Attachment B
2288ss03_Attachment B (002)_2018-06-07.docx
Pesticides Data Call In Program
Attachment B
OMB: 2070-0174
Attachment B (revised 6/08/2018)
Office of Pesticide Programs
2015 Revised General Methodology and Assumptions Used to
Estimate Paperwork Response Burden for Pesticide Data Call-In Recipients
November 2015
Introduction
In 2007, the United States Environmental Protection Agency (EPA or Agency) described the methodology and assumptions the EPA’s Office of Pesticide Programs (OPP) uses to calculate the related Paperwork Reduction Act (PRA) burden1 for industry to respond to Data Call-In (DCI) notices issued pursuant to Section 3(c)(2)(B) of the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA).
The methodology is entitled “General Methodology Used to Estimate Paperwork Burden Hours and Costs by the Office of Pesticide Programs for Submission of Required Data/Information for Responding to a Data Call-In Notice”2 Today, the Agency is redefining some of this methodology by revising the number of DCI recipient groups and calculations for those groups to reassess the PRA burden. This document revises and supplements the 2007 policy.
Industry Participation
To update the 2007 methodology, OPP conducted a reassessment of the information collection burden associated with the DCI response process. In December 2013, the Agency held a DCI Response Burden Assessment Workshop with industry stakeholders. As part of the reassessment, OPP consulted with industry about the Agency burden assumptions, the methodology used to estimate the burden, the time estimates for conducting PRA activities, and the accuracy of and appropriate distribution of the labor rates. 3 This document incorporates new burden and cost data obtained in part from workshop participants and other public comments. OPP’s burden reassessment of the DCI Information Collection Request (ICR) were limited only to PRA burdens for the following types of DCIs: Reregistration, Registration Review, Endangered Species Protection Program, Special Review Program, and the Tolerance Assessment Program including the Anticipated Residue/Percent Crop Treated Information.
Burden Assessment for the DCI ICR renewal
The PRA burden hour and cost estimates in this document are new and use the revised methodology. The Agency will use this revised methodology to update the burden and cost estimates in future DCI ICR renewal documents. The estimates in this document were used to update the DCI ICR document that is posted in the Docket EPA-HQ-OPP-2016-0109. The Agency seeks public comment on the new methodology and the new estimates. All public comment received will be posted to the docket for this action.
Section I: Background on Programs Involving Data Call-Ins (DCIs)
EPA uses its DCI authority to acquire the data that has been deemed necessary for the Agency’s statutorily mandated review of a pesticide’s registration, which require it to assess whether the continued registration of an existing pesticide causes an unreasonable adverse effect on human health or the environment and whether the Agency will pursue appropriate regulatory measures. The key OPP program areas which use the issuance of a DCI under FIFRA §3(c)(2)(B) include:
A. Reregistration Program
FIFRA §44 requires EPA to re-assess the health and safety data for all pesticide active ingredients registered before November 1, 1984, to determine whether these “older” pesticides meet the criteria for registration that would be expected of a pesticide being registered today for the first time. FIFRA §4 directs EPA to use FIFRA §3(c)(2)(B) authority to obtain the required data. While, the reregistration program reassessment process was completed by 2006 for food-use pesticide ingredients and 2008 for non-food use pesticide ingredients, the Agency will still issue certain types of product-specific and confirmatory/generic DCIs (PDCIs and GDCIs) in the future.
B. Registration Review Program
FIFRA §3(g)5 directs EPA to establish by regulation procedures for periodically reviewing pesticide registrations and to complete each pesticide's registration review, at least, every 15 years to assure that the pesticide continues to pose no unreasonable adverse effects on human health or the environment. The purpose of this review is to assure that a pesticide continues to meet the FIFRA standard for registration. The procedural regulations were promulgated in 2006 as 40 CFR Part 155, subpart C.6 FIFRA §3(g) instructs EPA to use the FIFRA §3(c)(2)(B) authority to obtain data determined to be necessary to complete the assessment, reviews, and decisions called for under FIFRA §3(g).
In addition, EPA intends these reviews to involve the review of data related to endangered species and endocrine effects:
Endangered Species Protection Program (ESPP). EPA conducts effects determinations (risk assessments) to ensure that protections are in place for populations of non-target species including endangered species as required under the Endangered Species Act (ESA)7, as part of the risk characterization of the pesticide under Registration Review. FIFRA §3(g) instructs EPA to use the FIFRA §3(c)(2)(B) authority to obtain the required data.
Endocrine Disruptor Screening Program (EDSP). EPA considers potential endocrine effects pursuant to Federal Food, Drug, and Cosmetic Act (FFDCA)§408(p)8 as part of the risk characterization of the pesticide under Registration Review. 9 FFDCA §408(p)(5) mandates the issuance of Orders requiring screening of substances for their potential endocrine disruptor effects. FIFRA §3(c)(2)(B) also provides a means of obtaining needed data for pesticides. Under the EDSP program, two types of data collection authorities allow the Agency to address endocrine disruptor screening, and testing data needs for DCIs and §408(p) Orders. In establishing the policy and procedures for issuing §408(p) Orders under the EDSP, EPA will integrate the considerations under the EDSP with the Registration Review activities whenever possible. EPA believes that doing this will provide efficiencies for everyone involved. Please note, however, the information collection activities associated with the issuance of §408(p) Orders are already covered by another ICR, identified under EPA ICR No. 2249 and approved under Office of Management and Budget (OMB) Control No. 2070-0176. As such, the issuance of §408(p) Orders for Registration Review chemicals is not currently covered by EPA ICR No. 2288 approved under OMB control No. 2070-0174.
C. Maintenance DCIs
Section 3(c)(2)(B) of FIFRA provides a means of obtaining needed additional data “to maintain in effect an existing registration of a pesticide.” A need for a data call-in may arise from evolving of scientific understanding and methodologies, changes in the discovery of deficiencies in previously submitted data, or from the new discovery of specific attributes of the pesticide or its ingredients. For example, such data may be needed in support of Agency enforcement cases resulting from consumer complaints about the product, its storage stability, the integrity of its container, or exaggerated advertising claims. A DCI might also be needed to confirm product performance of public health pesticides or a new type of manufacturing process may call into question data submitted for a pesticide registration using older manufacturing technologies no longer used today. These situations may give rise to new concerns such as observed or suspected adverse human health or environmental effects attributed to the use of a pesticide that were not present at the time of the original registration or from unanticipated circumstances such as changes in pathogens of public health concern, new EPA initiatives, or the evolution of scientific test methodologies or manufacturing technologies. This IC category replaces the old IC named “Enforcement and Unanticipated Incident” activities in an older ICR.
D. Tolerance Assessment Program (Anticipated Residue/Percent Crop Treated Information)
Under FFDCA §408, before a pesticide may be used on food or feed crops, the Agency must establish a tolerance for the pesticide residues on that crop or establish an exemption from the requirement to have a tolerance. In order to conduct the required evaluation, a Pesticide Registrant may be required to submit specific data necessary to demonstrate that residues do not exceed the residue levels used to establish the tolerance. Under the authority of FIFRA §3(c)(2)(B), the Agency will issue a DCI to obtain any additional data that is determined to be necessary for the decisions that must be made under this program. FFDCA §408(b)(2)(E) and (F) authorize the use of anticipated or actual residue (ARs) data, and percent crop treated (PCT) data to establish, modify, maintain, or revoke a tolerance for a pesticide. FFDCA requires that if AR data are used, data must be reviewed within five years after a tolerance is initially established. If PCT data are used, FFDCA affords EPA the discretion to obtain additional data if any or all of several conditions, including but not limited to the following, are met:
The existing data have been found unreliable;
Exposure estimates underestimate exposures for any significant population group; and
Dietary exposure must be re-evaluated periodically.
Section II: Data Collections Using a DCI
The data that EPA may collect and review under this ICR will likely vary for each DCI because the DCI is tailored to address the specific needs of the individual chemical or active ingredient under review. However, the data requested will be primarily based on the data requirements that are found in 40 CFR Part 158. In codifying the requirements in 40 CFR Part 158, EPA provided substantiation and support to demonstrate the need and practical utility for the data in terms of its use to assess the risks for particular chemicals based on the different use patterns and pesticides, and in order to make the required registration decisions about those pesticides. 40 CFR Part 158 also includes a provision that allows the Agency to seek additional non-codified data if it is determined to be necessary to make the risk-based decisions mandated by federal law.
A. History of the Methodology Used to Estimate the Paperwork Burden and Costs for DCIs
DCI Test Cost Burden Calculation Assumptions
Prior to 2007, EPA calculated the paperwork burden and costs for the data generation activities as a percentage of the testing costs. This percent-based estimate of paperwork associated with conducting a test was initially established in consultation with OMB in the 1980s in an effort to provide a reasonable estimate of the burden associated with the paperwork component of data generation, which may vary based on the complexity of the test performed. In 2007, EPA solicited public comment on this approach as described in detail in the document entitled “General Methodology Used to Estimate Paperwork Burden Hours and Costs by the Office of Pesticide Programs for Submission of Required Data/Information for Responding to a Data Call-In Notice.”10 Based on feedback received, EPA concluded that this approach appeared to be a reasonable and fair alternative to simply setting a single estimate for data generation burden or using set criteria of high, medium or low burden, neither of which may fairly reflect potential differences in burden.
To calculate the burden and costs associated with the paperwork activities involved in conducting the tests, the Agency starts with the cost of the test, typically the market price for the test as identified by laboratories that offer testing services. The Agency maintains an archive of the basic FIFRA study cost estimates that were developed through surveys of independent testing laboratories, Agency economic analyses, and registrant comments during ICR renewal periods. To the greatest extent possible, EPA uses multiple sources to provide test cost estimates, which are updated as needed.
EPA uses 35% of the estimated total test cost to calculate the total potential cost of the paperwork activities related to data generation. The 35% of test cost is disaggregated by labor category, and then burden hours are extrapolated by using the loaded labor rates. To disaggregate by labor category, the Agency considered the estimated distribution of paperwork activity across the labor categories represented and the existing methodology assumption that paperwork activities for data generation mostly involve the technical staff to perform the tests, with fewer activities related to management and clerical staff.
See Figure 1 for an illustrated outline of the Agency burden calculation process for data generation.
Figure 1 – Method for Calculating Paperwork Burden from Test Costs
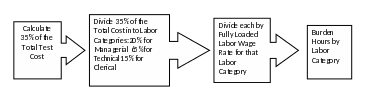
This approach assumes and incorporates the following core considerations:
Recipients generate all of the data as specified in the DCI and without any changes.
All data generation is performed by an independent laboratory.
Paperwork burden is disaggregated by labor category as follows:
Managerial (20%)
Technical (65%)
Clerical (15%)
Labor rates are fully loaded, meaning that they include the estimated costs of wages, overhead, and benefits paid to an employee.
