Disclosure Regarding Additional Risks in Direct-to-Consumer Prescription Drug Television Advertisements (Cognitive Interviews)
IMPROVE Study Phase 2
RISK DISCLOSURE - COGNITIVIE TESTING GUIDE 6-9-2015
Disclosure Regarding Additional Risks in Direct-to-Consumer Prescription Drug Television Advertisements (Cognitive Interviews)
OMB: 0910-0695
FDA Risk Disclosure Study
Cognitive Testing Guide
INTRODUCTION
Thank you for agreeing to participate in this study today.
Review following points:
RTI is a non-profit research organization. We are conducting a study about a health survey. The study is sponsored by a public health agency.
During our time today, you will be asked to view one ad on a laptop computer and I will ask you to read and answer some survey questions about what you viewed. We are interested in getting your feedback on the survey questions as well as the ad. Your feedback will help us improve the study.
Our discussion will take up to 60 minutes. At the end of our discussion you will receive $75 in appreciation for your participation.
Anything that you say today will be kept private. Your name and other identifying information will not be used in any reports that result from our discussion today.
We will be audio recording today’s discussion to help with a report we’re going to write after we finish talking with everyone. Recordings will be stored on a password protected computer and destroyed at the end of the project.
The most important thing I want you to know is that I didn’t write the survey questions or create the ad that we are going to look at today so I won’t be offended if you criticize them. If something is confusing it means that the writer didn’t do a good job writing the question and/or instructions. Your participation in this interview is very important because it will help improve the questionnaire so that it is as easy to complete as possible.
INFORMED CONSENT
Before we get started I’m going to give you an informed consent form that describes the study and what you’ll be asked to do in more detail. I’m going to give you two copies- one to sign for us and one for you to take home. Please take a few minutes to read the form and let me know if you have any questions or concerns.
PROCESS
[SHOW R LAPTOP] Please read the instructions on the first page and then I’ll show you the advertisement. After that I’m going to ask you to read and answer each question aloud. As you answer each question I’d like you to talk me through what you are thinking about. For example, if the question asked how many times you cooked dinner at home during the past week, rather than tell me that you made dinner five times, I want to know how you came up with that answer—what did you think about? Did you think about each day individually? Were there any days that you were unsure of? After some items I will ask you to answer some additional questions about the question and/or your answer. I’d also like you to point out any questions or answer choices that might be confusing.
Do you have any questions before we begin?
INTERVIEWER NOTES
For each question, probe as needed if R seems confused or unsure, or if his/her answer suggests the question was misinterpreted.
General probes to be used as needed:
In your own words, what is this question asking?
How did you choose your answer?
Other things to be aware of:
How R’s use answer scales
For questions where R is asked to answer “based on information in the ad” is he/she able to do so?
[SCRIPT] Thank you for agreeing to participate in this study today.
Make sure you are comfortable and can read the screen from where you sit. This study is about advertising for prescription medication. Your answers are private and will not be connected with your name. Your input is extremely valuable.
Let’s begin.
We will ask you questions about the ad after you have finished watching it. Do your best to remember details about the ad. We will ask you to explain your answers. Make sure your computer sound is turned on and set at a comfortable volume.
[PROGRAMMER: Screen 3. TIME SPENT ON EACH SCREEN IN MILLISECONDS]
[Next page: Screen 4]
[SCRIPT] Please click the link below to view the ad. It may take a minute or two for the video to begin. You should be able to see and hear the video when it begins to play.
[PROGRAMMER: Screen 4. TIME SPENT ON EACH SCREEN IN MILLISECONDS]
[Next page: Screen 5]
[SCRIPT] Please answer the following questions based on the ad you saw.
Q1. Were you able to view the entire ad for [DRUG]?
Yes
No [Skip back to ad]
Not sure [Skip back to ad]
[PROGRAMMER: If Q1=No or Not sure, replay the ad. If, after this, they still say No or Not sure to Q1, then terminate.]
Probe: What are your initial reactions to the ad you just watched?
Probe: Was there anything in the ad that was confusing or unclear? If so, what?
Probe: How different or similar is it to other prescription drug ads that you’ve seen?
What is similar or different from other prescription drug ads that you’ve seen?
Amount and type of information about the drug?
Graphics?
Narration? [INTERVIEWER: Looking to see whether R notices difference in how fast the risk information/disclosure statement is read]
(Information seeking/intention items)
Q2. I am interested in trying [ABILIFY/LUNESTA/CRESTOR].
