Protocol
Att5 Dimock EI Protocol Final.docx
ATSDR Exposure Investigations (EIs)
Protocol
OMB: 0923-0048
Exposure Investigation
Protocol
Drinking Water Investigation
Dimock, Pennsylvania
June 5, 2017
Cost Recovery: 3ATA00
Prepared by
Robert H. Helverson, MS
Karen Scruton, MS
Agency for Toxic Substances and Disease Registry (ATSDR)
Division of Community Health Investigations (DCHI)
ATSDR Eastern Branch (Region 3) and
Science Support Branch (SSB)
Table of Contents
What is an Exposure Investigation (EI)? 7
Investigators and Collaborators 8
Identification of Residences to be Included in the Sampling Program: 9
Collection of Demographic and Water Quality Information 10
Sampling and Analysis Plan (SAP) 11
Description of Risks and Benefits 14
Informed Consent Procedures 14
Protection of Confidentiality 15
Dissemination, Notification and Reporting of Results 16
Feasibility and Limitations 16
List of Tables
Table 1: Roles and Responsibilities
Table 2: Water and Indoor Air Sampling
Appendices
A EI Process: Four Questions
B Recruitment Invitation Letter and EI Fact Sheet
C Access/Consent Form
D Questionnaire
E Sampling and Analysis Plan
F Lists of Chemical Abstract Service (CAS) Number, Laboratory Reporting Limits, ATSDR Comparison Values, EPA Water Quality Values and Regional Screening Levels for Sampled Parameters
G Individual Results Letter Examples
H Data Management Plan
ACRONYMS
AROA A Record of Activity
ATSDR Agency for Toxic Substances and Disease Registry
CDC Centers for Disease Control and Prevention
CV Comparison Value
DCHI Division of Community Health Investigations
DMP Data Management Plan
EB Eastern Branch
EI Exposure Investigation
EPA (U.S.) Environmental Protection Agency
HC Health Consultation
LEL Lower Explosive Limit
NCEH National Center for Environmental Health
NRC National Response Center
OMB Office of Management and Budget
PADEP Pennsylvania Department of Environmental Protection
PADOH Pennsylvania Department of Health
QA/QC Quality Assurance/Quality Control
SAP Sampling and Analysis Plan
SSB Science Support Branch
SVOC Semi-volatile Organic Compound
VOC Volatile Organic Compound
Project Overview
Background
Unconventional natural gas drilling activities in the Dimock area have been ongoing for nearly 10 years. In October 2011, Dimock residents requested assistance from EPA and ATSDR Region 3 for their ongoing groundwater quality concerns from natural gas extraction operations and the installation of private water treatment systems (EPA 2011). In December 2011, ATSDR evaluated available data (pre-2012 data set) at Dimock and concluded there may be a public health threat from chronic exposure to analytes in area well water (ATSDR 2011). ATSDR supported a “Do Not Use Until Further Notice” and recommended further water testing.
In the first six months of 2012, EPA sampled 64 private water wells and analyzed contaminants that may be present due to natural gas drilling activities. Water quality data from both pre- and post-home water treatment sampling locations were evaluated, as available (EPA 2012a, 2012b). EPA requested ATSDR assistance in the analysis of the sampling results and ATSDR prepared a Health Consultation (HC) report (ATSDR 2016) that provided an analysis of exposure to identified analytes.
The 2016 HC also screened the pre-2012 data set which included analytical results collected from 18 of the 64 Dimock residential water wells sampled by EPA in 2012. When relevant for assessment of chronic exposure to specific chemicals, ATSDR used both the pre-2012 and EPA 2012 data sets, and discussed those analyses in the HC (ATSDR 2016).
The 2016 HC included the following recommendations:
People with elevated levels of inorganic analytes of concern in their well water should install a home treatment system for their well water or obtain an alternative source of drinking water.
People with high levels of methane in their well water should vent their well and home, and treat their water to eliminate the buildup of the flammable methane gas.
Additional sampling of the 64 tested wells be completed for further assessment of groundwater in the area.
Site conditions have changed since 2012, when the last groundwater data set was acquired by EPA. In August 2012, the Pennsylvania Department of Environmental Protection (PADEP) lifted the moratorium on completion of previously drilled natural gas wells in the area. In December 2012 and February 2013, several residents reported to PADEP and one resident reported to the National Response Center (NRC) visual changes in their private drinking water quality (turbidity, color changes, increased methane) (NRC 2012, verbal communications to ATSDR Region 3 from EPA Region 3 and residents).
In a follow-up to the release of the ATSDR 2016 Dimock HC, ATSDR met with community residents, PADEP, EPA and Pennsylvania Department of Health (PADOH). ATSDR met with these stakeholders to (1) discuss the current groundwater quality, (2) communicate the conclusions and recommendations of the HC, (3) determine whether any additional environmental sampling data were available from state agencies or residents that had not been evaluated and (4) further support and recommend additional groundwater and indoor air assessments to address data gaps regarding current exposures in the site area.
The data gaps identified from the 2016 HC and from communications with stakeholders include:
Retesting of private well water at residences tested in 2012 because conditions associated with natural gas extraction activities in the area have changed since 2012 and residents have concerns about the quality of their water.
Testing of water supplied to residents (bulk water) because the source of the water varies and some residents are concerned about the quality of the supplied water.
Testing radon in the home based on residents’ concern. Radon testing in both water and indoor air will be conducted in response to community concerns given that radon is naturally-occurring in the Dimock area. The radon testing in indoor air will be provided in the homes where water testing is completed.