For certain types of DCIs, paperwork burden calculations do not follow the current methodology—that is, they are not broken out by 20%-65%-15% Managerial-Technical-Clerical as is typically the case. This is because certain types of DCIs have paperwork burden that falls disproportionately on different labor categories and thus do not follow the EPA methodology for estimating paperwork burden hours for DCIs. For more information, see the case studies in the 2007 methodology document.11 Mailing costs for DCIs are not included in the estimates below as electronic submissions are now accepted, and it is typical practice for registrants to submit data using this method. In the case that DCI submissions are submitted using certified mail, it is estimated that the cost to submit an individual DCI would be no more than $20.12
B. Methodology for Calculating Labor Wage Rates
The Agency updates the estimated wages, benefits, and overhead for all labor categories for affected industries, state government, and EPA employees based on publicly available data from the U.S. Bureau of Labor Statistics (BLS). The formulas used to estimate the labor rates and formulas used to derive the fully loaded rates and overhead costs for the new and renewal ICRs are listed in Table 1.
Table 1: EPA Methodology Used to Determine Labor Rates
Methodology |
The methodology uses data on each sector and labor type for an Unloaded wage rate (hourly wage rate), and calculates the Loaded wage rate (unloaded wage rate + benefits), and the Fully loaded wage rate (loaded wage rate + overhead). Fully loaded wage rates are used to calculate respondent costs. This renewal uses 2015 base data. |
Unloaded Wage Rate |
Wages are estimated for labor types (management, technical, and clerical) within relevant sectors. The Agency uses average wage data for the relevant sectors available in the National Industry-Specific Occupational Employment and Wage Estimates from the (BLS) at http://www.bls.gov/oes/current/oes_nat.htm. |
Sectors |
The specific North American Industry Classification System (NAICS) code and website for each sector is included in that sector’s wage rate table. Within each sector, the wage data are provided by Standard Occupational Classification (SOC). The SOC system is used by Federal statistical agencies to classify workers into occupational categories for the purpose of collecting, calculating, or disseminating data (see http://www.bls.gov/oes/current/oes_stru.htm). |
Loaded Wage Rate |
Unless stated otherwise, all benefits represent 43% of unloaded wage rates, based on benefits for all civilian non-farm workers, from http://www.bls.gov/news.release/ecec.t01.htm. However, if other sectors are listed for which 43% is not applicable; the applicable percentage will be stated. |
Fully Loaded Wage Rate |
We multiply the loaded wage rate by 50% (EPA guidelines 20-70%) to calculate overhead costs and add this to the loaded wage rate to get the fully loaded wage rate. |
C. Information Collection Groups
Information Collection (IC) groups are a way of categorizing similar DCI requests into subprogram groupings. EPA projects 13 IC groups for this ICR renewal. The IC groups and subgroups are outlined in Table 2. In the previous DCI ICR, the IC group, “DCIs for Enforcement and Unanticipated Incidents” was included but EPA is removing this group in future ICRs because EPA has not issued, and does not foresee issuing, any DCIs for this category. The Special Review program section is also being removed from this ICR renewal. The program has not conducted a special review in over a decade and does not foresee any new special review program activity being conducted in the near future. Conversely one new IC group is being added, the Maintenance DCI group, which includes the paperwork burden and cost of non-target pollinator insect studies and efficacy studies. Detailed information about the specific IC program groups is in Section 1: Programs Involving Data Call-ins.
Table 2: Information Collection (IC) Groups
IC Group |
Activity |
|
Reregistration Program |
|
|
|
|
|
|
|
Maintenance DCIs |
|
Registration Review |
|
|
|
|
|
|
|
|
|
Tolerance Assessment Program (Anticipated Residue/Percent Crop Treated) |
|
|
|
Section III: Redefining the DCI Recipient Groups to Better Define Paperwork Burden
Each IC subprogram group described in Section II, has a unique set of DCI recipients with corresponding burden activities, see Table 3. The Agency is redefining the DCI recipient groups to better classify the activities and burdens associated with each group. These new definitions refine the existing burden methodology and will be used in future DCI PRA burden assessments. While each DCI involves the same basic paperwork activities, the burden associated with each recipient group can be very different. The new DCI recipient groups and their definitions are:
DCI Recipients
Data Generators
Consortium Participants
DCI Recipients - After receiving a DCI, each recipient has 90 days to provide the initial response indicating how the recipient plans to comply with the DCI. A recipient may avoid generating the data if they qualify for a generic data exemption, i.e., they use a registered pesticide as the source of the active ingredient in their own product, cancel the product’s registration, submit or cite existing data, or is granted a waiver by EPA in response to their request. Thus, not all DCI recipients will generate data in response to a DCI however, at a minimum, all recipients are assumed to be involved in the four burden activities listed in Table 3.
Data Generators - Regardless of the response option that the DCI recipient might select, the Agency has assumed that some data will be generated for each chemical. The data generator is assumed to be involved in the nine burden activities listed in Table 3. While Agency records indicate that not all the studies requested in a DCI are, in fact, generated (data generators can request waivers, submit or cite existing data like the DCI recipients), for the most part, the Data Generators group will assume the highest DCI response burden among the three respondent groups.
Consortium Participants - The Agency will assume that whenever more than one company receives identical DCIs for the same chemical, the companies will work together and participate in a consortium or task force to generate one set of data to respond to the DCI. Generally, the Agency calculations for DCI response burdens are accounted for as part of the 35% of the cost of generating studies.13 However, in addition to the burden of creating the DCI response and generating data, consortium participants are subject to the burden associated with operating a consortium or task force (e.g., communication, attending meetings, etc.). The seven additional consortium burden activities are listed in Table 3.
Table 3: New Recipient Groups - Activities and Corresponding Category.
DCI Recipient |
|
Collection Activity |
Collection Category |
|
Reporting |
|
|
|
|
|
Recordkeeping |
Data Generator |
|
Collection Activity |
Collection Category |
|
Reporting |
|
|
|
|
|
|
|
|
|
|
|
|
|
Recordkeeping |
|
|
Consortium Activities |
|
|
Paperwork burden associated with operating a consortium |
|
|
|
|
|
|
|
|
|
|
|
|
Section IV: Estimating the Burden and Cost for the Redefined DCI Recipient Groups
The estimated paperwork burden and costs for DCI recipients vary from DCI to DCI because of the variations in the individual studies that are part of the DCI and the combination of activities (waivers, exemptions, etc.) each DCI manifests. As discussed, there are multiple ways of responding to a DCI and not all DCI recipients will generate and submit data as part of the DCI response. Until the Agency receives the 90-day response letters to the DCI notice from the registrants indicating what studies, if any, they will conduct, it is not possible to accurately estimate the burden and costs of developing the data. Nor can the Agency accurately predict the number of DCI recipients who will generate data or the amount of data that might be submitted to EPA. The Agency’s burden estimates are based on past patterns of DCI response activities.
DCI Recipients - DCI recipients are subject to burden from having to provide an initial response to the EPA for a DCI regardless of whether or not they generate data. The methodology EPA used for calculating the burden for this group is derived from the 2007 Methodology, Phase 1 requirements outlined in Case Study #1, Attachment A, which reflects the activities that all DCI recipients would have to conduct regardless of whether or not they generate data.
Given that a single DCI can be sent to several companies, DCI recipient burden is calculated at the company level—not at the DCI level. To estimate the number of companies that are DCI recipients, EPA conducted a search of companies that received a DCI request in its Pesticide Registration Information System (PRISM) to determine the average annual number of impacted entities. The Agency estimates that 122 companies will receive a DCI request annually. For more information on methodologies used in estimating the total number of DCI recipients and burdens to DCI recipients, see Appendix A.
Data Generators – Generally, the paperwork burden and costs for data generators are based in part on the average cost of paying a laboratory to conduct the test(s) necessary to generate the data requested in the DCI. To estimate paperwork activities for each type of labor category (e.g., managerial, technical, and clerical), the disaggregated paperwork burden costs are multiplied by their corresponding labor category wage rates ($/hr). However, burden and cost calculations for some DCIs do not follow the Agency’s methodology of paperwork burden being categorized as 20%-65%-15% Managerial-Technical-Clerical, as certain Information Collection (IC) Groups have paperwork burden that falls disproportionately on different labor categories. For details regarding the methodology used for calculating data generation paperwork burden for each of the IC Groups, refer to Appendix B.
EPA has also assumed that for each DCI, companies are combining resources when responding to a DCI and data generation is necessary—thus, it is expected that only one set of data is being submitted to the EPA in response to each DCI request. EPA understands that this assumption may not be accurate and solicits industry input to clarify this assumption.
EPA expects to issue approximately 663 DCIs over the next three years that will require data generation. This represents an annual average of 221 DCIs issued. The estimate for data generators does not include voluntarily submitted data, as they are not DCIs (i.e., IC Groups 2, 3, 8, and 9 in Table 4 are excluded from this estimate).
Consortium Participants - Unlike typical data generators, consortiums face additional paperwork burden activities, such as meetings and correspondence to coordinate consortium activities. While consortium members encumber burden from consortium activities, the cost savings incurred by sharing the DCI response and data generation burden provides cost savings to industry. EPA assumes that no business would opt to join a consortium if the cost of consortium activities would result in a higher cost per DCI response. Thus for each consortium member, the upper bound (i.e., maximum) total cost per DCI submitted by a consortium is expected to be less than or equal to the per DCI cost incurred by a recipient who chooses to submit their DCI data independently – a.k.a., a Data Generator. Industry provided EPA with information to support that approximately 21 consortiums exist and the typical consortium activities that result in paperwork burden. Details on consortium activities and the methodology used for calculating total consortium paperwork burden are located in Appendix C.
The breakdown of the regulatory decisions for DCIs that EPA expects to make over the next three years (2018 – 2021) is as follows:
Table 4: Estimated Number of DCIs by IC Group
IC Number |
IC Group |
Total DCIs 1-Year Period* |
Total DCIs 3-Year Period* |
1 |
Reregistration DCIs: Confirmatory Data |
23.7 |
71 |
2 |
Reregistration: Voluntarily Submitted Data (Low Burden Studies) |
0.3 |
1 |
3 |
Reregistration: Voluntarily Submitted Data (High Burden Studies) |
||
4 |
Reregistration DCIs: Product Specific Data |
20.3 |
61 |
5 |
Maintenance DCI1 |
35 – NTIP 0.3 - Efficacy |
105 – NTIP 1 - Efficacy |
6 |
Registration Review DCIs |
61.3 |
184 |
7 |
Registration Review Resistance Management Plans |
79 |
237 |
8 |
Registration Review: Voluntarily Submitted Data (Low Burden Studies) |
50 |
150 |
9 |
Registration Review: Voluntarily Submitted Data (High Burden Studies) |
||
10 |
Anticipated Residue DCIs: Base Set of Data |
0.3 |
1 |
11 |
Anticipated Residue DCIs: Verification of Use Data |
0.3 |
1 |
12 |
Anticipated Residue DCIs: Updated Public Source Monitoring Data |
0.3 |
1 |
13 |
DCIs for Percent Crop Treated Estimates |
0.3 |
1 |
Total DCIs* |
221 |
663 |
|
Total Voluntarily Submitted Data |
50.7 |
151 |
|
1Includes Non-Target Insect Pollinator (NTIP) and Efficacy Studies
* Counts for IC Groups 2, 3, 8, and 9 are for voluntarily submitted data—i.e., they are not DCIs. Therefore, the total DCI count does not include these estimates.