1 |
2 |
3 |
4 |
5 |
Strongly disagree |
Somewhat disagree |
Neither agree nor disagree |
Somewhat agree |
Strongly agree |
[PROGRAMMER: Show Abilify for people in the depression condition. Show Lunesta for people in the insomnia condition. Show Crestor for people in high cholesterol condition]
Q3a. Have you seen this exact ad before?
Yes [Go to Q3b]
No [Continue to Q3c]
Not sure [Continue to Q3c]
Probe: [IF YES] Where did you see this ad before?
Probe: [IF NOT SURE] Have you seen any ads that were similar to this one, but not exactly the same? Please tell me about it.
Q3b. In the last 6 months, how often did you see this exact ad before?
Never
Rarely
Sometimes
Often
Very often
Q3c. Have you seen other ads for this product before?
Yes
No
Not sure
Q4. Please list the thoughts that were going through your mind as you viewed the ad for [ABILIFY/LUNESTA/CRESTOR] and list them below. Use one line for each thought.
[PROGRAMMER: Five separate text boxes]
[PROGRAMMER: Show Abilify for people in the depression condition. Show Lunesta for people in the insomnia condition. Show Crestor for people in high cholesterol condition]
|
|
|
|
|
Probe: [IF NEEDED] How did you decide what to write here? Did you have additional thoughts that you didn’t record? [INTERVIEWER: Note whether 5 spaces is sufficient to capture main thoughts]
[Next page: Screen 6]
Q5. What condition does [ABILIFY/LUNESTA/CRESTOR] treat?
[PROGRAMMER: randomize response options]
[PROGRAMMER: Show Abilify for people in the depression condition. Show Lunesta for people in the insomnia condition. Show Crestor for people in high cholesterol condition]
Seasonal allergies
Insomnia
Migraine headaches
High Cholesterol
Diabetes
Acid Reflux/GERD
Depression
Don't know
[PROGRAMMER: Counterbalance Q6 and Q7]
Q6. What are the benefits of [ABILIFY/LUNESTA/CRESTOR]? Use one line for each benefit you list. [Open-ended]
[PROGRAMMER: Provide 5 separate text boxes.
[PROGRAMMER: Show Abilify for people in the depression condition. Show Lunesta for people in the insomnia condition. Show Crestor for people in high cholesterol condition]
|
|
|
|
|
Q7. What are the risks and side effects of [ABILIFY/LUNESTA/CRESTOR]? Use one line for each risk or side effect you list. [Open-ended]
[PROGRAMMER: Provide 10 separate text boxes with an option to “click here for additional lines”.]
[PROGRAMMER: Show Abilify for people in the depression condition. Show Lunesta for people in the insomnia condition. Show Crestor for people in high cholesterol condition]
|
|
|
|
|
[Next page: Screen 7]
[PROGRAMMER: MATCH VERSION OF Q8 TO DISEASE AD SHOWN]
(Memory for benefits and risks)
Q8_1. Based on the information in the ad, please check which of the following are benefits or risks of taking ABILIFY. [Depression version]
[PROGRAMMER: randomize a-k]
|
Yes |
No |
a. Can improve symptoms as quickly as one to two weeks |
X |
|
b. Taken along with your antidepressant it can help improve symptoms of depression Probe: [IF NO] Why did you choose this response? How confident are you in your response? |
X |
|
c. There are no dietary restrictions while taking Abilify |
|
X |
d. Has been found to also treat restless leg syndrome |
|
X |
e. Can cause changes in behavior |
X |
|
f. Can increase the risk of suicidal thoughts |
X |
|
g. Can increase risk of stroke |
X |
|
h. Can cause uncontrollable muscle movements |
X |
|
i. Can cause nausea or vomiting |
|
X |
j. Can increase the risk of developing certain forms of cancer |
|
X |
k. Can cause liver problems such as yellowing of skin or eyes |
|
X |
Q8_2. Based on the information in the ad, please check which of the following are benefits or risks of taking LUNESTA. [Insomnia version]
[PROGRAMMER: randomize a-k]
|
Yes |
No |
a. Can help you fall asleep at night |
X |
|
b. Can help you sleep up to seven hours |
X |
|
c. Has been found to also help treat restless leg syndrome |
|
X |
d. Starts working within 30 minutes. |
|
X |
e. Can cause sleep walking and other activities |
X |
|
f. Can cause changes in behavior or mood such as aggressiveness Probe: [IF NO] Why did you choose this response? How confident are you in your response? |
X |
|
g. Can increase the risk of suicide |
X |
|
h. Can cause potentially fatal allergic reactions |
X |
|
i. Can cause nausea or vomiting |
|
X |
j. Can increase the risk of developing certain forms of cancer |
|
X |
k. Can cause liver problems such as yellowing of skin or eyes |
|
X |
Q8_3. Based on the information in the ad, please check which of the following are benefits or risks of taking CRESTOR. [High cholesterol version]
[PROGRAMMER: randomize a-h]
|
Yes |
No |
a. Can help reduce cholesterol |
X |
|
b. Has been found to work better than Lipitor to reduce cholesterol |
X |
|
c. No regular blood tests are needed |
|
X |
d. Has been found to relieve problems with urination |
|
X |
e. Can cause muscle problems such as pain or weakness Probe: [IF NO] Why did you choose this response? How confident are you in your response? |
X |
|
f. Can cause liver problems such as yellowing of skin or eyes Probe: [IF NO] Why did you choose this response? How confident are you in your response? |
X |
|
g. Can cause nausea or vomiting |
|
X |
h. Can increase the risk of developing certain forms of cancer |
|
X |
Probe: How difficult or easy was it to remember the information about benefits or risks? Was it easier to remember the benefits or to remember the risks?
