eSubmission Screenshots
eSubmission Screenshots.docx
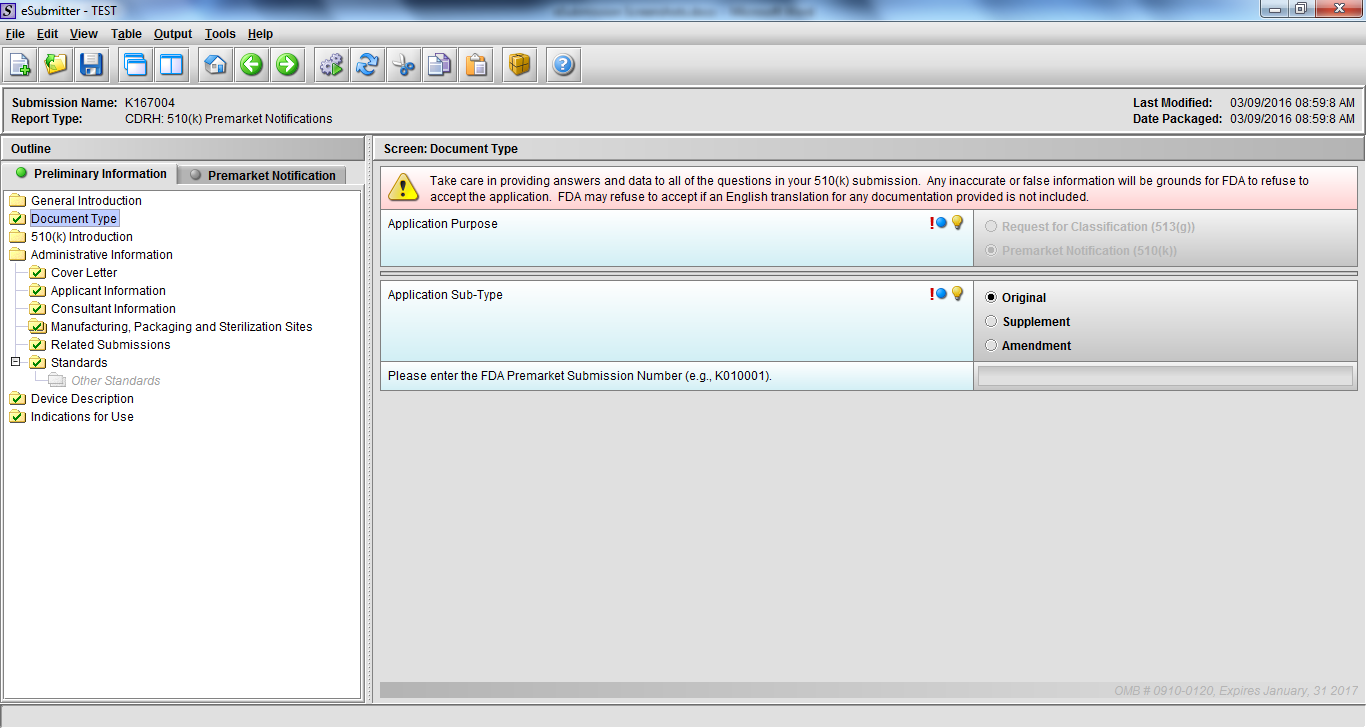
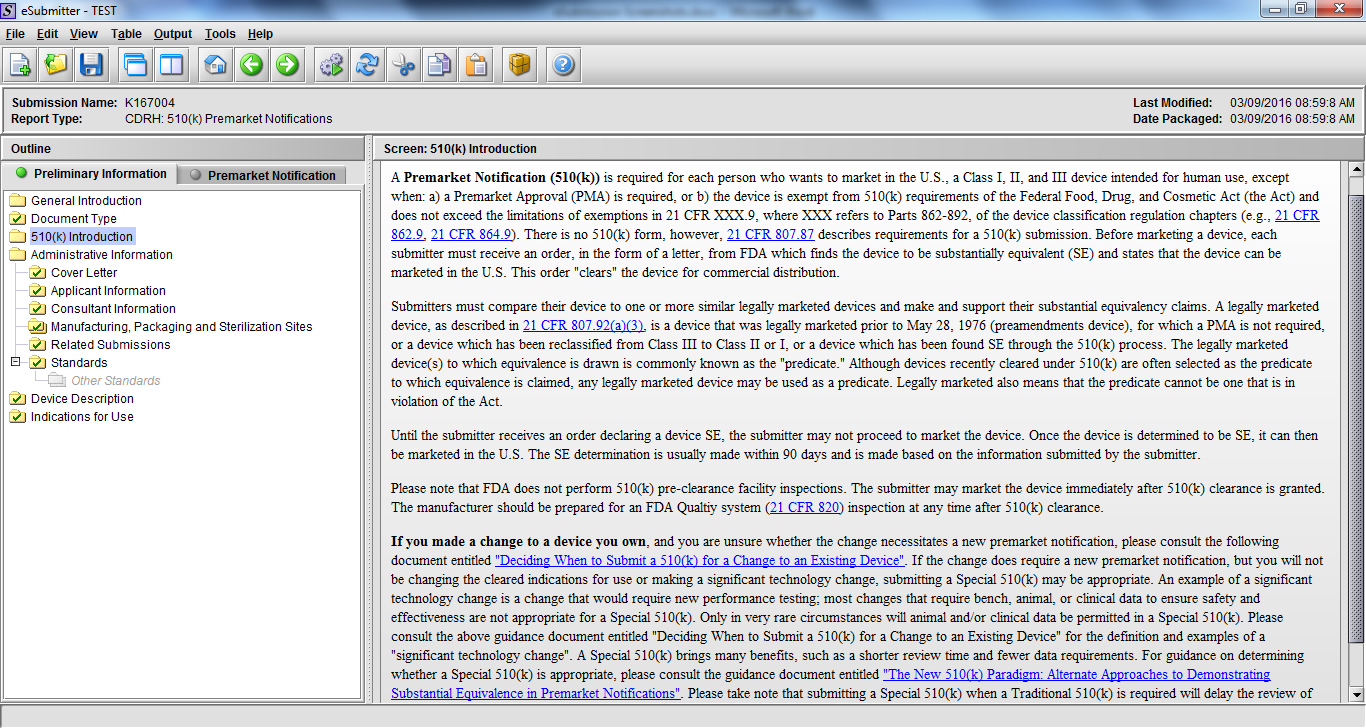
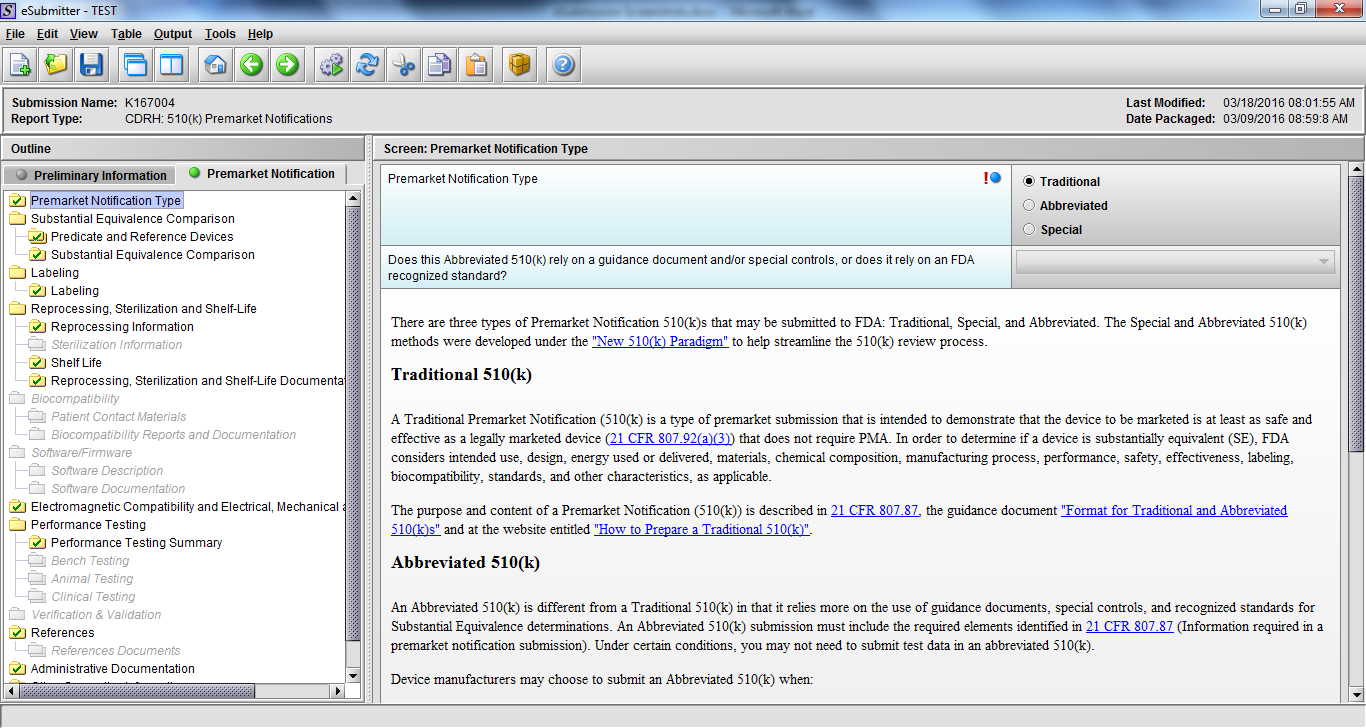
Premarket Notification Submission 510(k), Subpart E
eSubmission Screenshots
OMB: 0910-0120
⚠️ Notice: This form may be outdated. More recent filings and information on OMB 0910-0120 can be found here:
Document [docx]
Download: docx | pdf
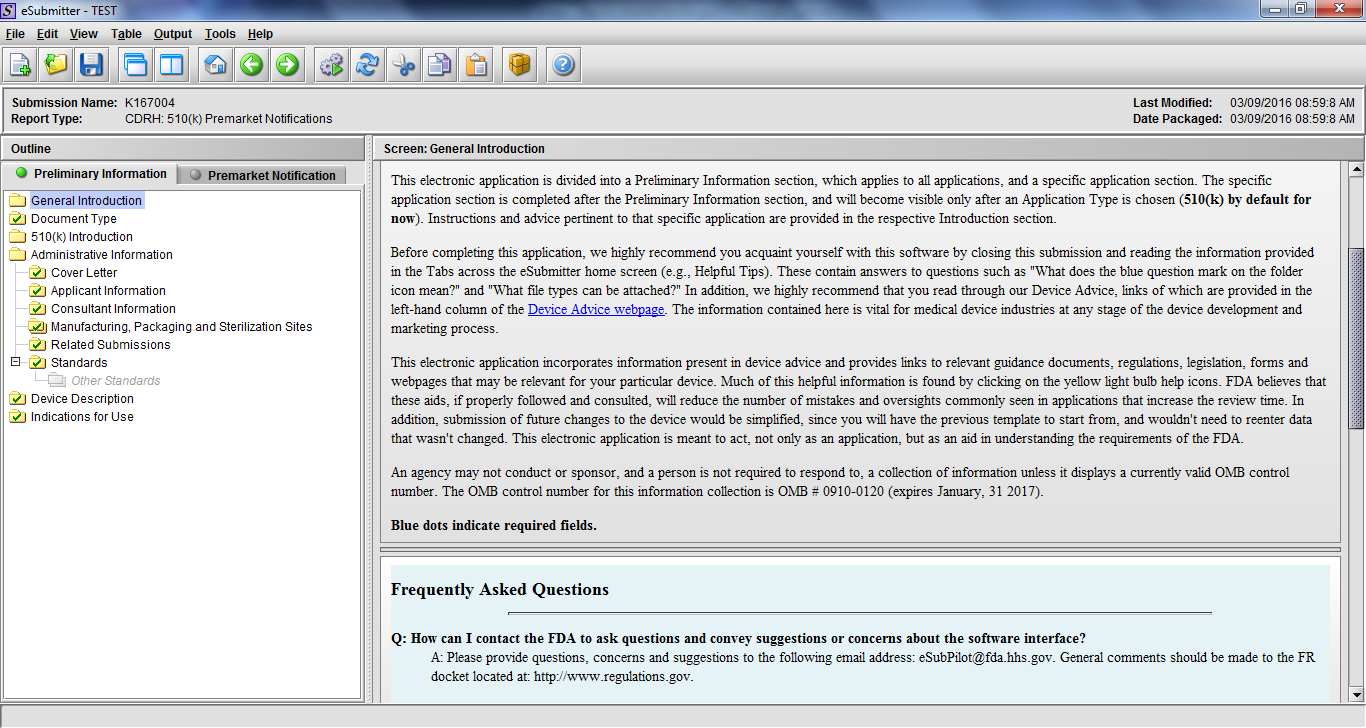
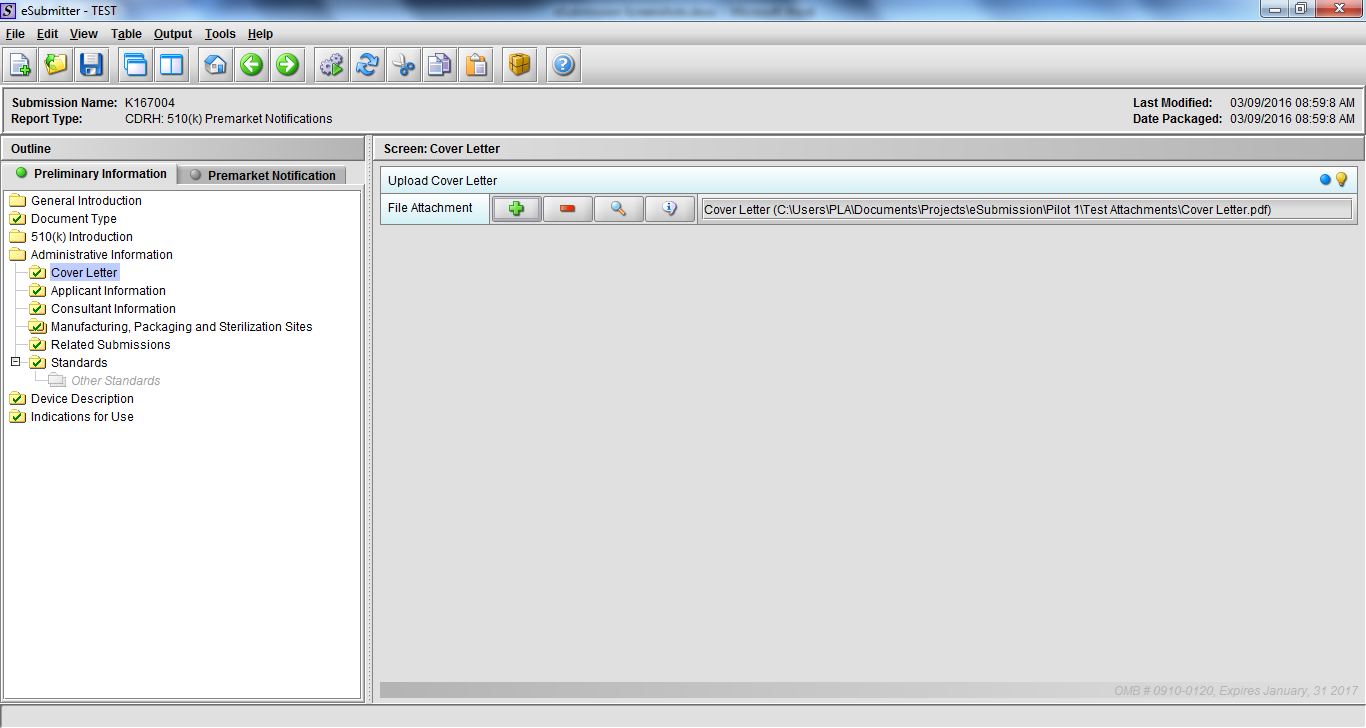
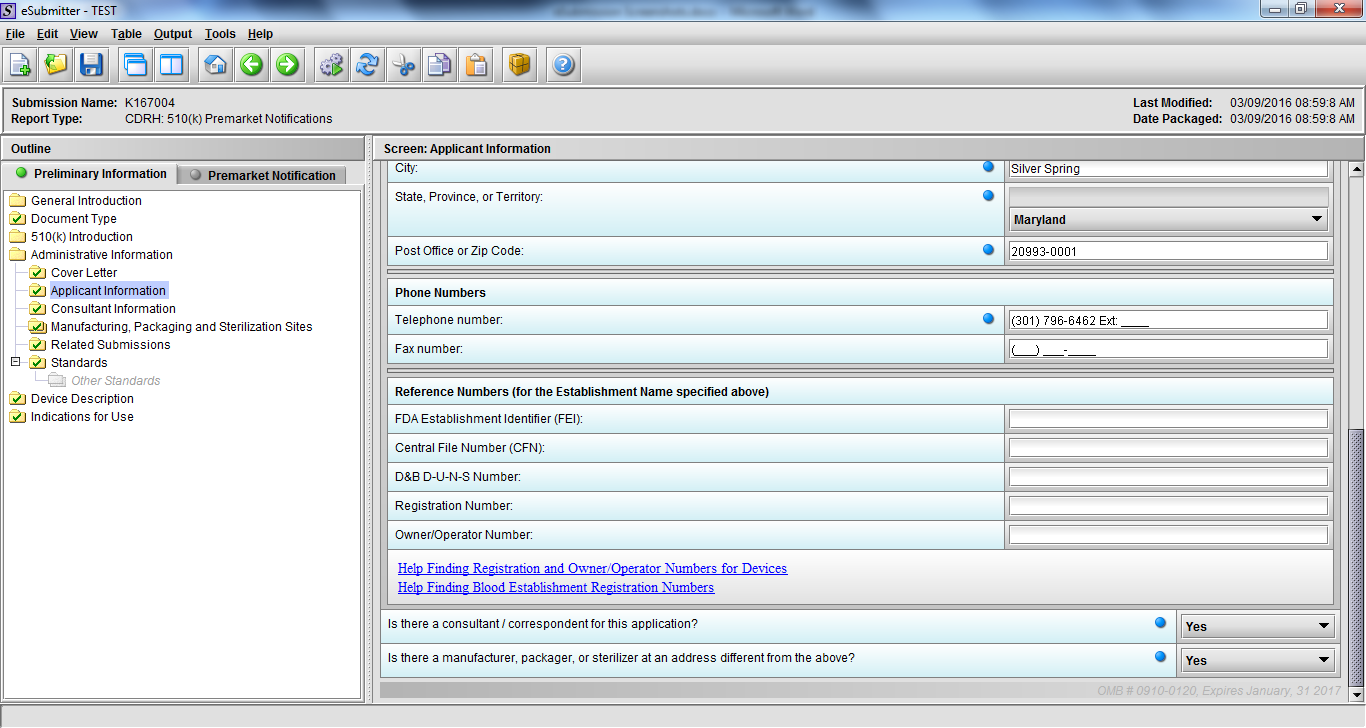
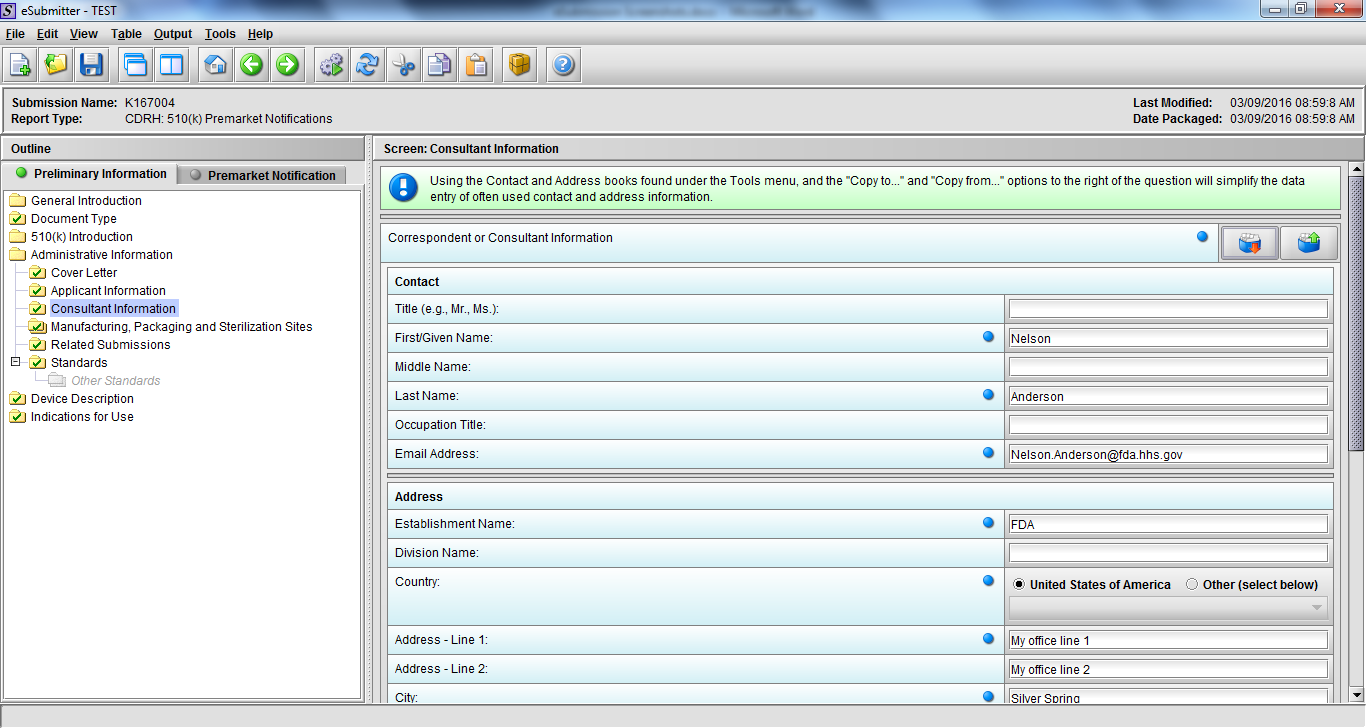
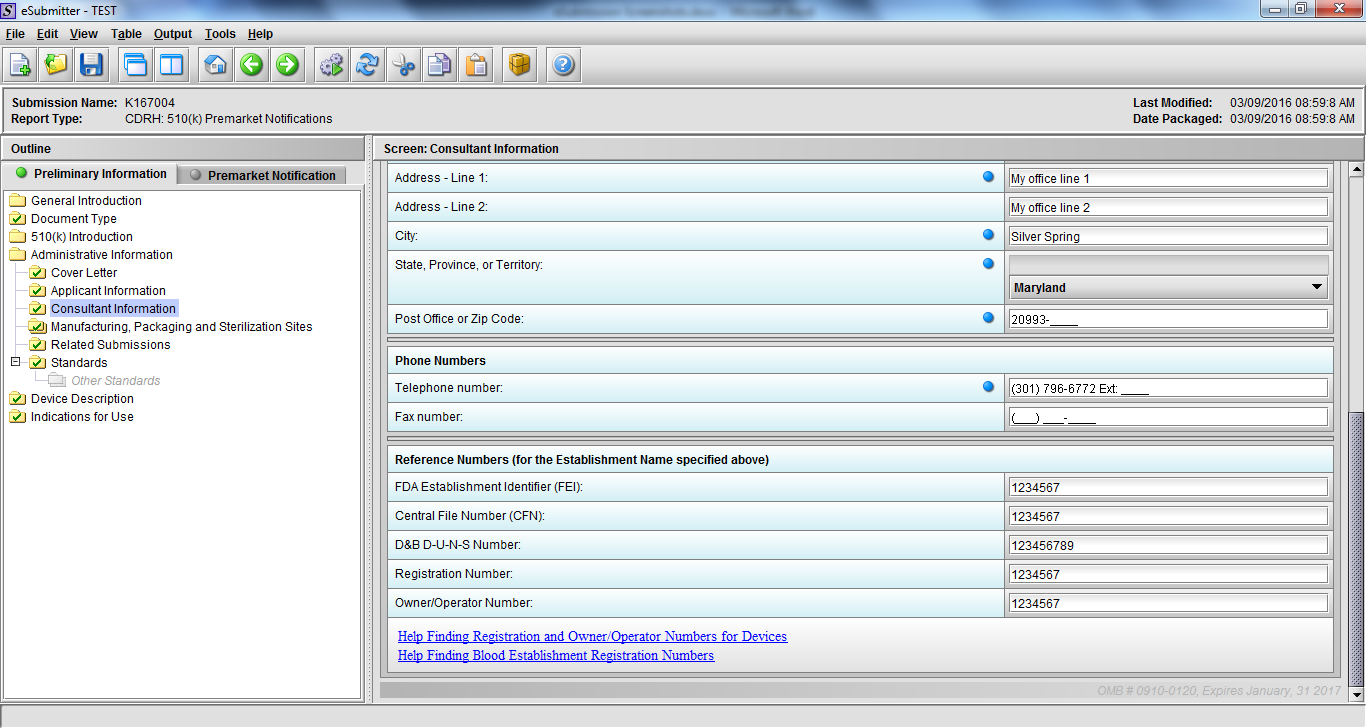
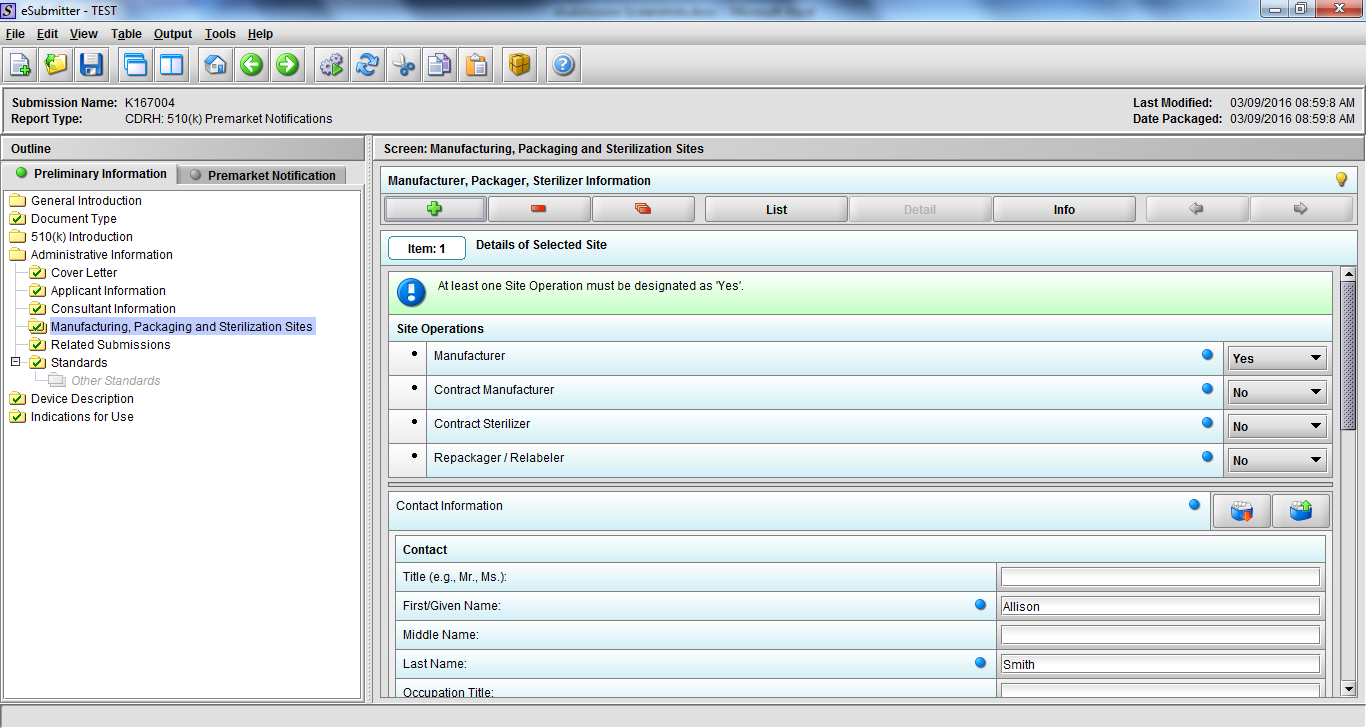
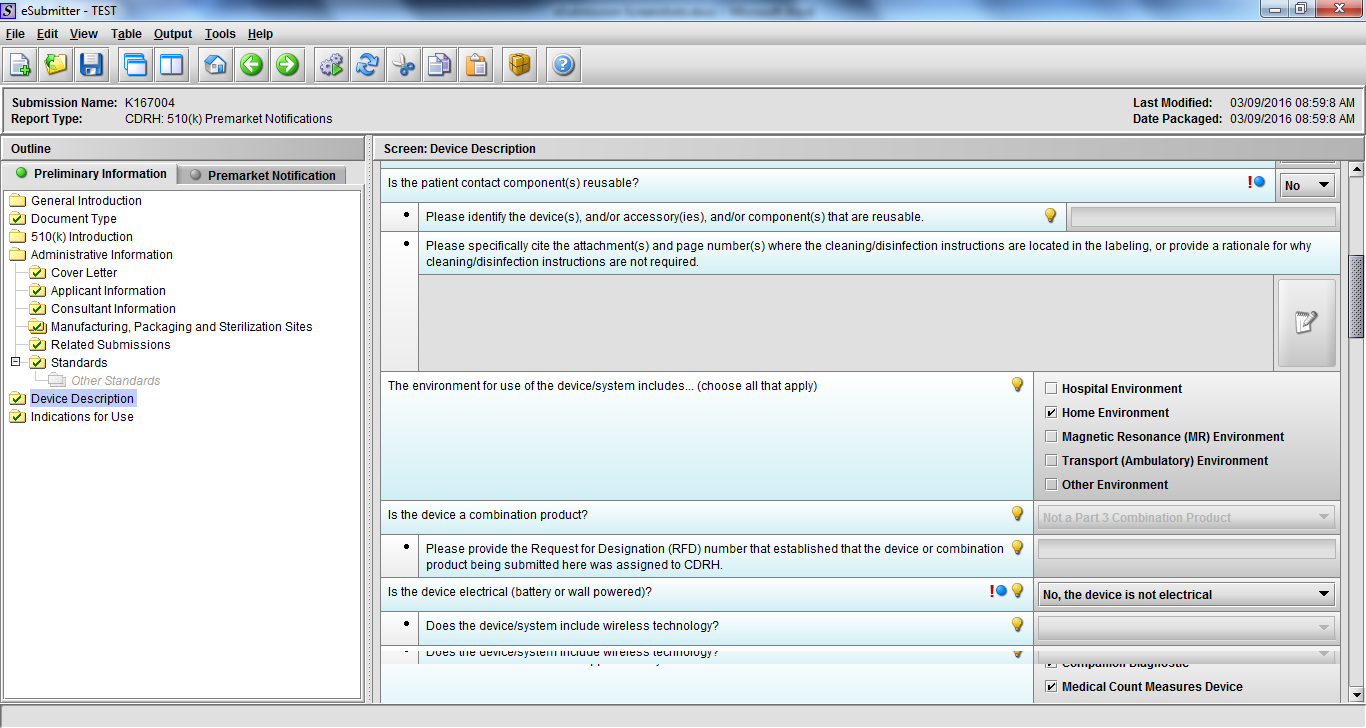
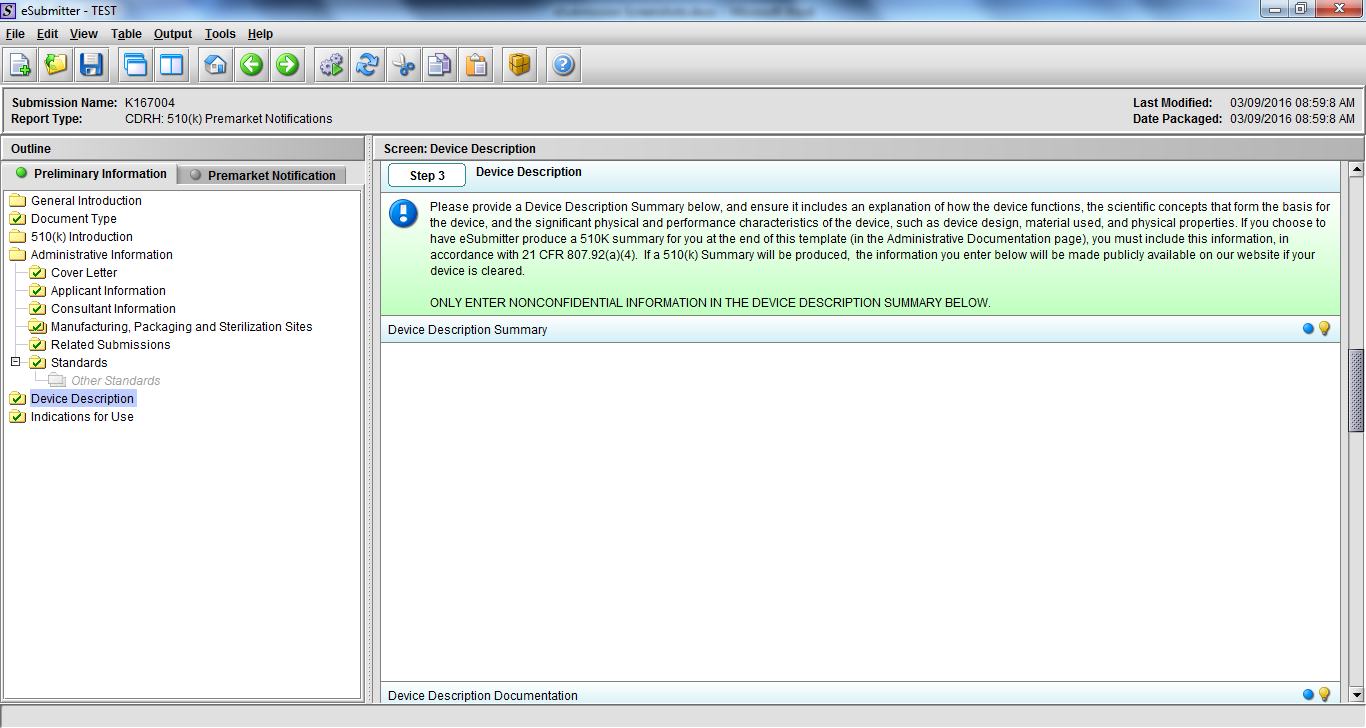