Numbers may not add due to rounding.
DCI-Related Respondent Burden and Cost Estimates
Tables 5 and 6 provide information on the burden and costs faced by DCI recipients, data generators, and consortium participants. Respondent costs are based on managerial, technical and clerical wage rates estimated at $126.56, $71.69, and $42.97 per hour, respectively. These wage rates are based on 2015 wage rates estimated by the Bureau of Labor Statistics (BLS) for the North American Industry Classification System (NAICS) for pesticide registrants (NAICS code 325300).
Table 5 outlines annual burden and costs to these three groups per company of DCI—that is, for DCI recipients, burden is estimated by company since companies are responsible for responding to the 90-day notice; for data generators, it is assumed that only one data package is being submitted by one or more (consortium participant) companies for each DCI.
Table 5: Estimated DCI-Related Annual Respondent Burden and Costs per Company/DCI*
Activity Category |
Clerical |
Technical |
Manager |
Totals |
||||
Hrs. |
$42.97/hr |
Hrs. |
$71.69/hr |
Hrs. |
$126.56/hr |
Burden (hrs) |
Costs ($) |
|
IC Category – DCI Recipients |
||||||||
Reporting |
0 |
$0 |
7 |
$502 |
12 |
$1,519 |
19 |
$2,021 |
Recordkeeping |
1 |
$43 |
0 |
$0 |
0 |
$0 |
1 |
$43 |
IC Category – Data Generators1 |
||||||||
Reregistration Program DCIs |
||||||||
1) Confirmatory DCIs |
||||||||
Reporting |
835 |
$35,886 |
4,070 |
$291,760 |
576 |
$72,852 |
5,480 |
$400,498 |
Recordkeeping |
732 |
$31,443 |
0 |
$0 |
134 |
$16,920 |
865 |
$48,363 |
2) Product Specific DCIs |
||||||||
Reporting |
127 |
$5,471 |
619 |
$44,348 |
86 |
$10,846 |
832 |
$60,665 |
Recordkeeping |
111 |
$4,764 |
0 |
$0 |
22 |
$2,799 |
133 |
$7,563 |
3) Reregistration: Voluntarily Submitted Low Burden Studies |
||||||||
Reporting |
87 |
$3,723 |
422 |
$30,252 |
65 |
$8,185 |
573 |
$42,160 |
Recordkeeping |
76 |
$3,258 |
0 |
$0 |
9 |
$1,123 |
85 |
$4,381 |
4) Reregistration: Voluntarily Submitted High Burden Studies |
||||||||
Reporting |
479 |
$20,577 |
2,334 |
$167,287 |
326 |
$41,317 |
3,139 |
$229,181 |
Recordkeeping |
420 |
$18,028 |
0 |
$0 |
80 |
$10,156 |
500 |
$28,184 |
Maintenance and Registration Review DCIs |
||||||||
5) Maintenance DCIs |
||||||||
Reporting |
450 |
$19,322 |
2,188 |
$156,889 |
320 |
$40,536 |
2,958 |
$216,747 |
Recordkeeping |
393 |
$16,883 |
0 |
$0 |
61 |
$7,737 |
454 |
$24,621 |
6) Registration Review DCIs |
||||||||
Reporting |
3,213 |
$138,095 |
15,916 |
$1,141,005 |
2,544 |
$321,951 |
21,673 |
$1,601,051 |
Recordkeeping |
2,846 |
$122,326 |
0 |
$0 |
525 |
$66,462 |
3,372 |
$188,787 |
7) Registration Review Resistance Management Plans |
||||||||
Reporting |
0 |
$0 |
30 |
$2,152 |
6 |
$755 |
36 |
$2,907 |
Recordkeeping |
0 |
$0 |
4 |
$302 |
0 |
$0 |
4 |
$302 |
8) Registration Review: Voluntarily Submitted Low Burden Studies |
||||||||
Reporting |
87 |
$3,723 |
422 |
$30,252 |
65 |
$8,185 |
573 |
$42,160 |
Recordkeeping |
76 |
$3,258 |
0 |
$0 |
9 |
$1,123 |
85 |
$4,381 |
9) Registration Review: Voluntarily Submitted High Burden Studies |
||||||||
Reporting |
479 |
$20,577 |
2,334 |
$167,287 |
326 |
$41,317 |
3,139 |
$229,181 |
Recordkeeping |
420 |
$18,028 |
0 |
$0 |
80 |
$10,156 |
500 |
$28,184 |
Anticipated Residue/Percent Crop Treated DCIs |
||||||||
10) AR DCIs: Base Set of Data |
||||||||
Reporting |
3 |
$114 |
11,898 |
$852,992 |
5 |
$598 |
11,906 |
$853,703 |
Recordkeeping |
1 |
$57 |
0 |
$0 |
0 |
$0 |
1 |
$57 |
11) AR DCIs: Verification-of-use Data |
||||||||
Reporting |
15 |
$651 |
36 |
$2,590 |
18 |
$2,308 |
70 |
$5,548 |
Recordkeeping |
2 |
$81 |
0 |
$0 |
0 |
$0 |
2 |
$81 |
12) AR DCIs: Updated Public Source Monitoring Data |
||||||||
Reporting |
14 |
$615 |
101 |
$7,216 |
16 |
$1,977 |
131 |
$9,808 |
Recordkeeping |
2 |
$77 |
0 |
$0 |
0 |
$0 |
2 |
$77 |
13) DCIs for Percent Crop Treated Estimates |
||||||||
Reporting |
3 |
$126 |
47 |
$3,403 |
1 |
$189 |
52 |
$3,718 |
Recordkeeping |
1 |
$63 |
0 |
$0 |
0 |
$0 |
1 |
$63 |
IC Category - Consortiums |
||||||||
Paperwork burden associated with operating a consortium |
510 |
$21,917 |
800 |
$57,351 |
810 |
$102,511 |
2,120 |
$181,779 |
* Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table.
Note that these estimates reflect burden and costs per company when referring to DCI recipients and per DCI when referring to data generators. Methods used for calculating the cost and burden for cases under each IC Group vary. For a review of methods used in these calculations, refer to Appendix A, B, and C.
Table 6 presents the total respondent burden hours for DCI recipients, data generators, and consortium participants (excluding voluntary data submissions). These calculations reflect recordkeeping, reporting, and total burden numbers for each IC group universe. Refer to Appendices A, B, and C for methodologies and formulas demonstrating how these estimates were calculated. For example, the supporting text under Table A-1 in Appendix A demonstrates how the total burden hours and costs were calculated for DCI recipients. Tables in Appendix B provide the same information for data generators by IC group. The 3-year total bottom-line paperwork burden is estimated at 1,877,007 burden hours which equates to $134,671,171 in paperwork burden costs.
Table 6: Summary of Registrant DCI Paperwork Burdens and Costs (3-year Totals)
|
Burden Hours |
Costs |
|||||
Reporting |
Recordkeeping |
Total |
Reporting |
Recordkeeping |
Total |
||
Data Recipients |
2,318 |
122 |
2,440 |
$246,502 |
$5,243 |
$251,745 |
|
Data Generators |
|||||||
Reregistration Program DCIs |
|||||||
|
Confirmatory DCIs |
129,705 |
20,480 |
150,185 |
$9,478,451 |
$1,144,593 |
$10,623,044 |
Product Specific DCIs |
16,910 |
2,704 |
19,613 |
$1,233,528 |
$153,774 |
$1,387,302 |
|
Voluntarily Submitted Low Burden Studies |
191 |
28 |
219 |
$14,053 |
$1,460 |
$15,514 |
|
Voluntarily Submitted High Burden Studies |
1,046 |
167 |
1,213 |
$76,394 |
$9,395 |
$85,788 |
|
Maintenance and Registration Review DCIs |
|||||||
|
Maintenance DCIs |
101,377 |
15,549 |
116,925 |
$7,427,823 |
$842,633 |
$8,270,456 |
Registration Review DCIs |
1,329,299 |
206,794 |
1,536,093 |
$98,197,781 |
$11,578,951 |
$109,776,732 |
|
Registration Review Resistance Management Plans |
2,843 |
333 |
3,176 |
$229,651 |
$23,860 |
$253,511 |
|
Registration Review: Voluntarily Submitted Low Burden Studies |
28,665 |
4,234 |
32,899 |
$2,107,986 |
$219,064 |
$2,327,050 |
|
Registration Review: Voluntarily Submitted High Burden Studies |
156,940 |
24,988 |
181,927 |
$11,459,042 |
$1,409,208 |
$12,868,250 |
|
Anticipated Residue/Percent Crop Treated DCIs |
|||||||
|
AR DCIs: Base Set of Data |
3,969 |
0.4 |
3,969 |
$284,568 |
$19 |
$284,587 |
AR DCIs: Verification-of-use Data |
23 |
0.6 |
24 |
$1,849 |
$27 |
$1,877 |
|
AR DCIs: Updated Public Source Monitoring Data |
44 |
0.6 |
44 |
$3,269 |
$26 |
$3,295 |
|
DCIs for Percent Crop Treated Estimates |
17 |
0.5 |
18 |
$1,239 |
$21 |
$1,260 |
|
|
|||||||
DCI Data Generator Total |
1,584,185 |
245,862 |
1,830,047 |
$116,858,160 |
$13,743,903 |
$130,602,062 |
|
|
Operating Activities Burden Hours |
Operating Activities Cost |
|||||
Consortium Members |
- |
- |
44,520 |
- |
- |
$3,817,364 |
|
Total Burden |
1,586,503 |
245,984 |
1,877,007 |
$117,104,661 |
$13,749,146 |
$134,671,171 |
|
Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table. Methods used for calculating the cost and burden for cases under each IC Group vary. For a review of methods used in these calculations, refer to Appendices A, B, and C.
Section V: Agency Activities and Estimated Costs
After initiating a statutorily mandated pesticide review, if additional data are needed, the Agency will issue a DCI when the need for additional data has been identified.
The functions and responsibilities associated with the EDSP under FFDCA §408(p) have been assigned to EPA’s Office of Chemical Safety and Pollution Prevention (OCSPP). Within OCSPP, OPP will be responsible for the administrative functions related to the issuance of the §408(p) Orders, receiving, processing, and maintaining records of responses to the §408(p) Orders, as well as, other administrative functions related to the §408(p) Orders for pesticide chemicals. The technical review of the data will reside with OPP and include consultation and coordination on an as-needed basis with staff from within OCSPP and other EPA offices (e.g., Office of Science Coordination and Policy, OSCP; Office of Water, OW; Office of Research and Development, ORD) as appropriate.
