
ASK: Respondents who participated in app-based data collection in the previous wave and have not changed phones or indicated they preferred not to answer whether they changed phones, and have logged out of the POSITEv app since thy downloaded it.
J6d. [IF J6c = 1] About how long ago did you log back into the POSITEv app? Please answer in days, weeks, or months.
____ Days (Range: 0 to 7)
____ Weeks (Range: 0 to 4)
____ Months (Range: 0 to 12)
999 Prefer not to answer
ASK: Respondents who participated in app-based data collection in the previous wave and have not changed phones or indicated they preferred not to answer whether they changed phones, have logged out of the POSITEv app since thy downloaded it, but have logged back into the POSITEv app.
J7. [IF J1 = 2 OR 999] Have you [IF IPHONE FILL: deleted; IF ANDROID FILL: uninstalled] the POSITEv app?
YES
NO
999 Prefer not to answer
ASK: Respondents who participated in app-based data collection in the previous wave and have not changed phones or indicated they preferred not to answer whether they changed phones.
J7a. [IF J7 = 1] Why did you [IF IPHONE FILL: delete; IF ANDROID FILL: uninstall] 1the POSITEv app? Select all that apply
1 Privacy reasons
2 Data usage
3 Battery usage
4 Concern from family member or friend
5 Memory issue
6 Something else (specify)______________
999 PREFER NOT TO ANSWER
ASK: Respondents who participated in app-based data collection in the previous wave and have not changed phones or indicated they preferred not to answer whether they changed phones, and indicated they deleted or uninstalled the POSITEv app.
J7b. [IF J7 = 1] How long ago did you [IF IPHONE FILL: delete; IF ANDROID FILL: uninstall] the POSITEv app? Please answer in days, weeks, or months.
____ Days (Range: 0 to 7)
____ Weeks (Range: 0 to 4)
____ Months (Range: 0 to 12)
999 Prefer not to answer
ASK: Respondents who participated in app-based data collection in the previous wave and have not changed phones or indicated they preferred not to answer whether they changed phones, and indicated they deleted or uninstalled the POSITEv app.
J7c. [IF J7 = 1] Have you downloaded the POSITEv app again?
YES
NO
999 Prefer not to answer
ASK: Respondents who participated in app-based data collection in the previous wave and have not changed phones or indicated they preferred not to answer whether they changed phones, and indicated they deleted or uninstalled the POSITEv app.
J7d. [IF J7c= 1] About how long ago did you download the POSITEv app again? Please answer in days, weeks, or months.
____ Days (Range: 0 to 7)
____ Weeks (Range: 0 to 4)
____ Months (Range: 0 to 12)
999 Prefer not to answer
ASK: Respondents who participated in app-based data collection in the previous wave and have not changed phones or indicated they preferred not to answer whether they changed phones, and indicated they deleted or uninstalled the POSITEv app but have since downloaded the POSITEv app again.
APP USABILITY QUESTIONS
J8. [IF (WAVE = 2 AND APP_CONSENT (WAVE 1) = 1) OR (WAVE = 3 AND FU_APP_CONSENT (Wave 2) = 1) OR (WAVE = 4 AND FU_APP_CONSENT (Wave 3) = 1)] Please tell us how much you disagree or agree with the following statements about using the POSITEv app.
|
|
Strongly Disagree |
Disagree |
Neither Agree or Disagree |
Agree |
Strongly Agree |
Not Applicable |
Prefer Not to Answer |
J8a. |
The POSITEv app was easy to install |
1 |
2 |
3 |
4 |
5 |
6 |
999 |
J8b. |
It was simple to complete the survey |
1 |
2 |
3 |
4 |
5 |
6 |
999 |
J8c. |
I have liked participating in the app-based part of the POSITEv study |
1 |
2 |
3 |
4 |
5 |
6 |
999 |
J8d. |
The app is an important part of the POSITEv study |
1 |
2 |
3 |
4 |
5 |
6 |
999 |
J8e. |
A $5 digital gift card is a good deal for taking a short survey |
1 |
2 |
3 |
4 |
5 |
6 |
999 |
ASK: All respondents who consented to app-based portion of study in the previous wave.