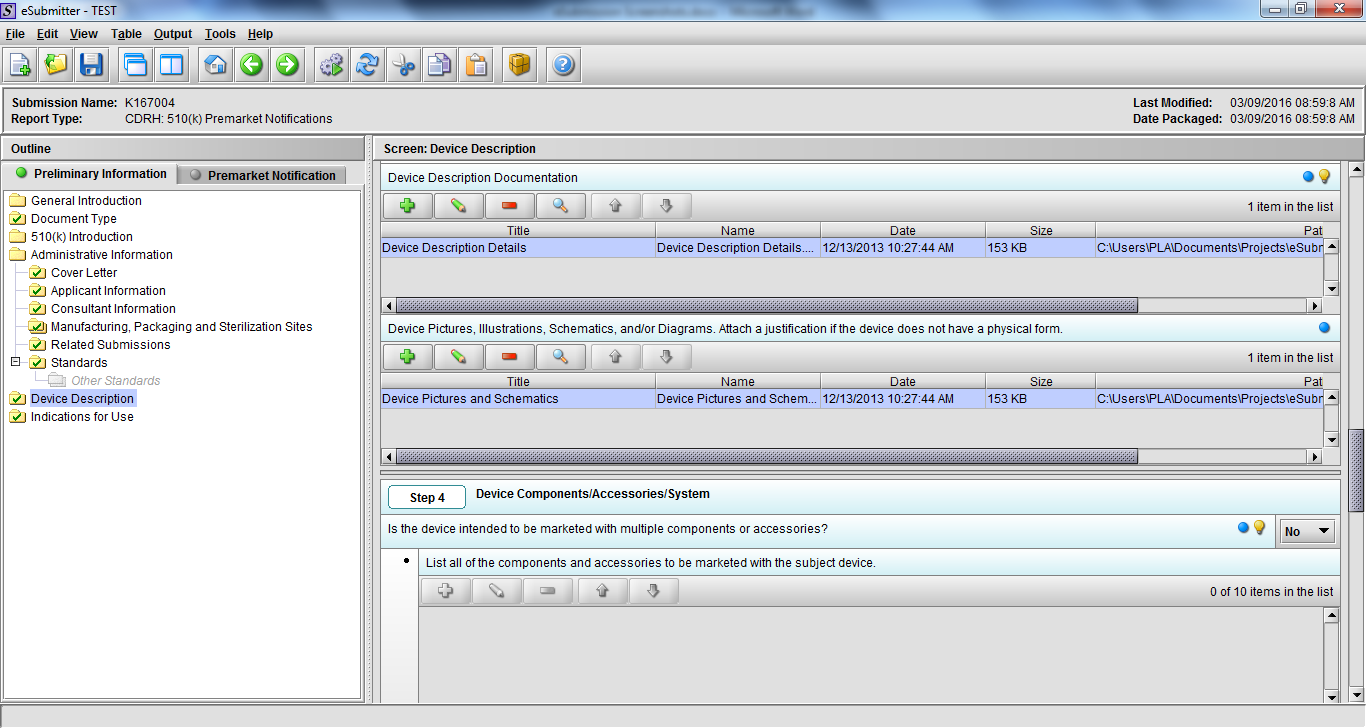
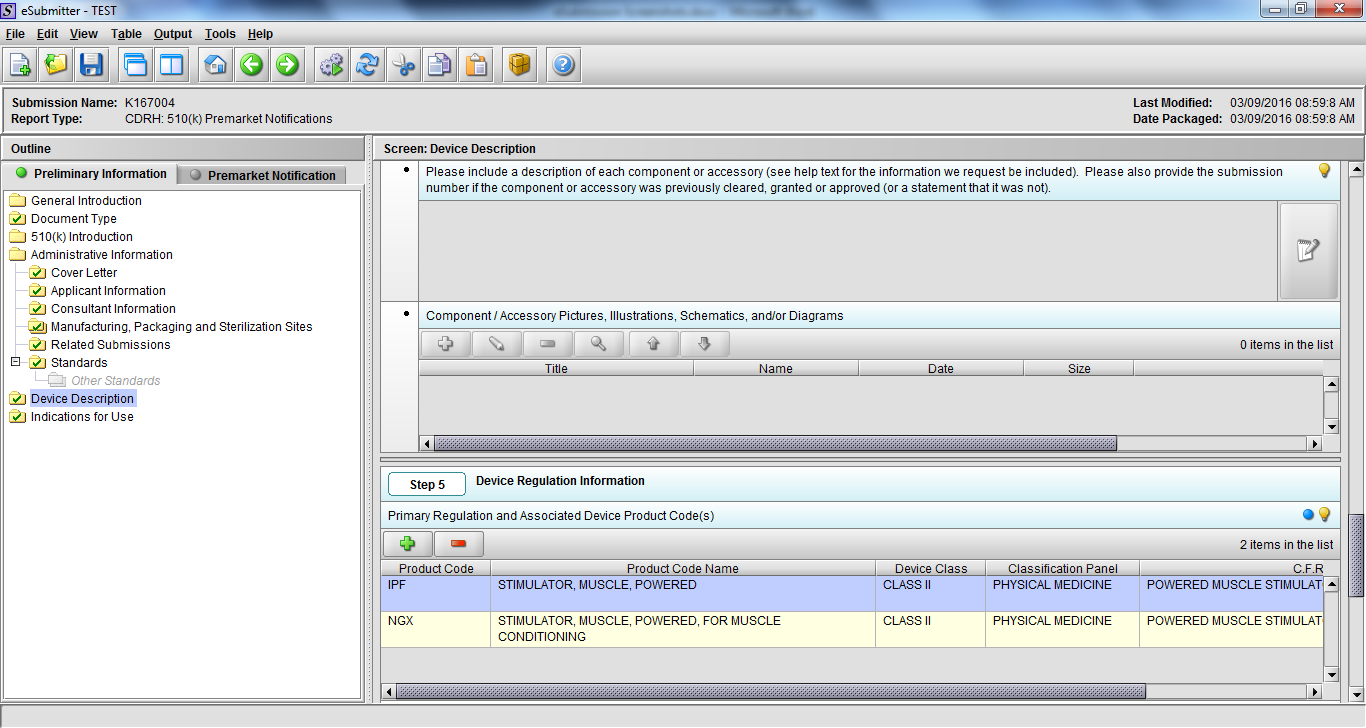
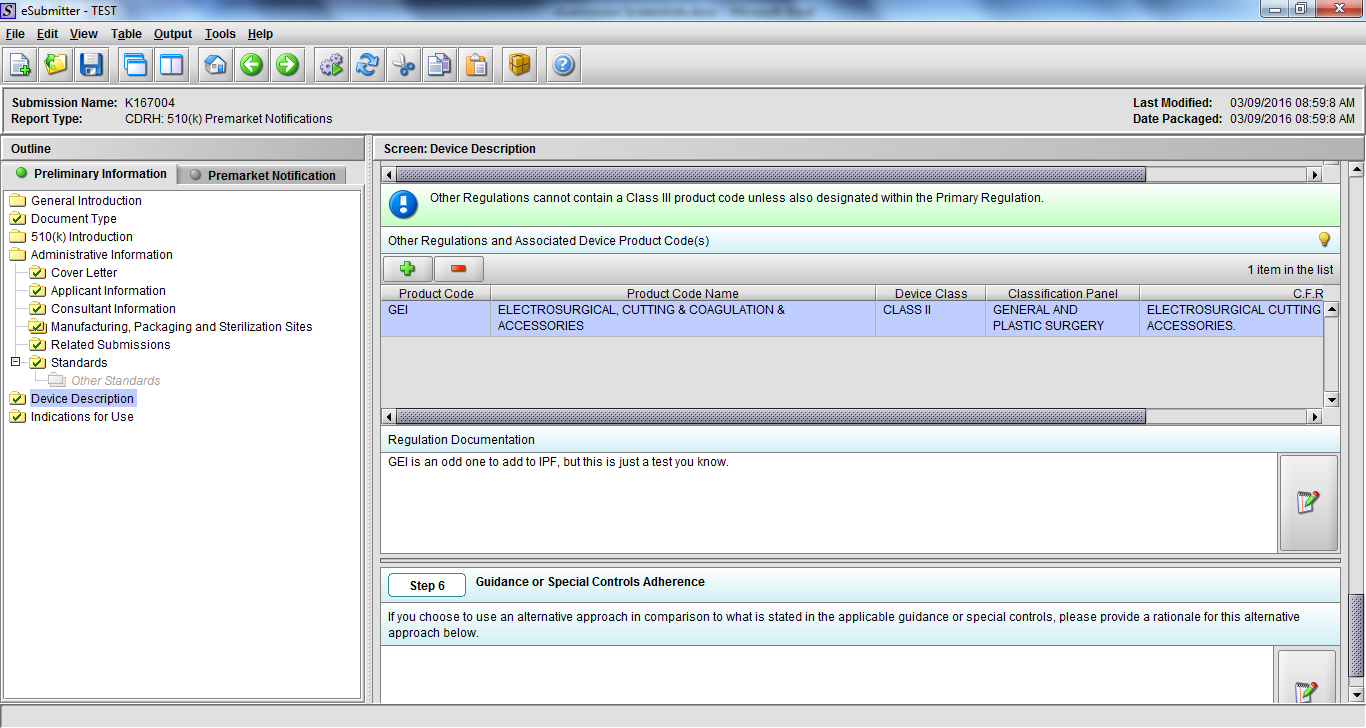
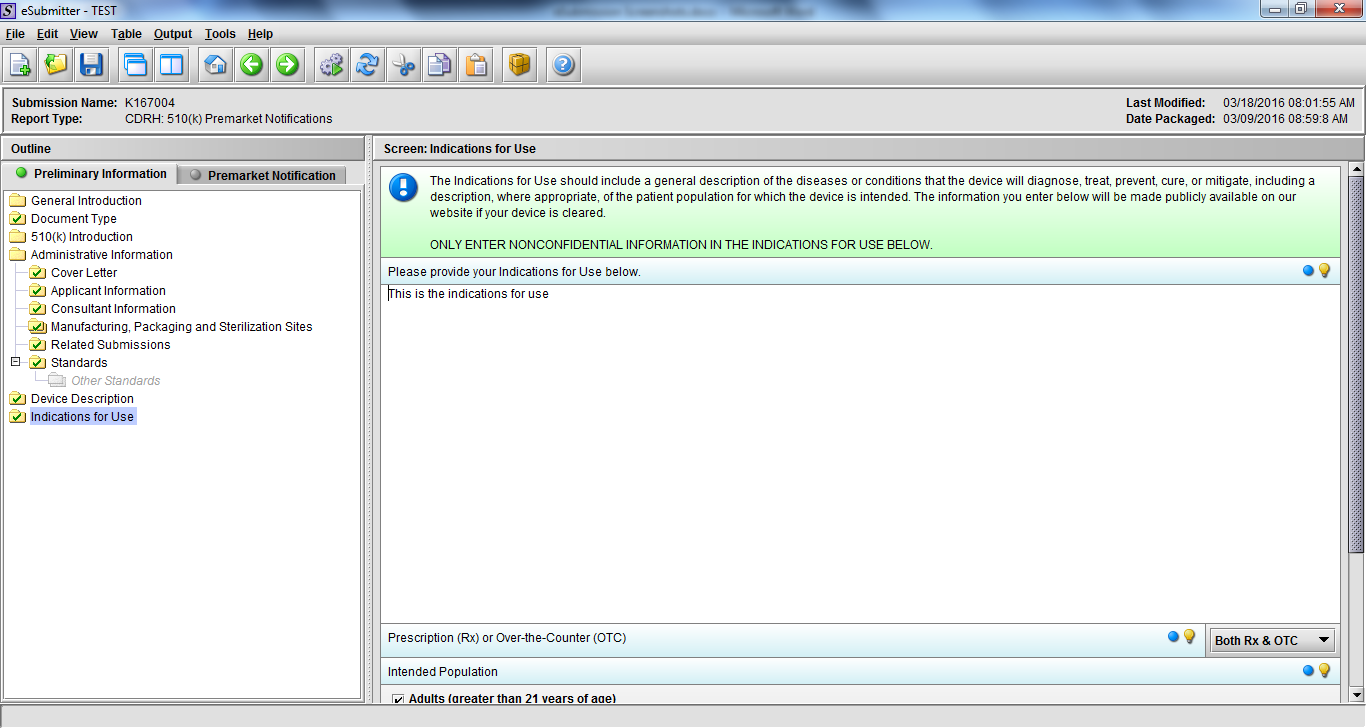
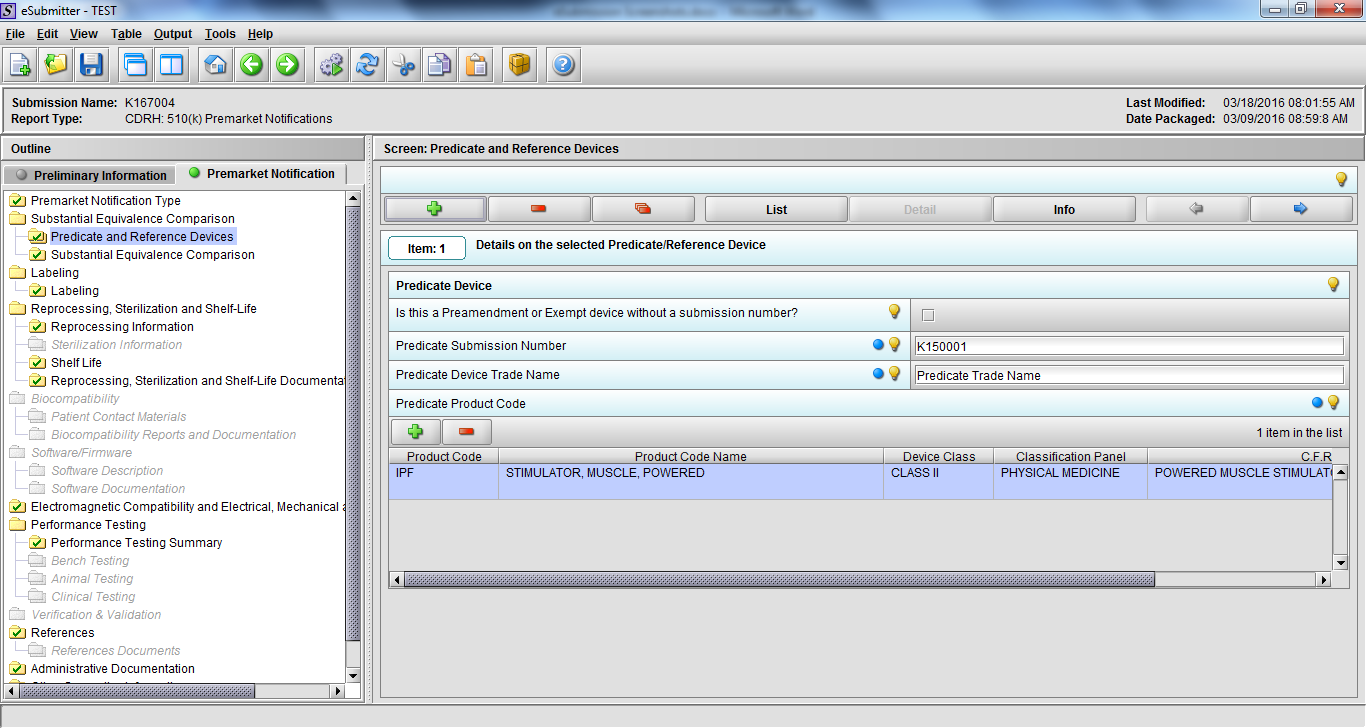
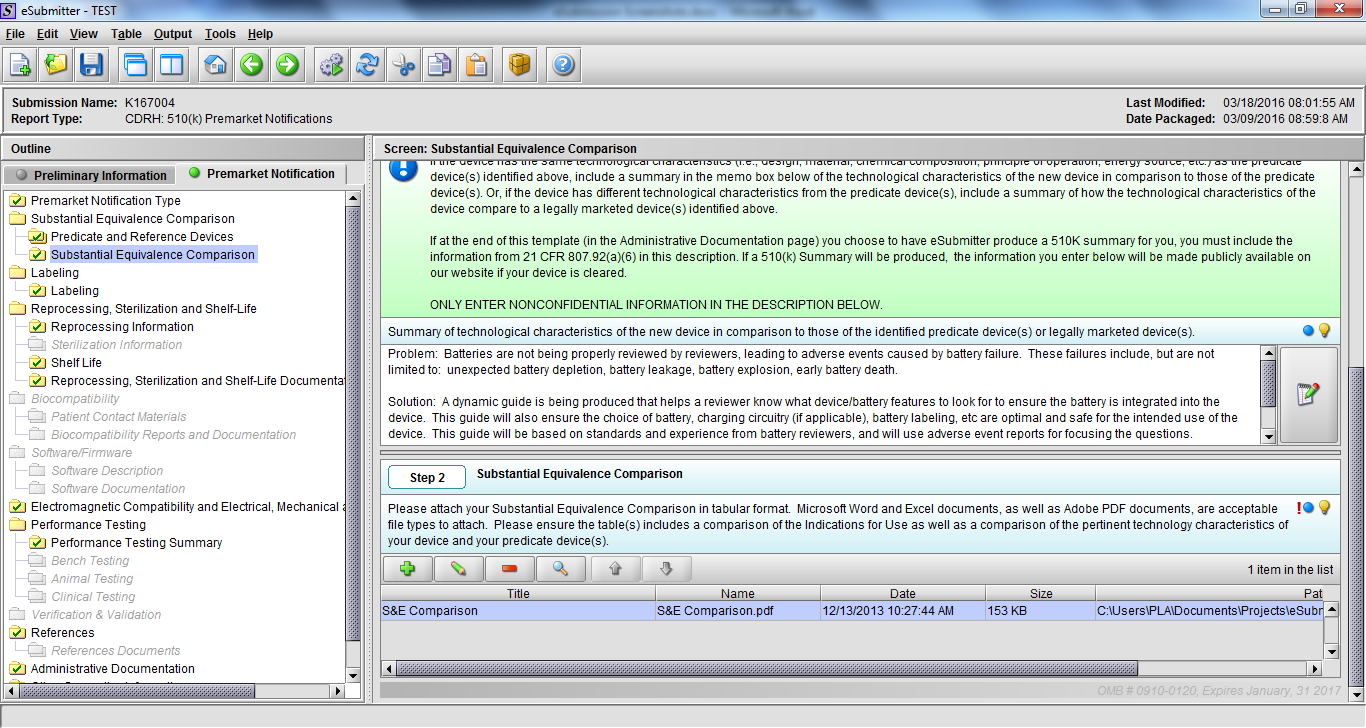
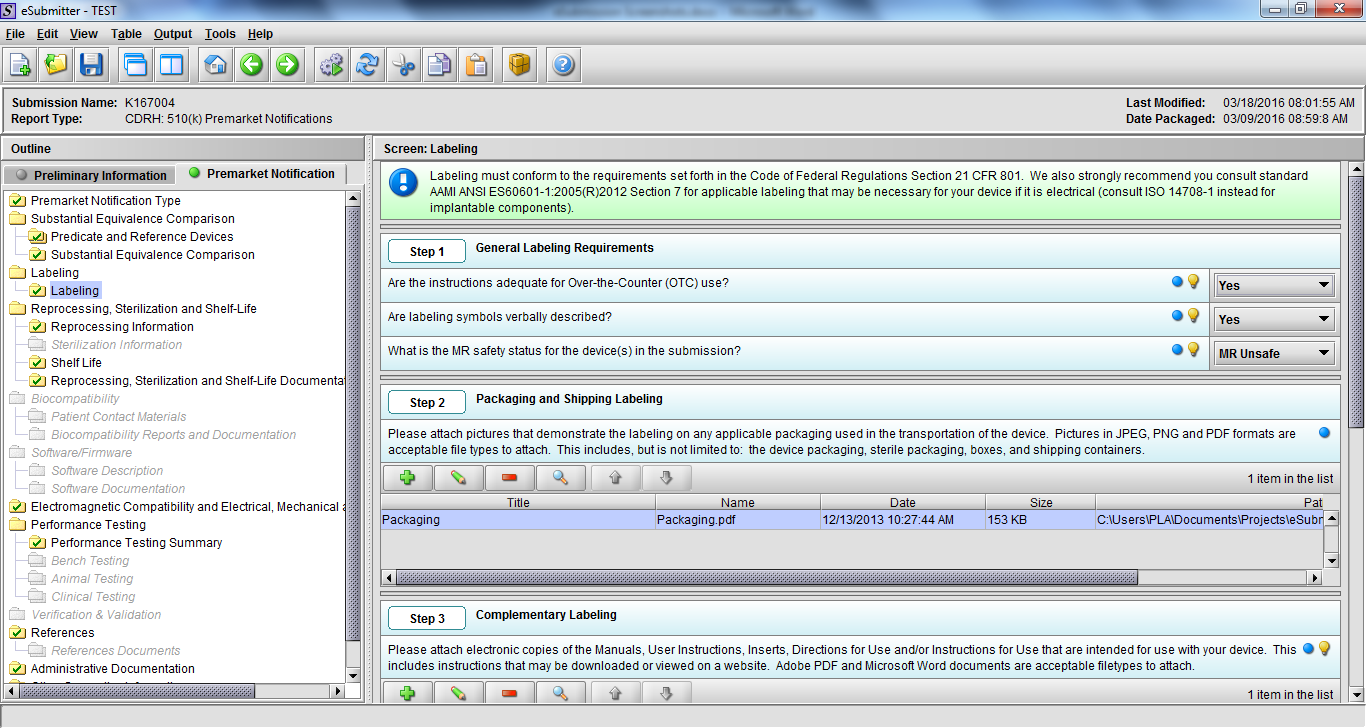
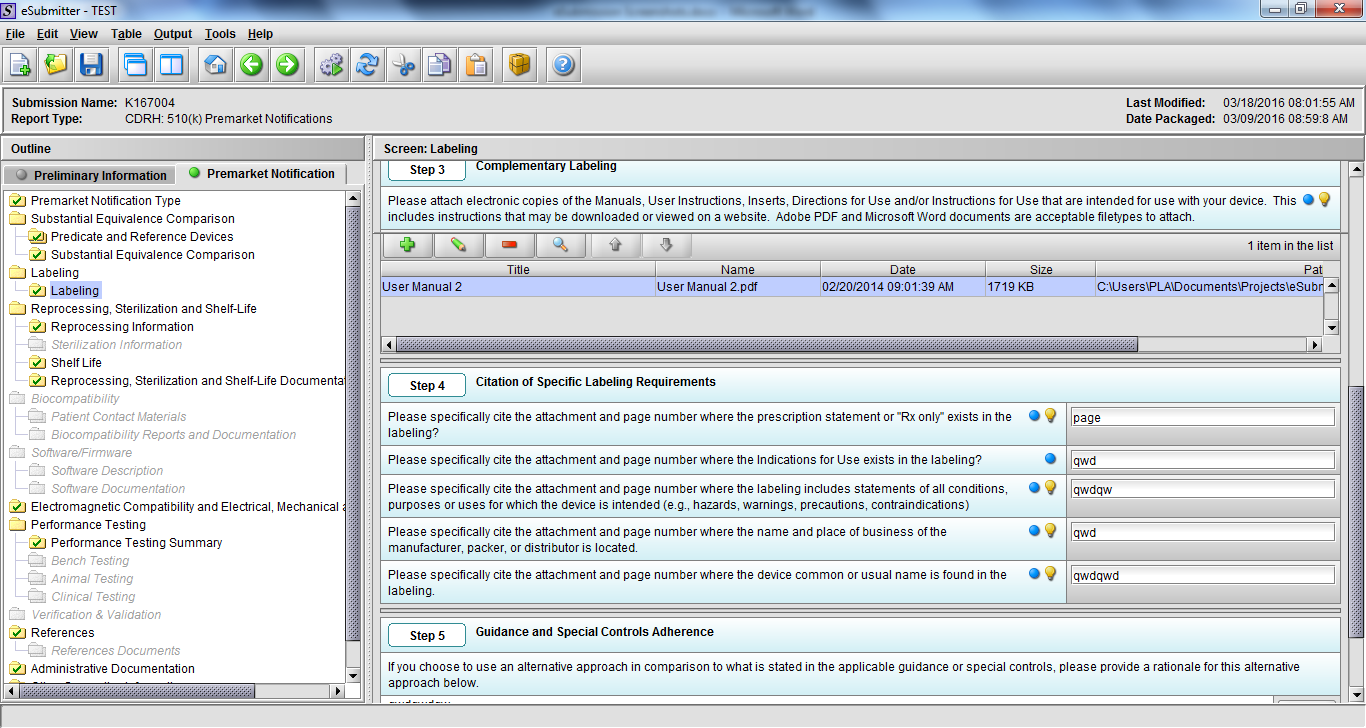
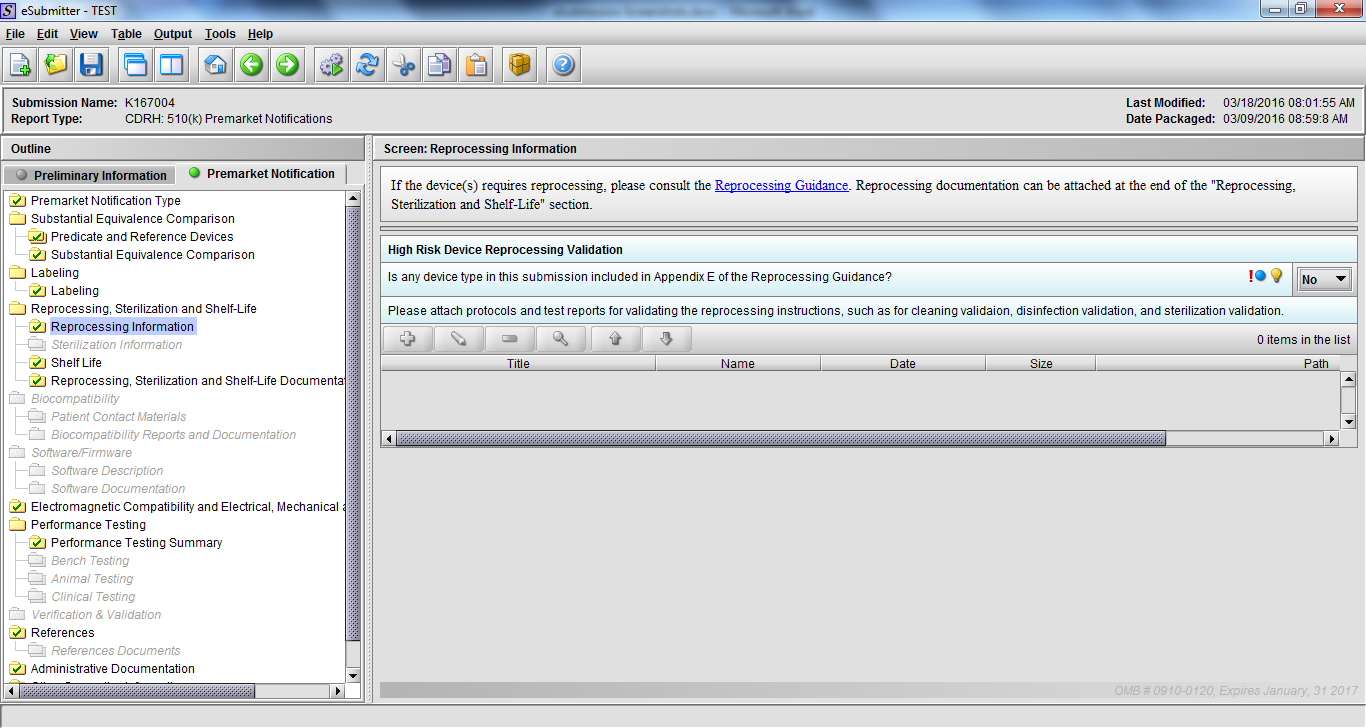