The Pesticide Registration Information System (PRISM) software application has been developed in OPP to integrate functionality necessary to support the Registration Review and EDSP (Endocrine Disrupter Screening Program) programs. PRISM supports many of the Registration Review and EDSP processes associated with tracking, including DCIs, §408(p) orders, and data submissions. PRISM serves as a replacement for the equivalent functionality provided by the Office of Pesticide Programs Information Network (OPPIN) application. PRISM was enhanced to accept electronic registration (e-Registration) documents. The e-Submission module of PRISM supports the processing of a number of specific application documents (FIFRA §3 new applications, §3 amendments, experimental use permits, petitions for tolerances, and applications for supplemental distributor products) required for pesticide applications. OPP continues to track Reregistration program information, including DCIs, registrant responses, and reregistration data submissions through OPPIN. Also currently, OPPIN lists the bibliography of data submitters for all the DCIs. All correspondence associated with the issuance and response to the DCI is filed in the master registration file or ‘registration jacket’ of affected products. Failures to comply with DCI requirements are referred to EPA's Office of Enforcement and Compliance Assurance for appropriate follow-up actions.
Although the Agency does not publish the submitted information, public access to the OPPIN bibliography is made through the National Pesticides Information Retrieval System (NPIRS). NPIRS supports searches of the OPPIN database by chemical, subject, submission date, laboratory, guideline number, and document type. The public may request copies of non-confidential studies through the Freedom of Information Act (FOIA).
In September 2015, OPP debuted a new electronic system for pesticide applications, the Pesticide Submission Portal (PSP). 14 In February 2016, EPA began to accept DCI response packages through the PSP. The electronic submission process is a combination of document file uploads and providing information online that is equivalent to existing OMB-approved forms that would otherwise be filled out, printed, and mailed to EPA. The current PSP leverages the Agency’s existing Central Data Exchange (CDX) to provide a secure method of submitting these documents and information within a secure online environment. CDX does require initial user registration for which the paperwork burden estimate is covered under “Cross-Media Electronic Reporting Rule” ICR, OMB No. 2025-0003; EPA No. 2002.26.
DCI-Related Agency Burden and Cost Estimates
Tables 7 and 8 provide information on the burden and costs faced by the Agency in requesting DCIs. Agency costs are based on managerial, technical and clerical wage rates estimated at $120.60, $78.24, and $44.61 per hour, respectively. These wage rates are based on 2015 wage rates estimated by the U.S. Bureau of Labor Statistics (BLS) for the North American Industry Classification System (NAICS) for the federal government (NAICS code 999100).
Table 7 outlines Agency burden and cost per DCI. Overall, average reporting hours range from 9 to 326 hours per DCI ($523 to $26,568), depending on the type of DCI. Average recordkeeping hours per DCI range from 2 to 45 hours ($104 to $3,664).
Table 7: Estimated DCI-Related Annual Agency Burden and Costs per DCI*
Category |
Clerical |
Technical |
Manager |
Totals |
||||
Hrs. |
$46.41/hr |
Hrs. |
$81.37/hr |
Hrs. |
$124.89/hr |
Burden (hrs) |
Costs ($) |
|
Reregistration |
||||||||
Reporting |
2.0 |
$93 |
321.1 |
$26,131 |
2.8 |
$343 |
326 |
$26,568 |
Recordkeeping |
4.5 |
$209 |
0.0 |
$0 |
0.0 |
$0 |
5 |
$209 |
Maintenance DCIs |
||||||||
Reporting |
1.0 |
$46 |
160.6 |
$13,066 |
1.4 |
$172 |
163 |
$13,284 |
Recordkeeping |
2.3 |
$104 |
0.0 |
$0 |
0.0 |
$0 |
2 |
$104 |
Registration Review DCIs |
||||||||
Reporting |
7.2 |
$332 |
28.6 |
$2,326 |
4.3 |
$536 |
40 |
$3,194 |
Recordkeeping |
5.3 |
$244 |
0.0 |
$0 |
0.0 |
$0 |
5 |
$244 |
Anticipated Residue/Percent Crop Treated DCIs |
||||||||
Reporting |
6.0 |
$278 |
2.2 |
$177 |
0.5 |
$67 |
9 |
$523 |
Recordkeeping |
1.1 |
$51 |
43.6 |
$3,546 |
0.5 |
$67 |
45 |
$3,664 |
* Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table. Methods used for calculating the cost and burden for cases under each IC Group vary. For a review of methods used in these calculations, refer to Appendix B.
Table 8 presents the total annual Agency burden hours. These calculations reflect recordkeeping, reporting, and total burden numbers for each type of DCI. Refer to Appendix B for methodologies and formulas demonstrating how these estimates were calculated for each type of DCI. Total bottom-line Agency paperwork burden is estimated at 28,232 burden hours which equates to $2,255,251 in paperwork burden costs.
Table 8: Summary of Agency DCI Paperwork Burdens and Costs (3-year Totals)
|
Burden Hours |
Costs |
||||
Reporting |
Recordkeeping |
Total |
Reporting |
Recordkeeping |
Total |
|
Reregistration Program DCIs |
14,339 |
198 |
14,537 |
$1,168,974 |
$9,190 |
$1,178,164 |
Maintenance DCI |
7,170 |
99 |
7,269 |
$584,487 |
$4,595 |
$589,082 |
Registration Review DCIs |
5,618 |
737 |
6,354 |
$448,228 |
$34,195 |
$482,423 |
Anticipated Residue/Percent Crop Treated DCIs |
12 |
60 |
72 |
$697 |
$4,886 |
$5,583 |
Total Agency Burden |
27,138 |
1,094 |
28,232 |
$2,202,386 |
$52,865 |
$2,255,251 |
Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table. Methods used for calculating the cost and burden vary for each type of DCI. For a review of methods used in these calculations, refer to Appendices B.
Appendix A
Estimated Burden Hours and Costs for DCI Recipients
This Appendix outlines the burden hours and costs for DCI recipients. The methodology EPA used for calculating the burden for this group is derived from the 2007 document entitled General Methodology Used to Estimate Paperwork Burden Hours and Costs by the Office of Pesticide Programs for Submission of Required Data/Information for Responding to a Data Call-In Notice, see Attachment 1, Case Study #1, Attachment A.
To calculate the universe of DCI recipients, EPA conducted a search of its Pesticide Registration Information System (PRISM) for all companies that received DCI requests in 2013. This number was 82; however, an influx of DCIs under reregistration began antimicrobials starting in 2014, with an estimated average of 20 confirmatory and 20 product-specific DCIs being sent out annually for the next three years. This would result in 122 DCIs being expected annually from 2018 to 2021.
Annual burden and costs is estimated at 20 hours or $2,063 for each DCI recipient; with 122 companies receiving DCIs annually, the total annual burden is 2,440 hours and $251,745.
Table A-1: Annual Burden and Costs per DCI Recipient
Activity |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
7 |
2 |
0 |
9 |
$1,029 |
|
3 |
3 |
0 |
6 |
$595 |
|
2 |
2 |
0 |
4 |
$396 |
|
0 |
0 |
1 |
1 |
$43 |
Totals |
12 |
7 |
1 |
20 |
$2,063 |
Estimated Total Burden & Costs Across all DCI Recipients:
Reporting (Collection Activities 1-3):
Burden: 19 hours per response x 122 responses = 2,318 burden hours
Costs: $2,021 per response x 122 responses = $246,502
Recordkeeping (Collection Activity 4):
Burden: 1 hour per response x 122 responses = 122 burden hours
Costs: $43 per response x 122 responses = $5,243
Total (Reporting + Recordkeeping):
Burden: 20 hours per response x 122 responses = 2,440 burden hours
Costs: $2,063 per response x 122 responses = $251,745
* Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table.
Appendix B:
Estimated Burden Hours and Costs for DCI Collection Activities for Data Generators, by IC Group
The estimated burden hours, collection activity costs, and total cost per DCI by IC Group using loaded labor rates for the labor categories are outlined in this Appendix. Please see Section 1(b)(i): Methodology Used to Estimate the Paperwork Burden and Costs for DCIs for an overview of how paperwork burden estimates in this section were calculated.
Section B-1: Estimating Paperwork Burden – Reregistration
Over the next three years, EPA expects to issue 44 reregistration DCIs for confirmatory and product specific data; a maximum of one voluntarily data submission is expected to be received by the Agency. This is a substantial decrease from previous DCI estimates as the reregistration process is transitioning to registration review. However, EPA’s Antimicrobials Division in the Office of Pesticide Programs still has several chemicals that are subject to reregistration which accounts for a majority of the reregistration DCIs to be issued.
Section B-1.1: Paperwork Burden Related to the Submission of Confirmatory Data Reregistration
Confirmatory data are required to complete registrant databases and to assist in the evaluation of risk findings. For DCIs involving confirmatory studies, EPA assumes that only one DCI recipient will provide the data requested. The total cost for the most burdensome confirmatory data DCI that the EPA has issued in the past three years was $1,282,460. The paperwork cost associated with this (35% of the total cost) is $448,861 and 6,346 total burden hours. EPA expects to issue 71 confirmatory DCIs over the next three years, which is an average of 23.7 confirmatory DCIs annually. Total annual burden across all Confirmatory Data DCIs is 150,185 burden hours or $10,623,048. See Table B-1 below for burden activity details.
Table B-1: Annual Respondent Paperwork Burden per DCI Involving Confirmatory Studies
Collection Activity |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
72 |
0 |
0 |
72 |
$9,165 |
|
36 |
63 |
0 |
99 |
$9,088 |
|
146 |
63 |
0 |
209 |
$22,953 |
|
110 |
3,128 |
627 |
3,864 |
$265,037 |
|
0 |
314 |
0 |
314 |
$22,525 |
|
212 |
502 |
0 |
714 |
$62,768 |
|
0 |
0 |
209 |
209 |
$8,962 |
|
67 |
0 |
418 |
485 |
$26,422 |
|
67 |
0 |
314 |
381 |
$21,941 |
Total |
709 |
4,070 |
1,567 |
6,346 |
$448,861 |
Estimated Total Annual Burden & Costs Across All Confirmatory Study DCI Data Generators:
Reporting (Collection Activities 1-7):
Burden: 5,480 hours per response x 1 response per DCI x 23.7 DCIs = 129,705 burden hours
Costs: $400,498 per response x 1 response per DCI x 23.7 DCIs = $9,478,451
Recordkeeping (Collection Activities 8-9):
Burden: 865 hours per response x 1 response per DCI x 23.7 DCIs = 20,480 burden hours
Costs: $48,363 per response x 1 response per DCI x 23.7 DCIs = $1,144,593
Total (Reporting + Recordkeeping):
Burden: 6,346 hours per response x 1 response per DCI x 23.7 DCIs = 150,185 burden hours
Costs: $448,861 per response x 1 response per DCI x 23.7 DCIs = $10,623,044
* Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table.
