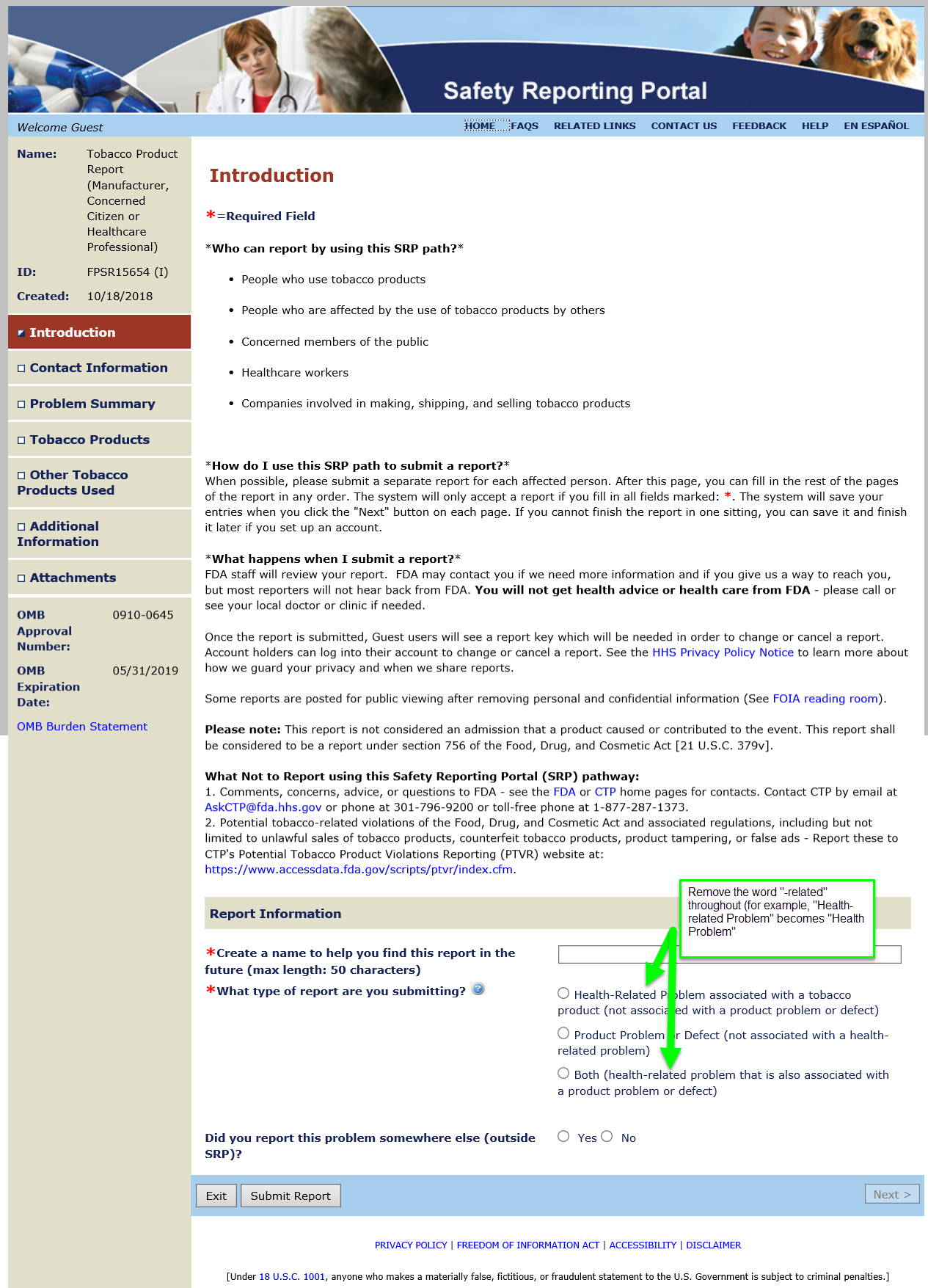
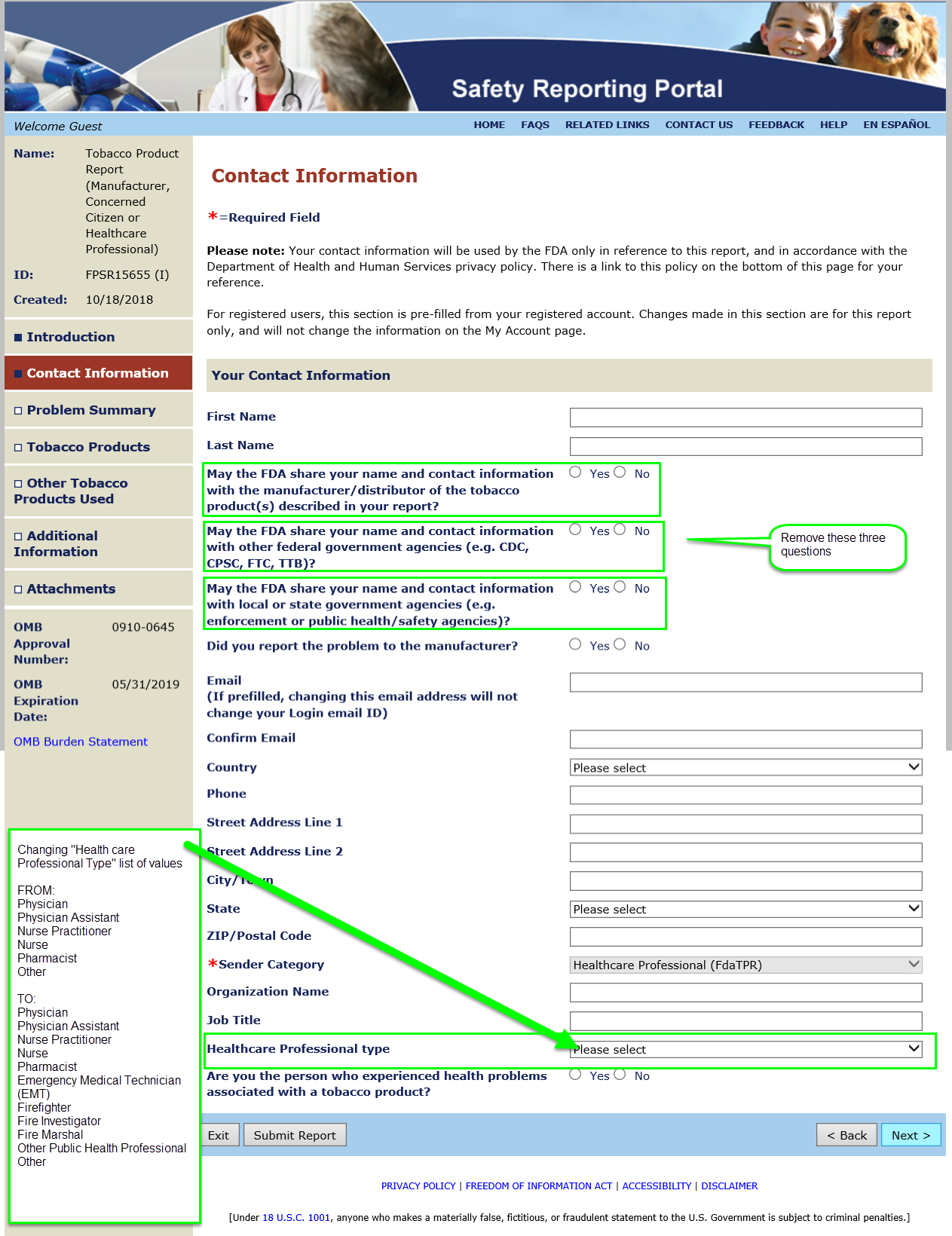
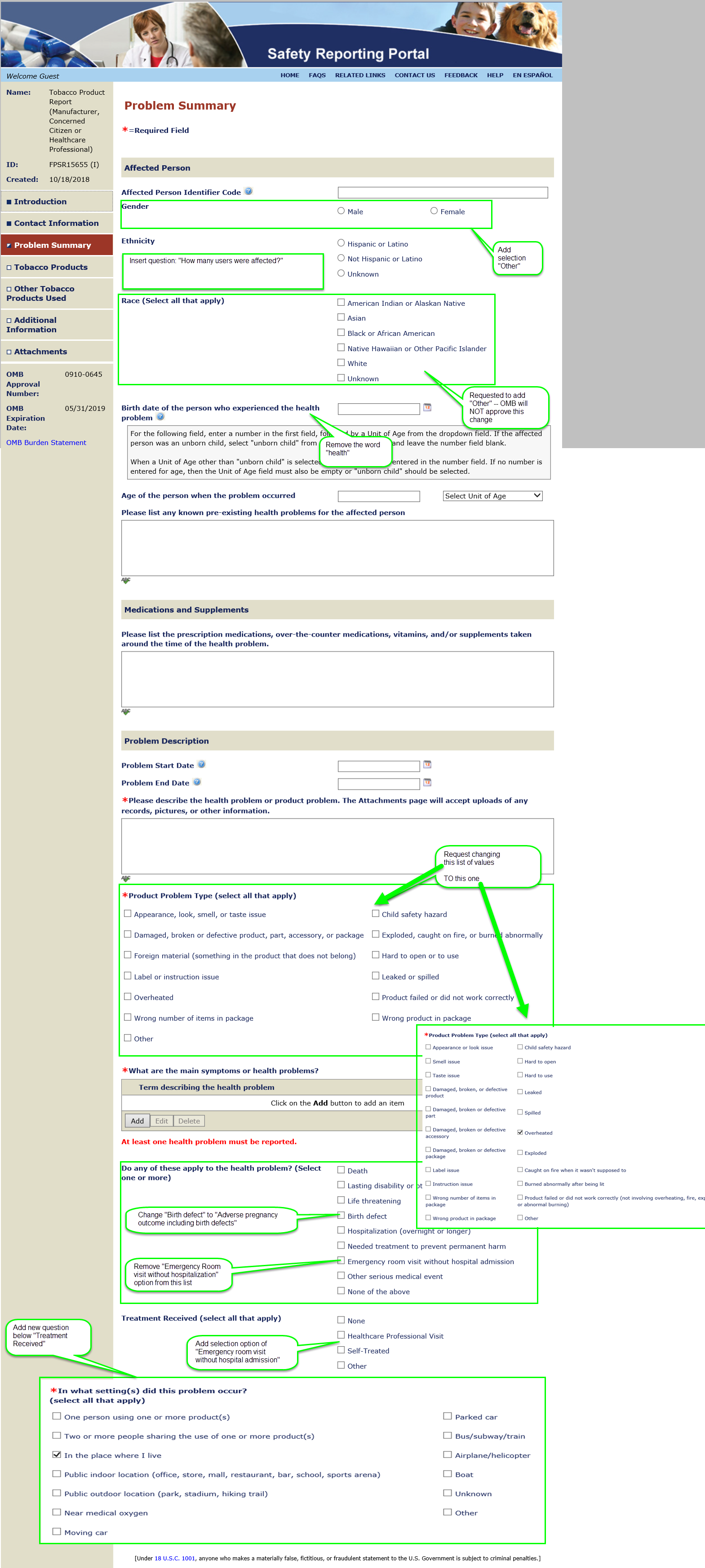
