Cognitive Interview Screener
Hearing, Aging, and Direct-to-Consumer Television Advertisements
Appendix C Cognitive Interview Documents 092515
Cognitive Interview Screener
OMB: 0910-0818
Cognitive Interviews:
Telephone Screener
Introduction
Ask to speak to someone 18 years or older.
Hello, my name is _______________ and I’m from [name of company]. I’m calling on behalf of RTI International, a non-profit research organization, about a study being sponsored by a public health agency. We’re calling to recruit people to take part in a research study about advertisements. I’m not selling or promoting any product. RTI will be conducting interviews with several people, and we would like to see if you are eligible. We have various time slots available and will work with you to find a time that fits your schedule. To see if you are eligible, I’d like to ask you some questions. If you are eligible and choose to participate, all of your comments will be kept private and we will reimburse $75 at the end of the interview.
May I continue?
Yes CONTINUE
No [Thank respondent and end call.]
CORE ELIGIBILITY CRITERIA
Have you participated in any market research interviews such as a focus groups or one-on-one interview in the past three months?
-
Yes
TERMINATE
No
CONTINUE
What is your current occupation?
-
Healthcare Provider (e.g., Physician, Nurse, Counselor, Physical therapist) TERMINATE
Pharmaceutical Employee (e.g., Pharma Rep) TERMINATE
Market Research employee/Advertising employee TERMINATE
All Other Occupations (including student, unemployed, or retired) CONTINUE
How old are you?
-
17 YEARS OR YOUNGER
TERMINATE
18-25 YEARS
CONTINUE
26-39 YEARS
TERMINATE
40-49 YEARS
TERMINATE
50-59 YEARS
TERMINATE
60-74 YEARS
CONTINUE
75 YEARS OR OLDER
TERMINATE
*RECORD TO FILL QUOTAS*
What is your sex?
-
Male
CONTINUE
Female
CONTINUE
SCREEN FOR A MIX
What is the highest level of education you have attained?
-
Less than high school
CONTINUE
High school graduate (or GED)
CONTINUE
Some college or technical school (No degree)
CONTINUE
College graduate (2- or 4-year degree)
CONTINUE
Some graduate school (No degree)
CONTINUE
Graduate school degree
CONTINUE
SCREEN FOR A MIX
Which of these racial groups best describes you? [Read options below]
-
White
CONTINUE
Black / African American
CONTINUE
American Indian or Alaskan Native
CONTINUE
Asian
CONTINUE
Native Hawaiian or Pacific Islander
CONTINUE
Other
CONTINUE
SCREEN FOR A MIX
Have you ever been diagnosed with a hearing loss or hearing impairment?
-
Yes
CONTINUE
No
CONTINUE
Do you currently use or wear a hearing aid or other listening device?
-
Yes
CONTINUE
No
CONTINUE
Interview Invitation:
Thank you for answering all of my questions. Based on your responses, you appear eligible to participate in our study.
Each interview will last about 60 minutes and should be very interesting. The study involves reviewing a few advertisements on a computer and answering some questions about what you watched. If you normally wear or use a hearing aid or listening device, it is important that you bring it with you that day.
No one will try to sell you anything, and no one will call you later because you participated. We will reimburse you $75 at the end of the discussion for your time and participation. If it’s okay, we would like to record the discussion. Can I schedule your participation?
The interviews will take place on [DATES AND TIMES TBD]. Which date and time would work best for you?
Your participation in this study is very important. If for some reason you will not be able to attend, please let us know right away. You can call us anytime at [insert phone number], and if we are not here, please leave a message.
Closing for Ineligible Participants:
I’m sorry, but you are not eligible for this study. There are many possible reasons why people are not eligible. These reasons were decided earlier by the researchers. However, thank you for your interest in this study and for taking the time to answer our questions today.
Participant Information
NAME: ________________________________________________________
ADDRESS: ________________________________________________________
CITY: ________________________________________________________
ZIP CODE: ________________________________________________________
EMAIL ________________________________________________________
What is the best time to reach you? What is the best telephone number to reach you at that time?