Is there a difference between risks and side effects, and if so, how do they differ?
Would you have answered this question any differently if it asked you to check off which of the following were benefits or side effects of taking [DRUG], instead of saying “benefits or risks”?
[Next page: Screen 8]
[PROGRAMMER: Randomize presentation order of the questions on screen 8]
(Perceived Benefit)
[SCRIPT] Please answer the following questions based on your impressions from the ad. Even though the ad didn’t tell you, please provide your best guess for the following questions
INTERVIEWER: Note the following and probe as needed for Q9-13
Is R able to answer questions based on their impression from the ad (can probe- “How did you choose your answer” if unsure)
Is R able to use ruler without difficulty?
Q9. In your opinion, if 100 people take [ABILIFY/LUNESTA/CRESTOR], how many of the 100 would benefit from taking the drug?
[PROGRAMMER: Participants can slide an arrow to their answer.]
[PROGRAMMER: Show Abilify for people in the depression condition. Show Lunesta for people in the insomnia condition. Show Crestor for people in high cholesterol condition]
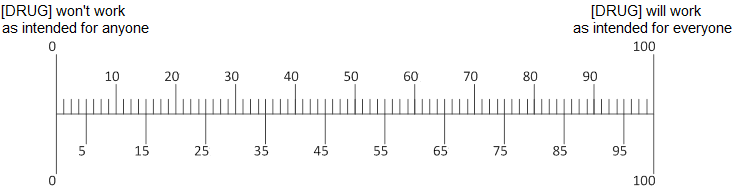
Q10. In your opinion, if [ABILIFY/LUNESTA/CRESTOR] did help a person’s [depression/insomnia/high cholesterol], how much would it help?
[PROGRAMMER: Show Abilify/depression for people in the depression condition. Show Lunesta/insomnia for people in the insomnia condition. Show Crestor/high cholesterol for people in high cholesterol condition]
1 |
2 |
3 |
4 |
5 |
Would help [condition] a little |
|
|
|
Would help [condition] a lot |
(Perceived Risk)
Q11. In your opinion, if 100 people take [ABILIFY/LUNESTA/CRESTOR], how many of the 100 would have any side effects?
[PROGRAMMER: Participants can slide an arrow to their answer.]
[PROGRAMMER: Show Abilify for people in the depression condition. Show Lunesta for people in the insomnia condition. Show Crestor for people in high cholesterol condition]
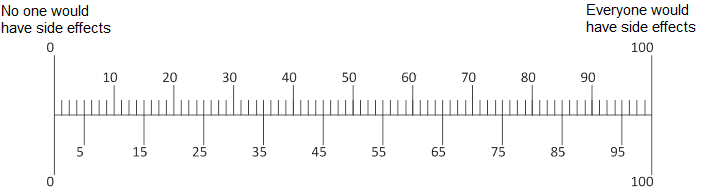
Q12. In your opinion, if [ABILIFY/LUNESTA/CRESTOR] did cause a person with [depression/insomnia/high cholesterol] to have side effects, how serious would they be?