ATSDR designed the EI to address these data gaps. A panel of water analytes potentially associated with natural gas production has been developed by ATSDR and is consistent with sampling performed by EPA in their 2012 sampling event. In addition, radon in water and indoor air will be tested. The EI report will summarize the data, present conclusions about potential health hazards, and provide recommendations for further actions by property owners, PADEP or local agencies, and the EPA. ATSDR will provide a health interpretation of individual sampling results to participants in the EI.
What is an Exposure Investigation (EI)?
An EI is an approach developed by ATSDR that employs targeted biologic and environmental sampling to determine whether people are or have been exposed to unusual levels of pollutants at specific locations (e.g., where people live, spend leisure time, or anywhere they might come into contact with contaminants under investigation).
ATSDR uses EIs to fill data gaps that are essential for evaluating community exposure pathways and determining if a health hazard is present. An EI is NOT a research study. Rather, it is a biased attempt at identifying the most highly exposed individuals and determining their exposure. The EI results are a public health service directed to individual participants and are not generalizable to other populations.
EIs must consider the following four questions:
Can an exposed population be identified?
Does a data gap exist that affects your ability to determine if a public health hazard exists?
Can an EI address the data gap?
How will the EI results impact public health decision making?
Responses to these four questions were used in the decision making process for this EI and are provided in Appendix A.
Investigators and Collaborators
ATSDR DCHI Science Support Branch (SSB) and Eastern Branch (EB) will be co-leads for the EI. PADEP and PADOH will assist ATSDR in the identification of participants and with community outreach. The ATSDR team will be accompanied by Eastern Research Group (ERG), their contractor, during field activities. The specific roles of the EI team partners are provided in Table 1.
Table 1
Roles and Responsibilities for Partners
Agency |
Roles and Responsibilities |
ATSDR – SSB and EB |
|
PADEP, PADOH |
|
EPA Region 3 |
|
EI Design
Identification of Residences to be Included in the Sampling Program:
EI participants will be selected from the 64 residences tested by EPA in 2012 with a maximum of 20 homes being included in the EI. An invitation letter and fact sheet will be sent to the 64 potential participants that outlines the EI objectives. The invitation letter and EI fact sheet are provided in Appendix B. The residences to be included in the EI will be identified using the following criteria:
Residences included in the 2012 EPA testing event that have
private well water identified as a health hazard in the 2016 HC that continues to be used as a source of potable water in the home, or
an existing concern about the quality of their water, e.g., detections near or exceeding level of concern, discoloration or bubbling of water, and proximity of well to natural gas production wells.
If greater than 20 residences meet the criteria, participants will be selected based on the results of the 2012 EPA testing: Those with well water with the highest number and/or concentration of analytes of concern will be given priority for inclusion in the EI.
ATSDR will conduct a site visit prior to the testing to accomplish the following:
Obtain written consent from the property owner for accessing and testing water at the residence (Appendix C). The consent includes granting permission to share well water and indoor air monitoring results with Pennsylvania state agencies and other federal agencies,
Assess the accessibility at the residence to test raw water if a treatment system is in place, and
Complete a site-specific questionnaire about water quality in the home to assist in the sampling effort. The questionnaire is provided in Appendix D and is discussed below.
Collection of Demographic and Water Quality Information
During the site visit, a questionnaire about water quality in the home and basic demographic information will be administered by the EI team (Appendix D). The questionnaire will take approximately 20 minutes and will be administered during the site visit to provide information to ATSDR and ERG for conducting the water sampling at the residence.
Questionnaire responses will be entered directly into a password protected computer. Only ATSDR personnel will enter data and have access to the computer. Information from the questionnaire will be used for interpreting analytical results. The water use habits of each participant, including the use of supplied bulk water and the amount and quality of filtering systems, if any, may impact the potential for exposure to identified analytes. Information on characteristics found on a participant’s property (e.g., septic tanks, natural gas well pads, etc.) might provide information to help explain the results. Demographic information will be used to qualitatively describe the EI participants. In addition, the question about length of residence will define the potential duration of exposure, if any, for each resident.
Sampling and Analysis Plan (SAP)
The Sampling and Analysis Plan (SAP) (Appendix E) provides the methods and the Quality Control/Quality Assurance (QA/QC) plan used in this EI. Tap water, raw groundwater and supplied bulk water will be sampled and analyzed. A maximum of 20 of the 64 private wells sampled in 2012 will be retested for naturally occurring analytes as well as those associated with natural gas extraction activities.
ATSDR will also measure radon in water and indoor air. Although the source of the radon in air cannot be determined because it is naturally occurring in the Dimock area, the radon test kits will be placed in up to two locations: one in the kitchen to evaluate radon potentially being released during water use, and one in the basement, if available, to evaluate radon entering from underlying sources. If the home does not have a basement, the second test kit will be located near a slab or a vapor entry point to the house.