BEST TIME TO BE REACHED: ________________________________________
BEST PHONE NUMBER: ________________________________________
Is there another time and number we can try if we miss you?
ALTERNATE PHONE NUMBER:
Recruiter: ____________________
Cognitive Interviews:
Informed Consent
Introduction and Purpose:
Thank you for agreeing to participate in this research study. The purpose of the study is to get feedback on survey questions about prescription drug advertisements to make sure that the questions are easy to understand. You will see an advertisement before looking at the survey questions.
RTI International, a non-profit research organization in North Carolina, is conducting the study. We will be conducting interviews in Raleigh, NC, and Baltimore-Washington, DC. You are one of approximately 18 people being asked to participate in this phase of the study.
Procedures:
If you agree to participate, you will watch an ad and answer some interview questions. During the interview, we will ask you to complete a survey asking about your reactions to, preferences for, and understanding of the ad. We will ask you to “think aloud” as you answer the survey questions. This helps us to test the survey and ensure that it is understandable. The ad viewing and interview will last approximately 1 hour.
Benefits:
There is no direct benefit to you for participating. However, you may find the interview to be informative or interesting.
Risks:
There are no known risks to participating in this study. While the survey questions we ask are not meant to be sensitive, there is always a chance that you may feel uncomfortable with some of the questions. You do not have to answer any question that you don’t want to answer.
Confidentiality:
We will try to keep the information you share in this study confidential. The study team will not disclose your name or any of your comments, and your personal information (name, address, phone number) will not be linked to any of your responses.
With your permission, we will audio-tape the interview portion of the study to supplement our notes. Recordings will not include full names and will be stored on password protected computers that only project staff can access. At the end of the project, we will destroy the recordings. All hardcopy forms will be kept in a locked file cabinet that only project staff can access.
Observation:
Some project staff may observe the interview portion of the study behind a one way mirror. They will not record your name and will keep all of your comments confidential.
Reimbursement:
In appreciation for your time and travel, we will reimburse you $75 at the end of the interview.
Right to Refuse or Withdraw:
Your participation in this study is voluntary. You can choose not to talk about any topic, and you can withdraw from the study for any reason at any time without penalty.
Persons to Contact:
If you have questions about the study, you can call the associate project director, Sarah Ray, at 1-800-334-8571, ext. 24934. She can be reached between 9:00 AM and 5:00 PM Eastern Time Monday to Friday. If you have questions about your rights as a participant, you can call RTI’s Office of Research Protection toll-free at 1-866-214-2043.
I had a chance to ask questions, and my questions were answered. I was given a copy of this consent form. I agree to participate in the study.
_________________________________ _________________
Signature of Participant Date
_________________________________ _________________
Signature of Person Obtaining Consent Date
Cognitive Interviews:
Thank you for agreeing to participate in this study today. During today’s interview, we will show you a television advertisement and ask you to complete a survey afterwards. Your feedback will help us to strengthen the survey.
[Note: Participants will be blind to FDA’s sponsorship]
This survey is being conducted by RTI International (RTI), an independent nonprofit research organization, on behalf of a government agency.
[PROGRAMMER: The OMB control number and expiration date should appear at the bottom of every screen. It should be as unobtrusive as possible.]
This study involves advertising for a new product. You will watch a television advertisement twice and then will be asked to answer the questions that follow.
To begin, please enter your participant ID in the following box.
Participant
ID

Interviewer: Note any confusion as the participant is reading and answering the participant ID question.
|
[NEW SCREEN]
Let’s go to the first set of instructions on the computer screen. Could you please read those instructions?
[PROGRAMMER: Begin playing audio recording of instructions as soon as the page opens. Play the instructions on a loop.]
[AUDIO FILE]
Make sure you are comfortable and can read the screen from where you sit. Because the survey will include some audio, we first want to be sure the sound on your computer is active and you can hear the advertisement.
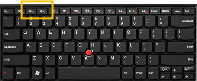
To adjust the volume, please use the laptop keyboard. There is an image of your laptop keyboard at the bottom of this page. You can scroll down to see it. The yellow box on the image of the keyboard shows where the volume keys are located.
