Supporting Statement B
Supporting Statement B.docx
"Cohort Study of HIV, STIs and Preventive Interventions among Young MSM in Thailand
OMB: 0920-1191
Cohort Study of HIV, STIs and Preventive Interventions
among Young MSM in Thailand
OMB No. 0920-New
Supporting Statement B
August 29, 2016
Contact Information:
Program Official/Project Officer: Eileen F. Dunne MD, MPH
Medical Officer, Division of HIV/AIDS Prevention, NCHHSTP, CDC
Phone Number: +66 081-848-0834, +66 2 580-0669 ext 454
Email address: dde9@cdc.gov
Fax number: +66 2 580-0712
Table of Contents
1. Respondent Universe and Sampling Methods
2. Procedures for the Collection of Information
3. Methods to maximize response rates and deal with no response
4. Tests of procedures or methods to be undertaken
5. Individual consulted on statistical aspects and individuals collecting and/or analyzing data
1. Respondent Universe and Sampling Methods
The respondent universe is men who have sex with men (MSM) and transgender women (TGW) who are HIV-negative in Bangkok, Thailand ages 15-29 years.
There are two components of the study: the cohort study and the qualitative data collection. For the cohort study, young MSM and TGW will be enrolled ages 15-29 years. For the qualitative study there are two populations: adolescents ages 15-18 years and community leaders age >18 years. The community leaders will be leaders in the community on adolescent issues.
The number of persons in the FGD will be 30 in total and number of persons for KII will be 12. The number of these men enrolled in the cohort study at SCC @TropMed will be 500. Information from previous studies indicates that we will need to screen approximately 900 MSM and TGW over a 2 year period in order to have 500 MSM and TGW for our cohort study. The active follow-up will occur in 3 years, and so the total study period is 5 years.
We will use a convenience sampling as the respondent selection method. MSM/TGW will present for the study through walk-in HIV voluntary counseling and testing services (VCT) in the clinical setting, through outreach working with NGOs and other partners, and through the Internet and other key websites. All MSM and TGW potentially interested in the study will first have a screening visit with the study nurse. The study nurse will review the screening eligibility checklist, and if he/she is eligible, he/she will complete the consent for screening and consent for enrollment (or assent if <18 years), and process for other screening and enrollment activities. This convenience sampling method had previously been used in the Bangkok Men who Have Sex with Men Cohort Study (BMCS), a cohort study conducted at SCC @TropMed from April 2006 to November 2010. Based on this study, we expect that less than 10% of young men eligible will not enroll in this YMSM cohort study.
2. Procedures for the Collection of Information
The data will be collected by study staff located at Silom Community Clinic @Tropical Medicine (SCC@ TropMed) located in Bangkok, Thailand. The collection will occur using electronic records, and using Standard Operating Procedures (SOP). The study staff are trained in Good Clinical Practice (GCP) and have sensitivity, counseling and other required training. Men will be screened based on designated eligibility criteria. The potential participants can come to the clinic during operating hours (Tuesday-Saturday, 4-9 pm) and appointments for screening and enrollment will be made. A quality control plan for the collection of data will be implemented, in the form of an SOP. All instruments are attached to the protocol, and will be administered in Thai.
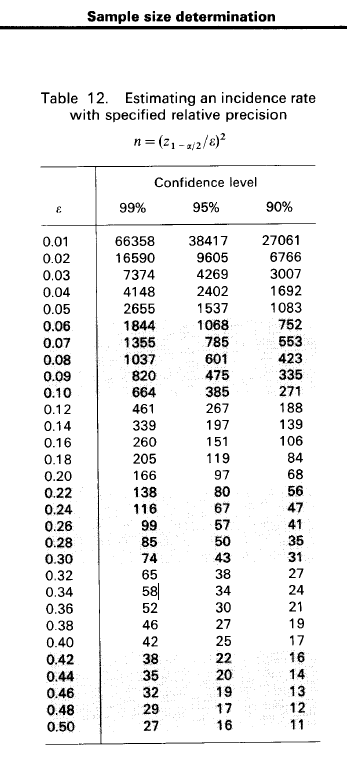
Statistical justification for the sample size of 500 is based on the following:
The figure below shows that for relative precision of 10% and a confidence interval of 95%, a sample size of 385 would be needed for the study. Other adjustments included expected loss to follow-up since enrollment during 36-months of follow-up of 20% and assuming non-participation proportion 10%. The total number of young men required at SCC @TropMed = 500.