Information collection and environmental sampling will be conducted by ATSDR and Eastern Research Group (ERG), and samples will be analyzed by TestAmerica (constituents in water), Microbac (bacteria in water) and AccuStar Labs (radon test kits). Field work will be completed by July 20, 2017. A minimum of two sampling teams will be identified for the effort. Each team will use the following field equipment: a multi-parameter meter to measure basic water properties, such as pH, specific conductance, temperature, and dissolved oxygen; a photoionization detector/flame ionization detector (PID/FID) for monitoring total VOCs; a lower explosive limit (LEL) monitor for assessing combustible gas; radon test kits, and dedicated field sampling equipment and shipping supplies, as needed. Water and indoor air samples from residences will be collected and evaluated as follows in Table 2:
Table 2: Water and Indoor Air Sampling
Media |
Location |
Purpose |
Parameters Tested |
Water |
Kitchen Tap |
Water will be tested at the primary drinking water location |
|
Raw Water:
|
Water will be purged prior to sampling to reflect groundwater in the area |
||
Bulk Water |
If bulk water used, it will be tested |
||
Indoor Air |
Inside home |
To monitor airborne levels of total VOCs and combustible gas during water sampling to ensure safety of sampling team |
Field Measurements: total VOCs (PID/FID) and combustible gases (LEL monitor) |
Radon inside home (test kit left for up to one week):
|
To sample the level of radon in indoor air |
The two test kits will assess whether the radon may be associated with water use (high water use location) or vapor intrusion from naturally-occurring radon (basement test), although a definitive source cannot be identified. Test kits will be analyzed by AccuStar Labs. |
Analysis of Data
The field team will notify residents if the combustible gas monitoring indicates a hazardous condition during the water sampling.
Drinking water from the tap, raw local groundwater (taken prior to any treatment system) and supplied bulk water will be sampled and analyzed as part of this EI. The analytical results will be compared to appropriate health-based comparison values (CVs) if available. If appropriate CVs are not available, the results may be compared to maximum contaminant levels (MCLs), EPA’s regional screening levels (RSLs), and other health-based screening values. The radon levels detected from the radon test kits will be compared to the EPA action level of 4 pCi/L. All available screening values are listed in Appendix F. Concentrations of analytes below the screening value are not considered to be a health concern. For those analytes that exceed the screening value, ATSDR will assess the potential health impact of the analytes and make recommendations to reduce exposures to radon in indoor air and to chemicals detected in private water wells or bulk water.
ATSDR will provide each participant a letter with their test results and our interpretation of the data within three months of the testing. An example of the results letter is provided in Appendix G. Each participant will also receive a copy of the health consultation with overall interpretation of the results.
EI Timeline
Week 1: Invitation letters and the fact sheet will be provided to 64 residents.
Week 2: ATSDR personnel will conduct a site visit to identify residents that will participate in the testing.
Week 4: Water and indoor air sampling will be conducted.
Week 8: Laboratory analysis of the samples will be completed.
Week 16: Letters will be sent to EI participants providing water sample and radon test results.
Week 70: The exposure investigation (health consultation) will be released.
Human Subjects
This EI will conduct environmental sampling and not biological testing; therefore, human subjects considerations are not an issue for this investigation. An Institutional Review Board (IRB) waiver is included in the OMB package.
Description of Risks and Benefits
The potential benefit to the participants of this EI is that they will learn if they have analytes in their drinking water or analytes in the air at levels of public health or safety concern. If elevated levels are found, appropriate recommendations will be made to the residents that can reduce participants’ exposures.
Homeowners may be required to disclose their water and radon sampling test results if they sell their home. Participants may be inconvenienced prior to and during the water and indoor air testing because ATSDR will need to access their home. In addition, the home owner will need to complete the survey questionnaire which will be administered by a member of the EI team. The survey questionnaire will be completed electronically (laptop) and will take about 20 minutes to complete. Completing both the questionnaire and water sampling will take about one hour. Staging and collection of radon equipment will take less than an hour. An additional visit will be required within one week after the water sampling to retrieve the radon test kits for analysis.
Informed Consent Procedures
This is a voluntary activity. Potential participants will have an opportunity to discuss the risks and benefits of participating in this investigation with a member of the EI team in person and/or via telephone. In addition, a fact sheet describing the exposure investigation will be provided to potential participants (Appendix B). A resident who meets the criteria for participation and wants to participate must provide written Informed Consent (Appendix C) for water and indoor air testing, and complete a brief questionnaire (Appendix D). Participants will have the opportunity to discuss the investigation throughout the EI. They can decide not to participate at any time without penalty.