To adjust the volume “up,” use the + key. To adjust the volume “down,” use the – key.
Please scroll down to the bottom of this screen and answer the volume question.
.

Q1. As you adjust the volume, you will see a number bar on your screen. When the volume is at a comfortable level, please record the volume number in the box below.
Volume number

I do not want to change the volume
Cognitive Testing Probes
Interviewer: Note any confusion as the participant is reading the instructions and answering the question. |
[NEW SCREEN]
The study will take about 20 minutes to complete. We ask you to complete the study in one sitting (without taking any breaks) in order to avoid distractions.
On the next screen, you will see the television advertisement. The ad may take 15-30 seconds to start playing.
Now I would like you to view the ad.
[SHOW THE AD ONCE]
[NEW SCREEN]
We would like you to watch the ad a second time. Please click the Next button to view the ad.
[SHOW THE AD A SECOND TIME]
[NEW SCREEN]
Q2. Did you change the volume after seeing the ad?
Turned volume up
Turned volume down
Did not change volume
Interviewer: Note any confusion as the participant is reading the instructions and answering the question. |
COGNITIVE TESTING INSTRUCTIONS:
Now that you have viewed the ad, I would like to ask you some questions about it.
What did you think about seeing the ad at the beginning of this survey?
Did it seem abrupt or natural to view the ad right after you started?
What were your general impressions of that ad?
How did the ad compare to other ads you have seen for prescription medications?
Was it similar or different?
Was the ad believable?
Is there anything about the ad you would change?
COGNITIVE TESTING INSTRUCTIONS:
Now I’d like to ask you to answer some survey questions.
As we review the survey, I’d like you to read the instructions and questions aloud and then “think aloud” as you answer each question. This may feel a little unnatural, but it will help us to understand how you think about and answer each question. Here’s an example:
Question: How many times did you go to a doctor’s office for a scheduled appointment in the past three months?
Answer: Well, I see my heart doctor every month, so that’s three visits. I see my primary care doctor twice a year, but I didn’t go in the last three months. I also had to go to urgent care last week for a sinus infection…but that wasn’t a scheduled appointment. I guess my answer is three visits.
After each survey question, I also may ask you some follow-up questions. We are interested in your initial impressions and honest opinions. There are no right or wrong answers, and it is ok to have strong opinions. Please feel free to use the entire range of response options. Let’s begin…
[MAIN MESSAGE RECALL – GIST MEMORY]
Q3. What was the main message of the ad you saw?
[OPEN-ENDED RESPONSE]
Cognitive Testing Probes
|
[CONFIDENCE IN MEMORY JUDGMENTS – MAIN MESSAGE RECALL]
Q4. How confident are you that you were able to correctly remember the main message of the ad?
0% confident
25% confident
50% confident
75% confident
100% confident
Cognitive Testing Probes
|
[BRAND RECOGNITION – VERBATIM MEMORY]
Q5. Which of the following drugs did you see advertised?
[PROGRAMMER: RANDOMIZE ORDER, BUT KEEP “NONE OF THE ABOVE” AT THE END OF THE LIST]
- GILARIX
- PEXACOR
- VOTREA
- None of the above
Cognitive Testing Probes
|
[RECALL OF RISKS – GIST MEMORY]
The ad you saw was about a prescription drug named VOTREA.
Q6. Based on the ad, what are the side effects of VOTREA? Please list as many side effects as you can remember.
[OPEN-ENDED RESPONSE]
Cognitive Testing Probes
|
[CONFIDENCE IN MEMORY JUDGMENTS – RISKS RECALL]
Q7a. How confident are you that you were able to correctly remember the side effects of VOTREA?
Not at all confident
Slightly confident
Moderately confident
Very confident
Extremely confident
Q7b. Using a different scale, how confident are you that you were able to correctly remember the side effects of VOTREA?
0% confident
25% confident
50% confident
75% confident
100% confident
Cognitive Testing Probes
|
[RECALL OF BENEFITS – GIST MEMORY]
Q8. Based on the ad, what are the benefits of VOTREA? Please list as many benefits as you can remember.