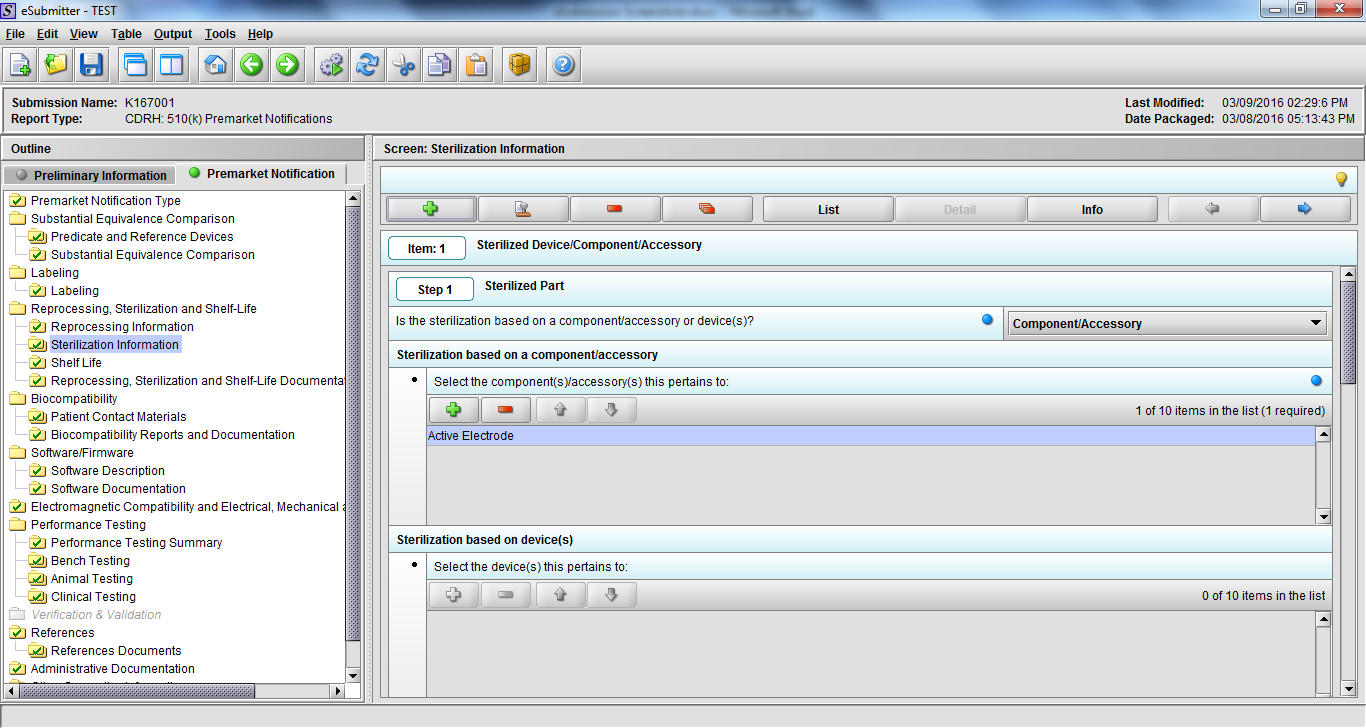
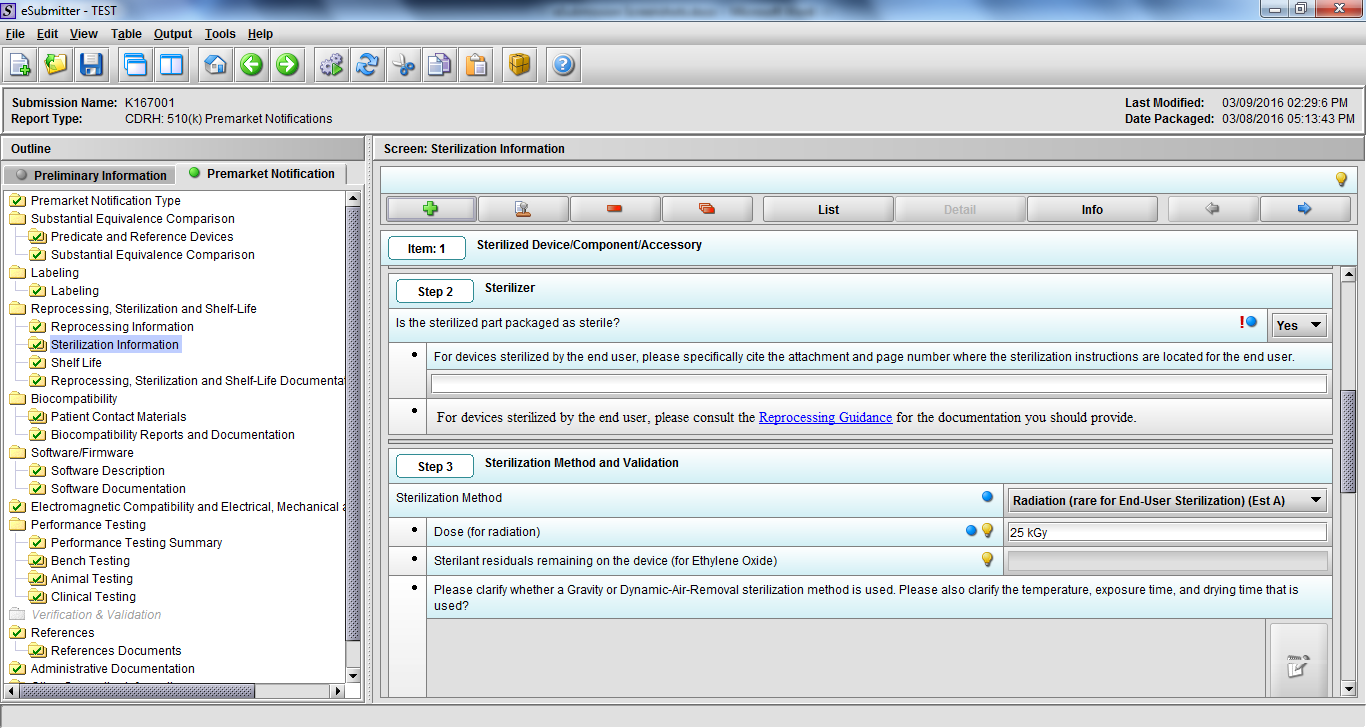
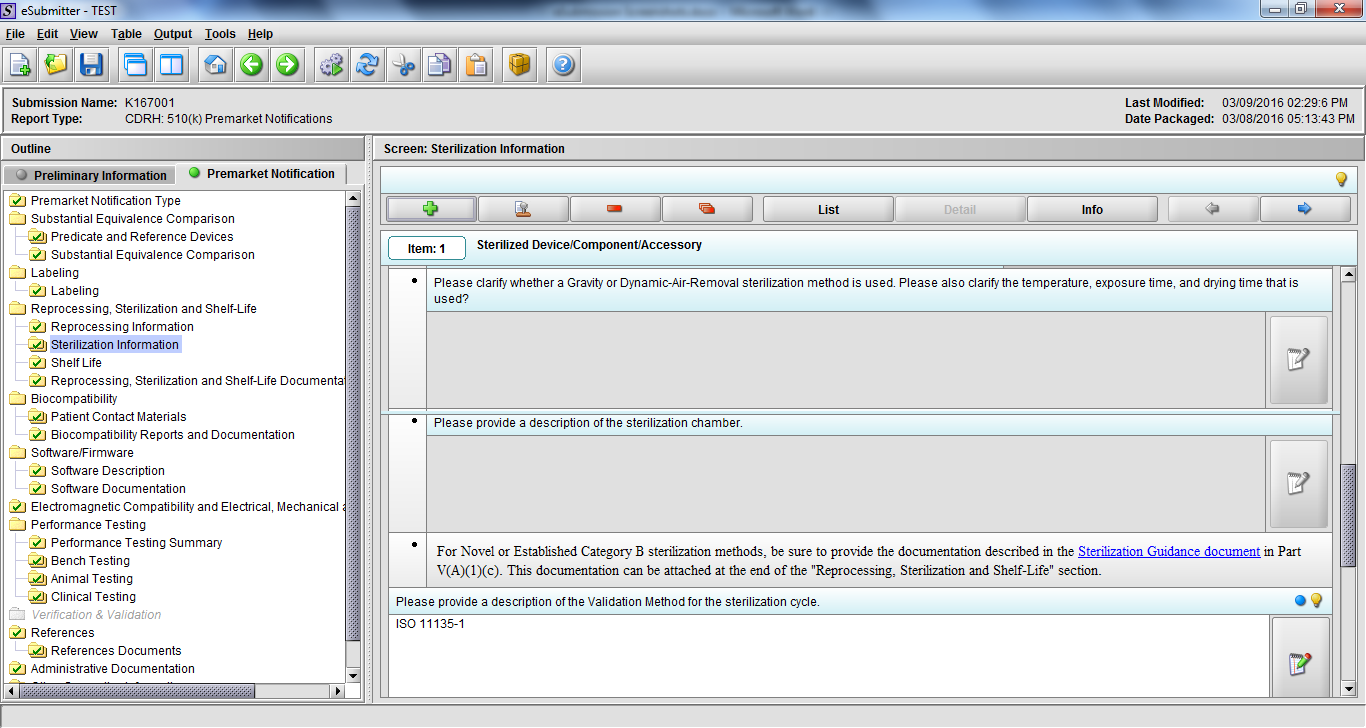
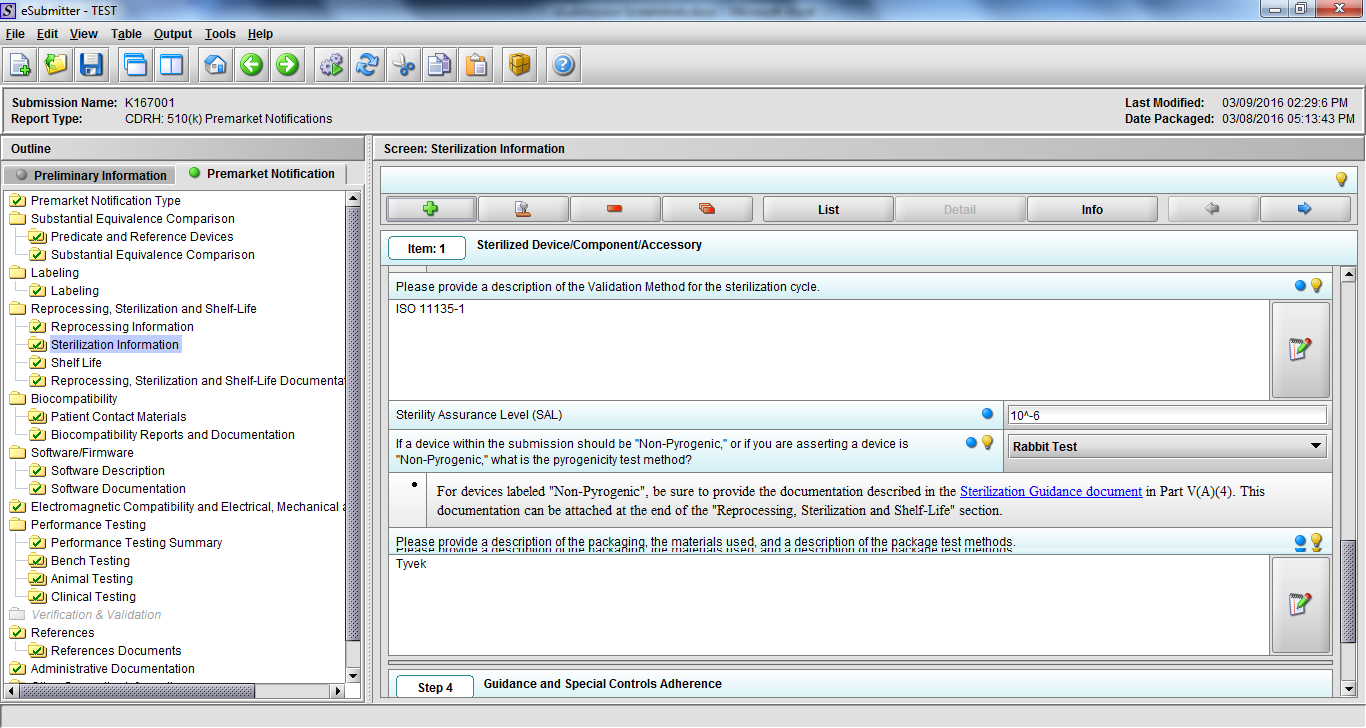
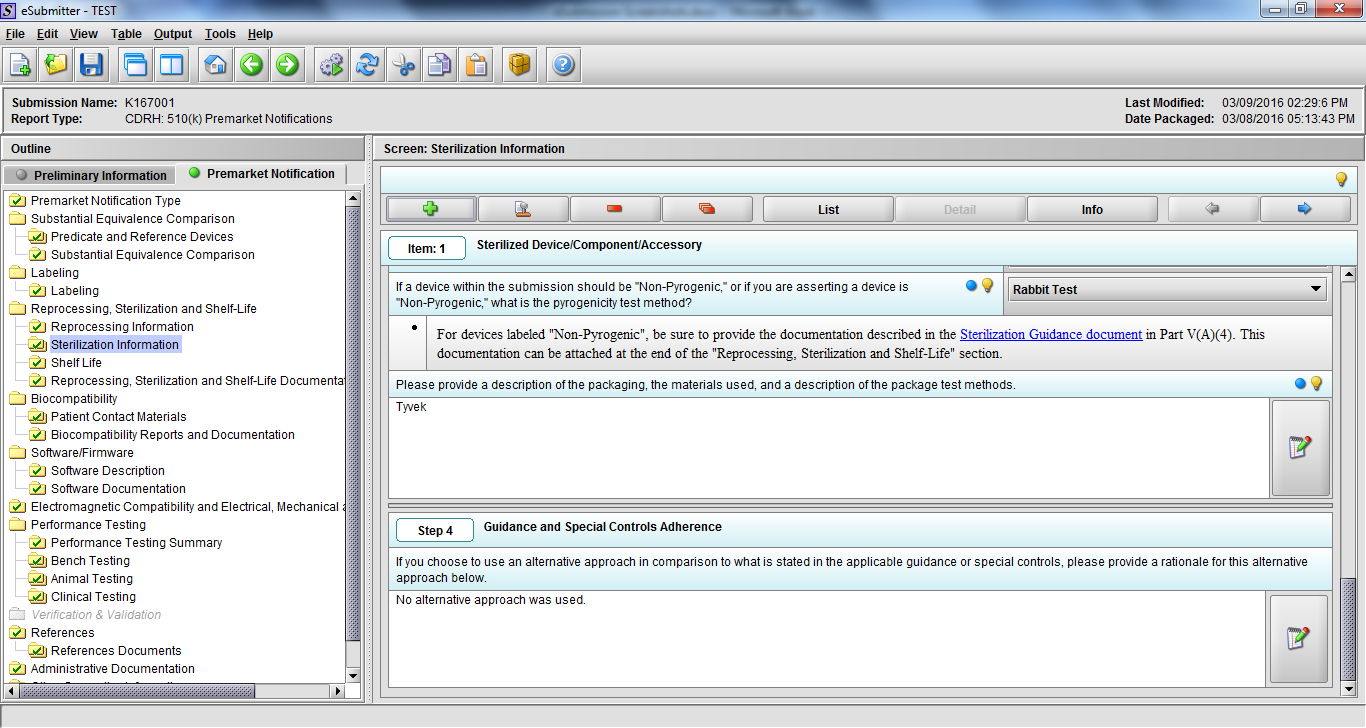
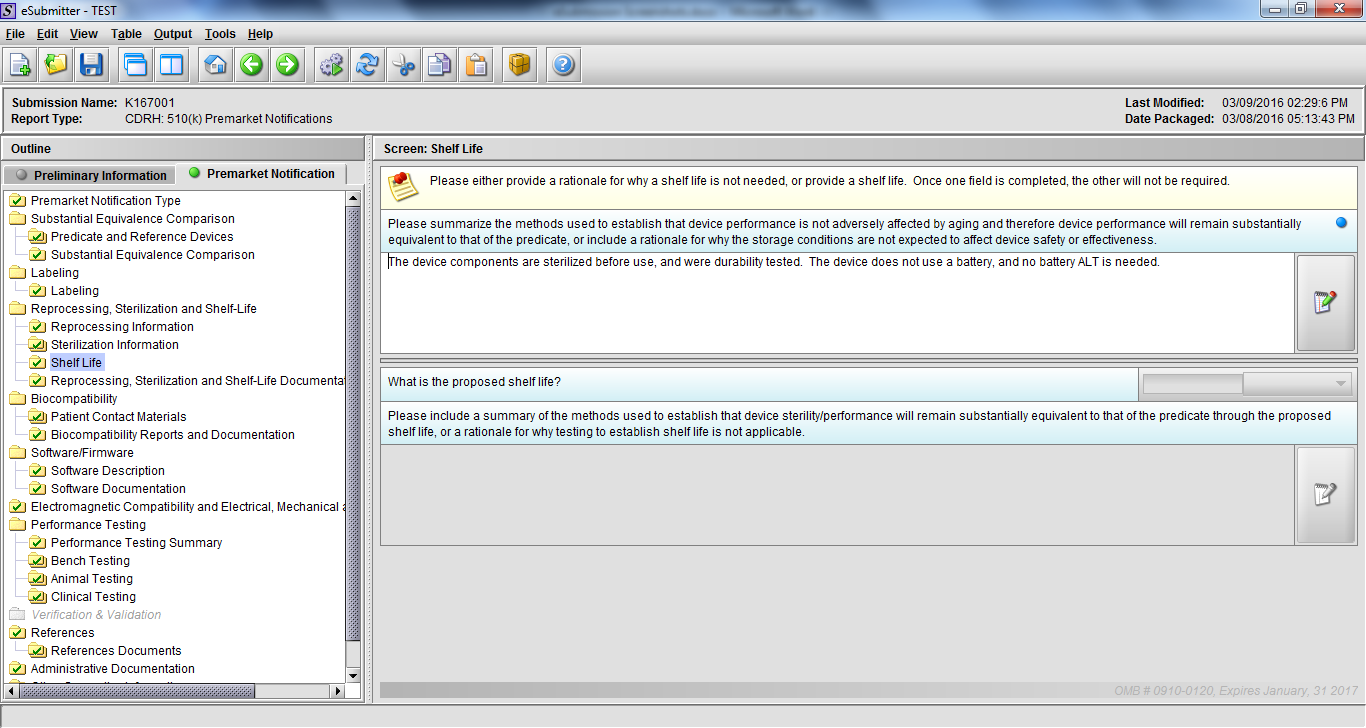
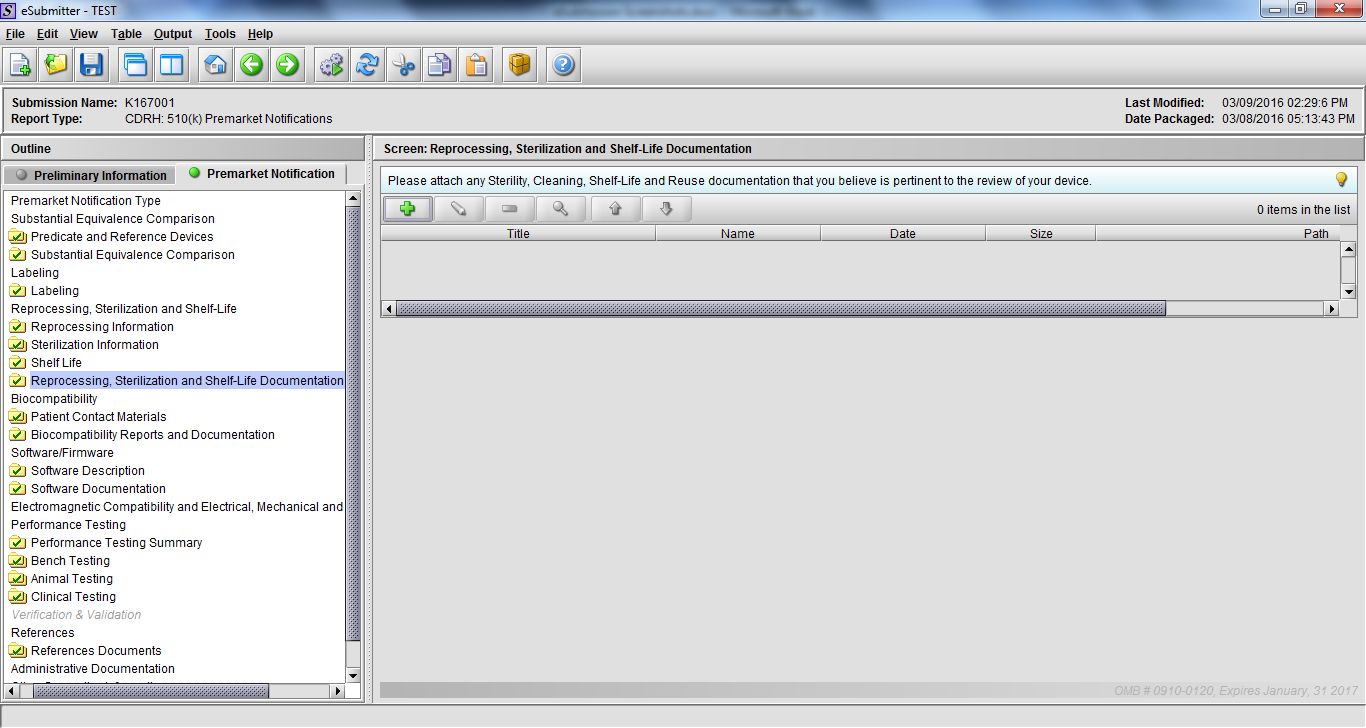
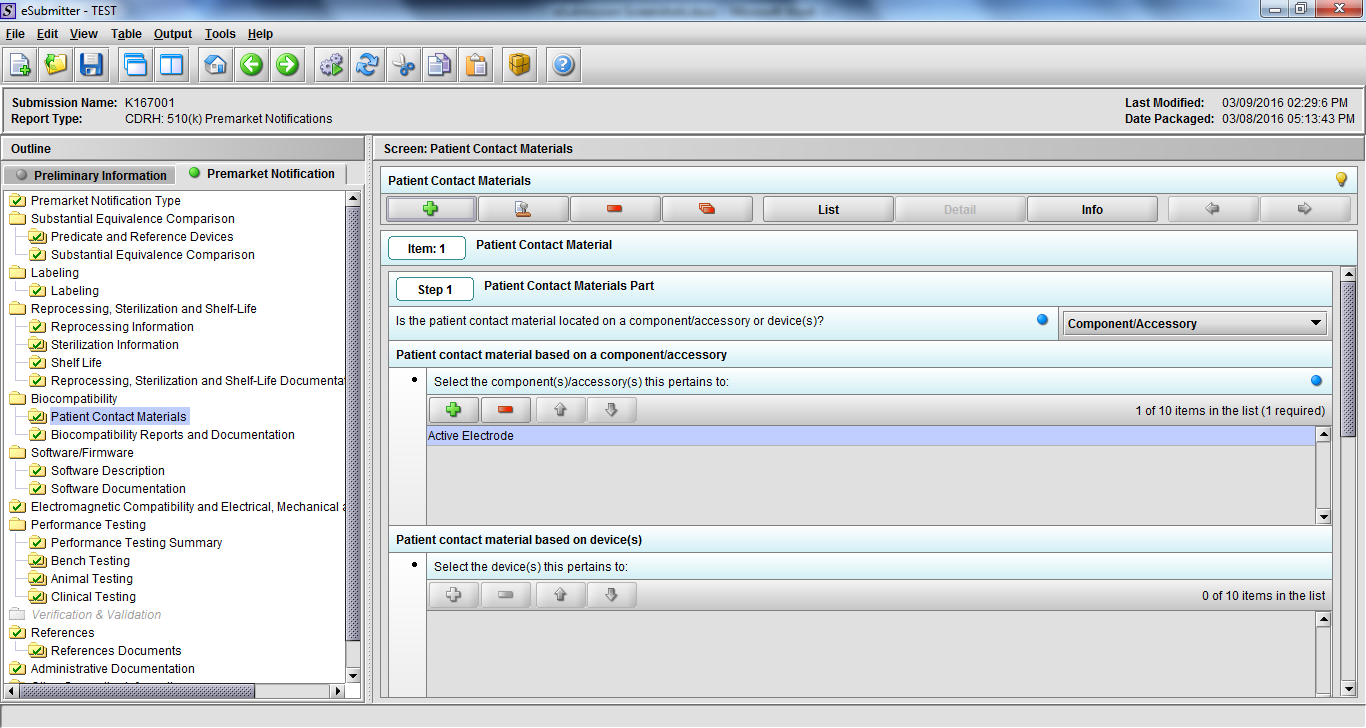
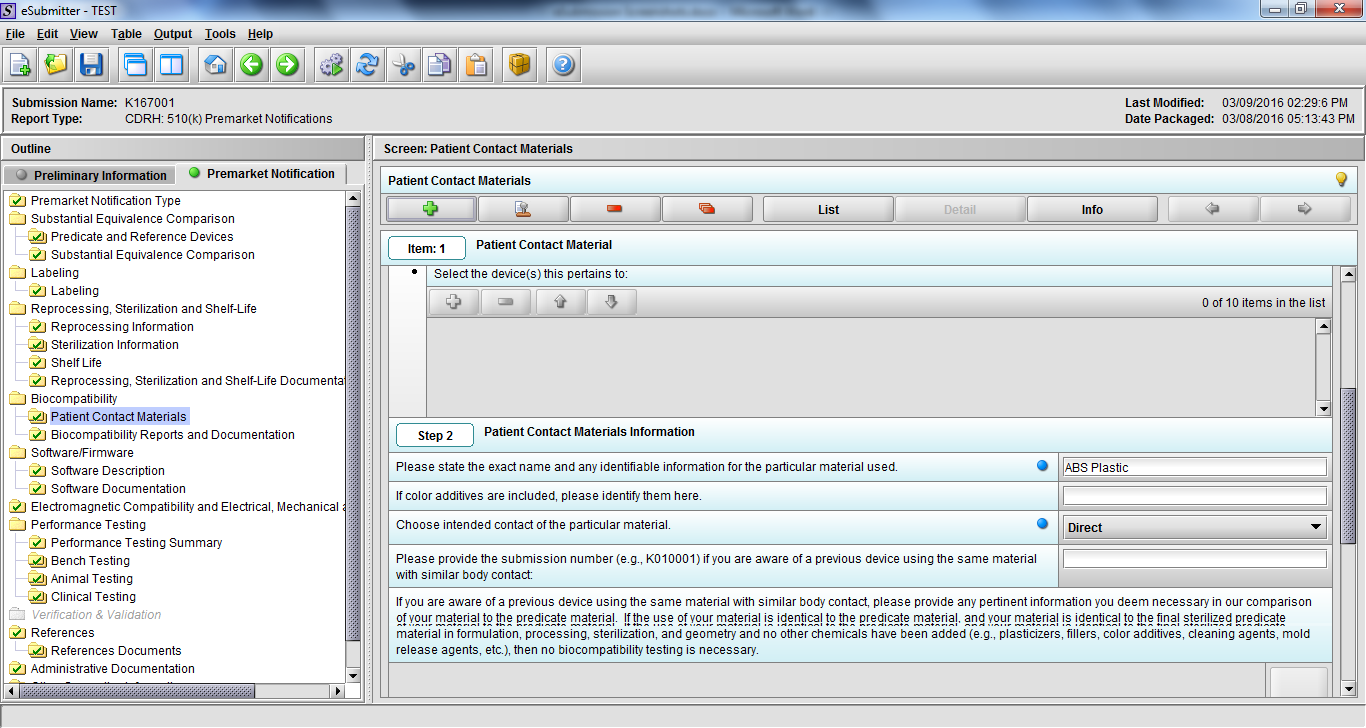