Protection of Confidentiality
Privacy will be protected to the fullest extent possible by law. ATSDR will share information about well water and indoor air results and personal contact information based on each participant’s selections on the signed Informed Consent (Appendix C). Individual test results that include personal contact or identifying information may be released to other federal, state, and local public health and environmental agencies only with the consent of the participant. These agencies must also protect this private information to the extent the law allows. ATSDR will keep all personal information pertaining to the identification of owner’s names and addresses protected. Electronic data will be kept by ATSDR in a password protected computer. All laboratory samples will be coded so personal information is not available to the laboratory or in the report.
ATSDR will prepare a report summarizing the findings of the investigation. Reports produced about this investigation will give only group information and will not identify specific individuals by name or address.
Data Management
The ATSDR principal investigator will be responsible for data management in accordance with the ATSDR Data Management Plan (DMP) (Appendix H). This includes storing, organizing and securing the results of laboratory analyses, the field determinations data, and associated meta-data (GPS position information, chains of custody, shipping records, logbook data) and quality-control information. A unique record will be established for each water sample and the field determinations and analytical data will be archived for each record. ATSDR project team members will provide data entry, retrieval, compilation, and verification.
We will only share information about participants’ results with other state and federal environmental and public health agencies if the participant provides consent. ATSDR will keep all personal information pertaining to the identification of owner’s names and addresses confidential. Electronic data will be kept by ATSDR in a password protected computer. All laboratory samples will be coded so personal information is not available to the laboratory or in the report. If there is a need for ATSDR to share information with other agencies, we will not provide personal contact information (name, address, telephone number, email) without prior consent of the participating property owner. The EI report that is provided to the public will include aggregate/summary data but not individual results to protect the privacy of EI participants.
Dissemination, Notification and Reporting of Results
ATSDR will send a letter (Appendix G) to each participant with their test results, health interpretation, and our contact information for participants to call us if they have questions about their results. The letter may also include recommendations for how exposures may be reduced.
ATSDR will also prepare a written report presenting the overall EI findings. This report will not contain individual identifiers. The report will be available to the public and will be shared with Dimock participants, and federal, state, and local environmental and public health agencies. Following completion of the final report, a public availability session will be held in Dimock, PA to give participants and other interested parties an opportunity to discuss the report and ask related questions.
Feasibility and Limitations
ATSDR staff has experience in this type of investigation and has previously worked on water and indoor air exposure investigations. ATSDR is well positioned to address these issues and to respond to community concerns that may be voiced during this EI.
Potential limitations include:
The concentrations of analytes in water cannot be used to predict the future occurrence of disease nor be attributed as the cause of current health problems.
Groundwater conditions in the Dimock area may have changed since 2012 due to changes in natural gas drilling activities. Therefore, it is not known whether the EI participants chosen based on the results of the 2012 sampling effort represent the most highly exposed individuals.
Given that radon is naturally-occurring in the Dimock area, the source of radon that may be detected during the air testing cannot be attributed to natural gas drilling activities.
The number of participants recruited may not delineate the extent of contamination and exposure.
The results of this EI will be applicable only to the residences tested and cannot be generalized to other residences.
Handling Unexpected or Adverse Events
There is a small chance of unexpected or adverse events occurring during the course of this EI. The SAP provides information on the identification of potential safety issues associated with the testing (Appendix E). The most likely adverse events would be:
inconvenience of the participants during the sampling, and
malfunctioning of the sampling equipment resulting in data not being attainable.
If any adverse event should occur, the sampling team will notify the principal investigator and the malfunction will be noted.
References
ATSDR. 2011. ATSDR Record of Activity (AROA) – Historic Data Technical Assistance Document issued to EPA Region 3. Atlanta, GA: U.S. Department of Health and Human Services. December 28.
ATSDR 2016. ATSDR Dimock Groundwater Site Health Consultation. Released May 24, 2016. Online: https://www.atsdr.cdc.gov/hac/pha/DimockGroundwaterSite/Dimock_Groundwater_Site_HC_05-24-2016_508.pdf
EPA. 2011. Formal (Email) Request from EPA Jon Capacasa to ATSDR Lora Werner for ATSDR Health Consultation - Dimock PA Drinking Water/Marcellus Shale. December 7.
EPA. 2012a. Dimock Residential Ground Water Site Executive Summary of the Data Collection, Validation and Review Process. July 24. Online at http://epaosc.org/sites/7555/files/Executive%20Summary%20of%20Data%20Collection,%20Validation%20and%2 0Review%20Process%20FINAL%20072412.pdf
EPA. 2012b. Individual toxicological memoranda issued to homeowners summarizing private well data.