J9 [IF ((WAVE = 2 OR 3) AND (J1=1 OR 999)) OR ((WAVE = 2 OR 3) AND (J7=1 AND J7c = 2))] We are inviting you to continue to participate in the smartphone application-based component of our study. As a reminder, this is a new approach to research that uses your phone’s technology to determine how often you go to stores that sell tobacco products. If you choose to download the smartphone application (or app), it will use your phone’s location services to record the time, date, and your location whenever you go into and exit these kinds of stores. The app will also ask you to complete a short questionnaire (5 minutes or less) [IF WAVE = 2 FILL: two times; IF WAVE = 3 FILL: once] over the next few months. After you complete [IF WAVE = 2 FILL: each questionnaire; IF WAVE = 3 FILL: the questionnaire] with the app, you will receive a $5 electronic gift card that can be redeemed from an online vendor. You will receive an e-mail containing instructions on how to redeem your gift card. The app will not interact with, obtain information from, or transmit information to any of the other apps installed on the phone. Although the app will access location data from the phone, no location data will be logged except data for the mapped convenience stores. The data obtained from the app will not be used for any purpose except for analysis.
Would you like to keep participating in this part of the study?
YES
NO APP_REFUSAL
ASK: All respondents who participated in the previous wave and have gotten a new phone since the previous wave of data collection or declined to answer the question about getting a new phone or reported uninstalling or deleting the app since they first downloaded it.
APP_INSTRUCTIONS1 [IF J9 = 1 OR FU_APP_CONSENT = 1]
Thank you for agreeing to participate in the smartphone app portion of the study!
The app uses location services and specific settings to determine how often you go into stores that sell tobacco products. The app will not work if you delete the app, change the app settings, or turn off location services. As a result, we hope that you will not delete the app, change any of the app’s settings, or turn off location services while participating in this study.
[IF CAPI FILL: INTERVIEWER: RECORD THE RESPONDENT’S USER ID (ON THIS SCREEN, BELOW) ON THE BLUE SMARTPHONE APP INSTRUCTIONS SHEET AND GIVE TO RESPONDENT.]
Please take a moment to download the app. If you have any questions about the instructions, [IF CAPI FILL: you may ask me; IF WEB FILL: please contact us at 1-800-957-6457.]
[IF CAPI FILL: INTERVIEWER: ASSIST THE RESPONDENT, AS NEEDED, WITH FINDING THE "APP STORE” (IF R HAS IPHONE) OR THE “PLAY STORE” (IF R HAS ANDROID).]
Here are the instructions:
[IF J2 = 1 (iPhone) FILL:
1. Tap on the “App Store” icon.
2. Search for POSITEv.
3. Click the cloud icon to the right of the app name (“POSITEv”). A square should appear with a circle around it in place of the cloud. The circle should slowly change from white to blue.
If you are prompted for your Apple password, enter it.
Once the download is complete, the word “open” will appear to the right of the app name. Click “Open” (or find the app on your home screen).
Click “Allow” when you see the following message: “POSITEv” Would Like to Send You Notifications. Notifications may include alerts, sounds, and icon badges. These can be configured in Settings.”
The first screen you will see is a “Welcome” screen. Swipe from right to left to get to the “Introduction” screen. In the bottom right hand corner, click on the box that reads “Get Started”.
Enter your “RTI ID” and “Password”:
User ID: [Display RTI-assigned ID number]
Password: fda$tudy
Click “return” on your phone’s keyboard or the button labeled “Sign In” under the login boxes.
After entering your RTI ID and Password the following question should appear via a pop-up: “Allow “POSITEv” to access your location? ? This app requires location access to track your visits to stores that sell tobacco products as a voluntary part of your participation in the POSITEv research study.”
Please select “Always Allow”]
[IF J2 = 2 (Android) FILL:
Tap on the “Play Store” icon and select “APPS.”
Search for POSITEv.
If under the search bar you see “showing results for positive” click “search instead for positev”
When you find the app, tap on it and click “Install.”
Click “Open” once download is complete (or find the app on your home screen).
You will see a “Welcome” screen, followed by an “Introduction” screen. On the “Introduction” screen, click on “Get Started”
Enter your “RTI ID” and “Password”:
User ID: [Display RTI-assigned ID number]
Password: fda$tudy
After entering your RTI ID and Password the following question should appear via a pop-up: “Allow “POSITEv” to access this device’s location?”
Please select “Allow”]
[IF CAPI FILL: INTERVIEWER: THE FIRST SCREEN IN THE APP IS A “WELCOME” SCREEN. R SHOULD:
SWIPE FROM RIGHT TO LEFT TO THE “INTRODUCTION” SCREEN
CLICK ON “GET STARTED”
ENTER USER ID AND PASSWORD AND CLICK ‘SIGN IN’
CLICK ”ALWAYS ALLOW”/”ALLOW” WHEN ASKED TO ALLOW ACCESS TO THE DEVICE’S LOCATION.