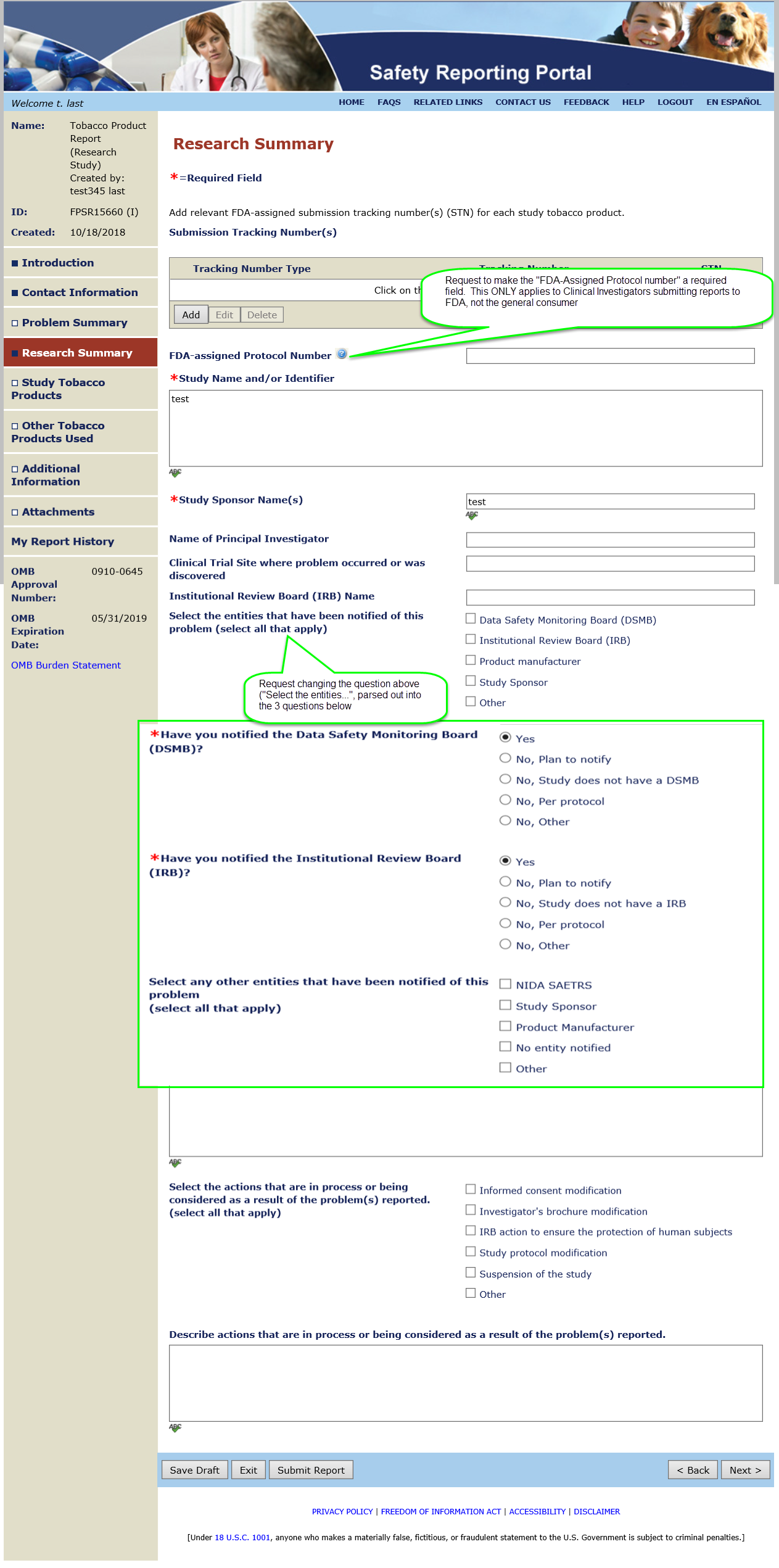
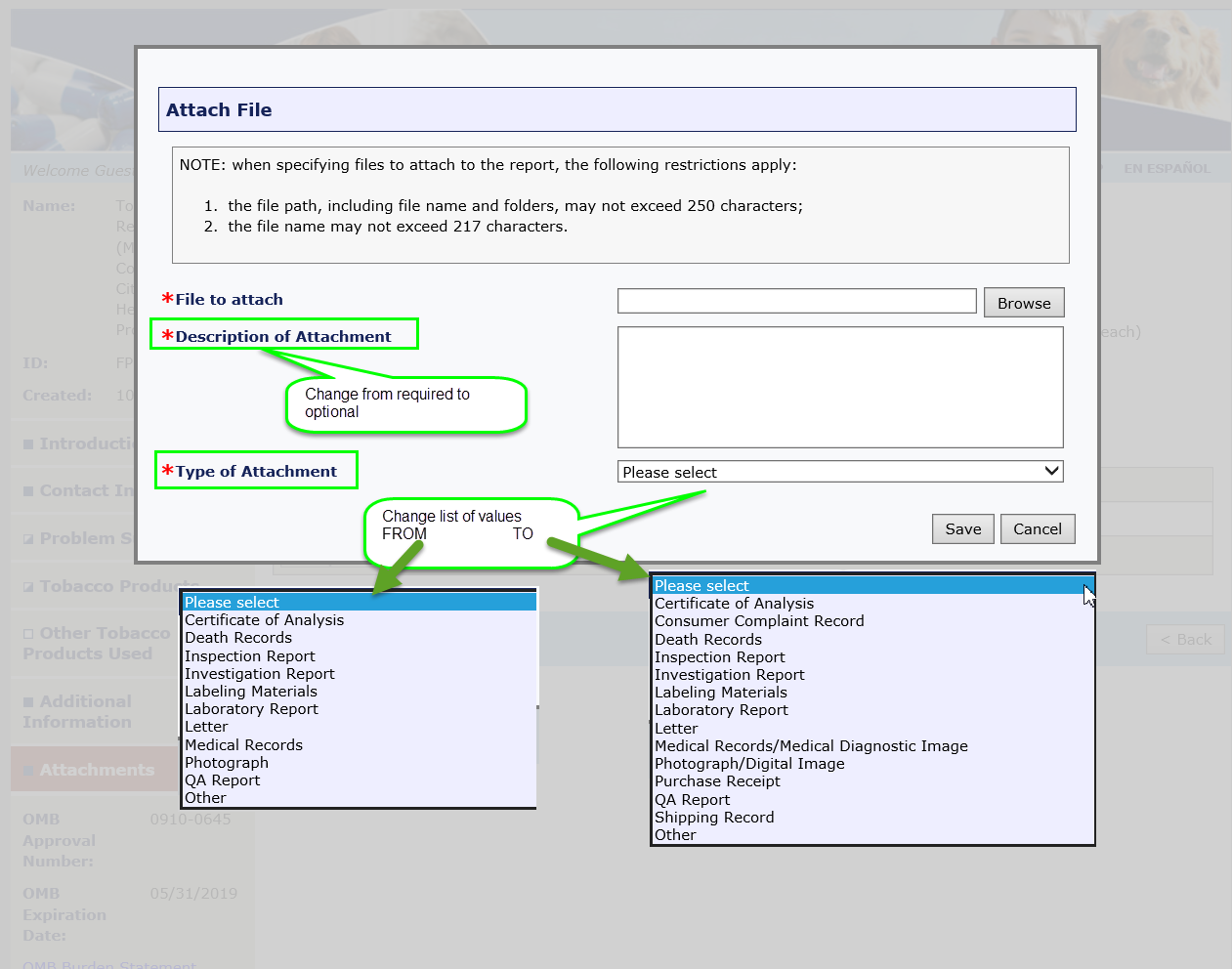
Summary of Changes
0645 Summary of SRP changes for OMB.docx
FDA Adverse Event and Products Experience Reports; Electronic Submissions
Summary of Changes
OMB: 0910-0645
⚠️ Notice: This form may be outdated. More recent filings and information on OMB 0910-0645 can be found here:
© 2026 OMB.report | Privacy Policy