National Response Center (NRC). 2012. Incident Report # 1033706. Incident report dated December 19, 2012.
Appendix A
EI Process: Four Questions
Appendix A: EI Process: Four Questions
For the Dimock EI, the four EI questions are answered as follows:
Can an exposed population be identified?
Yes – EPA sampling conducted in 2012 identified constituents in private well water that may result in health effects or a safety hazard (ATSDR 2016). Dimock residents have expressed concern about exposure to their groundwater since 2009 and some residents continued to express concern in 2016.
2. Does a data gap exist that affects your ability to determine if a public health hazard exists?
Yes - There are insufficient data to assess current exposures to analytes in private well water. A limited amount of data has been made available for certain Dimock private wells. However, the last comprehensive sampling activities were conducted over four years ago when EPA sampled 64 wells in the Dimock area. Without current groundwater assessment data, and given the considerable changes in the area (e.g., completion activities including hydraulic fracturing), ATSDR cannot determine whether unhealthy exposures to chemicals in groundwater are occurring. Residents have also had complaints regarding the quality of the bulk water that has been supplied as an alternate source of drinking water. The results of the drinking water and bulk water testing will assist regulators in determining whether residents should obtain or continue using alternate sources of drinking water. Residents have also been concerned about radon in indoor air in the area. Radon is found naturally in the Dimock area and will be tested as a public service in homes where water testing will be conducted.
3. Can an EI address the data gap?
Yes - The sampling of groundwater, bulk water and radon and an analysis of the constituents detected in these exposure media will provide a health-based evaluation for residents in the community. Screening indoor air in the field for total VOCs and combustible gas emissions is included to ensure the safety of personnel during water sampling because methane has been detected in elevated concentrations in past sampling events. Testing of radon in indoor air will be provided in those homes where water testing will be conducted.
4. How will the EI results impact public health decision making?
More information on the chemical composition of area well water will provide important information to guide public health recommendations and actions. Assessment of bulk water will determine the quality of these replacement supplies that have been provided to numerous residents in the Dimock area. Although municipal water is not available to residents in this area, point of use and whole house water treatment and other alternative water supplies can be considered as an exposure mitigation alternative. Indoor air testing for radon will provide important information regarding exposures to a known carcinogen. Information from this EI and the subsequent health consultation may also be useful in triggering remedial actions to mitigate exposures or safety hazards.
Appendix B
Recruitment Invitation Letter and EI Fact Sheet
The 64 potential participants will receive the invitation letter via U.S. Mail. The fact sheet will be included in the mailing. These materials will be sent to residents prior to the site visit in an effort to identify participants. If residents do not respond, they will be contacted during the site visit. If residents are not at their residence during the site visit, the fact sheet will be left at the home and ATSDR will continue to try contact the residents via phone or another home visit.
Fact Sheet will be put into final form by Creative Services
DATE
From: Robert Helverson, MS
Region 3 Representative
Agency for Toxic Substances and Disease Registry
1650 Arch Street, Philadelphia, PA 19103
To: ADD RECIPIENT’S ADDRESS
Re: ATSDR Dimock Drinking Water Exposure Evaluation
Mr. or Ms. NAME:
The Agency for Toxic Substances and Disease Registry (ATSDR) is a federal public health agency that determines whether concentrations of contaminants in the environment may pose a public health risk. ATSDR has received complaints by residents in your area about the quality of their private well water.
In 2012, the U.S. Environmental Protection Agency (EPA) collected samples from 64 private water wells in Dimock, including yours, and asked the ATSDR to evaluate the EPA sampling data. Based on the health assessment and community concerns, ATSDR recommended that further drinking water and indoor air sampling be completed in the Dimock area.
As follow-up to the 2016 report, ATSDR is going to test up to 20 private water wells, supplied bulk water (if you use it), and radon and combustible gas at residences in the area during the summer of 2017. We will be testing water to see if there are water quality issues (such as salt, pH, bacteria), gases or chemicals in the water that may be a health or safety concern for people. We will also be conducting radon air tests in the homes when we conduct the water testing. Your residence has been identified as eligible for additional testing.
A fact sheet is enclosed that provides information about our plans. If you want to be included or need more information, please call me, Robert Helverson, at 215-814-3139. If I am not in, please leave a message and I will return your call.
ATSDR will be in the Dimock area in June 2017 to talk to people about the proposed testing.
ATSDR will be in Dimock,
PA to conduct free testing of drinking water and indoor air.
ATSDR will
Contact
you to talk about the sampling
Test
your drinking water, bulk water (if you use it) and radon in
indoor air in summer 2017
Who
are we? (font
smaller in mock up to fit on page)
The
Agency for Toxic Substance and Disease Registry (ATSDR) – a
public health agency which is part of the Centers for Disease
Control and Prevention (CDC)
Local
office in Philadelphia,
PA