[PROGRAMMER: Show Abilify/depression for people in the depression condition. Show Lunesta/insomnia for people in the insomnia condition. Show Crestor/high cholesterol for people in high cholesterol condition]
1 |
2 |
3 |
4 |
5 |
Not at all serious |
|
|
|
Very serious |
(Risk/benefit tradeoff)
Q13. Thinking overall about the risks and benefits of [ABILIFY/LUNESTA/CRESTOR], would you say it has:
[PROGRAMMER: Show Abilify for people in the depression condition. Show Lunesta for people in the insomnia condition. Show Crestor for people in high cholesterol condition]
1 |
2 |
3 |
4 |
5 |
Many more risks than benefits |
Somewhat more risks than benefits |
Equal risks and benefits |
Somewhat more benefits than risks |
Many more benefits than risks |
Probe: How did you choose your answer? What specific risks and benefits did you think about?
Probe to see if people were still basing answers from the impression they got from the ad and not from personal experience
(Perceptions of risk and benefit statements)
[PROGRAMMER: Randomize order of Q14a-g.]
[PROGRAMMER: Show Abilify for people in the depression condition. Show Lunesta for people in the insomnia condition. Show Crestor for people in high cholesterol condition]
Q14. Please rate your agreement or disagreement with each of the following statements.
|
Strongly disagree |
Somewhat disagree |
Neither disagree nor agree |
Somewhat agree |
Strongly agree |
|
|
|
|
|
|
|
|
|
|
|
|
Probe: What does it mean to “evenly” balance the risks and benefits of [DRUG]? |
|
|
|
|
|
Probe: IF R AGREES: What information do you think was missing/what else would you want to know about the possible benefits and positive effects of [DRUG]? |
|
|
|
|
|
Probe: IF R AGREES: What information do you think was missing/what else would you want to know about the possible risks and side effects of [DRUG]? |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Q15. In my opinion, the prescription drug ad mentioned….
1 |
2 |
3 |
4 |
5 |
Not enough risks and side effects |
|
|
|
Too many risks and side effects |
Probe: How did you choose your answer?
Q16. To what extent do you agree or disagree that the risks and side effects were:
[PROGRAMMER: Randomize order of Q16a-f.]
|
Strongly disagree |
Somewhat disagree |
Neither disagree nor agree |
Somewhat agree |
Strongly agree |
|
|
|
|
|
|
Probe: What does it mean for the risks and side effects to be “actionable”? |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Probe: What does it mean for the risks and side effects to be “important”? Important for them personally or for all people that watch the ad? |
|
|
|
|
|
Probe: IF R AGREES: What information do you think was missing? |
|
|
|
|
|
Q17. Overall, the risks and side effects mentioned in the prescription drug ad were….
1 |
2 |
3 |
4 |
5 |
Not at all serious |
|
|
|
Very serious |
Probe: Is this question different from the one you already answered asking “In your opinion, if [DRUG] did cause a person with [condition] to have side effects, how serious would they be?” Why or why not?
Q18_1. In your opinion, how serious are the following risks and side effects? [Depression version]
[PROGRAMMER: Randomize order of Q18_1.a-g.]
[[PROGRAMMER: Only show this item for people in depression condition]
|
Not at all serious |
|
|
|
Very serious |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Q18_2. In your opinion, how serious are the following risks and side effects? [Insomnia version]
[PROGRAMMER: Randomize order of Q18_2.a-d.]
[[PROGRAMMER: Only show this item for people in insomnia condition]
|
Not at all serious |
|
|
|
Very serious |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Q18_3. In your opinion, how serious are the following risks and side effects? [High cholesterol version]
[PROGRAMMER: Randomize order of Q18_3.a-b.]
[[PROGRAMMER: Only show this item for people in high cholesterol condition]
|
Not at all serious |
|
|
|
Very serious |
|
|
|
|
|
|
|
|
|
|
|
|
Q19_1. How likely is it that you would experience any of the following risks and side effects if you took ABILIFY? [Depression version]
[PROGRAMMER: Randomize order of Q19_1.a-g.]
[[PROGRAMMER: Only show this item for people in depression condition]
|
Not at likely |
|
|
|
Very likely |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Q19_2. How likely is it that you would experience any of the following risks and side effects if you took LUNESTA? [Insomnia version]
[PROGRAMMER: Randomize order of Q19_2.a-d.]
[[PROGRAMMER: Only show this item for people in insomnia condition]
|
Not at likely |
|
|
|
Very likely |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Q19_3. How likely is it that you would experience any of the following risks or side effects if you took CRESTOR? [High cholesterol version]
[PROGRAMMER: Randomize order of Q19_3.a-b.]
