Measuring Accuracy - Facial Examiners - Informed Consent - Template
0693-0043-MeasuringAccuracy-FacialExaminers-InformedConsent-Template12-14-17.docx
NIST Generic Clearance for Usability Data Collections
Measuring Accuracy - Facial Examiners - Informed Consent - Template
OMB: 0693-0043
Attachment BI – ITL-0021
Informed Consent to Participate in Research
Principal Investigator: P. Jonathon Phillips, Ph.D.
Study Title: Measuring the accuracy of facial forensics comparisons
Study Site(s): Laboratory or office location of the participants.
Introduction
You are being asked to take part in a research study. Research studies include only people who choose to take part. This document is called an informed consent form. Please read this information carefully and take your time making your decision. Ask the researcher or study staff to discuss this consent form with you, please ask him/her to explain any words or information you do not clearly understand. The nature of the study, risks, inconveniences, discomforts, and other important information about the study are listed below.
The person who is in charge of this research study is P. Jonathon Phillips, Ph.D. This person is called the Principal Investigator (PI). However, other NIST research staff may be involved and can act on behalf of the person in charge.
This research is being sponsored by the Federal Bureau of Investigation (FBI).
Purpose of the study
Previously, you participated in our study to measure the accuracy of facial forensic examiners. In the study, you were asked to compare 20 pairs of face images. This study focused on one aspect of face recognition. However, there are many other aspects of face recognition. To gain a more complete understanding of the face recognition ability of facial forensic examiners, we are conducting a follow-up study. The follow-up study consists of five experiments that test different aspects of face recognition. The five experiments do not necessarily test the professional skills that facial examiners use when making comparisons in their laboratory.
Why are you being asked to take part?
We are asking you to take part in this research study because you completed the previous part of the study. To be eligible to participate you need to have access to email and the World Wide Web (the Web). If you are using your employer’s computers, email, laboratory tools, methods, or procedures, as applicable, to participate, you must have your employer’s permission. You must also have your employer’s permission if you will participate in study activities during your normal working hours. The access to email and the Web is required for communicating with participants, taking the questionnaire and submitting test results.
Study Procedures:
If you take part in this study, you will be asked to:
Schedule an interview with a NIST researcher
Confirm you have your employer’s permission to use your employer’s computers and email.
Review, prior to the interview, the consent form. We will send to you a copy of the consent form by email or fax.
Participate in a phone interview in which we will:
Review the goals of the project.
Explain the consent form.
Answer any questions you may have.
Ask you if you want to sign the consent form. You will choose to sign or not to sign the form. Then the form is either scanned and securely emailed or faxed to NIST. The NIST researcher who explained the consent form to you signs the form and sends the form to you by email or fax. If the form is faxed, arrangements will be made so that you are present when the form is faxed.
Participate in five experiments. Each experiment will start by clicking on a link to an online survey, which will be run by Survey Gizmo.
One experiment measures your ability to remember faces.
This experiment contains a practice section where you will learn how the experiment is run.
In each trial, you will be asked to memorize up to 6 faces at a time. The faces will be shown for up to 20 seconds.
Then a set of faces will be presented. You will be asked to identify a face that you just memorized.
The second experiment will ask you to compare two faces that are shown on your screen.
Experiment consists of 40 pairs of faces.
On the screen, two faces will be presented side-by-side for up to 30 seconds.
You will be asked to answer if the faces are from the Same or Different people.
A pair of faces will be displayed until you enter a rating or for 30 seconds. If you take more than 30 seconds to make a response, the faces will disappear from the screen, and you will need to enter a response. The next pair of images will not be shown until you enter a response.
The other three experiments will ask you to compare two faces that are shown on your screen.
Each experiment consists of at most 156 pairs of faces.
On the screen, two faces will be presented side-by-side for up to 30 seconds.
Rate the similarity between each pair on the following scale:
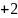 :
Sure they are the same.
:
Sure they are the same.
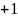 :
Think they are the same.
:
Think they are the same.
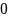 :
Do not know.
:
Do not know.
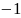 :
Think they are not the same.
:
Think they are not the same.
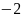 :
Sure they are not the same.
:
Sure they are not the same.A pair of faces will be displayed until you enter a rating or for 30 seconds. If you take more than 30 seconds to make a decision, the faces will disappear from the screen and you will need to enter a rating. The next pair of images will not be shown until you enter a rating.
Please complete each experiment in a single session. You may withdraw from this study until you click the final submit button on the fifth experiment you take.
Please do not consult or discuss this study with friends, family, or colleagues from your institution or other institutions.
You will have four weeks to complete the online experiments. If you do not finalize any submission within four weeks, we will withdraw you from this portion of the study.
You will receive an email for each experiment from the PI once a week reminding you about the study. You will also receive an email one day before the deadline. Once you have completed an experiment, you will not receive any reminder emails.
We will link your answers to these five experiments to your answers submitted to the earlier part of the study through your study number. We will keep this data and your previous data to perform detailed analysis and for re-analysis and meta-analysis at a later date.
Total Number of Participants
The study is being administered by NIST and up to 300 individuals will take part in this study.