Section B-1.2: Paperwork Burden Related to Voluntarily Submitted Data - Reregistration
Voluntary data consist of studies not required by the Agency. Instead voluntary data is submitted by registrants to supplement a pesticide database. The Agency does not expect to receive more than one voluntary data submission over the next three years under the reregistration program. Despite the small chance of such an occurrence, the Agency has provided high and low burden estimates given the variable nature of the expected cost of voluntarily submitted data.
In the last several years, no voluntary submissions have been received for the reregistration program. Therefore, the Agency used the burden hour cost calculated in the previous DCI ICR—adjusted for wage rate changes—and backed out the proportional burden hour estimates for each of the three labor groups (i.e., 20% managerial, 65% technical, 15% clerical). This method was used both for low and high burden voluntary submission burden hour estimates. The labor wage rates were updated and indexed to 2015, thus, for the new ICR (2018 – 2021) the Agency is projecting a total data cost per voluntary submission is expected to range from $117,862 to $731,969. This translates to paperwork burden estimates for voluntary submissions ranging from 583 to 3,622 burden hours, or $41,252 to $256,189 per voluntary submission. EPA adopted industry recommended adjustments to the burden hour estimates, which puts the range of possible paperwork burden per voluntary submission at 658 to 3,639 hours, or $46,541 to $257,365. Since only one submission is expected every three years, the expected range of potential annual burden from a voluntary submission is 219 to 1,213 burden hours or $15,514 to $85,788 in burden cost.
Table B-2. Annual Respondent Paperwork Burden per Submission of Voluntary Studies (Low Burden)
Collection Activity |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
4 |
0 |
0 |
4 |
$562 |
|
3 |
7 |
0 |
9 |
$815 |
|
9 |
7 |
0 |
16 |
$1,617 |
|
7 |
324 |
65 |
396 |
$26,875 |
|
25 |
32 |
0 |
58 |
$5,537 |
|
13 |
52 |
0 |
65 |
$5,422 |
|
3 |
0 |
22 |
25 |
$1,332 |
|
4 |
0 |
43 |
48 |
$2,423 |
|
4 |
0 |
32 |
37 |
$1,958 |
Total |
74 |
422 |
162 |
658 |
$46,541 |
Estimated Total Annual Burden & Costs Across All Voluntary Low Burden Studies:
Reporting (Collection Activities 1-7):
Burden: 573 hours per response x 1 response per DCI x 0.33 DCIs = 191 burden hours
Costs: $42,160 per response x 1 response per DCI x 0.33 DCIs = $14,053
Recordkeeping (Collection Activities 8-9):
Burden: 85 hours per response x 1 response per DCI x 0.33 DCIs = 28 burden hours
Costs: $4,381 per response x 1 response per DCI x 0.33 DCIs = $1,460
Total (Reporting + Recordkeeping):
Burden: 658 hours x 1 response x 0.33 submissions = 219 burden hours
Costs: $46,541 x 1 response x 0.33 submissions = $15,514
* Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table.
Table B-3. Annual Respondent Paperwork Burden per Submission of Voluntary Studies (High Burden)
Collection Activity |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
40 |
0 |
0 |
40 |
$5,078 |
|
20 |
36 |
0 |
56 |
$5,118 |
|
79 |
36 |
0 |
115 |
$12,619 |
|
59 |
1,794 |
359 |
2,213 |
$151,554 |
|
9 |
180 |
0 |
189 |
$14,047 |
|
119 |
288 |
0 |
406 |
$35,631 |
|
0 |
0 |
119 |
119 |
$5,135 |
|
40 |
0 |
240 |
280 |
$15,385 |
|
40 |
0 |
180 |
220 |
$12,799 |
Total |
407 |
2,334 |
898 |
3,639 |
$257,365 |
Estimated Total Annual Burden & Costs Across All Voluntary High Burden Studies:
Reporting (Collection Activities 1-7):
Burden: 3,139 hours per response x 0.33 submissions = 1,046 burden hours
Costs: $229,181 per response x 0.33 submissions = $76,394
Recordkeeping (Collection Activities 8-9):
Burden: 500 hours per response x 0.33 submissions = 167 burden hours
Costs: $28,184 per response x 0.33 submissions = $9,395
Total (Reporting + Recordkeeping):
Burden: 3,639 hours x 1 response x 0.33 submissions = 1,213 burden hours
Costs: $257,365 x 1 response x 0.33 submissions = $85,788
* Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table.
Section B-1.3: Paperwork Burden Related to the Submission of Product-Specific Data – Reregistration
The Agency projects 61 Product-Specific DCIs (PDCIs) will be called-in over the next three years (20.3 annually) and that each PDCI will generate one response. Table B-4 provides details for each burden activity. Although a PDCI has not been issued recently, total test cost estimates are available from 2011 for four product chemicals. The average total test cost for each of these chemicals is $194,937. Using the 2015 revised general methodology and assumptions burden estimate methodology, the paperwork cost associated with this (35% of the total cost) is $68,228 or 965 burden hours. Total annual paperwork burden across all PDCI’s is 19,613 burden hours or $1,387,302. See Table B-4 below for burden activity details.
Table B-4. Annual Respondent Burden Estimates per Product Specific DCI
Collection Activity |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
11 |
0 |
0 |
11 |
$1,400 |
|
6 |
10 |
0 |
15 |
$1,388 |
|
21 |
10 |
0 |
31 |
$3,371 |
|
16 |
476 |
95 |
586 |
$40,166 |
|
0 |
48 |
0 |
48 |
$3,440 |
|
32 |
76 |
0 |
108 |
$9,524 |
|
0 |
0 |
32 |
32 |
$1,377 |
|
11 |
0 |
63 |
74 |
$4,116 |
|
11 |
0 |
48 |
59 |
$3,446 |
Total |
108 |
619 |
238 |
965 |
$68,228 |
Estimated Total Annual Burden & Costs Across All Product-Specific DCI Data Generators:
Reporting (Collection Activities 1-7):
Burden: 832 hours per response x 1 response per DCI x 20.3 DCIs = 16,910 burden hours
Costs: $60,665 per response x 1 response per DCI x 20.3 DCIs = $1,233,528
Recordkeeping (Collection Activities 8-9):
Burden: 133 hours per response x 1 response per DCI x 20.3 DCIs = 2,704 burden hours
Costs: $7,563 per response x 1 response per DCI x 20.3 DCIs = $153,774
Total (Reporting + Recordkeeping):
Burden: 965 hours per response x 1 response per DCI x 20.3 DCI = 19,613 burden hours
Costs: $68,228 per response x 1 response per DCI x 20.3 DCI = $1,387,302
Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table.
Section B-1.4: Estimating Agency Paperwork Burden – Reregistration
The Agency’s annual burden hours and costs for developing DCI correspondence, communication with registrants, developing documents, tracking and storing the evaluation of the data submissions, and other DCI processing activities are detailed in Table B-5 below. Overall, the average annual Agency paperwork burden is 330 hours ($26,776) per DCI.
Table B-5: Annual Agency Paperwork Burden – Reregistration
Collection Activities |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
2.0 |
20.0 |
2.0 |
2.0 |
$1,970 |
|
0.3 |
10.0 |
0.0 |
0.3 |
$845 |
|
0.5 |
291.1 |
0.0 |
0.5 |
$23,753 |
|
0.0 |
0.0 |
4.0 |
0.0 |
$186 |
|
0.0 |
0.0 |
0.5 |
0.0 |
$23 |
Total Annual Agency Burden |
3 |
321 |
7 |
330 |
$26,776 |
Estimated Total Annual Agency Burden & Costs Across All Reregistration:
Reporting (Collection Activities 1-3):
Burden: 326 hours per response x 1 response per DCI x 44 DCIs = 14,339 burden hours
Costs: $26,568 per response x 1 response per DCI x 44 DCIs = $1,168,974
Recordkeeping (Collection Activities 4-5):
Burden: 5 hours per response x 1 response per DCI x 44 DCIs = 198 burden hours
Costs: $209 per response x 1 response per DCI x 44 DCIs = $9,190
Total (Reporting + Recordkeeping):
Burden: 330 hours per response x 1 response per DCI x 44 DCIs = 14,537 burden hours
Costs: $26,776 per response x 1 response per DCI x 44 DCIs = $1,178,164
Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table. Note that the number of reregistration DCIs for reregistration reported here do not include voluntarily submitted data.
Section B-2: Estimating Respondent Burden – Maintenance DCIs
Section 3(c)(2)(B) of FIFRA provides a means of obtaining needed additional data “to maintain in effect an existing registration of a pesticide.” A need for a data call-in may arise from evolving of scientific understanding and methodologies, changes in the discovery of deficiencies in previously submitted data, or from the new discovery of specific attributes of the pesticide or its ingredients. For example, such data may be needed in support of Agency enforcement cases resulting from consumer complaints about the product, its storage stability, the integrity of its container, or exaggerated advertising claims. A DCI might also be needed to confirm product performance of public health pesticides or a new type of manufacturing process may call into question data submitted for a pesticide registration using older manufacturing technologies no longer used today. These situations may give rise to new concerns such as observed or suspected adverse human health or environmental effects attributed to the use of a pesticide that were not present at the time of the original registration. Therefore, the need for data arise not from a mandated review program like the programs described in paragraphs above, but from unanticipated circumstances such as changes in pathogens of public health concern, new EPA initiatives, or the evolution of scientific test methodologies or manufacturing technologies.
The Agency projects 105 Non-Target Insect Pollinator (NTIP) studies and 1 Efficacy Study will be called-in over the next three years (35 and 0.3 studies, respectively annually) and that each study DCI will generate one response. Table B-6 combines the studies (burden hours and costs) and provides details for each burden activity. The average combined total study cost is $689,623 (NTIP = $675,000 and Efficacy = $14,623). Using the 2015 revised general methodology and assumptions burden estimate methodology, the paperwork cost associated with this (35% of the total cost) is $241,368 or 3,412 burden hours. Total annual paperwork burden across all Maintenance DCIs is 116,925 burden hours or $8,270,456. See Table B-6 below for burden activity details.