[[PROGRAMMER: Only show this item for people in high cholesterol condition]
|
Not at likely |
|
|
|
Very likely |
|
|
|
|
|
|
|
|
|
|
|
|
In your opinion, how actionable are the following risks and side effects? We define ‘actionable’ as a symptom you would know you are at risk for (e.g., because of pre-existing condition or allergy) or recognize (e.g., because it is an observable physical or mental system) and can act upon to help lessen the risk (e.g., get immediate medical help to prevent a bad outcome).
Probe: How helpful is this definition “actionable”? Is there anything confusing or difficult to understand about it? [NOTE THAT R WAS PREVIOUSLY ASKED TO DEFINE ACTIONABLE FOR Q16B. HOW DOES THEIR DEFINITION COMPARE TO THIS?]
Q20_1 [PROGRAMMER: Randomize order of Q20_1.a-g.] [Depression version]
[[PROGRAMMER: Only show this item for people in depression condition]
|
Not at all actionable |
|
|
|
Very actionable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Q20_2 [PROGRAMMER: Randomize order of Q20_2.a-d.] [Insomnia version]
[[PROGRAMMER: Only show this item for people in insomnia condition]
|
Not at all actionable |
|
|
|
Very actionable |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Q20_3 [PROGRAMMER: Randomize order of Q20_3.a-b.] [High cholesterol version]
[[PROGRAMMER: Only show this item for people in high cholesterol condition]
|
Not at all actionable |
|
|
|
Very actionable |
|
|
|
|
|
|
|
|
|
|
|
|
[Next page: Screen 9]
[PROGRAMMER: counterbalance 21/22 and 23/24]
Q21. How likely are you to talk to your doctor about [ABILIFY/LUNESTA/CRESTOR]?
[PROGRAMMER: Show Abilify for people in the depression condition. Show Lunesta for people in the insomnia condition. Show Crestor for people in high cholesterol condition]
1 |
2 |
3 |
4 |
5 |
Very unlikely |
Somewhat unlikely |
Neither likely nor unlikely |
Somewhat likely |
Very likely |
Q22. How likely are you to read the patient labeling for more information about [ABILIFY/LUNESTA/CRESTOR]?
[PROGRAMMER: Show Abilify for people in the depression condition. Show Lunesta for people in the insomnia condition. Show Crestor for people in high cholesterol condition]
1 |
2 |
3 |
4 |
5 |
Very unlikely |
Somewhat unlikely |
Neither likely nor unlikely |
Somewhat likely |
Very likely |
Probe: What “patient labeling” are you thinking about? Where would you find the patient labeling?
Q23. How likely are you to look for more information about [ABILIFY/LUNESTA/CRESTOR]?
[PROGRAMMER: Show Abilify for people in the depression condition. Show Lunesta for people in the insomnia condition. Show Crestor for people in high cholesterol condition]
1 |
2 |
3 |
4 |
5 |
Very unlikely |
Somewhat unlikely |
Neither likely nor unlikely |
Somewhat likely |
Very likely |
Probe: If R says somewhat or very likely, where would they look for information?
Q24. How likely are you to look for more information about [depression/insomnia/high cholesterol]?
[PROGRAMMER: Show depression for people in the depression condition. Show insomnia for people in the insomnia condition. Show high cholesterol for people in high cholesterol condition]
1 |
2 |
3 |
4 |
5 |
Very unlikely |
Somewhat unlikely |
Neither likely nor unlikely |
Somewhat likely |
Very likely |
Q25. If one of your family members or close friends had [depression/insomnia/high cholesterol], how likely would you be to mention [ABILIFY/LUNESTA/CRESTOR] to them?
[PROGRAMMER: Show Abilify for people in the depression condition. Show Lunesta for people in the insomnia condition. Show Crestor for people in high cholesterol condition]
1 |
2 |
3 |
4 |
5 |
Very unlikely |
Somewhat unlikely |
Neither likely nor unlikely |
Somewhat likely |
Very likely |
[Next page: Screen 10]
[PROGRAMMER: Rotate order of Q26/Q26a and Q27/Q27a]
(Perceived Comparative Benefit and Risk)
[SCRIPT] For the next two questions, please think about other medicines you know of that treat [depression/insomnia/high cholesterol]. If you are not aware of other medicines that treat [depression/insomnia/high cholesterol], please choose the answer option “Neither disagree nor agree.”
When answering these questions, please base your impressions on the prescription drug ad you saw.
[PROGRAMMER: Show depression for people in the depression condition. Show insomnia for people in the insomnia condition. Show high cholesterol for people in high cholesterol condition]
INTERVIEWER: Note whether R seems to base their impressions only on the drug ad or if they mention personal experience or other sources of information.