CLICK “ALLOW” WHEN ASKED TO ALLOW NOTIFICATIONS, OR CONFIGURE THIS IN SETTINGS.]
[IF WEB FILL: Would you like assistance downloading and installing the POSITEv app? If so, please contact us at 1-800-957-6457, or if you would rather we contact you, please mark the box below:
__ Please call me to assist me in downloading and installing the POSITEv app.]
ASK: Respondents who participated in the previous wave of app-based data collection and have gotten a new phone since the previous wave of data collection or declined to answer the question about getting a new phone or reported uninstalling or deleting the app since they first downloaded it, but consented to continue participating in the app-based portion of the study, and respondents who did not participate in the previous wave of app-based data collection but agree to participate in Wave 2 or 3 or 4.
APP_INSTRUCTIONS2 [If J9 = 1 OR FU_APP_CONSENT = 1]
[IF CAPI FILL: INTERVIEWER: PLEASE ENSURE THE FOLLOWING:
YOU PROVIDED R WITH SMARTPHONE APP INSTRUCTIONS SHEET THAT INCLUDES USER ID
R HAS DOWNLOADED/INSTALLED POSITEV APP
R HAS SIGNED INTO APP WITH USER ID AND PASSWORD
R HAS ALLOWED app ACCESS TO LOCATION AND NOTIFICATIONS]
You may close the app. The app will continue to collect information from your phone, specifically the date, time, and location when you enter into and exit stores that sell tobacco products. The app may ask you to complete [IF WAVE = 2 FILL: two very brief questionnaires; IF WAVE = 3 FILL: one very brief questionnaire] over the next several months. You will receive a $5 gift card for [IF WAVE = 2 FILL: each completed questionnaire; IF WAVE = 3 FILL: completing the questionnaire]. [IF WAVE = 2 FILL: Each time you complete a questionnaire; IF WAVE = 3 FILL: After you complete the questionnaire], you will receive an e-mail describing how to redeem your gift card online.
[IF CAPI FILL: Press NEXT to continue / IF WEB FILL: Press NEXT to continue]
GO TO EXIT 3
ASK: Respondents who participated in the previous wave of app-based data collection and have gotten a new phone since the previous wave of data collection or declined to answer the question about getting a new phone or reported uninstalling or deleting the app since they first downloaded it, but consented to continue participating in the app-based portion of the study, and respondents who did not participate in the previous wave of app-based data collection but agree to participate in Wave 2 or 3 or 4.
APP_INSTRUCTIONS3 [((IF J3 OR J4 OR J5 OR J6 = 1) AND (J7 = 2 OR J7c = 1)]
Thank you for agreeing to participate in the smartphone app portion of the study!
To make sure that it works properly we recommend that you:
Go to ‘Settings’ on your phone and find ‘POSITEv’
Make sure ‘location access’ is set to ‘Always’
Make sure ‘Notifications’ are set to ‘Allow’
ASK: Respondents who participated in the previous wave of app-based data collection but changed the settings or deleted/uninstalled and then reinstalled the app.
APP_EMAIL [IF (WAVE = 2 OR 3 AND FU_APP_CONSENT = 1) AND ((AL_EPEM1 = 2 OR 999) OR (AL_EPEM2 = 999))]
We need your e-mail address to contact you about the study, such as providing information about the gift card that you will receive for completing a short questionnaire. What is your personal (non-work) e-mail address that you use at home?
INTERVIEWER:
E![]() NTER
THE ADDRESS AS: _________@__ [ALLOW 40 CHARACTERS]
GO TO EXIT 3
NTER
THE ADDRESS AS: _________@__ [ALLOW 40 CHARACTERS]
GO TO EXIT 3
I![]() do not have an e-mail address
GO TO EXIT4
do not have an e-mail address
GO TO EXIT4
9![]() 99
PREFER NOT TO ANSWER
GO TO EXIT4
99
PREFER NOT TO ANSWER
GO TO EXIT4
ASK: Respondents who agreed to app-based data collection but did not provide an e-mail address.