Figure 1 Sample size determination for incidence rate studies by Lwanga & Lemeshow Calculation
3. Methods to maximize response rates and handle non-response
There will be a standard operating procedure (SOP) for maximizing response rates, and dealing with non-response. A reminder system will be used to facilitate appointments, and the study staff will contact the participant up to 3 times for follow-up. In addition a calendar will be provided to the participant.
From a previous cohort conducted at SCC @TropMed, we experienced a non-response rate of less than 5%, however, we had an overall 10% of loss-to-follow-up (LTFU, i.e. never returned any follow-up visit since enrollment). We found that young age (18-21 years) had a higher LTFU. In this study, we estimate a non-response rate of less than 10% but LTFU could be as high as 20%. We have a plan for analyzing LTFU bias as the following:
To determine whether LTFU is associated with differences in exposure-outcome effects, we will evaluate factors associated with prevalent HIV infection at enrollment between participants initially enrolled in the study, and those who are retained to at least one follow-up visit (or a pre-defined definition). The primary outcome of this evaluation will be to quantify HIV incidence and its predictors; however, HIV status at the follow-up visits among the LTFU will not be available; thus, prevalent HIV infection will be used as a proxy for incident infection. Following Nohr et al., 2006 we will define adjusted relative odds ratio (adjusted ROR) as the ratio of the adjusted OR retained participant/adjusted OR all participant, and calculate the confidence interval by using the equation method previously described by Following Nohr et al., 2006 and Osler et al., 2008. We expect the LTFU not to effect statistical measures of association, and the impact of LTFU on estimated associations from this study will be published in order to validate study findings.
4. Tests of procedures or methods to be undertaken
We conducted as small pilot test to assess the content validity of the risk assessment questionnaire as the following:
Approximately 5 MSM and TGW aged 15-29 years were asked to score the level of understanding to the questions in the CASI questionnaire (Attachment 7_Screening CASI and Attachment 11 Follow-up CASI). The Likert scale of 3 where 1 = “Difficult to understand” and 3 = ”Easy to understand”. Questions that were identified with a score of 1 were revised.
In addition, we evaluated the instrument for HIV positive MSM and TGW and pretested with a nurse and study staff to ensure content validity and logical sequence.
5. Individuals consulted on statistical aspects and individuals collecting and/or analyzing data
Dr. Sarika Pattanasin, Lead of the Epidemiology Unit, HIV/STD Research Program, Thailand MOPH-US CDC Collaboration, email vpv6@cdc.gov, will be leading the activity. Dr. Sarika Pattanasin received Bachelor degree in Medical Technology from Chulalongkorn University, Master of Science in Biostatistics and Doctoral of Public Health from Mahidol University. During 2004-2007, she worked as data analyst at the Thai FETP program to assist FETP trainees to apply statistical method that are appropriate to epidemiological study design. After that, she worked as statistician at the Thailand MOPH-US CDC Collaboration from 2008-2012. She analyzed many Global AIDS Program (GAP) data sets collected from MOPH hospitals and the National Health Security Office (NHSO).
Philip A. Mock, Chief of Information Technology, Thailand MOPH-US CDC Collaboration, email pgm6@cdc.gov, will be consultant on data management and statistical aspects. Mr. Mock has been involved as the statistician in many scientific publications from the Thailand MOPH-US CDC Collaboration. He was the statistical mentor to Dr. Sarika Pattanasin and previously supervised her statistical analysis and data management.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Eileen Dunne |
| File Modified | 0000-00-00 |
| File Created | 2021-01-23 |
© 2026 OMB.report | Privacy Policy