Q26. [ABILIFY/LUNESTA/CRESTOR] is more effective than other medicines that treat [depression/insomnia/high cholesterol].
Strongly disagree
Somewhat disagree
Neither disagree nor agree
Somewhat agree
Strongly agree
[PROGRAMMER: Show Abilify/depression for people in the depression condition. Show Lunesta/insomnia for people in the insomnia condition. Show Crestor/high cholesterol for people in high cholesterol condition]
Q26a. What other medicines were you thinking about?
[PROGRAMMER: Add fill space for write-ins]
Q27. [ABILIFY/LUNESTA/CRESTOR] is safer than other medicines that treat [depression/insomnia/high cholesterol].
Strongly disagree
Somewhat disagree
Neither disagree nor agree
Somewhat agree
Strongly agree
[PROGRAMMER: Show Abilify/depression for people in the depression condition. Show Lunesta/insomnia for people in the insomnia condition. Show Crestor/high cholesterol for people in high cholesterol condition]
Q27a. What other medicines were you thinking about?
[PROGRAMMER: Add fill space for write-ins]
INTERVIEWER: Note whether R chose “Neither disagree nor agree” if they did not list other medicines here.
PROBE: IF R mentions different medicines in Q26a and Q27a, probe on why that is.
(Perceived Quality)
Q28. Based on the information in the prescription drug ad, how would you rate the quality of [ABILIFY/LUNESTA/CRESTOR]?
[PROGRAMMER: Show Abilify for people in the depression condition. Show Lunesta for people in the insomnia condition. Show Crestor for people in high cholesterol condition]
1 |
2 |
3 |
4 |
5 |
Poor quality |
|
|
|
Good quality |
Probe: How did you choose your answer?
(Attitude toward Ad. From Bhutada et al., 2009, Shen & Chen, 2007, and MacKenzie & Lutz, 1989)
[PROGRAMMER: Randomize order of Q29a-d]
Q29. In my opinion, the ad for [ABILIFY/LUNESTA/CRESTOR] was:
a. 1 2 3 4 5
Good Bad
b. 1 2 3 4 5
Pleasant Unpleasant
c. 1 2 3 4 5
Favorable Unfavorable
d. 1 2 3 4 5
Very Not at all
persuasive persuasive
[PROGRAMMER: Show Abilify for people in the depression condition. Show Lunesta for people in the insomnia condition. Show Crestor for people in high cholesterol condition]
Probe: How did you choose your answers to this set of questions?
What do you think about the scales used for this question- e.g., favorable-unfavorable, etc. ? Do they make sense for the ad you saw ?
(Self-reported attention)
Q30. How much attention did you pay to the prescription drug ad when you were watching it?
1 |
2 |
3 |
4 |
5 |
A little |
|
|
|
Probe: What does [ANSWER] mean? If you were completing this survey on your own do you think you would have paid the same amount of attention to the ad?
[Next page: Screen 11]
(Notice Disclosure)
[PROGRAMMER: Control condition and Revised Risk Alone condition SKIP to Q33]
Q31. Do you remember the ad saying “This is not a full list of risks and side effects. Talk to your doctor and read the patient labeling for more information.”?
Yes
No [PROGRAMMER: IF checked and in Original Risk +Disclosure Condition or Revised Risk + Disclosure Condition, display the following script: “This ad did say that “This is not a full list of risks and side effects. Talk to your doctor and read the patient labeling for more information.”]
Not sure [PROGRAMMER: IF checked and in Original Risk +Disclosure Condition or Revised Risk + Disclosure Condition, display the following script: “This ad did say that “This is not a full list of risks and side effects. Talk to your doctor and read the patient labeling for more information.”]
[SCRIPT] Now we will show you the ad again. This ad said “This is not a full list of risks and side effects. Talk to your doctor and read the patient labeling for more information.” When answering the next few questions, please think only about that statement.
[Next page: Screen 12]
(Inferences about Statement)
Q32a. In your own words, list all your thoughts, reactions and ideas that went through your mind when the statement, “This is not a full list of risks and side effects. Talk to your doctor and read the patient labeling for more information.” was presented. Please use a separate line for each thought.
[PROGRAMMER: Seven separate text boxes]
|
|
|
|
|
|
|
Q32b. For each of the thoughts, reactions and ideas you listed, indicate whether the thought was positive, negative or neutral.