FU_APP_REFUSAL [IF FU_APP_CONSENT = 2]
IF CAPI FILL: INTERVIEWER: WHY DID THE PARTICIPANT REFUSE TO DOWNLOAD THE APP? SELECT ALL THAT APPLY / IF WEB FILL: We are interested in learning why people don’t want to participate in app-based data collection. Please select the reasons you are not interested in participating in this research]
[PROGRAM SO THAT INTERVIEWERS CAN SELECT MORE THAN ONE RESPONSE]
[IF CAPI FILL: DOES NOT HAVE A SMARTPHONE, AFTER ALL/ IF WEB FILL: I DO NOT HAVE A SMARTPHONE]
CONCERNED ABOUT PRIVACY
CONCERNED ABOUT DATA USAGE
[IF CAPI FILL: PARTICIPANT REFUSED AFTER DIFFICULTY IN DOWNLOADING AND INSTALLING THE APP / IF WEB FILL: IT IS TOO DIFFICULT TO DOWNLOAD AND/OR INSTALL THE APP]
THE INCENTIVE IS TOO SMALL
SOME OTHER REASON (SPECIFY)
GO TO EXIT 2
ASK: Asked of interviewers or web respondents when refuse to participate in the app-based portion of the study.
FU_APP_REFOTR [IF FU_APP_REFUSAL = 6]
[IF CAPI FILL: INTERVIEWER: SPECIFY WHY PARTICIPANT REFUSED TO DOWNLOAD THE APP / IF WEB FILL: PLEASE SPECIFY WHY YOU DO NOT WANT TO PARTICIPATE IN THE APP-BASED DATA COLLECTION.]
SPECIFY:_____________[ALLOW 50 ALPHA CHARACTERS]
ASK: Asked of interviewers or web respondents that indicate there is another reason the participant/they refused to participate in the app-based portion of the study.
APP_INSTRUCTIONS1
[INSERT APP INSTRUCTIONS]
[IF (AL-ECPH1A = 2 OR 999) OR (AL_ECPH1 = 2 or 999)]
EXIT 1: Thank you for answering all of our questions.
[IF CAPI: Here is $25 in cash as a token of our appreciation for your participation.
FI PROVIDE RESPONDENT WITH $25 CASH.
I also need to fill out a receipt to certify you received (or declined) the cash incentive. Since maintaining the privacy of your information is important to us, I will not include your name on the receipt.
FI FILL OUT RECEIPT AND PROVIDE RESPONDENT WITH PINK COPY OF RECEIPT.
Here is a copy of the receipt for your records. Thank you again for answering all of our questions.
1 RESPONDENT ACCEPTS INCENTIVE
2 RESPONDENT DECLINES INCENTIVE]
OMB cONTROL NO. 0910-0851 EXPIRATION DATE: 4/30/2021
Paperwork Reduction Act Statement: The public reporting burden for this collection of information has been estimated to average 40 minutes per response (time to read and agree to the assent/consent and respond to the questionnaire). Send comments regarding this burden estimate or any other aspects of this collection of information, including suggestions for reducing burden to PRAStaff@fda.hhs.gov.
ASK: Respondents reaching the end of the survey
[IF W2_APP_CONSENT = 2]
EXIT 2: Thank you for considering this part of our study. [IF CAPI FILL: Here is $25 in cash as a token of our appreciation for your participation.
FI PROVIDE RESPONDENT WITH $25 CASH.
I also need to fill out a receipt to certify you received (or declined) the cash incentive. Since maintaining the privacy of your information is important to us, I will not include your name on the receipt.
FI FILL OUT RECEIPT AND PROVIDE RESPONDENT WITH PINK COPY OF RECEIPT.
Here is a copy of the receipt for your records. Thank you again for answering all of our questions.
1 RESPONDENT ACCEPTS INCENTIVE
2 RESPONDENT DECLINES INCENTIVE]
OMB cONTROL NO. 0910-0851 EXPIRATION DATE: 4/30/2021
Paperwork Reduction Act Statement: The public reporting burden for this collection of information has been estimated to average 40 minutes per response (time to read and agree to the assent/consent and respond to the questionnaire). Send comments regarding this burden estimate or any other aspects of this collection of information, including suggestions for reducing burden to PRAStaff@fda.hhs.gov.
PROGRAMMER: COMPLETED CASE THAT WILL NOT PARTICIPATE IN APP-BASED DATA COLLECTION SHOULD DISPOSITION AS 2690.
ASK: Respondents reaching the end of the survey who have a smartphone but do not want to participate in the app-based portion of the study
[W2_APP_CONSENT = 1 AND (AL_EPEM2 NE 999 OR (APP_EMAIL NE 999 OR “I do not have an email address”))]
EXIT 3: Thank you for answering our questions.
[IF CAPI FILL: Here is $25 in cash as a token of our appreciation for your participation.
FI PROVIDE RESPONDENT WITH $25 CASH.