Table B-6: Estimated Annual Paperwork Burden Hours and Cost Estimates for Maintenance DCIs per Respondent
Collection Activities |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
30.6 |
0.0 |
0.0 |
31 |
$3,877 |
|
15.3 |
33.9 |
0.0 |
49 |
$4,368 |
|
62.0 |
33.9 |
0.0 |
96 |
$10,278 |
|
46.7 |
1,681.9 |
337.0 |
2,066 |
$140,969 |
|
74.5 |
168.6 |
0.0 |
243 |
$21,515 |
|
91.1 |
270.2 |
0.0 |
361 |
$30,900 |
|
0.0 |
0.0 |
112.6 |
113 |
$4,840 |
|
30.6 |
0.0 |
224.4 |
255 |
$13,511 |
|
30.6 |
0.0 |
168.5 |
199 |
$11,110 |
Total |
381 |
2,188 |
842 |
3,412 |
$241,368 |
Estimated Total Annual Burden & Costs Across All Maintenance DCI Data Generators:
Reporting (Collection Activities 1-7):
Non-Target Pollinator Burden: 2,896 hours per response x 1 response per DCI x 35 DCIs = 101,356 burden hours
Non-Target Pollinator Costs: $212,180 per response x 1 response per DCI x 35 DCIs = $7,426,301
Efficacy Burden: 62 hours per response x 1 response per DCI x 0.3 DCIs = 21 burden hours
Efficacy Costs: $4,567 per response x 1 response per DCI x 0.3 DCIs = $1,522
Recordkeeping (Collection Activities 8-9):
Non-Target Pollinator Burden: 444 hours per response x 1 response per DCI x 35 DCIs = 15,545 burden hours
Non-Target Pollinator Costs: $24,070 per response x 1 response per DCI x 35 DCIs = $842,449
Efficacy Burden: 10 hours per response x 1 response per DCI x 0.3 DCIs = 3 burden hours
Efficacy Costs: $551 per response x 1 response per DCI x 0.3 DCIs = $184
Total (Reporting + Recordkeeping):
Non-Target Pollinator Burden: 3,340 hours per response x 1 response per DCI x 35 DCIs = 116,901 burden hours
Non-Target Pollinator Costs: $236,250 per response x 1 response per DCI x 35 DCIs = $8,268,750
Efficacy Burden: 72 hours per response x 1 response per DCI x 0.3 DCIs = 24 burden hours
Efficacy Costs: $5,118 per response x 1 response per DCI x 0.3 DCIs = $1,706
* Numbers may not add due to rounding.
Section B-2.1: Estimating Agency Paperwork Burden – Maintenance DCIs
The Agency’s annual burden hours and costs for developing DCI correspondence, communication with registrants, developing documents, tracking and storing the evaluation of the data submissions, and other DCI processing activities are detailed in Table B-7 below. Overall, average annual Agency paperwork burden is 165 hours ($13,388) per DCI or 7,269 burden hours ($589,082) annually.
Table B-7: Annual Agency Burden – Maintenance DCIs
Collection Activities |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
1.0 |
10.0 |
1.0 |
12 |
$985 |
|
0.1 |
5.0 |
0.0 |
5 |
$422 |
|
0.3 |
145.6 |
0.0 |
146 |
$11,876 |
|
0.0 |
0.0 |
2.0 |
2 |
$93 |
|
0.0 |
0.0 |
0.3 |
0 |
$12 |
Total Annual Agency Burden |
1 |
161 |
3 |
165 |
$13,388 |
Estimated Total Annual Agency Burden & Costs Across All Maintenance DCIs:
Reporting (Collection Activities 1-3):
Burden: 163 hours per response x 1 response per DCI x 44 DCIs = 7,170 burden hours
Costs: $13,284 per response x 1 response per DCI x 44 DCIs = $584,487
Recordkeeping (Collection Activities 4-5):
Burden: 2 hours per response x 1 response per DCI x 44 DCIs = 99 burden hours
Costs: $104 per response x 1 response per DCI x 44 DCIs = $4,595
Total (Reporting + Recordkeeping):
Burden: 165 hours per response x 1 response per DCI x 44 DCIs = 7,269 burden hours
Costs: $13,388 per response x 1 response per DCI x 44 DCIs = $589,082
Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table.
Section B-3: Estimating the Respondent Burden – Registration Review Program
Data that is submitted under the Registration Review Program includes those submitted through DCIs for Registration Review, and Voluntarily Submitted Data. As the burden associated with Voluntarily Submitted Data can be highly variable, low and high burden estimates are provided.
Section B-3.1: Estimating Respondent Burden – Registration Review
The total estimated cost for all studies requested under the most burdensome registration review DCI that EPA has issued in the past three years was $4,916,432. The paperwork cost associated with this (35% of the total cost) was $1,789,838 or 25,045 total burden hours. EPA expects to issue 184 Registration Review DCIs over the next three years, which equals an average of 61.3 registration review DCIs annually. These DCIs are also to include requests for the new suite of non-target insect pollinator studies that EPA plans to codify in the coming year.
Table B-8: Estimated Annual Respondent Paperwork Burden Hours and Costs for Registration Review DCIs
Collection Activity |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
356 |
0 |
0 |
356 |
$44,998 |
|
178 |
330 |
0 |
508 |
$46,183 |
|
711 |
330 |
0 |
1,041 |
$113,680 |
|
394 |
12,100 |
2,440 |
14,933 |
$1,022,105 |
|
89 |
1,214 |
0 |
1,303 |
$98,309 |
|
776 |
1,942 |
0 |
2,718 |
$237,445 |
|
40 |
0 |
774 |
814 |
$38,331 |
|
263 |
0 |
1,627 |
1,889 |
$103,131 |
|
263 |
0 |
1,220 |
1,483 |
$85,656 |
Total |
3,069 |
15,916 |
6,060 |
25,045 |
$1,789,838 |
Estimated Total Annual Burden & Costs Across All Registration Review DCI Data Generators:
Reporting (Collection Activities 1-7):
Burden: 21,673 hours per response x 1 response per DCI x 61.3 DCIs = 1,329,299 burden hours
Costs: $1,601,051 per response x 1 response per DCI x 61.3 DCIs = $98,197,781
Recordkeeping (Collection Activities 8-9):
Burden: 3,372 hours per response x 1 response per DCI x 61.3 DCIs = 206,794 burden hours
Costs: $188,787 per response x 1 response per DCI x 61.3 DCIs = $11,578,951
Total (Reporting + Recordkeeping):
Burden: 25,045 hours per response x 1 response per DCI x 61.3 DCIs = 1,536,093 burden hours
Costs: $1,789,838 per response x 1 response per DCI x 61.3 DCIs = $109,776,732
* Numbers may not add due to rounding.
Section B-3.3: Estimating the Respondent Burden – Registration Review: Resistance Management Plans
The average paperwork burden associated with developing a resistance management plan is estimated to be 40 hours or $3,209. A resistance management plan typically includes a remedial action plan and the estimate of burden assumes the completion of both a resistance management plan and remedial action plan. EPA expects to request 237 resistance management plans to be completed over the next three years, which equates an average of 79 requests annually. See Table B-11 below for burden activity details. Across all requests, annual burden hours associated with resistance management plans are estimates at $253,511 annually or 3,176 burden hours.
Table B-11: Estimated Annual Respondent Burden Hours and Costs for Registration Review: Resistance Management Plans
Collection Activity |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
1 |
0 |
0 |
1 |
$151 |
|
1 |
1 |
0 |
2 |
$151 |
|
2 |
1 |
0 |
3 |
$302 |
|
2 |
16 |
0 |
18 |
$1,359 |
|
0 |
6 |
0 |
6 |
$453 |
|
0 |
6 |
0 |
6 |
$415 |
|
1 |
0 |
0 |
1 |
$76 |
|
0 |
2 |
0 |
2 |
$151 |
|
0 |
2 |
0 |
2 |
$151 |
Total |
6 |
34 |
0 |
40 |
$3,209 |
Estimated Total Annual Burden & Costs Across All Voluntary Low Burden Studies:
Reporting (Collection Activities 1-7):
Burden: 36 hours per response x 1 response per request x 79 requests = 2,843 burden hours
Costs: $2,907 per response x 1 response per request x 79 requests = $229,651
Recordkeeping (Collection Activities 8-9):
Burden: 4 hours per response x 1 response per request x 79 requests = 333 burden hours
Costs: $302 per response x 1 response per request x 79 requests = $23,860
Total (Reporting + Recordkeeping):
Burden: 40 hours per response x 1 response x 79 requests = 3,176 burden hours
Costs: $3,209 x 1 response x 79 request = $253,511
* Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table.
Section B-3.4: Estimating the Respondent Burden – Registration Review: Voluntary Submission of Data
Given that the Agency has completed all Reregistration Eligibility Decisions (REDs), voluntary data submissions to the EPA are now being received under the Registration Review Program. The Agency has provided high and low burden estimates as the expected cost of voluntarily submitted data is variable in nature.
Since no data is available on the burden per submission for voluntary data under Registration Review, burden hours and cost were adopted from estimates for voluntary data under Reregistration Review. The method used for Reregistration Review burden calculations used the burden hours and costs calculated in the previous DCI ICR—adjusted for wage rate changes—to back out the proportional burden hour estimates for each of the three labor groups (i.e., 20% managerial, 65% technical, 15% clerical). The total test cost per voluntary submission is updated with 2015 labor wage rates and expected to range from $117,862 to $731,969. This translates to paperwork burden estimates for voluntary submissions ranging from 664 to 4,126 burden hours, or $41,252 to $256,189 per voluntary submission. EPA adopted industry recommended adjustments to these estimates, which puts the range of potential paperwork burden per voluntary submission at 658 to 3,639 hours, or $46,541 to $257,365. The Agency anticipates one voluntary submission per chemical case (or 50 submissions per year), with the expected range of potential annual burden from voluntary submissions from 32,899 to 181,927 burden hours or $2,327,050 to $12,868,250 in burden cost.
Table B-12: Estimated Annual Respondent Burden Hours and Costs for Registration Review: Voluntarily Submitted Data (Low Burden)
Collection Activity |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
4 |
0 |
0 |
4 |
$562 |
|
3 |
7 |
0 |
9 |
$815 |
|
9 |
7 |
0 |
16 |
$1,617 |
|
7 |
324 |
65 |
396 |
$26,875 |
|
25 |
32 |
0 |
58 |
$5,537 |
|
13 |
52 |
0 |
65 |
$5,422 |
|
3 |
0 |
22 |
25 |
$1,332 |
|
4 |
0 |
43 |
48 |
$2,423 |
|
4 |
0 |
32 |
37 |
$1,958 |
Total |
74 |
422 |
162 |
658 |
$46,541 |
Estimated Total Annual Burden & Costs Across All Voluntary Low Burden Studies:
Reporting (Collection Activities 1-7):
Burden: 573 hours per response x 1 response per DCI x 50 DCIs = 28,665 burden hours
Costs: $42,160 per response x 1 response per DCI x 50 DCIs = $2,107,986
Recordkeeping (Collection Activities 8-9):
Burden: 85 hours per response x 1 response per DCI x 50 DCIs = 4,234 burden hours
Costs: $4,381 per response x 1 response per DCI x 0.33 DCIs = $219,064
Total (Reporting + Recordkeeping):
Burden: 658 hours x 1 response x 50 submissions = 32,899 burden hours
Costs: $46,541 x 1 response x 0.33 submissions = $2,327,050
* Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table.