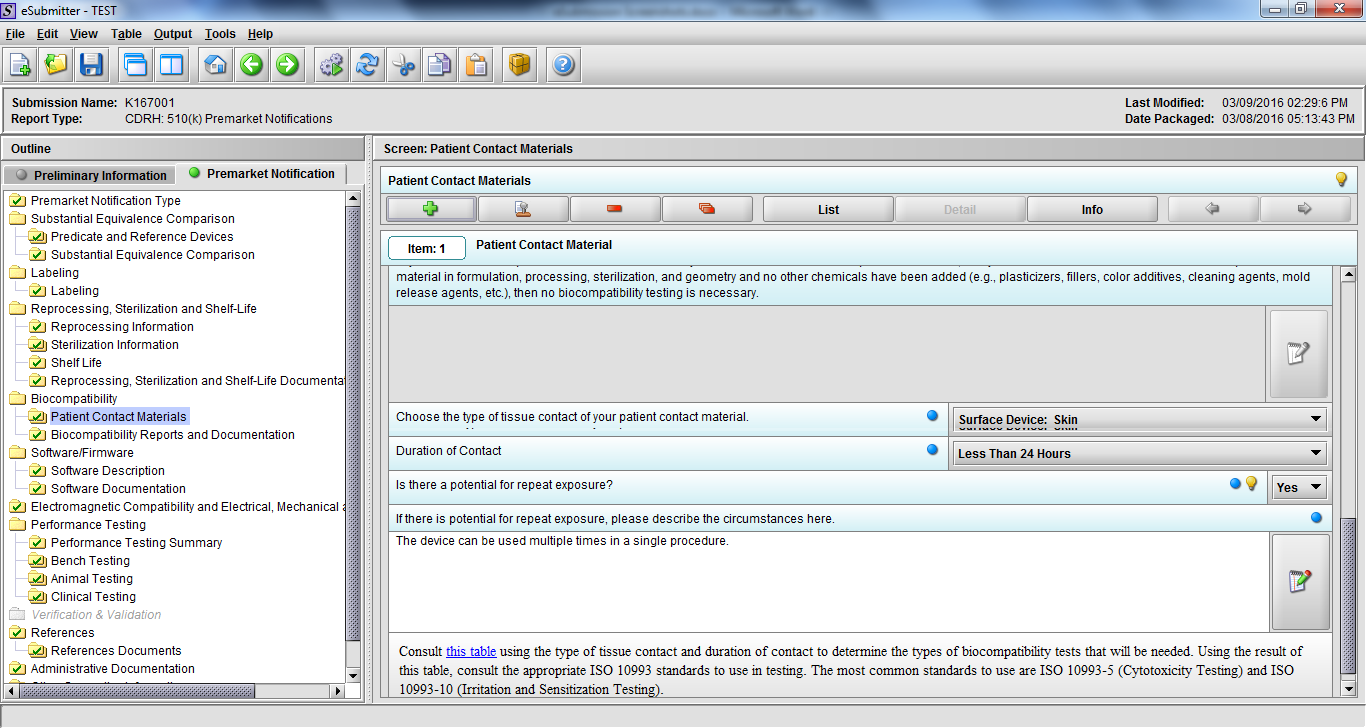
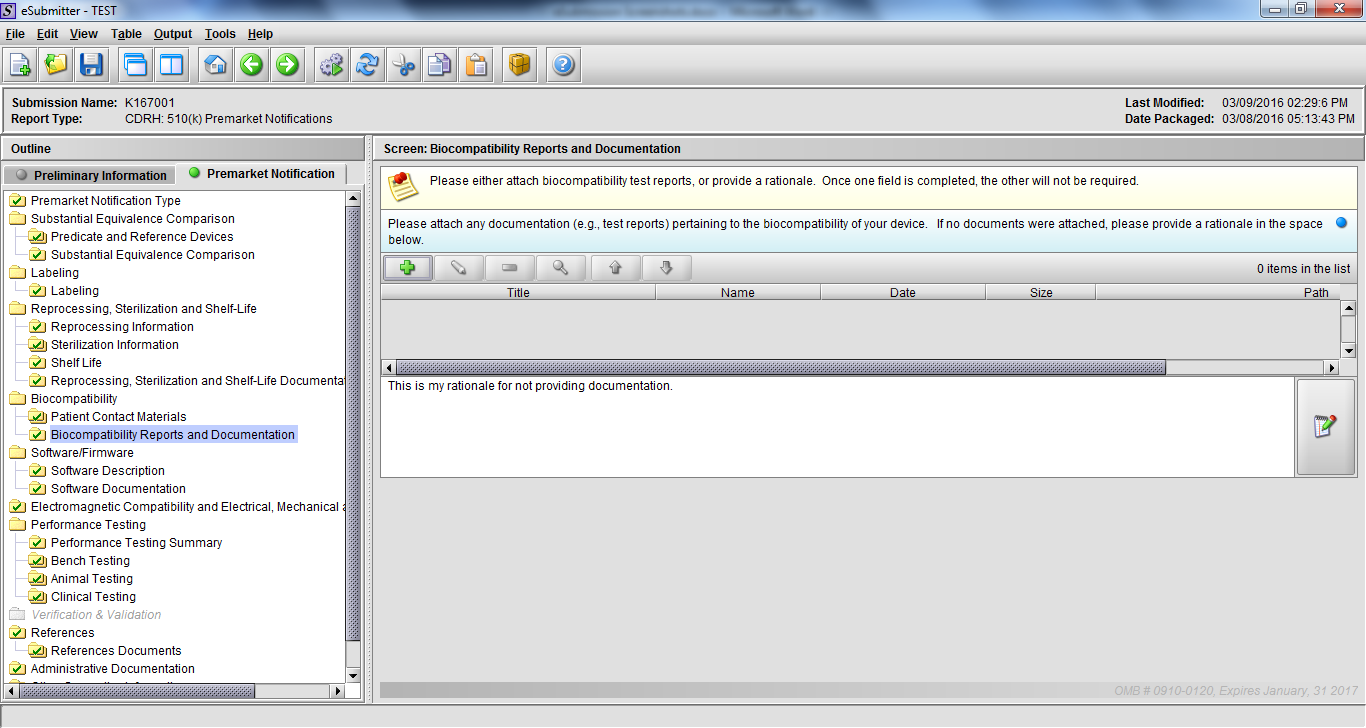
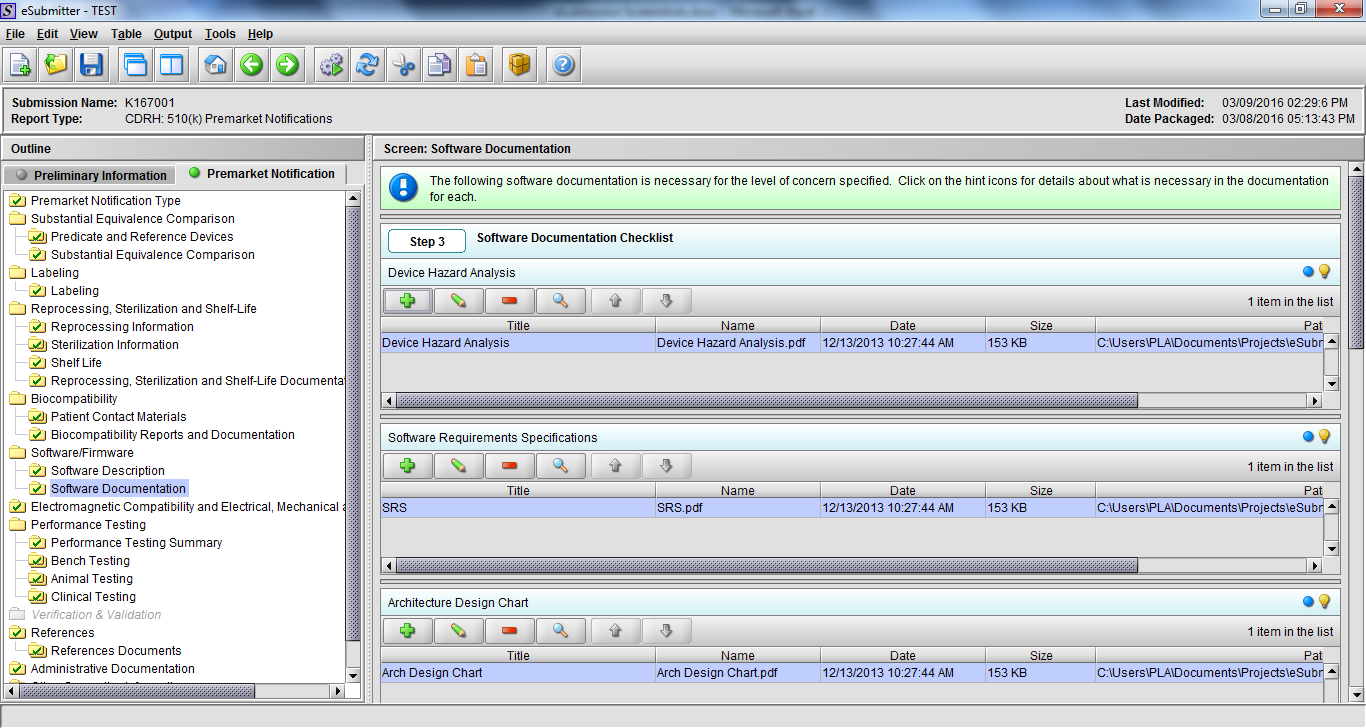
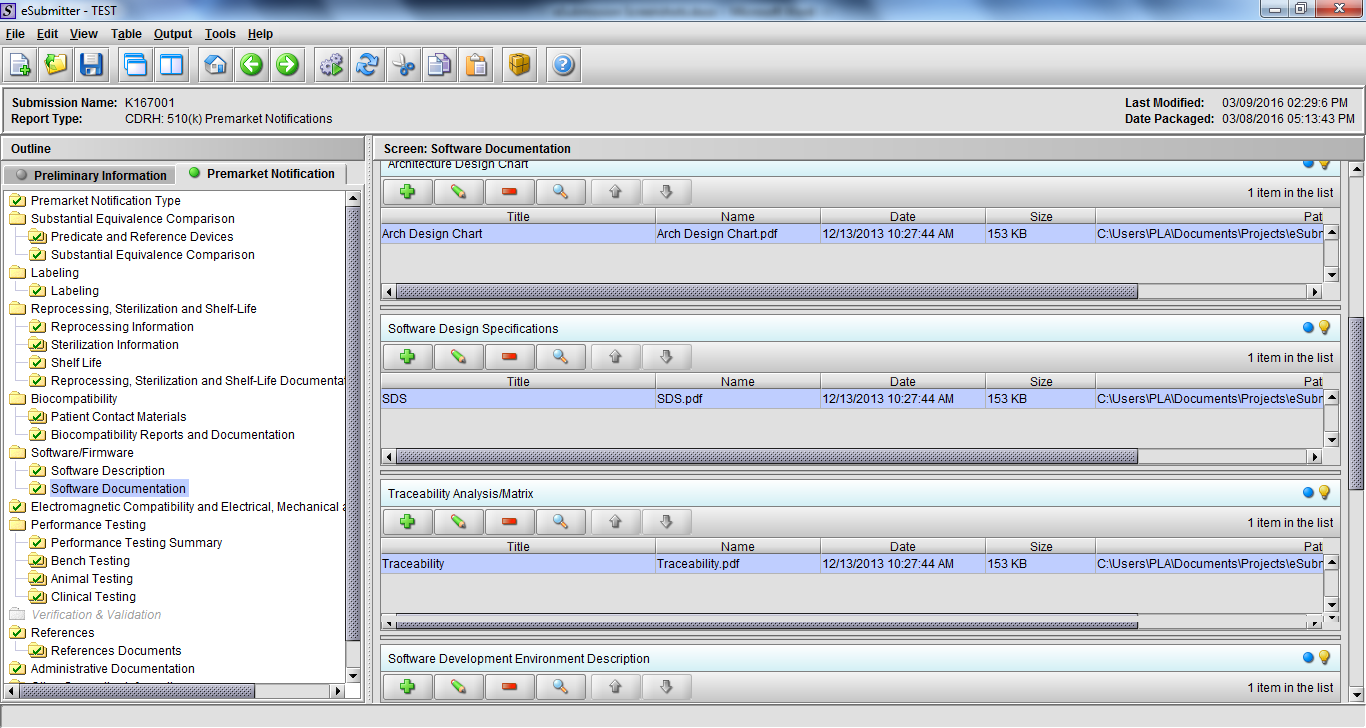
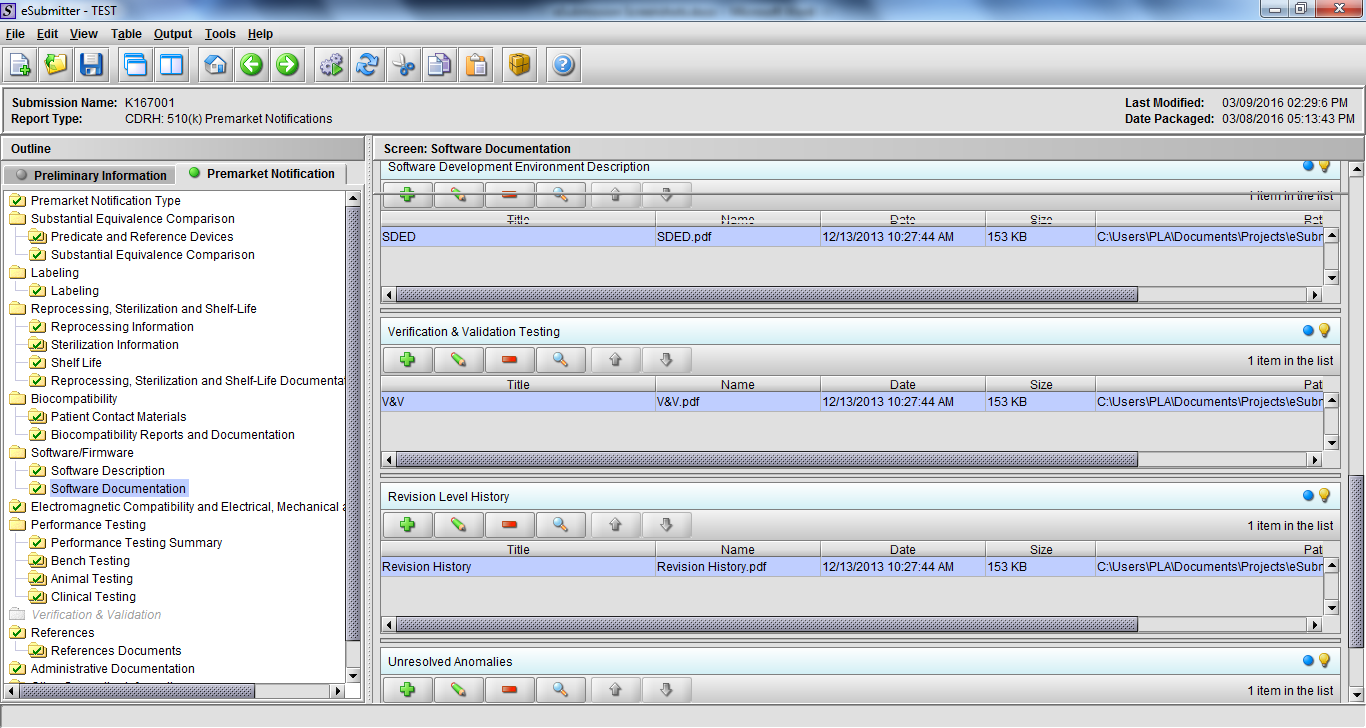
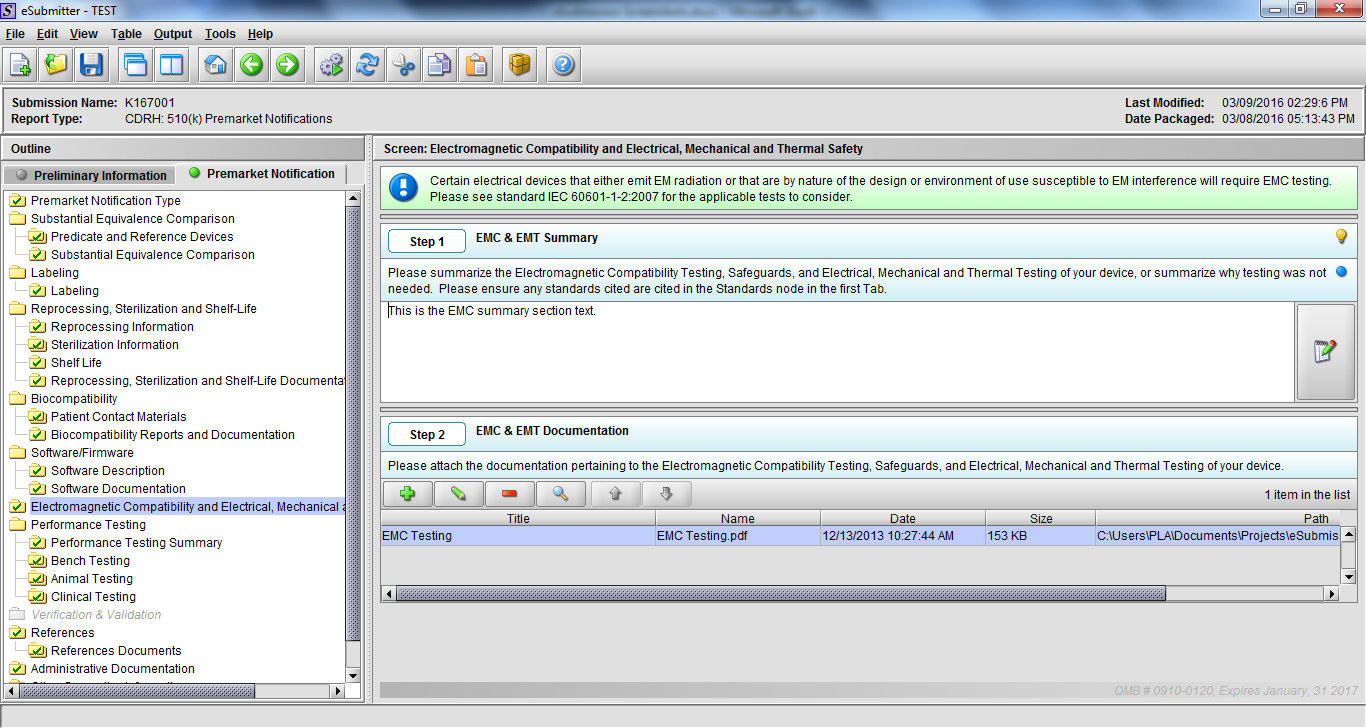
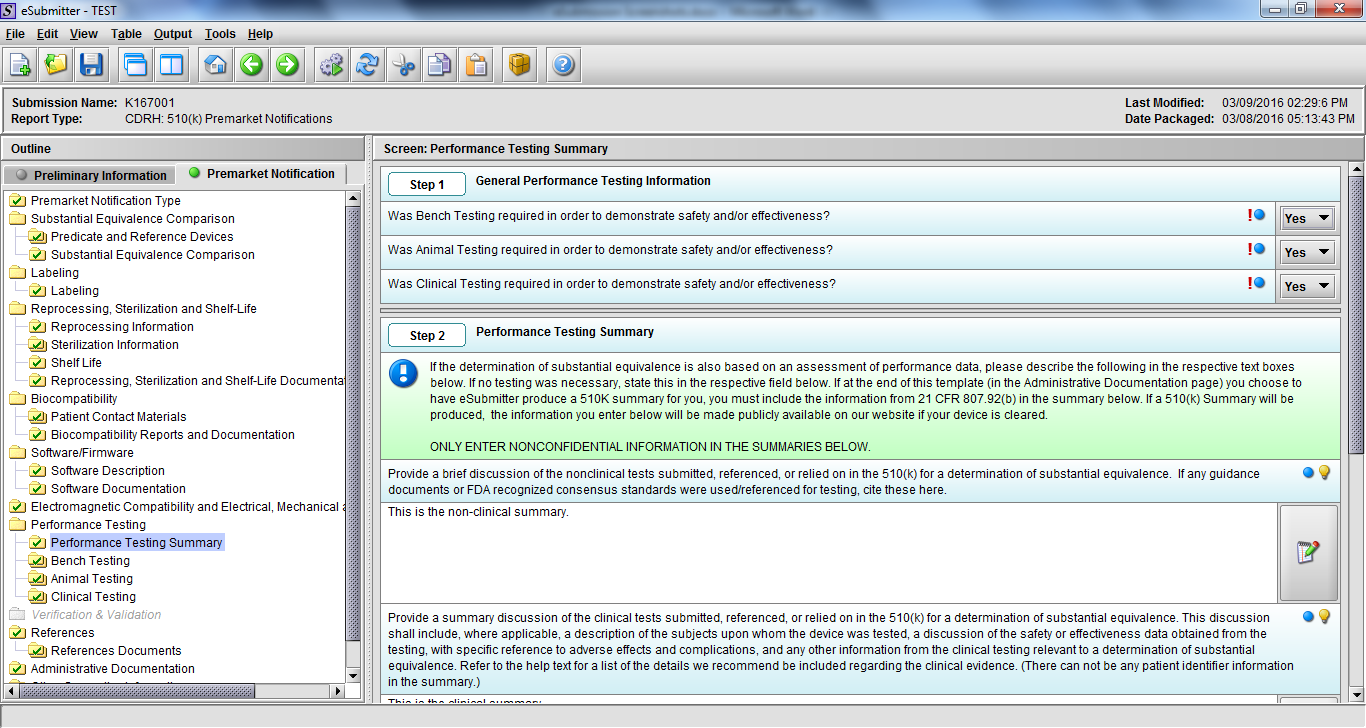
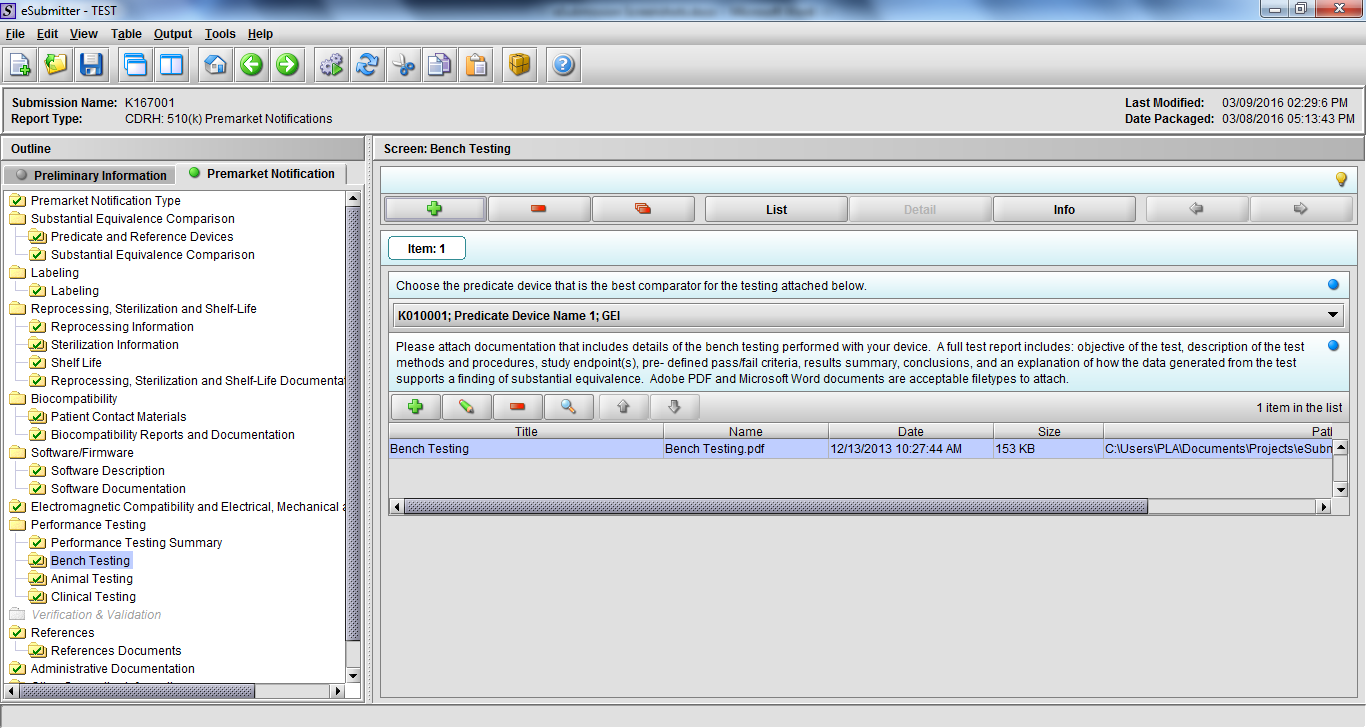
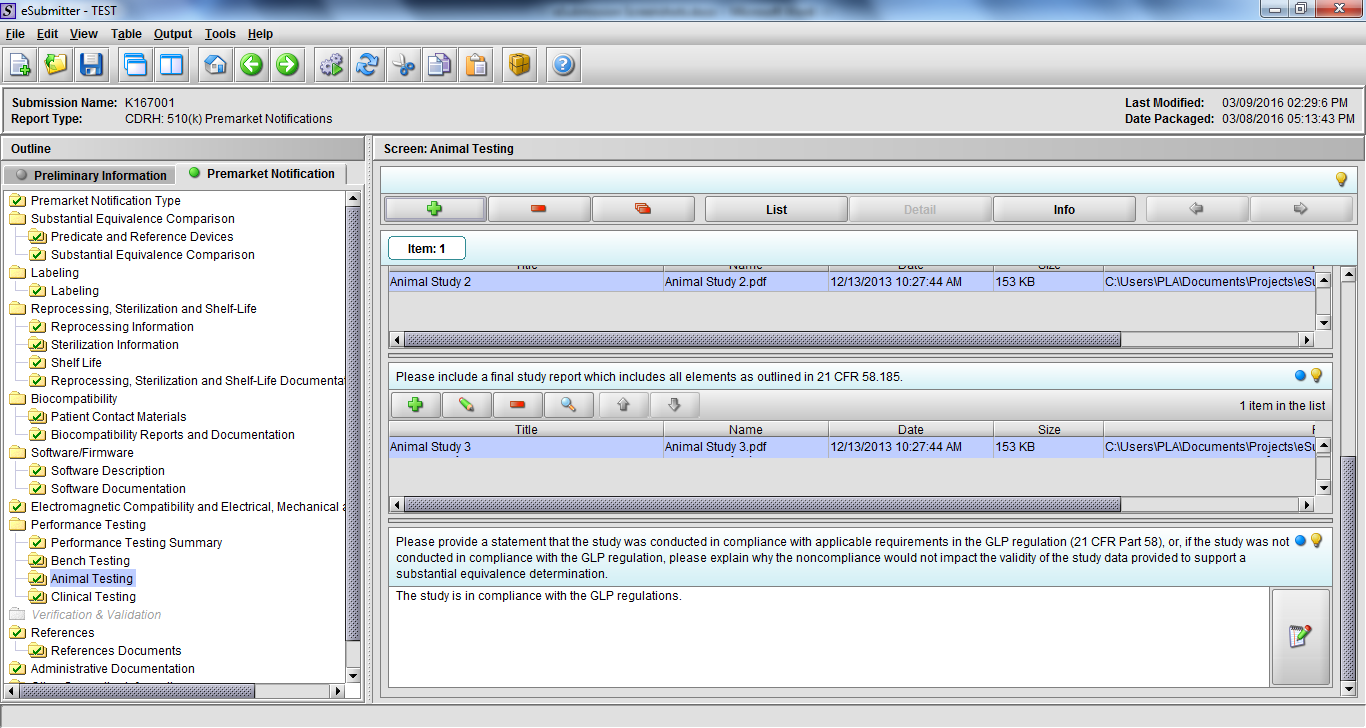