[OPEN-ENDED RESPONSE]
Cognitive Testing Probes
|
[CONFIDENCE IN MEMORY JUDGMENTS – BENEFITS RECALL]
Q9. How confident are you that you were able to correctly remember the benefits of VOTREA?
0% confident
25% confident
50% confident
75% confident
100% confident
Cognitive Testing Probes
|
[PERCEIVED RISK – LIKELIHOOD AND MAGNITUDE]
Please answer the following questions to the best of your ability, even if you have never taken the drug.
Q10. In your opinion, if 100 people take VOTREA, how many will have any side effects? Please enter a number in the box below.
__ people
Cognitive Testing Probes
|
Q11. In your opinion, if VOTREA did cause you to have side effects, how serious would they be?
1 2 3 4 5 6
Not at all Very
serious serious
Cognitive Testing Probes
|
[BENEFIT RECALL]
Please answer the following questions to the best of your ability, even if you have never taken the drug.
Q12. In your opinion, if 100 people take VOTREA, for how many will the drug work? Please enter a number in the box below.
__ people
Cognitive Testing Probes
|
[PERCEIVED EFFICACY – MAGNITUDE]
Q13. In your opinion, if you took VOTREA, how effective do you think VOTREA would be in helping to lower your cholesterol?
1 2 3 4 5 6
Not at all Very
effective effective
Cognitive Testing Probes
|
[CLAIM RECOGNITION – VERBATIM MEMORY]
INTERVIEWER – If the participant sees an ad with a “simple” voiceover then have them answer the first question (Q14a) and skip the second question (Q14b).
If the participant sees an ad with a “complex” voiceover then skip the first question (Q14a) and have them answer the second question (Q14b).
[PROGRAMMER: IF COMPLEXITY CONDITION = SIMPLE, SHOW Q14A ONLY. IF COMPLEXITY CONDITION = COMPLEX, SHOW Q14B ONLY]
The next few questions ask about information that may or may not have been in the advertising. Please answer each question, even if you do not remember the information.
[SIMPLE]
Q14a. Which of the following claims, if any, were in the ad you saw? Check all that apply.
[PROGRAMMER: RANDOMIZE ORDER, BUT KEEP “NONE OF THE ABOVE” AT THE END OF THE LIST]
[CORRECT] VOTREA reduces bad cholesterol for people with several common risk factors for heart disease.
[CORRECT] Women who could become pregnant should not take VOTREA.
[CORRECT] TTP can occur within two weeks of taking VOTREA.
[CORRECT] Muscle pain is a rare but serious side effect of taking VOTREA.
[FOIL] Blurry vision is a side effect of taking VOTREA.
[FOIL] People with kidney problems should not take VOTREA.
[FOIL] None of the above
Cognitive Testing Probes
|
[COMPLEX]
Q14b. Which of the following claims, if any, were in the ad that you saw? Check all that apply.
[PROGRAMMER: RANDOMIZE ORDER, BUT KEEP “NONE OF THE ABOVE” AT THE END OF THE LIST]
[CORRECT] VOTREA reduces bad cholesterol for people with several common risk factors for heart disease.
[CORRECT] VOTREA is not for people who could become pregnant.
[CORRECT] TTP can occur within two weeks of taking VOTREA.
[CORRECT] A rare but serious side effect of taking VOTREA is muscle pain.
[FOIL] Blurry vision is a side effect of taking VOTREA.
[FOIL] People with kidney problems should not take VOTREA.
[FOIL] None of the above
Cognitive Testing Probes
|
[CONFIDENCE IN MEMORY JUDGMENTS – CLAIM RECOGNITION]
Q15. How confident are you that you were able to correctly remember the claims in the ad?
0% confident
25% confident
50% confident
75% confident
100% confident
Cognitive Testing Probes
|
[CONFIDENCE IN COMPREHENSION JUDGMENTS – CLAIM RECOGNITION]
Q16. How confident are you that understood the claims in the ad?