[PROGRAMMER: Display responses from Q32a. For each response, include a drop-down choice box or other appropriate choice box with the choices POSITIVE, NEGATIVE, NEUTRAL]
You said… |
Is it… |
[insert each response from Q32a above in a separate box] |
POSITIVE NEGATIVE NEUTRAL |
|
|
|
|
|
|
|
|
|
|
|
|
INTERVIEWER: After R answers Q32a and Q32b review each entry to understand thought and why R coded as positive, negative, or neutral.
Probe: Did you have any additional thoughts that you did not record? If so, what were they?
(Peripheral Cue)
Q33. To what extent do you agree or disagree that the statement about the list of risks and side effects (“This is not a full list of risks and side effects. Talk to your doctor and read the patient labeling for more information”) was:
[PROGRAMMER: Randomize order of Q33a-g.]
|
Strongly disagree |
Somewhat disagree |
Neither agree nor disagree |
Somewhat agree |
Strongly agree |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Probe for each question: How did you choose your answer?
In addition, probe:
IF R DISAGREES: What could be done to make the information more noticeable? |
IF DISAGREE: What wasn’t believable about it? |
|
IF DISAGREE: Why do you think the statement is not important? IF AGREE: Why do you think the statement is important? |
IF DISAGREE: How can the statement be changed to make it clearer? |
IF AGREE: How could it be shortened? |
IF DISAGREE: What would make it more helpful? IF AGREE: What was helpful about it? |
[Next page: Screen 13]
(FDA Perceptions/Knowledge)
Q34. Please rate your agreement or disagreement with each of the following statements.
[PROGRAMMER: Randomize order of Q34a-h]
|
Strongly disagree |
Somewhat disagree |
Neither disagree nor agree |
Somewhat agree |
Strongly agree |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[Next page: Screen 14]
[SCRIPT] Now we will ask a few questions to help us describe our sample of participants.
(Subjective Health Literacy)
Q35. How often do you have someone (like a family member or friend) help you read instructions, pamphlets, or other written material from your doctor or pharmacy?
1 |
2 |
3 |
4 |
5 |
Never |
Occasionally |
Sometimes |
Often |
Always |
Q36. How confident are you filling out medical forms by yourself?
1 |
2 |
3 |
4 |
5 |
Not at all confident |
A little bit |
Somewhat |
Quite a bit |
Extremely confident |
(Perceived illness knowledge)
Q37. In general, how much do you feel you know about [depression/insomnia/high cholesterol]?
1 |
2 |
3 |
4 |
5 |
Nothing at all |
Only a slight amount |
Some |
A good bit |
A lot |
[PROGRAMMER: Show depression for people in the depression condition. Show insomnia for people in the insomnia condition. Show high cholesterol for people in high cholesterol condition]
Q38. In general, how much do you feel you know about treatments for [depression/insomnia/high cholesterol]?
1 |
2 |
3 |
4 |
5 |
Nothing at all |
Only a slight amount |
Some |
A good bit |
A lot |
[PROGRAMMER: Show depression for people in the depression condition. Show insomnia for people in the insomnia condition. Show high cholesterol for people in high cholesterol condition]
Q39. In what year were you diagnosed with [depression/insomnia/high cholesterol]?
_______
INTERVIEWER: Note how challenging it is for the R to remember the year he/she was diagnosed.
[PROGRAMMER: Show depression for people in the depression condition. Show insomnia for people in the insomnia condition. Show high cholesterol for people in high cholesterol condition]
Q40. Are your currently taking a prescription drug to treat your [depression/insomnia/high cholesterol]?
Yes
No [Skip to Q43]
[PROGRAMMER: Show depression for people in the depression condition. Show insomnia for people in the insomnia condition. Show high cholesterol for people in high cholesterol condition]
Q41. How long have you been taking prescription drugs for [depression/insomnia/high cholesterol]?
Less than 2 weeks
At least 2 weeks but less than 2 months
At least 2 months but less than 6 months
At least 6 months but less than 1 year
At least 1 year but less than 5 years
At least 5 years
[PROGRAMMER: Show depression for people in the depression condition. Show insomnia for people in the insomnia condition. Show high cholesterol for people in high cholesterol condition]
Q42. Are you currently taking [ABILIFY/LUNESTA/CRESTOR]?
Yes
No
Don’t Know
[PROGRAMMER: Show Abilify for people in the depression condition. Show Lunesta for people in the insomnia condition. Show Crestor for people in high cholesterol condition]
Q43. Are you now covered by any form of health insurance or health plan? This includes any private insurance plan through your employer or a plan that you purchased yourself, as well as a government program like Medicare or Medicaid.