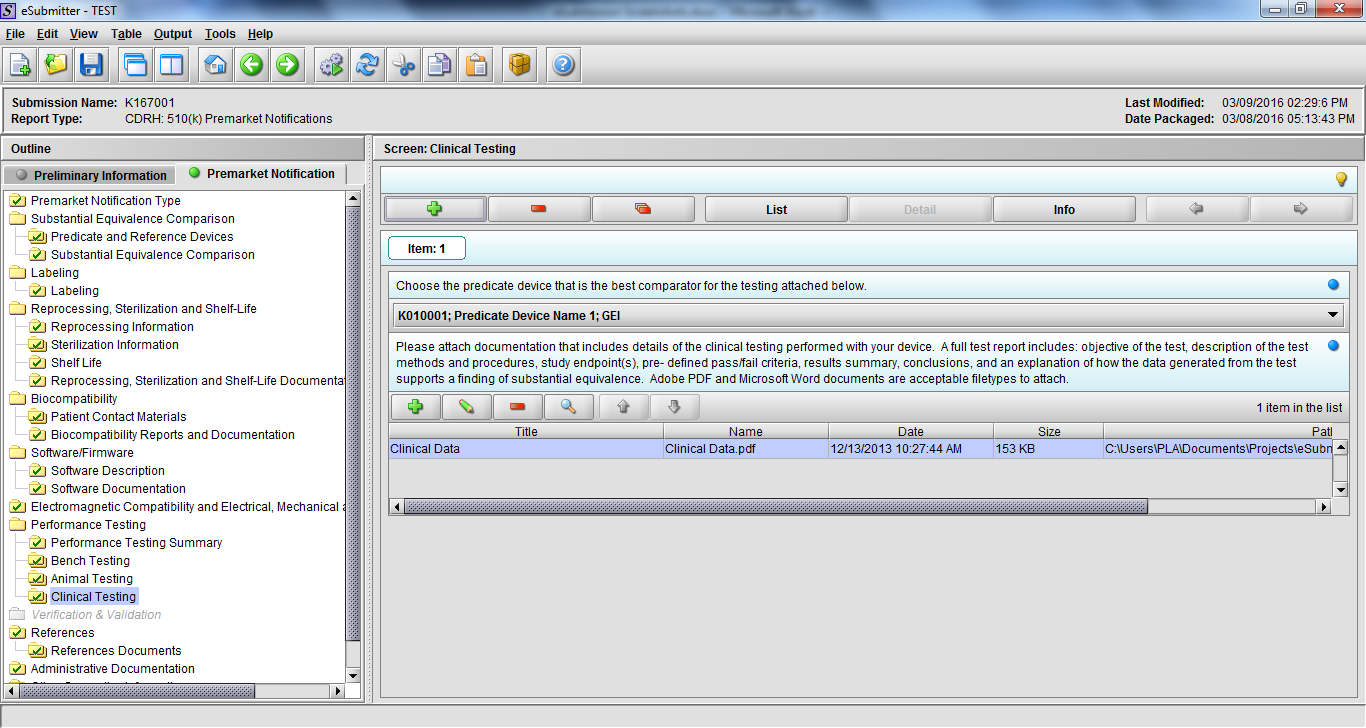
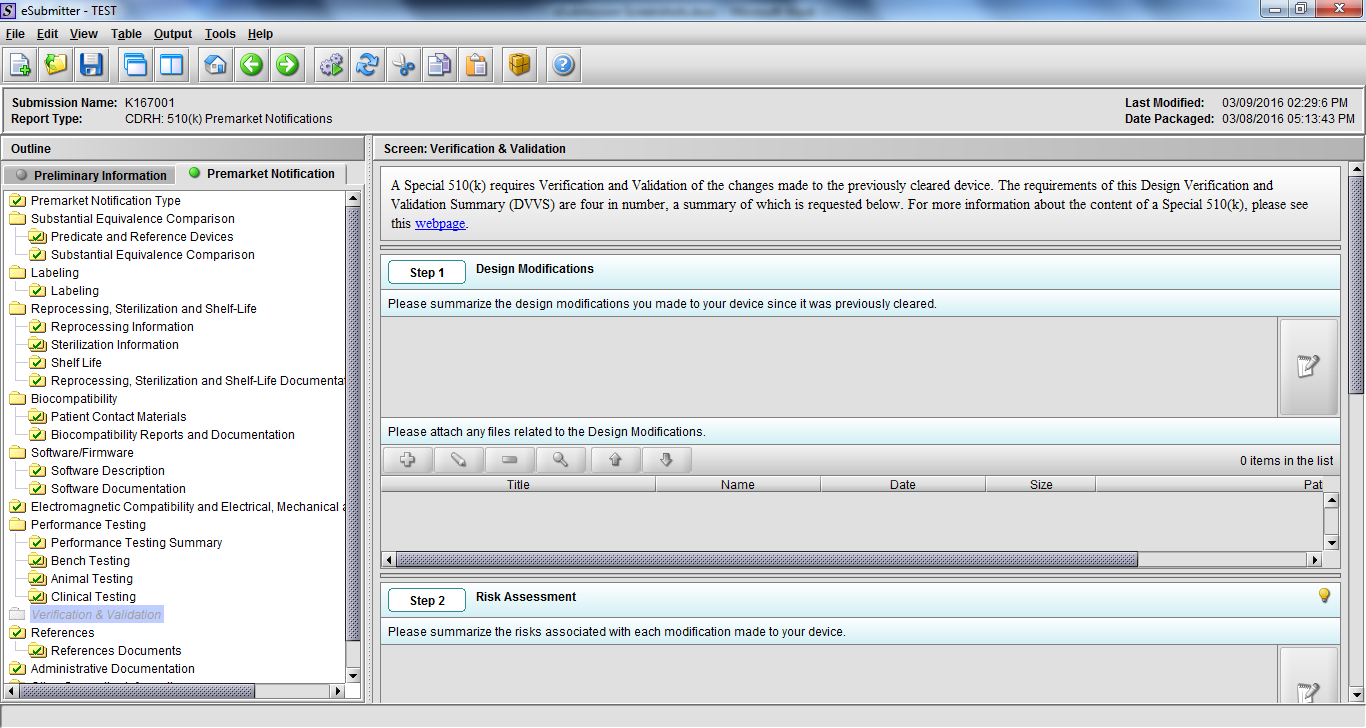
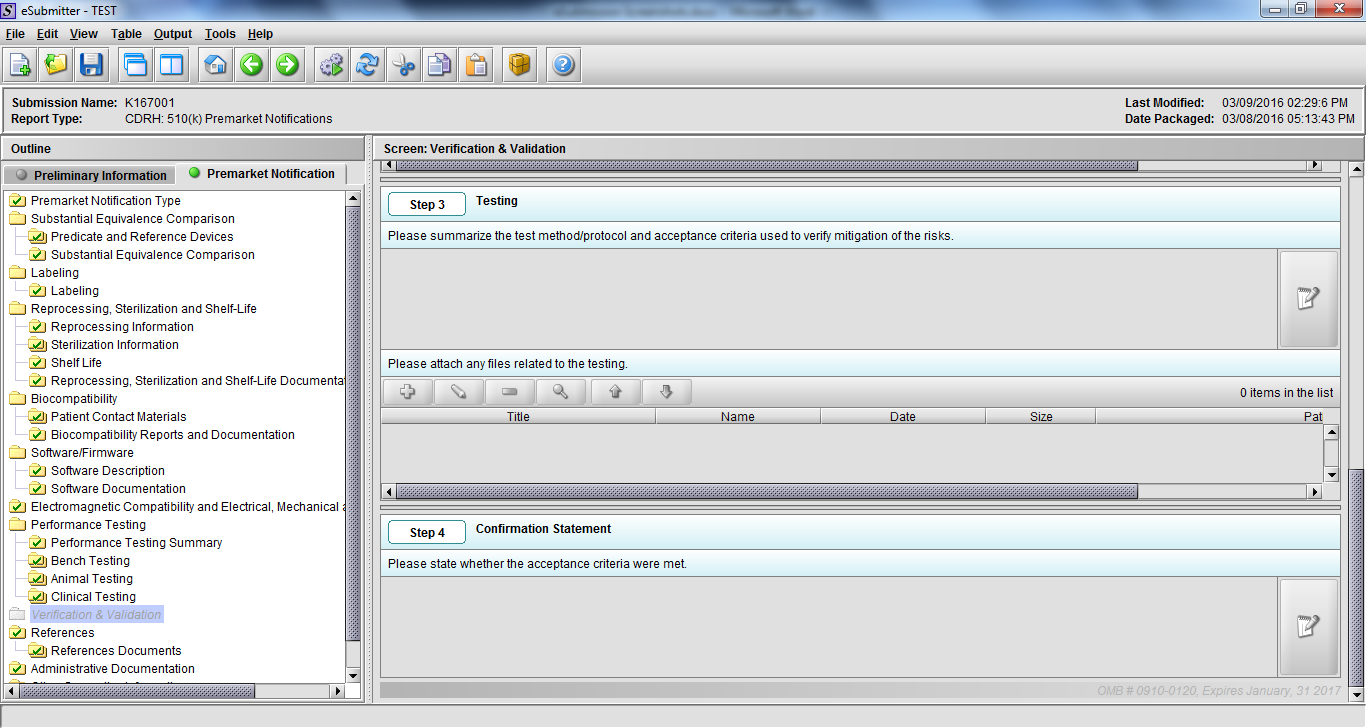
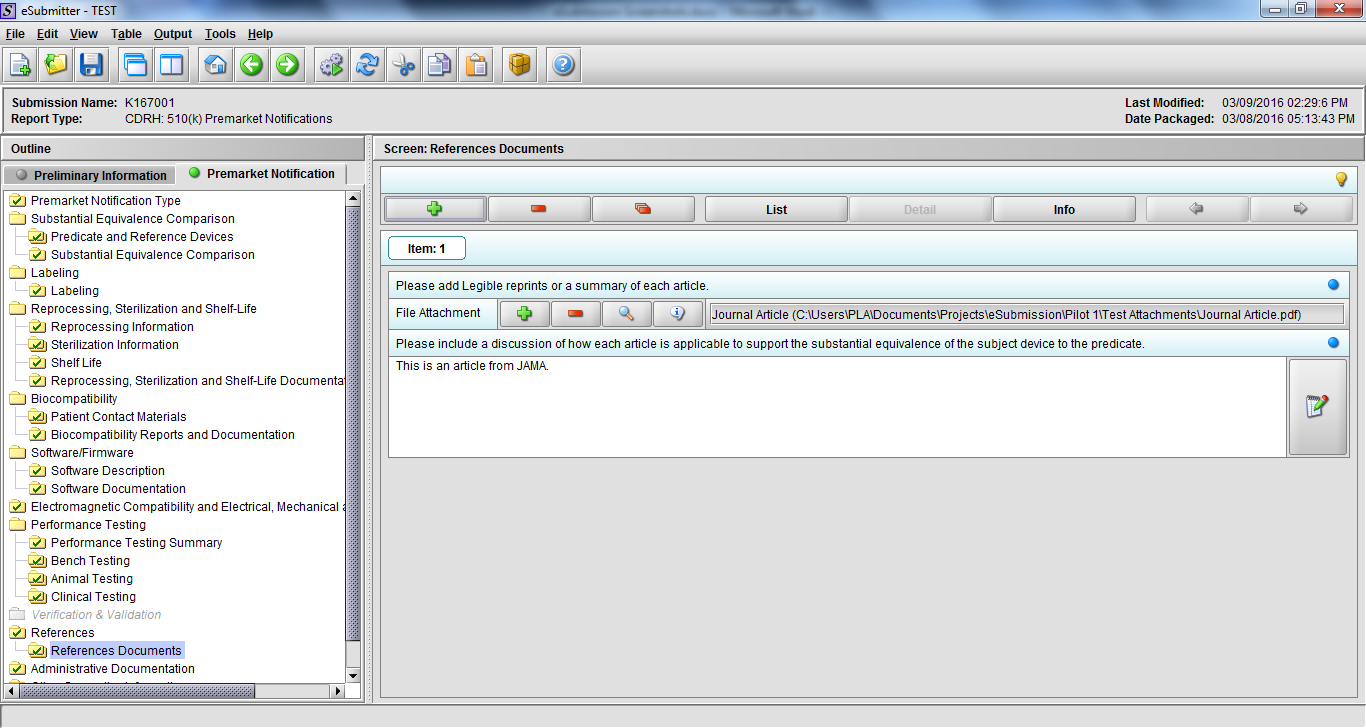
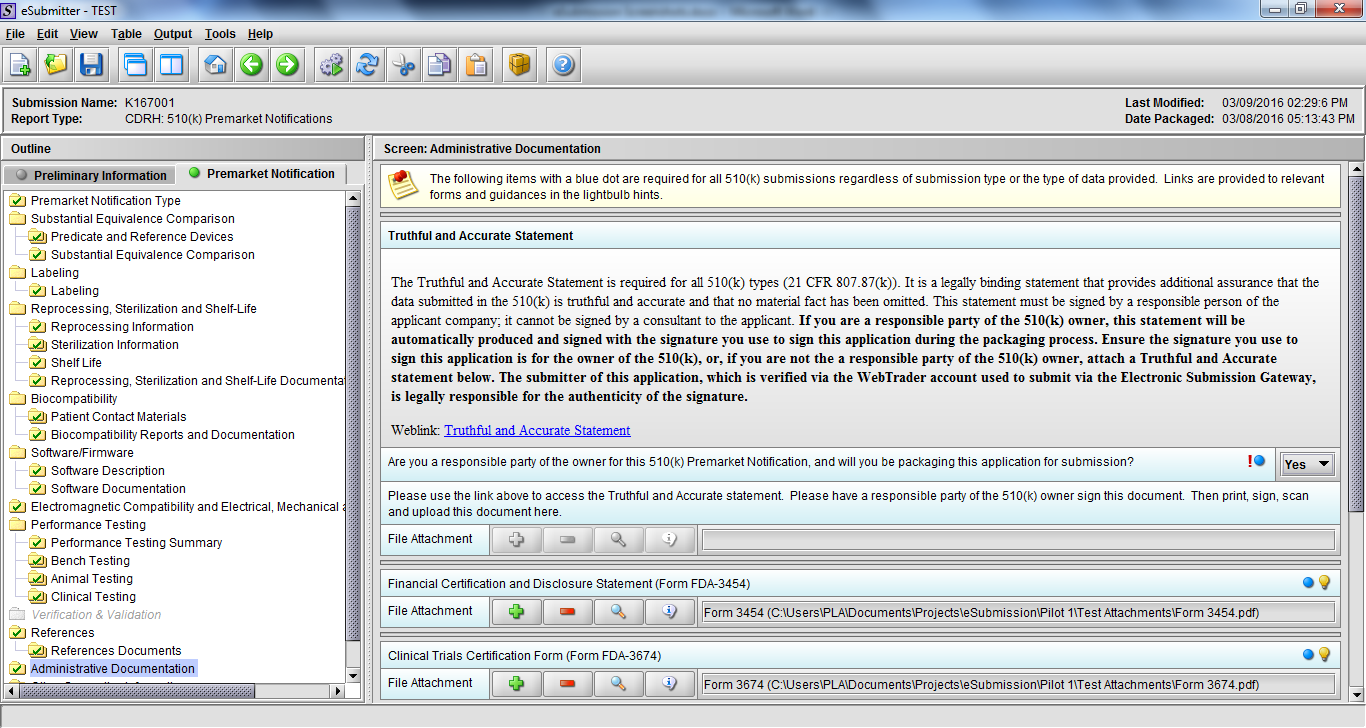
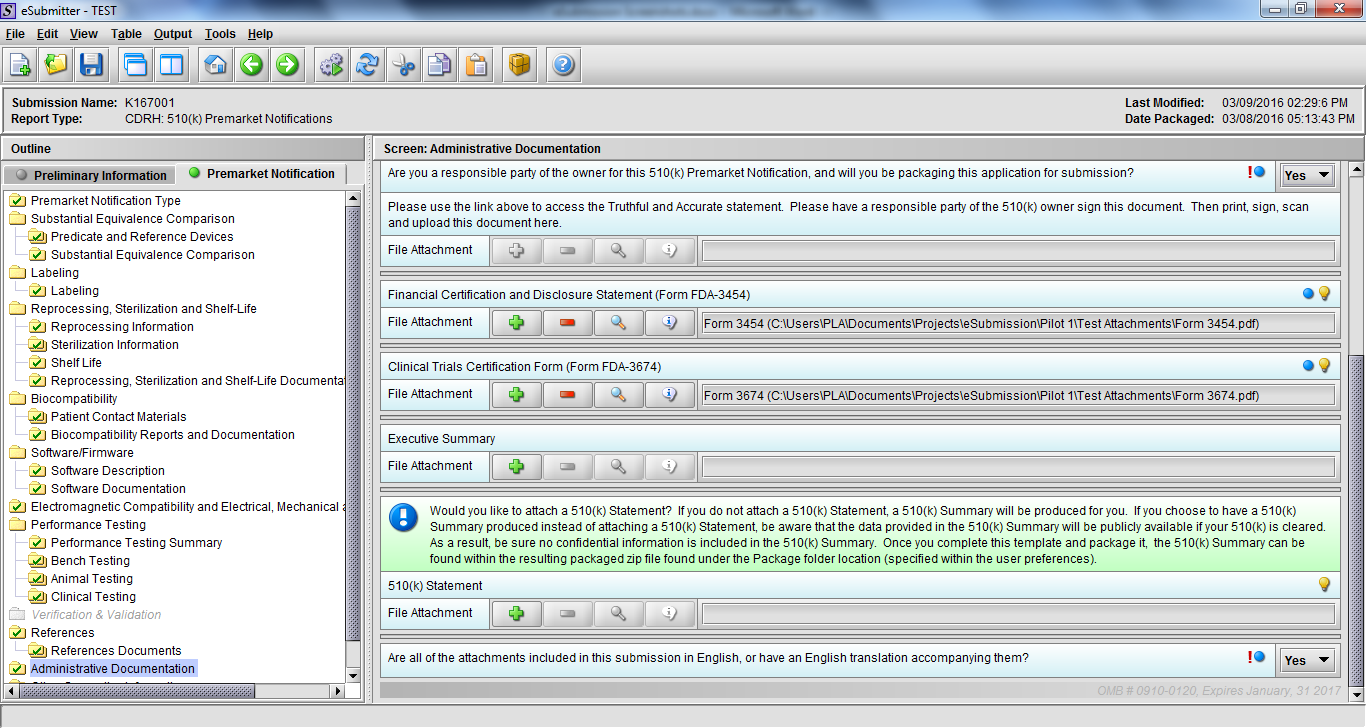
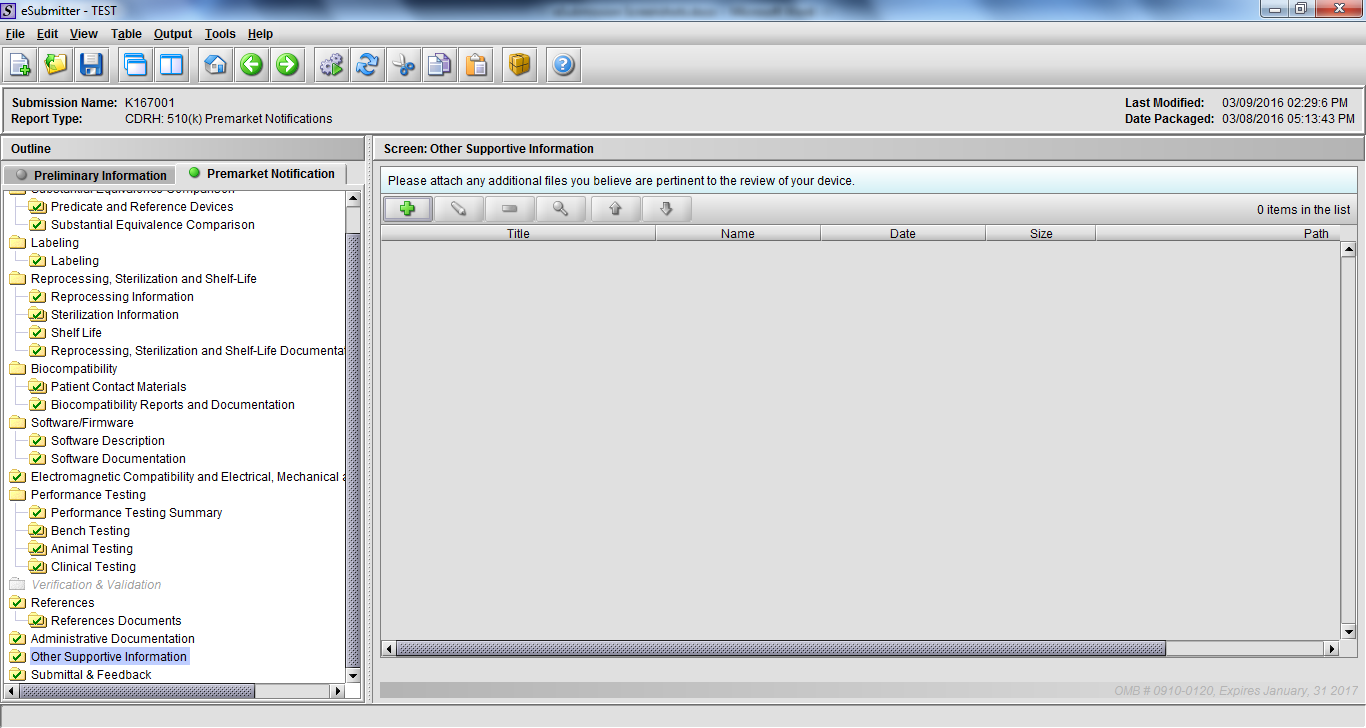
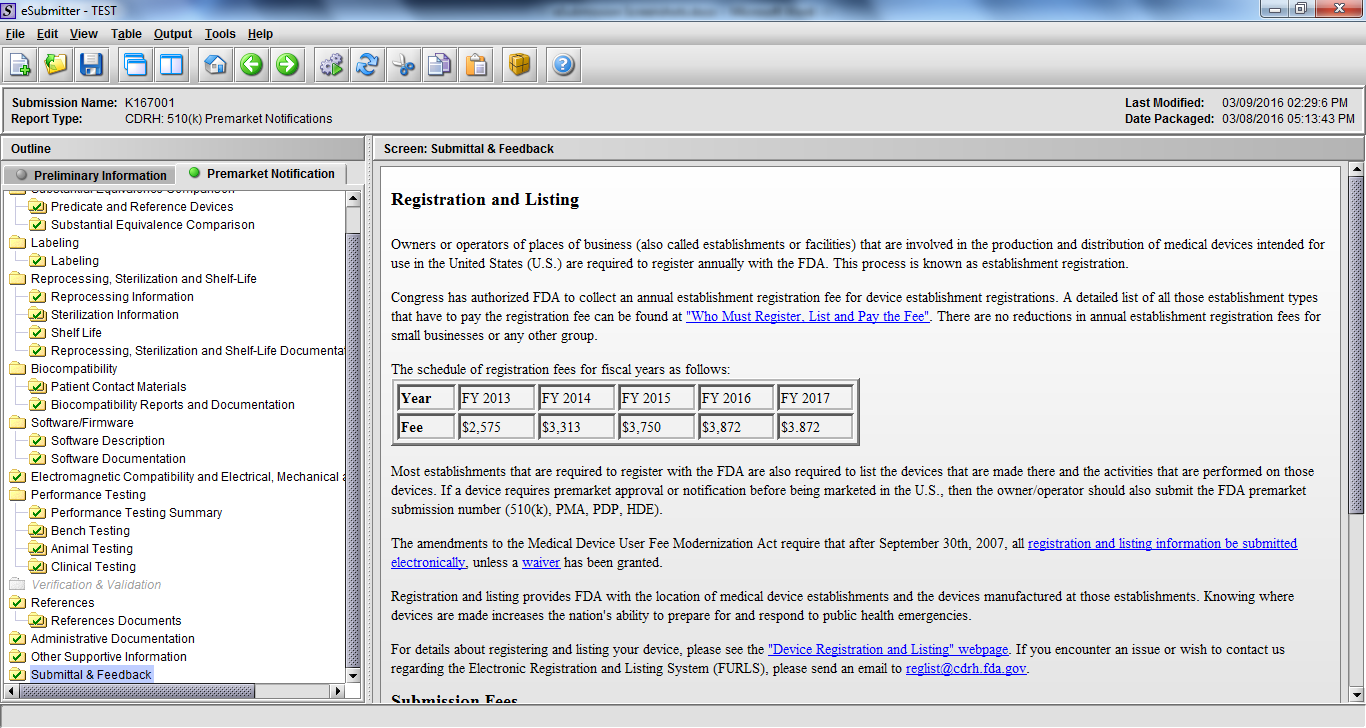
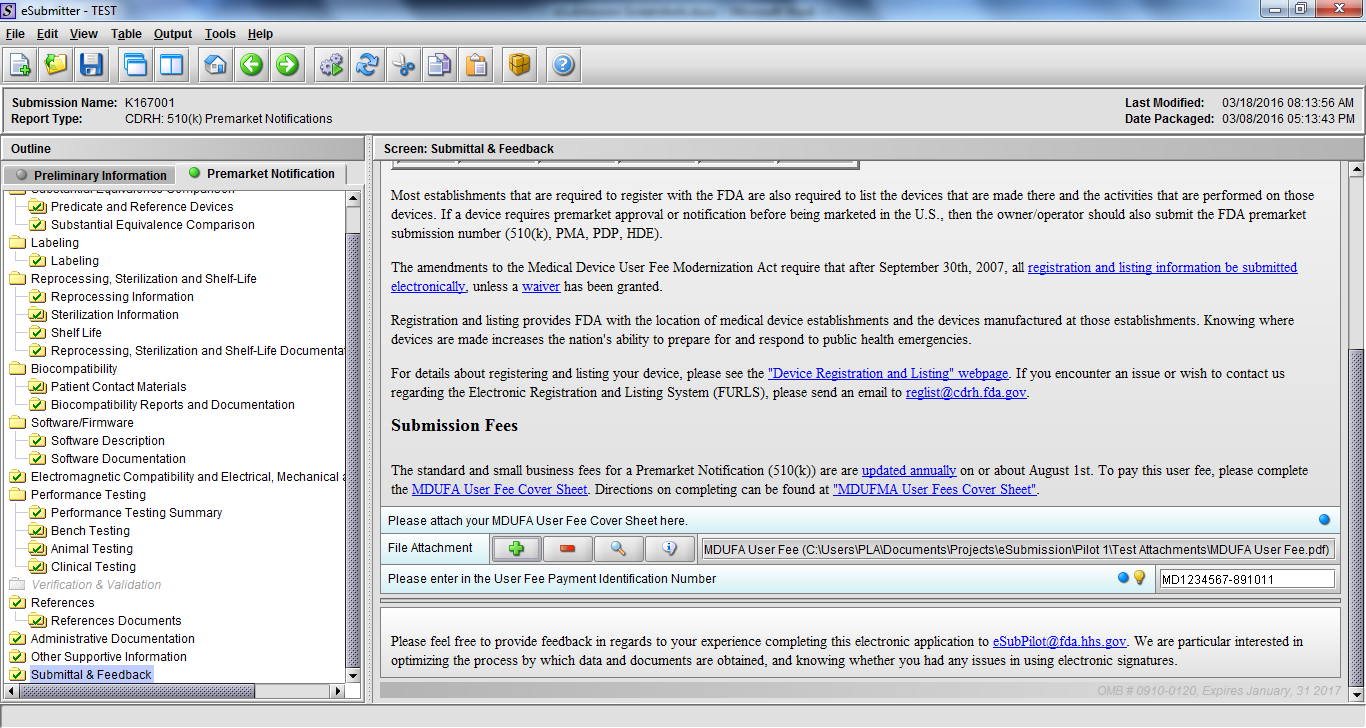
Note: Certain sections aren’t needed depending on how certain questions are answered. For example, the sterilization section, biocompatibility section, software section, etc aren’t necessary if the applicants indicate the device isn’t sterilized, doesn’t contact the patient, or doesn’t use software, respectively. The Verification and Validation section on page 25 – 26 below is only needed for Special 510(k)s. Other sections will also not be required depending on how certain questions are answered (e.g., labeling, performance testing, clinical testing, etc).







| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Axtell, Patrick L |
| File Modified | 0000-00-00 |
| File Created | 2021-01-24 |
© 2026 OMB.report | Privacy Policy