Form 2 ClinRegs Focus Group
Generic Clearance for the Collection of Qualitative Feedback on Agency Service Delivery (NIAID)
2018-03-14_ClinRegs PRA App - Focus Group
NIAID ClinRegs - Private Sector
OMB: 0925-0668
1. When the user is on ClinRegs, a message will pop up that gives them the opportunity to sign up for an online focus group.
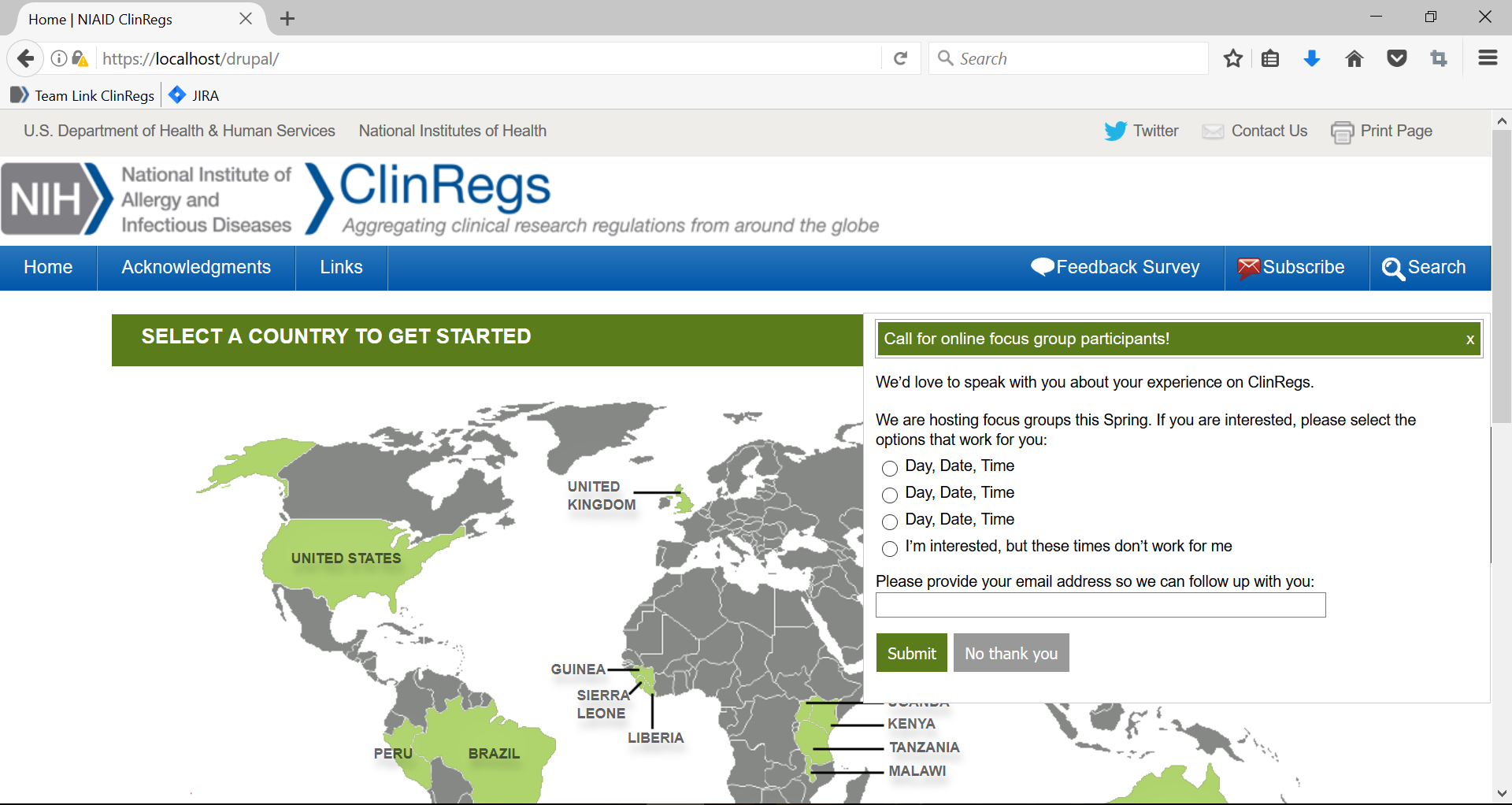
2. If the user clicks the “x” in the upper right hand corner or “No thank you,” the form will close to the right side of the page.