Yes
No [Skip to Q45]
Don’t Know [Skip to Q45]
Q44. Does your current insurance plan help pay for prescription drugs?
Yes
No
Don’t Know
[Next page: Screen 15]
(Attitudes toward Disclosures; From Thomas et al., 2013)
[SCRIPT] We would now like to ask you some questions about how you feel about the use of disclosures.
A disclosure qualifies, clarifies, or otherwise limits a specific advertising claim.
Examples of disclosures include:
“Certain rules and restrictions apply”
“Results not typical”
“This is not a full list of risks and side effects. Talk to your doctor and read the patient labeling for more information”
“Past performance is not necessarily indicative for future performances and transactions in financial products (including but not limited to securities, futures, options and other financial instruments) give rise to risks,”
“This statement has not been evaluated by the Food and Drug Administration”
“Prices include all cost to be paid by individual except for taxes, licensing, and registration”.
“Pay no interest for one year. If you transfer a balance from another card, you will be charged a 3% fee.”
Probe: In your own words, what does the term “disclosure” mean?
Probe: How well do you think the definition of a “disclosure” [A disclosure qualifies, clarifies, or otherwise limits a specific advertising claim.] explains this concept?
Probe: How helpful are the examples? Which of the examples have you seen or heard before? Where did you see/hear them? Were any examples not helpful or confusing? Which?
What are some other examples of disclosures that you are familiar with?
Q45. Please indicate your level of agreement with each of the following items.
[PROGRAMMER: Randomize order of Q45a-h]
|
Strongly disagree |
|
|
Neither disagree nor agree |
|
|
Strongly agree |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INTERVIEWER: For each of the above probe as needed to determine how R chooses their answer and what they are thinking about.
[Next page: Screen 16]
Q46. Would you like to receive the patient labeling for [ABILIFY/LUNESTA/CRESTOR] at the end of this survey to learn more about the complete list of risks and side effects?
Yes
No
[PROGRAMMER: Show Abilify for people in the depression condition. Show Lunesta for people in the insomnia condition. Show Crestor for people in high cholesterol condition]
Q47. What did you use to complete today’s survey?
INTERVIEWER: Ask R if they were taking this on their own, which would they most likely take the survey on?
Desktop computer
Laptop computer
Tablet computer (e.g., Apple iPad, Samsung Galaxy Tab)
Mobile phone or smartphone
Other: ________________
Q48. What is your gender?
Male
Female
Q49. Are you:
Hispanic or Latino
Not Hispanic or Latino
Q50. What is your race? You may select one or more races.
American Indian or Alaska Native
Asian
Black or African American
Native Hawaiian or other Pacific Islander
White
Q51. What is your household income?
Less than $30,000 per year
$30,001 - $75,000 per year
$75,001 - $150,000 per year
$150,001+ per year
[End time: ___________________ ]
[Next page: Screen 17]
[SCRIPT] The purpose of this research is to learn about consumer reactions to prescription drug advertising. In order to get your realistic reaction to this information, we used a real product; however the prescription drug ad was modified for the purpose of this study. Use of the brand name does not imply endorsement of the product by FDA. Please see your healthcare professional for questions about [depression/insomnia/high cholesterol].
[PROGRAMMER: Show depression for people in the depression condition. Show insomnia for people in the insomnia condition. Show high cholesterol for people in high cholesterol condition]
You have been very helpful. Thank you very much for your participation!
[PROGRAMMER: If Respondent chooses “Yes” to Q46, at the end of the survey, after the debriefing, provide a link [or attach a PDF] to the patient labeling for the drug and include the following script: [SCRIPT] You indicated that you would like to receive the patient labeling for [ABILIFY/LUNESTA/CRESTOR] at the end of this survey to learn more about the complete list of risks and side effects. In the link below you will find the patient labeling for [ABILIFY/LUNESTA/CRESTOR]. Please see your healthcare professional for questions about [depression/insomnia/high cholesterol].
[PROGRAMMER: Show Abilify/depression for people in the depression condition. Show Lunesta/insomnia for people in the insomnia condition. Show Crestor/high cholesterol for people in high cholesterol condition]
Complete: Congratulations! You have fully qualified and completed this research study. Your account has been credited the full credit amount.
END
OMB Control # _____ Expires _____
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | APPENDIX 2 |
| Author | Aikin |
| File Modified | 0000-00-00 |
| File Created | 2021-01-23 |
© 2026 OMB.report | Privacy Policy