0% confident
25% confident
50% confident
75% confident
100% confident
Cognitive Testing Probes
|
[OVERALL AD COMPREHENSION]
Q17. Which of the following choices best summarizes the information from the ad?
[PROGRAMMER: RANDOMIZE RESPONSE OPTIONS]
[CORRECT] VOTREA is a treatment for bad cholesterol, but not all people with bad cholesterol should take VOTREA. VOTREA has both common and uncommon side effects.
[FOIL] VOTREA is a treatment for bad cholesterol, but serious side effects from taking VOTREA are common. If you are taking VOTREA, it is necessary to see your doctor regularly to monitor the serious side effects.
[FOIL] VOTREA is a treatment for bad cholesterol, but if you have liver disease, you should get blood tests to check your liver while taking VOTREA.
[FOIL] VOTREA is a treatment for bad cholesterol. VOTREA has some side effects, but none of the side effects are life threatening.
Cognitive Testing Probes
|
[CONFIDENCE IN COMPREHENSION JUDGMENTS – CLAIM COMPREHENSION]
Q18a. How confident are you that you understood the information in the ad?
0% confident
25% confident
50% confident
75% confident
100% confident
Q18b. On a scale from 1 to 5, how confident are you that you understood the information in the ad?
1 2 3 4 5
Not at all Extremely
confident confident
Cognitive Testing Probes
|
[INTENTION FOR DRUG USE]
Q19. Based on the ads, please rate how likely or unlikely you are to do the following behaviors.
|
1 Not at all likely |
2 |
3 |
4 |
5 |
6 Extremely Likely |
b. Ask your doctor to prescribe VOTREA |
|
|
|
|
|
|
e. Take VOTREA if your doctor prescribed it |
|
|
|
|
|
|
Cognitive Testing Probes
|
[ATTITUDE TOWARD USING DRUG]
Q20. Please tell us how you feel about using VOTREA. Mark the number that most closely indicates your response.
Using VOTREA would be…
|
|
1 |
2 |
3 |
4 |
5 |
6 |
|
A |
Bad |
|
|
|
|
|
|
Good |
B |
Not useful |
|
|
|
|
|
|
Useful |
Cognitive Testing Probes
|
[PERCEIVED ATTENTION TO AD]
Q21. How much attention did you pay to the ad you saw about VOTREA?
None
Very little
Some
Quite a bit
A great deal
Cognitive Testing Probes
|
[COGNITIVE ABILITY – SELF-REPORTED]
Q22. How would you rate your ability to think quickly at the present time? Would you say your ability is…
Excellent
Very good
Good
Fair
Poor
Cognitive Testing Probes
|
Q23. How would you rate your memory at the present time? Would you say your memory is…
Excellent
Very good
Good
Fair
Poor
Cognitive Testing Probes
|
[NEW SCREEN]
Next you will see instructions for a new task.
[NEW SCREEN]
[COGNITIVE ABILITY LETTER DIGIT SUBSTITUTION TASK]
Please look at the key below. This key includes pairs of letters and numbers. For example “W” and “1” are a pair, “B” and “2” are a pair, and so on.
KEY
W |
B |
T |
P |
V |
D |
G |
C |
J |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
Use the key as a guide to fill in each blank square with the number that pairs with the letter above it. Try a practice round:
T |
W |
C |
G |
J |
V |
B |
D |
P |
V |
|
|
|
|
|
|
|
|
|
|
Cognitive Testing Probes
|
[NEW SCREEN]
KEY
W |
B |
T |
P |
V |
D |
G |
C |
J |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
Over the next few screens you will see the same key and more blank squares. Continue to use the key as a guide to fill in each blank square with the number that pairs with the letter above it. You will be timed. Fill in as many squares as you can in 30 seconds.
When you are ready, click “Next” and the timer will begin.
[CLICK TO NEXT SCREEN]
[PROGRAMMER: This page should have a 30 second timer (that the participant cannot see). At the end of 30 seconds, advance to the next screen automatically.]
KEY
W |
B |
T |
P |
V |
D |
G |
C |
J |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
Q24. Fill in the blank squares using the key.