Table B-13: Estimated Annual Respondent Burden Hours and Costs for Registration Review: Voluntarily Submitted Data (High Burden)
Collection Activity |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
40 |
0 |
0 |
40 |
$5,078 |
|
20 |
36 |
0 |
56 |
$5,118 |
|
79 |
36 |
0 |
115 |
$12,619 |
|
59 |
1,794 |
359 |
2,213 |
$151,554 |
|
9 |
180 |
0 |
189 |
$14,047 |
|
119 |
288 |
0 |
406 |
$35,631 |
|
0 |
0 |
119 |
119 |
$5,135 |
|
40 |
0 |
240 |
280 |
$15,385 |
|
40 |
0 |
180 |
220 |
$12,799 |
Total |
407 |
2,334 |
898 |
3,639 |
$257,365 |
Estimated Total Annual Burden & Costs Across All Voluntary High Burden Studies:
Reporting (Collection Activities 1-7):
Burden: 3,139 hours per response x 50 submissions = 156,940 burden hours
Costs: $229,181 per response x 50 submissions = $11,459,042
Recordkeeping (Collection Activities 8-9):
Burden: 500 hours per response x 50 submissions = 24,988 burden hours
Costs: $28,184 per response x 50 submissions = $1,409,208
Total (Reporting + Recordkeeping):
Burden: 3,639 hours per response x 50 submissions = 181,927 burden hours
Costs: $257,365 x 1 response x 50 submissions = $12,868,250
* Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table.
Section B-3.5: Estimating Agency Paperwork Burden – Registration Review DCIs
The Agency’s annual burden hours and costs for developing DCI correspondence, communication with registrants, developing documents, tracking and storing the evaluation of the data submissions, and other DCI processing activities is detailed in Table B-13 below. Overall, annual average Agency paperwork burden is 45 hours ($3,438) per DCI or 6,354 burden hours ($482,423) annually for 140.3 DCIs (61.3 DCIs and 79 Use Summary Tables). Note that there is no Agency burden associated with resistance management plans as they will not require Agency review.
Table B-14: Annual Agency Burden – Registration Review
Collection Activities |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
3.8 |
19.1 |
7.2 |
30.0 |
$2,359 |
|
0.2 |
9.5 |
0.0 |
9.8 |
$805 |
|
0.2 |
0.0 |
0.0 |
0.2 |
$30 |
|
0.0 |
0.0 |
4.8 |
4.8 |
$221 |
|
0.0 |
0.0 |
0.5 |
0.5 |
$22 |
Total Annual Agency Burden |
4.3 |
28.6 |
12.4 |
45.3 |
$3,438 |
Estimated Total Annual Agency Burden & Costs Across All Registration Review DCIs:
Reporting (Collection Activities 1-3):
Burden: 40 hours per response x 1 response per DCI x 140.3 DCIs = 5,618 burden hours
Costs: $3,194 per response x 1 response per DCI x 140 DCIs = $448,228
Recordkeeping (Collection Activities 4-5):
Burden: 5 hours per response x 1 response per DCI x 140.3 DCIs = 737 burden hours
Costs: $244 per response x 1 response per DCI x 140.3 DCIs = $34,195
Total (Reporting + Recordkeeping):
Burden: 45 hours per response x 1 response per DCI x 140.3 DCIs = 6,354 burden hours
Costs: $3,438 per response x 1 response per DCI x 140.3 DCIs = $482,423
Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table. Note that the number of registration review DCIs reported here include DCIs for Use Summary Tables but do not include voluntarily submitted data.
Section B-4: Estimating Respondent Burden – Anticipated Residues/Percent Crop Treated DCIs (AR/PCT)
The Anticipated Residue and Percent Crop Treated (AR/PCT) review programs require the Agency to reevaluate previous Agency decisions regarding the establishment of a tolerance (maximum residue limit) for pesticide residues on food or feed crops. The law also requires that tolerance decisions based on ARs or PCT data be verified to ensure that residues in or on food are not above the residue levels relied on for establishing the tolerance.
Section B-4.1: Estimating Respondent Burden - Anticipated Residues/Percent Crop Treated DCIs
There are four IC groups associated with AR/PCT, including:
DCI for anticipated residues requiring a base set of data,
DCI for anticipated residues for verification of use data,
DCI for anticipated residues collected from publicly available sources, and
DCI for percent crop treated using existing information.
After re-evaluating the burden hours from the last DCI ICR, the Agency is not changing the AR/PCT burden hour estimates for the 2018 – 2021 ICR renewal document (see Section II of this document for additional information). The labor burden breakout assumption for the AR/PCT programs does not follow the 20%-65%-15% Managerial-Technical-Clerical percentages most other DCI program burdens follow. Instead, the AR/PCT DCI paperwork burden falls disproportionately in the technical labor category, i.e., 72%-99% AR/PCT technical labor versus the standard 65% technical labor. Additionally, paperwork burden for anticipated residues verification of use data disproportionately elevates the management labor category (approximately 40% managerial labor versus the standard 20% managerial labor). These labor burden breakout assumptions were confirmed by industry at the DCI Response Burden Assessment Workshop.
In the previous collection activities associated with the AR/PCT DCIs, the burden definitions were less detailed and did not consider the newly defined burden categories discussed in Section III, Table 3 - Data Generator collection activities. To standardize burden activities, the Agency has redefined the AR/PCT burden activities to reflect the same the nine collection activities discussed in Table 3. Table B-15 provides a breakdown comparison of the old collection activity burden groups and the new redefined burden groups.
Table B-15: Change in Collection Activity Groupings for Anticipated Residues/Percent Crop Treated DCIs
Previous Collection Activity Groupings for AR/PCT DCI IC Groupings |
New Collection Activity Groupings for AR/PCT DCI IC Groupings |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following presents the Agency’s burden estimates for each type of AR/PCT DCI.
Section B-4.2: Anticipated Residue DCIs: Base Set of Data
As explained in Section B-4.1, the Agency is not changing the burden hour estimates for any of the AR/PCT DCIs from the previous (20%-65%-15% Managerial-Technical-Clerical) ICR burden estimate. For the AR base set of data, the Managerial-Technical-Clerical paperwork burden breakout for each type of labor is 0.07% managerial, 99.91% technical, and 0.02% clerical.
The total test cost for an anticipated residue DCI requiring a base set of data is estimated at $2,439,314. Respondent burden hours for generating and submitting data in response to a DCI for anticipated residues requiring a base set of data to be submitted are estimated at 11,907 burden hours, or $853,760, per response.
In most cases, registrants will be able to get the information from federal and state monitoring programs, thus the Agency estimates that no more than one registrant might generate their own monitoring data in response to the DCI every three years which would result in 3,969 burden hours or $284,587 in costs annually, as shown in B-16.
Table B-16: Anticipated Residue DCIs: Base Set of Data Annual Burden/Cost Estimates, per DCI
Collection Activity |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
1 |
0 |
0 |
1 |
$133 |
|
|||||
|
2 |
0 |
0 |
2 |
$266 |
|
0 |
11,877 |
0 |
11,877 |
$851,489 |
|
0 |
14 |
0 |
14 |
$1,002 |
|
1 |
7 |
0 |
8 |
$567 |
|
1 |
0 |
3 |
4 |
$247 |
|
0 |
0 |
1 |
1 |
$57 |
|
|||||
Total |
5 |
11,898 |
4 |
11,907 |
$853,760 |
Estimated Total Annual Burden & Costs Across All Anticipated Residue (Base Set of Data) DCIs:
Reporting (Collection Activities 1-7):
Burden: 11,906 hours per response x 1 response per DCI x 0.33 DCIs = 3,969 burden hours
Costs: $853,703 per response x 1 response per DCI x 0.33 DCIs = $284,568
Recordkeeping (Collection Activities 8-9):
Burden: 1 hour per response x 1 response per DCI x 0.33 DCIs = 0.4 burden hours
Costs: $57 per response x 1 response per DCI x 0.33 DCIs = $19
Total (Reporting + Recordkeeping):
Burden: 11,907 hours per response x 1 response per DCI x 0.33 DCIs = 3,969 burden hours
Costs: $853,760 per response x 1 response per DCI x 0.33 DCIs = $284,587
* Numbers may not add due to rounding.
Section B-4.3: Anticipated Residue DCIs: Verification of Use Data
As explained in Section B-4.1, the Agency is not changing the burden hour estimates for any of the AR/PCT DCIs from the original (20%-65%-15% Managerial-Technical-Clerical) paperwork burden breakouts breakout. For AR verification of use data, the Managerial-Technical-Clerical paperwork burden breakout for each type of labor is 41% managerial, 46% technical, and 13% clerical.
The total test cost for an anticipated residue DCI requiring a verification of use data is estimated at $16,085. The Agency estimates that the verification for updating use information is 71 burden hours or $5,630 per response and that no more than one respondent every three years will comply with a DCI by submitting a base set of data for updating use information, which equates to 24 burden hours annually ($1,877). Refer to Table B-17 for details.
Table B-17: Anticipated Residue DCIs: Verification of Use Data Annual Burden/Cost Estimates, per DCI
Collection Activity |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
5.2 |
0.0 |
0.0 |
5.2 |
$659 |
|
|||||
|
10.4 |
0.0 |
0.0 |
10.4 |
$1,319 |
|
0.0 |
0.0 |
0.0 |
0.0 |
$0 |
|
0.0 |
18.1 |
0.0 |
18.1 |
$1,295 |
|
1.3 |
18.1 |
0.0 |
19.4 |
$1,460 |
|
1.3 |
0.0 |
15.1 |
16.4 |
$815 |
|
0.0 |
0.0 |
1.9 |
1.9 |
$81 |
|
|||||
Total |
18.2 |
36.1 |
17.0 |
71.4 |
$5,630 |
Estimated Total Annual Burden & Costs Across All Anticipated Residue (Verification of Use) DCIs:
Reporting (Collection Activities 1-7):
Burden: 70 hours per response x 1 response per DCI x 0.33 DCIs = 23 burden hours
Costs: $5,592 per response x 1 response per DCI x 0.33 DCIs = $1,864
Recordkeeping (Collection Activities 8-9):
Burden: 2 hours per response x 1 response per DCI x 0.33 DCIs = 0.6 burden hours
Costs: $81 per response x 1 response per DCI x 0.33 DCIs = $27
Total (Reporting + Recordkeeping):
Burden: 71 hours per response x 1 response per DCI x 0.33 DCIs = 24 burden hours
Costs: $5,630 per response x 1 response per DCI x 0.33 DCIs = $1,877
* Numbers may not add due to rounding.
Section B-4.4: Anticipated Residue DCIs: Updated Public Source Monitoring Data
As explained in Section B-4.1, the Agency is not changing the burden hour estimates for any of the AR/PCT DCIs from the original (20%-65%-15% Managerial-Technical-Clerical) paperwork burden breakouts breakout. For updated public source monitoring data, the Managerial-Technical-Clerical paperwork burden breakout for each type of labor is 20% managerial, 73% technical, and 7% clerical.