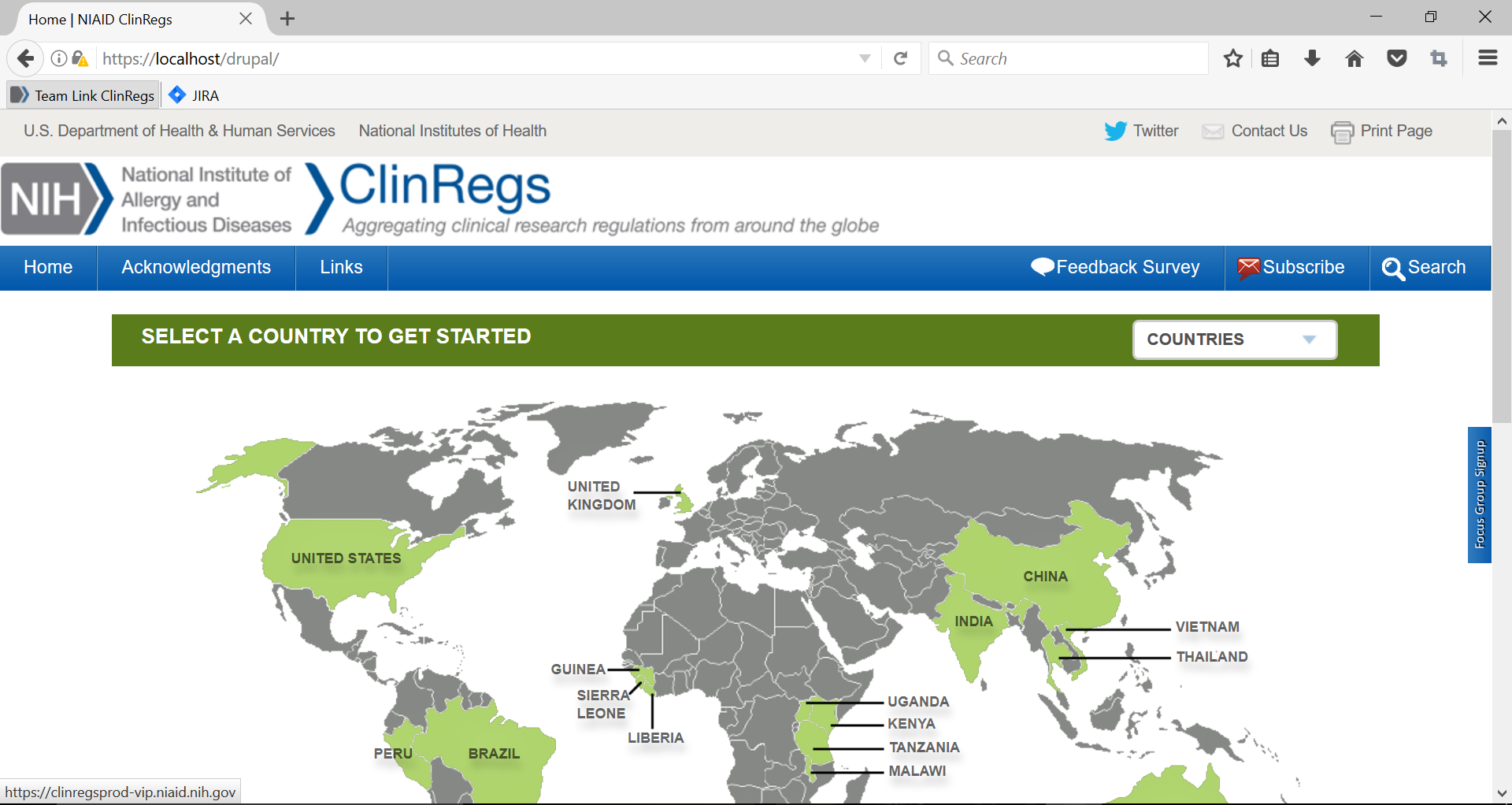
3. The form can be reopened by clicking the blue box on the right, labeled, “Focus Group Signup.”
4. If the user completes the form and clicks “Submit,” the following message will be diplayed.
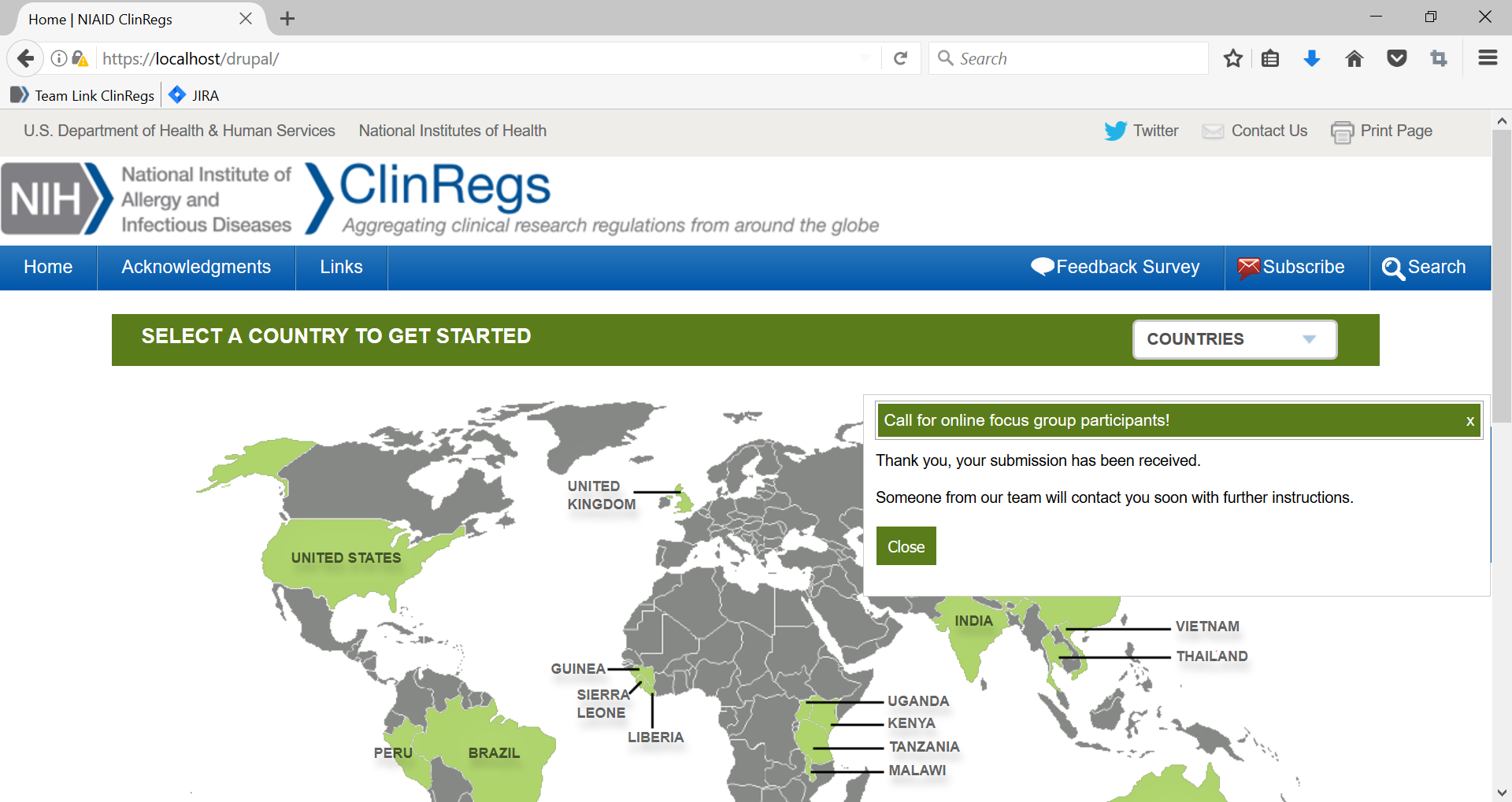
5. The user can then close the box by clicking “close” or by clicking the “x” in the upper right hand corner.
OMB #: 0925-0668
Expiration Date: 2/28/2019
Burden Disclosure: Public reporting burden for this collection of information is estimated to average 3 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to: NIH, Project Clearance Branch, 6705 Rockledge Drive, MSC 7974, Bethesda, MD 20892-7974, ATTN: PRA# 0925-0668. Do not return the completed form to this address.
NIAID ClinRegs Focus Group Script
Introduction (10 minutes)
Introduce leaders of the focus group and roles of each in supporting the meeting. Briefly describe that ClinRegs is an online database of country-specific clinical research regulatory information designed to assist in planning and implementing international clinical research.
[Don’t provide too much detail here; it may limit their thinking too much.]
We are conducting this focus group to better understand your experiences with ClinRegs. This focus group involves having you answer a few questions to learn about how ClinRegs has impacted your work. Specifically, we want to learn more about what you thought was particularly useful/helpful and how we can make it more relevant to the work you do. There are no wrong answers–we want your honesty and we expect differences in opinion. And there are no bad questions, so please feel free to speak up. The only limitation is to help us stay on time, so we can cover all our questions.
Your participation with the focus group is voluntary and confidential. Any input gathered will not be attributed to you individually, but will be combined with others for a fuller picture of the issues. We are recording the session to be sure our notes are accurate, but again, we will not connect any names to any comments. Please let me know if there are any concerns about this process.