Why is ATSDR testing
drinking water and radon in Dimock?
Since
2011, residents in the area have been concerned about possible
water contamination caused by natural gas drilling in the area.
In
2012, EPA sampled 64 private wells, including yours.
ATSDR
looked at the water sampling results to determine whether water in
the area could harm health. A report called a “health
consultation (HC)” was completed by ATSDR.
The
HC reported that contaminants that cause health problems were
found in the water that was sampled.
The
HC recommended further water testing for contaminants
that might be
associated with nearby natural gas well activities. We will also
test for bacteria in the water.
Residents
have also been concerned about radon in their homes, so ATSDR will
test radon in water and indoor air.
What harmful substances
may be found in drinking water?
EPA water testing in 2012 found levels of contaminants that may be
harmful to your health, including
Arsenic
May
affect your skin and can increase the risk of cancer
Manganese
May
affect the nervous systems in bottle-fed infants
Sodium
May
be harmful to those on a low-sodium diet
Iron
Affects
the taste of water and may cause staining
Methane
gas
May
be an explosive hazard if it builds up inside your home


What will ATSDR do?
Choose
participants from homes that were tested by EPA in 2012 and still
have problems with their well water.
Conduct
free
water testing of
tap water, groundwater and bulk water (if you use it).
Use
air monitors during the water sampling since gases may build up
when water is run for a long time.
Ask
some questions about people’s wells and their water use.
Send
water to a laboratory for analysis.
Send
water testing results and our interpretation to the participants
within about 3 months after sample collection.
Make
recommendations for reducing exposure to contaminants in water.
How will ATSDR test radon
in indoor air?
Although EPA did not test for radon in 2012, ATSDR will test radon
in water and indoor air.
ATSDR staff members will
Leave
radon sampling devices in these homes and pick them up in three
days.
What should you do now?
If
you have questions, please contact Robert
Helverson with the ATSDR Region 3 office at 215-814-3139 or by
email at gfu6@cdc.gov.