P |
D |
V |
B |
T |
D |
P |
W |
B |
J |
D |
T |
C |
V |
G |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
J |
P |
W |
C |
B |
V |
J |
D |
P |
C |
G |
W |
T |
B |
V |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
T |
G |
V |
B |
P |
W |
C |
V |
D |
J |
W |
J |
G |
D |
C |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
G |
T |
J |
C |
W |
C |
G |
D |
J |
P |
B |
V |
T |
C |
B |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[AUTOMATICALLY MOVE TO NEXT SCREEN]
Time is up. On the next screen, you will have 30 seconds to do a similar task.
When you are ready, click “Next” and the timer will begin.
[CLICK TO NEXT SCREEN]
[PROGRAMMER: This page should have a 30 second timer (that the participant cannot see). At the end of 30 seconds, advance to the next screen automatically.]
KEY
W |
B |
T |
P |
V |
D |
G |
C |
J |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
Q25. Fill in the blank squares using the key.
W |
P |
G |
V |
B |
J |
C |
P |
T |
C |
G |
W |
J |
D |
V |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
J |
P |
G |
D |
G |
B |
J |
C |
W |
V |
T |
B |
D |
T |
W |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
T |
V |
G |
W |
D |
P |
V |
D |
B |
J |
G |
T |
J |
P |
B |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
W |
C |
T |
V |
P |
B |
J |
G |
W |
D |
V |
C |
T |
P |
G |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
P |
T |
D |
C |
B |
|
|
|
|
|
[AUTOMATICALLY MOVE TO NEXT SCREEN]
Time is up. Click next to continue with the rest of the survey.
Cognitive Testing Probes
|
[SUBJECTIVE HEALTH LITERACY]
Q26. How often do you have problems learning about any of your medical conditions because of difficulty understanding written information?
Never
Rarely
Sometimes
Often
Always
Cognitive Testing Probes
|
[ILLNESS DIAGNOSIS]
Q27. Have you ever been diagnosed with high cholesterol by a physician or other qualified medical professional?
Yes
No
Don’t know
INTERVIEWER: Note the skip question here.
[PROGRAMMER NOTE: IF Q27=YES, ASK Q28. OTHERWISE, SKIP TO Q31]
[TIME SINCE TARGETED CONDITION DIAGNOSIS]
Q28. When did a healthcare professional first tell you that you had high cholesterol?
Six months ago or less
More than six months ago but less than a year ago
A year ago or more but less than 5 years
Five years ago or longer
[REPORTED IMPACT OF HIGH CHOLESTEROL]
Q29. How much does having high cholesterol affect your daily activities?
1 2 3 4 5 6
Not at all A great
deal
Cognitive Testing Probes
|
[CURRENT PRESCRIPTION STATUS]
Q30. Are you currently taking, or have you ever taken, any prescription drugs to lower your cholesterol?
Currently taking
Have taken in the past, but not currently taking
Have never taken
[FALSE AD RECOGNITION TENDENCY]
Q31. Have you ever seen any advertising for VOTREA before today?
Yes
No
Not sure
[GENERAL PERCEPTION OF DRUG ADVERTISING]
Q32. In general, how do you feel about ads on television for prescription drugs? Would you say the ads are…
1 2 3 4 5 6
Not at all Very useful
useful
Cognitive Testing Probes
|
[SELF-REPORTED HEARING LOSS]
Q33. Do you feel you have a hearing loss?
Yes
No
Don’t know
Cognitive Testing Probes
|
Q34. Do you need the television volume turned up loud when you are watching the television?