The total test cost for an anticipated residue DCI requiring public source monitoring data is estimated at $28,242. The average respondent burden for submitting a base set of data for updating monitoring information is estimated at 132 burden hours or $9,885 per year. The Agency estimates that an average of one respondent every three years is likely to be able to comply with a DCI by submitting data from publicly available sources. As such, the total annual respondent burden for this type of DCI is estimated to be 44 burden hours ($3,295). See Table B-18.
Table B-18: Anticipated Residue DCIs: Updated Public Source Monitoring Data Annual Burden/Cost Estimates, per DCI
Collection Activity |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
4.5 |
0.0 |
0.0 |
4.5 |
$565 |
|
|||||
|
8.9 |
0.0 |
0.0 |
8.9 |
$1,130 |
|
0.0 |
0.0 |
0.0 |
0.0 |
$0 |
|
0.0 |
60.4 |
0.0 |
60.4 |
$4,329 |
|
1.1 |
40.3 |
0.0 |
41.4 |
$3,028 |
|
1.1 |
0.0 |
14.3 |
15.4 |
$756 |
|
0.0 |
0.0 |
1.8 |
1.8 |
$77 |
|
|||||
Total |
15.6 |
100.7 |
16.1 |
132.4 |
$9,885 |
Estimated Total Annual Burden & Costs Across All Anticipated Residue (Public Source Monitoring Data) DCIs:
Reporting (Collection Activities 1-7):
Burden: 131 hours per response x 1 response per DCI x 0.33 DCIs = 44 burden hours
Costs: $9,808 per response x 1 response per DCI x 0.33 DCIs = $3,269
Recordkeeping (Collection Activities 8-9):
Burden: 2 hours per response x 1 response per DCI x 0.33 DCIs = 0.6 burden hours
Costs: $77 per response x 1 response per DCI x 0.33 DCIs = $26
Total (Reporting + Recordkeeping):
Burden: 132 hours per response x 1 response per DCI x 0.33 DCIs = 44 burden hours
Costs: $9,885 per response x 1 response per DCI x 0.33 DCIs = $3,295
* Numbers may not add due to rounding.
Section B-4.5: DCIs for Percent Crop Treated Estimates
As explained in Section B-4.1, the Agency is not changing the burden hour estimates for any of the AR/PCT DCIs from the original (20%-65%-15% Managerial-Technical-Clerical) paperwork burden breakouts breakout. For percent crop treated estimates, the Managerial-Technical-Clerical paperwork burden breakout for each type of labor is 5% managerial, 90% technical, and 5% clerical.
The estimated total test cost for a DCI requiring percent crop treated estimates is $10,802. The annual per respondent burden for generating percent crop treated estimates using existing information is estimated to be 53 burden hours ($3,781). Percent crop treated estimates are generally conducted within the Agency, and only in rare instances would a registrant need to gather the information; one DCI every three years impacting one respondent is likely an overestimation. If this were the case, however, the annual burden estimates is 18 hours or $1,260 in paperwork burden cost. The estimated costs assume that the cost of purchasing or obtaining percent crop treated information is obtaining data from existing, contracted data sources. See Table B-19.
Table B-19: DCIs for Percent Crop Treated Estimates Annual Respondent Burden/Cost Estimates, per DCI
Collection Activity |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
0.5 |
0.9 |
0.0 |
1.4 |
$127 |
|
|||||
|
0.0 |
1.8 |
0.0 |
1.8 |
$128 |
|
0.0 |
7.2 |
0.0 |
7.2 |
$514 |
|
0.0 |
19.7 |
0.0 |
19.7 |
$1,412 |
|
0.5 |
17.9 |
0.0 |
18.4 |
$1,347 |
|
0.5 |
0.0 |
2.9 |
3.4 |
$189 |
|
0.0 |
0.0 |
1.5 |
1.5 |
$63 |
|
|||||
Total |
1.5 |
47.5 |
4.4 |
53.4 |
$3,781 |
Estimated Total Annual Burden & Costs Across All Anticipated Residue (Public Source Monitoring Data) DCIs:
Reporting (Collection Activities 1-7):
Burden: 52 hours per response x 1 response per DCI x 0.33 DCIs = 17.3 burden hours
Costs: $3,718 per response x 1 response per DCI x 0.33 DCIs = $1,239
Recordkeeping (Collection Activities 8-9):
Burden: 1.5 hours per response x 1 response per DCI x 0.33 DCIs = 0.5 burden hours
Costs: $63 per response x 1 response per DCI x 0.33 DCIs = $21
Total (Reporting + Recordkeeping):
Burden: 53 hours per response x 1 response per DCI x 0.33 DCIs = 17.8 burden hours
Costs: $3,781 per response x 1 response per DCI x 0.33 DCIs = $1,260
* Numbers may not add due to rounding.
Section B-4.6: Estimating Agency Paperwork Burden – Anticipated Residues/Percent Crop Treated DCIs (AR/PCT)
The Agency’s annual burden hours and costs for developing DCI correspondence, communication with registrants, developing documents, tracking and storing the evaluation of the data submissions, and other DCI processing activities is detailed in Table B-120 below. Overall, the annual average Agency paperwork burden ranges between 12 and 72 burden hours annually for 1.33 DCIs.
Table B-20: Annual Agency Burden – Anticipated Residues/Percent Crop Treated DCIs (AR/PCT)
Collection Activities |
Burden Hours |
Total |
|||
Management |
Technical |
Clerical |
Hours |
Costs |
|
|
3.8 |
19.1 |
7.2 |
30.0 |
$2,359 |
|
0.2 |
9.5 |
0.0 |
9.8 |
$805 |
|
0.2 |
0.0 |
0.0 |
0.2 |
$30 |
|
0.0 |
0.0 |
4.8 |
4.8 |
$221 |
|
0.0 |
0.0 |
0.5 |
0.5 |
$22 |
Total Annual Agency Burden |
4.3 |
28.6 |
12.4 |
45.3 |
$3,438 |
Estimated Total Annual Agency Burden & Costs Across All AR/PCT DCIs:
Reporting (Collection Activities 1-3):
Burden: 9 hours per response x 1 response per DCI x 1.33 DCIs = 12 burden hours
Costs: $523 per response x 1 response per DCI x 1.33 DCIs = $697
Recordkeeping (Collection Activities 4-5):
Burden: 45 hours per response x 1 response per DCI x 1.33 DCIs = 60 burden hours
Costs: $3,664 per response x 1 response per DCI x 1.33 DCIs = $4,886
Total (Reporting + Recordkeeping):
Burden: 54 hours per response x 1 response per DCI x 1.33 DCIs = 72 burden hours
Costs: $4,187 per response x 1 response per DCI x 1.33 DCIs = $5,583
Numbers may not add due to rounding. Please refer to text for information on calculations presented in this table.
Appendix C:
Estimated Burden Hours and Costs for Consortium Activities
Consortium activities
In addition to the cost of data generation, consortium participants are subject to costs associated with operating a consortium or task force (e.g., communication, attending meetings, etc.). As shown in Table C-1, average annual paperwork burden associated with running a consortium totals 2,120 hours or $181,779. With an estimated 21 consortiums in existence, total paperwork burden across all consortiums is 44,520 hours or $3,817,364. This accounts for two annual meetings, which is typical for consortiums. Note that burden estimates provided in Table C-1 represent the average paperwork burden associated with consortium activities, as consortium activity costs can greatly vary depending on factors such as workload levels and number of participants. Consortium activity costs and the number of consortiums in existence quoted in this ICR were estimates provided as part of the industry response to the DCI Response Burden Assessment Workshop.15
Table C-1: Annual Consortium Activities Burden/Cost Estimates, per Consortium
Consortium Activity |
Management ($126.56) |
Technical ($71.69) |
Clerical ($42.97) |
Total |
|
Hours |
Costs |
||||
|
350 |
0 |
40 |
390 |
$46,014 |
|
50 |
400 |
100 |
550 |
$39,301 |
|
200 |
0 |
100 |
300 |
$29,609 |
|
0 |
0 |
50 |
50 |
$2,149 |
|
100 |
0 |
80 |
180 |
$16,094 |
|
100 |
400 |
100 |
600 |
$45,629 |
|
10 |
0 |
40 |
50 |
$2,985 |
Total |
810 |
800 |
510 |
2,120 |
$181,779 |
Estimated Total Annual Burden & Costs Across All Consortiums:
Burden: 2,120 hours per response x 21 consortiums = 44,520 burden hours
Costs: $181,779 per consortium x 21 consortiums = $3,817,364
* Numbers may not add due to rounding.
1 The PRA, 44 U.S.C 3501 et seq., requires federal agencies to estimate the “paperwork burden” for “information collection” activities. Under the PRA, “paperwork burden” means the total time, effort, or financial resources expended by persons to generate, maintain, retain, or disclose or provide information to or for a Federal Agency.
2 General Methodology Used to Estimate Paperwork Burden Hours and Costs by the Office of Pesticide Programs for Submission of Required Data/Information for Responding to a Data Call-In Notice was originally published as Attachment G to a DCI ICR renewal document posted under the docket identification #EPA-HQ-OPP-2007-0923-0005. See Attachment A.
3 On December 12, 2013, The Office Pesticide Programs sponsored a DCI Response Burden Assessment Workshop. Industry participants included, but were not limited to: representatives from BASF, the DOW Chemical Company, the American Chemistry Council Biocides Panel, Steptoe and Johnson, LLP, Technology Sciences Group Inc., Monsanto, and SC Johnson. Meeting materials and Industry comments are part of the docket for the ICR renewal at: EPA-HQ-OPP- 2016-0109.
7 For information about the EPA’s responsibilities and implementation of ESPP, go to http://www.epa.gov/espp/.
9 For information about the EDSP, go to http://www2.epa.gov/endocrine-disruption/endocrine-disruptor-screening-program-edsp-21st-century.
10 This document was originally published as Attachment G to a DCI ICR renewal document posted under the docket identification #EPA-HQ-OPP-2007-0923-0005. See docket at Regulations.gov.
11 Ibid, see the case studies in Attachment A.
12 EPA’s Pesticide Re-evaluation Division found that typical GDCI packets averaged 40 pages; certified mailing cost for this size package are approximately $20.
13 As part of the 2007 methodology, the Agency identified three response phases: Phase 1: the initial response; Phase 2: data generation and Phase 3: data submission to EPA. The Phase 1, Phase 2 and Phase 3 response activity burden hours and costs are accounted for as subsets of the paperwork burden estimates for information collection activities that are related to generating data to respond to a DCI. These burdens are accounted for as part of the 35% of the test burden and cost.
15 See footnote 3 on page 1.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Sells, Dexter |
| File Modified | 0000-00-00 |
| File Created | 2021-01-21 |
© 2026 OMB.report | Privacy Policy