Appendix C
Access/Consent Form
US Department of Health and Human Services (DHHS)
Agency for Toxic Substances and Disease Registry (ATSDR)
Dimock, Susquehanna County Drinking Water Exposure Investigation (EI)
Adult Access/Consent Form
Fleisch-Kincaid 9.0
Who are we and why we are doing this EI? |
|
What will we be testing? |
|
What does this involve? |
If you would like to participate, we need your written permission to test your drinking water and indoor air and ask you some questions.
This will take an hour and few minutes of your time.
Drinking Water Testing:
|
|
Bulk Water Testing:
Indoor Air Radon Testing:
Answering Questions:
Test Results:
|
When will I get my results? |
|
What are the benefits from being in this EI? |
|
|
What are the risks of this EI? |
|
|
What if I have questions? |
|
|
What about privacy? |
|
|
Voluntary Consent |
|
|
Signature of Resident |
YES__________NO_________
YES_________, NO________
I have read this form or it has been read to me. I give my permission to have my water and indoor air tested and to answer the questions ATSDR asks me.
_____________________________ _______________ Signature of Person Given Consent Date
________________________________________ Printed Name of Person Given Consent
Age _________
Street Address (If this address has another defining number or letter, please provide that now): ______________________________
______________________________
______________________________
Mailing Address (If different from Street Address):
______________________________
______________________________
______________________________
Telephone__________________ Cell phone _________________
Email Address: ________________________________________
|
Signature of Property Owner |
YES__________NO_________
YES_________, NO________
I have read this form or it has been read to me. I give my permission to have my water and indoor air tested and to answer the questions ATSDR asks me.
_____________________________ _______________ Signature of Property Owner Date
________________________________________ Printed Name of Property Owner
Age _________
Street Address of property being tested: ______________________________
______________________________
______________________________
Mailing Address of property owner:
______________________________
______________________________
______________________________
Telephone__________________ Cell phone _________________
Email Address: ________________________________________
|
Signature of Consent Form Administrator |
Certification of Consent Form Administrator:
I have read the consent form to the person name above. They have had the opportunity to ask questions about the EI and had the questions answered.
___________________________________ ___________ Signature of person administering consent Date
_________________________________________ Printed Name of person administering consent
|
Appendix D
Questionnaire
Form approved
09230048
Exp Date
03/31/2019
Questionnaire for the Dimock Drinking Water Exposure Investigation
OMB #0923-0048
Name of Surveyor: Date:
I just want to repeat my name is #######. Now since we have your permission, we would now like to ask you some questions.
Water History:
What is the main source of drinking water in your home?