I also need to fill out a receipt to certify you received (or declined) the cash incentive. Since maintaining the privacy of your information is important to us, I will not include your name on the receipt.
FI FILL OUT RECEIPT AND PROVIDE RESPONDENT WITH PINK COPY OF RECEIPT.
Here is a copy of the receipt for your records. Thank you again for answering all of our questions.
1 RESPONDENT ACCEPTS INCENTIVE
2 RESPONDENT DECLINES INCENTIVE]
OMB cONTROL NO. 0910-0851 EXPIRATION DATE: 4/30/2021
Paperwork Reduction Act Statement: The public reporting burden for this collection of information has been estimated to average 40 minutes per response (time to read and agree to the assent/consent and respond to the questionnaire). Send comments regarding this burden estimate or any other aspects of this collection of information, including suggestions for reducing burden to PRAStaff@fda.hhs.gov.
PROGRAMMER: COMPLETED CASE THAT AGREES TO APP-BASED DATA COLLECTION SHOULD DISPOSITION AS 2691.
ASK: Respondents reaching the end of the survey who want to participate in the app-based portion of the study
EXIT4 [IF APP_EMAIL = 999 OR “I do not have an e-mail address”] We provide electronic gift cards for participation in the app surveys via email. If you aren’t comfortable providing your e-mail address we can help you uninstall the app.
[IF CAPI FILL: INTERVIEWER: ASK RESPONDENT IF S/HE WOULD LIKE HELP UNINSTALLING THE APP.
Thank you for answering all of our questions. Here is $25 in cash as a token of our appreciation for your participation.
FI PROVIDE RESPONDENT WITH $25 CASH.
I also need to fill out a receipt to certify you received (or declined) the cash incentive. Since maintaining the privacy of your information is important to us, I will not include your name on the receipt.
FI FILL OUT RECEIPT AND PROVIDE RESPONDENT WITH PINK COPY OF RECEIPT.
Here is a copy of the receipt for your records. Thank you again for answering all of our questions.
1 RESPONDENT ACCEPTS INCENTIVE
2 RESPONDENT DECLINES INCENTIVE]
OMB cONTROL NO. 0910-0851 EXPIRATION DATE: 4/30/2021
Paperwork Reduction Act Statement: The public reporting burden for this collection of information has been estimated to average 40 minutes per response (time to read and agree to the assent/consent and respond to the questionnaire). Send comments regarding this burden estimate or any other aspects of this collection of information, including suggestions for reducing burden to PRAStaff@fda.hhs.gov.
PROGRAMMER: COMPLETED CASE THAT WILL NOT PARTICIPATE IN APP-BASED DATA COLLECTION SHOULD DISPOSITION AS 2690.
ASK: Respondents who agreed to app-based data collection but would not provide an e-mail address.
EXIT5_REFUSAL [IF SECA_CONSENT = 2]
FI: ATTEMPT TO CONVERT THE REFUSAL. IF CONVERT GO BACK TO INFORMED CONSENT AND UPDATE RESPONSE TO ‘YES’. IF UNABLE TO CONVERT, WHY DID THE PARTICIPANT REFUSE? SELECT ALL THAT APPLY
[PROGRAM SO THAT INTERVIEWERS CAN SELECT MORE THAN ONE RESPONSE]
CONCERNED ABOUT CONFIDENTIALITY OR PRIVACY
SAID THE INCENTIVE IS TOO SMALL
Too busy/no time/did too many already
Surveys/Govt. invasive
“Nothing in it for me”/Uncooperative
SOME OTHER REASON (SPECIFY)
OMB cONTROL NO. 0910-0851 EXPIRATION DATE: 4/30/2021
Paperwork Reduction Act Statement: The public reporting burden for this collection of information has been estimated to average 40 minutes per response (time to read and agree to the assent/consent and respond to the questionnaire). Send comments regarding this burden estimate or any other aspects of this collection of information, including suggestions for reducing burden to PRAStaff@fda.hhs.gov.
ASK: Respondents who do not consent to the interview
REFOTR [IF EXIT5_REFUSAL = 6]
INTERVIEWER: SPECIFY WHY PARTICIPANT REFUSED
SPECIFY:_____________[ALLOW 25 ALPHA CHARACTERS]
ASK: Asked of interviewers that indicate there is another reason the participant refused to participate
OMB No. 0910-0851
Expiration Date: 04/30/2021
RIHSC No. 17-082CTP
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Dutra, Lauren |
| File Modified | 0000-00-00 |
| File Created | 2021-01-15 |
© 2026 OMB.report | Privacy Policy