Yes
No
Cognitive Testing Probes
|
[SELF-REPORTED SPEECH RECOGNITION]
The following questions ask about your ability and experience hearing and listening in different situations. On a scale from 0 to 10, how well can you follow along in each situation?
|
Not at all |
|
|
|
|
|
|
|
|
|
Perfectly |
0 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
Q35a. You are talking with one other person and there is a TV on in the same room. Without turning the TV down, can you follow that the person you’re talking to says? |
|
|
|
|
|
|
|
|
|
|
|
Q35b. You are in a group of about five people in a busy restaurant. You can see everyone else in the group. Can you follow the conversation? |
|
|
|
|
|
|
|
|
|
|
|
Q35c. You are listening to someone talking to you, while at the same time trying to follow the news on TV. Can you follow what both people are saying? |
|
|
|
|
|
|
|
|
|
|
|
Cognitive Testing Probes
|
Q35d. Do you frequently have to ask people to repeat themselves or misunderstand what they say?
Yes
No
Not sure
Cognitive Testing Probes
|
Q35e. Do you have to concentrate hard when listening to a conversation or to the TV?
Yes
No
Not sure
Cognitive Testing Probes
|
[HEARING LOSS DIAGNOSIS]
Q36. Before today, has a doctor or other health professional ever diagnosed you with a hearing loss?
Yes
No
Don’t know
[HEARING AID USE]
Q37. On an average day, do you use any of the following hearing devices? Check all that apply.
One or more hearing aids
Cochlear implant
Personal FM System
TV Ears (or a voice clarifying headset)
Other
None of the above
Cognitive Testing Probes
|
INTERVIEWER: Note the skip questions here.
[PROGRAMMER: IF PARTICIPANT CHECKS ONLY “NONE OF THE ABOVE” IN Q37, SKIP Q38 – Q40.]
[FREQUENCY OF HEARING AID USE]
Q38. Think about how much you used any hearing device over the past two weeks. On an average day, how many hours did you use the hearing device?
None
Less than 1 hour a day
1 to 4 hours a day
4 to 8 hours a day
More than 8 hours a day
Cognitive Testing Probes
|
[HEARING AIDS DURING STUDY]
[PROGRAMMER: IF PARTICIPANT CHECKS MORE THAN ONE HEARING DEVICE IN Q37, ASK Q39A AND SKIP Q39B. IF PARTICIPANT CHECKS ONLY ONE HEARING DEVICE IN Q37, SKIP Q39A AND ASK Q39B.]
Q39a. Are you using one of your hearing devices now (during this study)?
Yes
No
Q39b. Are you using your hearing device now (during this study)?
Yes
No
INTERVIEWER: For cognitive interviews, skip Q40 and demographics questions but ask intention question at the end.
[PROGRAMMER: FOR COGNITIVE INTERVIEWS, SKIP Q40 FOR EVERYONE. PARTICIPANTS WILL NOT WEAR HEADPHONES DURING COGNITIVE INTERVIEWS.]
Q40. Did the headphones that you wore for this study interfere with your hearing device at any time?
Yes
No
[GENDER]
Q41. What is your sex?
Male
Female
[AGE]
Q42. Please tell us your age.
___ years old.
[EDUCATION]
Q43. What is the highest level of education you have completed?
Less than high school
High school graduate (high school diploma or GED)
Some college, but no degree
Associate’s degree (2-year)
Bachelor’s degree (4-year) (example: BA, BS)
Advanced or postgraduate degree (example: MA, MD, DDS, JD, PhD, EdD)
[RACE/ETHNICITY]
Q44. What is your race? (Select all that apply)
American Indian or Alaska Native
Asian
Black or African-American
Native Hawaiian or Other Pacific Islander
White
Some Other Race
Q45. Are you:
Hispanic or Latino
Not Hispanic or Latino
[DRUG INFORMATION SEARCH BEHAVIOR]
Q46. Would you like to see more information about VOTREA?
Yes, look for more information now
Yes, look for more information later
No, do not look for more information
[DEBRIEFING]
Thank You!
The purpose of this research is to learn about how people feel about information provided in ads and to learn how they use this information to understand how well prescription drugs work. VOTREA is not a real product and is not for sale. Please see your healthcare professional for questions about your health and your medical conditions.
You may exit the survey room to receive your reimbursement.
Cognitive Testing Probes
|
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Ray, Sarah |
| File Modified | 0000-00-00 |
| File Created | 2021-01-24 |
© 2026 OMB.report | Privacy Policy