Let’s do brief introductions–please tell us your name, organization, and what you do.
We will use your input to make decisions about revising the website, including what revisions need to be made to content and format, as well as about what new topics need to be developed, to better support you in your work.
Any questions before we begin?
Questions (48 minutes)
How did you initially hear about ClinRegs?
How is ClinRegs impacting your work?
Why do you use ClinRegs and has it made your job easier?
Can you share an example of when ClinRegs has saved you time?
ClinRegs Country Content
Do you find the information on the ClinRegs site to be trustworthy? If so, what made you trust it? And if not, then how can we improve your trust?
Does our inclusion of links to the primary regulation documents increase your trust of the site? Do you ever click on the links in the citations?
Are there any regulatory topics that you think are missing?
Is the information contained in ClinRegs covered at the correct level of detail?
Do you have suggestions for improving the readability of the content?
Are you aware of the comparison functionality?
If so, do you have any feedback on the comparison chart and its utility?
Are you aware of the quick facts chart? Has it been able to quickly provide an answer to a question you have?
How could we improve ClinRegs?
Closing (2 minutes)
We’re done! Thank you very much for your time. Your input and comments are very helpful. Do you have any questions for us?
| File Type | application/msword |
| File Title | Generic Clearance Submission Template |
| Subject | Generic Clearance Submission Template |
| Author | OD/USER |
| Last Modified By | SYSTEM |
| File Modified | 2018-04-02 |
| File Created | 2018-04-02 |
© 2026 OMB.report | Privacy Policy