Voluntary Participation / Withdrawal
You should only take part in this study if you want to volunteer. You should not feel that there is any pressure to take part in the study. You are free to participate in this research or withdraw at any time. There will be no penalty if you stop taking part in this study. A decision not to participate will not affect your job status.
You may withdraw from the follow-up study until you click the final submit button on the fifth experiment. If you wish to withdraw from the study, you will need to email or phone the PI. If you choose to withdraw, all of your data from this portion, including any completed experiments, will be destroyed. Withdrawing from this follow-up portion of this study will not withdraw you from forensic portion of study and your data from the forensic portion of the study will not be destroyed.
Benefits
The potential benefits of participating in this research study include contributing to the body of knowledge of psychology and forensics sciences in understanding how facial forensic examiners process faces. In addition, the knowledge gained from this experiment could contribute to develop more accurate procedures for performing facial forensic identification.
In the previous portion of the study, you were able to request your scores if you wished. However, NIST does not own all these additional experiments. Moreover, these experiments are still used in the field. Therefore, we will not be releasing the answers for these experiments.
Risks or Discomfort
This research is considered minimal risk. That means that the risks associated with this study are the same as what you may encounter every day (e.g., working on an office computer, or taking an online survey). There are no known additional risks to those who take part in this study.
Compensation
You will receive no payment or other compensation for taking part in this study.
Costs
It will not cost you anything to take part in the study.
Privacy and Confidentiality
We will keep your study records private and confidential. Certain people may need to see your study records. Anyone who looks at your records must keep them confidential. These individuals include:
The NIST research team, including the Principal Investigator, study coordinator, and all other NIST research staff.
Certain government people who need to know more about the study, and individuals who provide oversight to ensure that we are doing the study in the right way.
Any agency of the federal, state, or local government that regulates this research such as the FBI and Office for Human Research Protections (OHRP) within the Department of Health and Human Services (DHHS).
To communicate with you for administering the experiment and providing test scores, we have collected your name, email, and postal address; please let us know if any information needs to be updated. You will be assigned the same study number as previously in order to link your performance on this portion of the study with the previous forensic portion of the study. Concurrently with publishing the results of the forensic portion of the study the key linking study numbers to subjects’ identities (name, email, postal address) will be destroyed.
A master list linking your personally identifiable information to the study number will be kept in an encrypted disk on a government computer located in a locked office at NIST or on a government encrypted disk in a locked cabinet at NIST. The master list will link the previous background surveys and previous scores to the new scores; the master list will not contain any names, emails, or postal addresses. The master list will be kept since there is the potential for this data to be useful many years beyond the point of study completion. The file that includes your study code and other study information is called the de-identified data set. The de-identified data set will be made available to other researchers including Prof. Alice J. O’Toole of the University of Texas at Dallas and Dr. David White of the University of New South Wales, Australia.
Future research: We will keep all data, including identifiable data, for future research. Future research will be reviewed by an IRB that has the responsibility to make sure research studies are conducted in an ethical manner that protects the rights and welfare of subjects. For future research, only the de-identified data set will be shared outside NIST; no one outside the study team will receive the master list.
Confidentiality of your records will be protected to the extent possible under existing regulations and laws including the Freedom of Information Act, but cannot be guaranteed. We may publish what we learn from this study. If we do, we will not include your name. We will not publish anything that would let people know who you are.
You can get the answers to your questions, concerns, or complaints
If you have any questions, concerns or complaints about this study, or experience an unanticipated problem, or research-related injury, call P. Jonathon Phillips, Ph.D., at 301-975-5348.
If you have questions about your rights as a participant in this study, or have complaints, concerns or issues you want to discuss with someone outside the research, call the NIST Human Subjects Protection Office at (301) 975-5445.
You will receive a copy of this signed consent form.
Consent to Take Part in this Research Study
I freely give my consent to take part in this study. I understand that by signing this form I am agreeing to take part in research. I also confirm the following by initialing the box next to each item that applies to my participation in the study:
 I
have my employer’s permission to perform study activities in
the course of my
I
have my employer’s permission to perform study activities in
the course of my
work duties.
 I
have my employer’s permission to use my work computer and email
address to
I
have my employer’s permission to use my work computer and email
address to
perform study activities.
I have received a copy of this form to take with me.
_____________________________________________ ____________
Signature of Person Taking Part in Study Date
_____________________________________________
Printed Name of Person Taking Part in Study
Statement of Person Obtaining Informed Consent
I have carefully explained to the person taking part in the study what he or she can expect from their participation. I confirm that this research subject demonstrated proficiency in English, the language that was used to explain this research, and is receiving an informed consent form in English. This research subject has provided legally effective informed consent.
______________________________________________ ____________
Signature of Person obtaining Informed Consent Date
______________________________________________
Printed Name of Person Obtaining Informed Consent
Version #
Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Subject | USF IRB Form, informed consent, template, adult, social, behavioral |
| Author | California Family Health Council, John Arnaldi, Norma Epley |
| File Modified | 0000-00-00 |
| File Created | 2021-01-20 |
© 2026 OMB.report | Privacy Policy