Private Well

City or County (public)

Spring

Pond

Cistern

Supplied Bulk

Bottled

Other: (specify)

Don’t know

Refused
ATSDR estimates the average public reporting burden for this collection of information as 20 minutes per response, including the time for reviewing instructions, searching existing data/information sources, gathering and maintaining the data/information needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Information Collection Review Office, 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA (09230048). |
Has the water from your private well ever been tested at any time other than by EPA in 2012?

Yes

No

Don’t know

Refused
If “yes” do you know the date it was tested who did the testing, whether it was tested for bacterial and/or chemical contamination, and the results?
______________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
What is your main source of water for cooking?

Private Well

City or County (public)

Spring

Pond

Cistern

Supplied Bulk

Bottled

Other: (specify)

Don’t know

Refused
What is the main source of water for bathing and showering?

Private Well

City or County (public)

Spring

Pond

Cistern

Supplied Bulk

Bottled

Other: (specify)

Don’t know

Refused
What is the main source of water for pools and/or hot tubs (to include “kiddie” or wading pools)?

Private Well

City or County (public)

Spring

Pond

Cistern

Supplied Bulk

Bottled

Other: (specify)

Don’t know

Refused
List all of the water treatment devices for your drinking water or water used for mixing drinks (e.g. baby formula, juices)

None

Charcoal Filter/Granular Activated

Ceramic Filter

Reverse Osmosis

Water Softener

Distillation

Sediment Filter

Aerator

Water Filter system (e.g. Brita, Pur, etc.)

Iron Removal System

Chlorinator

Don’t know

Refused
List all of the water treatment devices for your water used for cooking.

None

Boil water

Charcoal Filter/Granular Activated

Ceramic Filter

Reverse Osmosis

Water Softener

Distillation

Sediment Filter

Aerator

Water Filter system (e.g. Brita, Pur, etc.)

Iron Removal System

Chlorinator

Don’t know

Refused
List all of the water treatment devices for your bathing and showering water.

None

Charcoal Filter/Granular Activated

Ceramic Filter

Reverse Osmosis

Water Softener

Boil water

Distillation

Sediment Filter

Aerator

Water Filter system (e.g. Brita, Pur, etc.)

Iron Removal System

Chlorinator

Don’t know

Refused
If you use filters, do you maintain them according to the manufacturers recommendations or if you have a whole house filter do you have a contractor maintain them?

Yes

No

Don’t know

Refused
If you use a Water Softener, do you regularly maintain it?


Yes

No

Don’t know
Refused
If yes, what is the brand and age of the Water Softener?

Brand: __________________________________________________________

Age: __________________________________________________________

Don’t know

Refused
Do you know the depth of your water well or have any records of the well history of your private water well?

Yes

No

Don’t know

Refused
If yes, please provide details (type, age, depth of well)
____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Do you obtain bulk water for drinking or household use?

Yes

No

Don’t know
If you answered “yes”, how long have you used bulk water?____________________________
What is the source of your bulk water?______________________________________________
What do you use bulk water for (e.g., drinking, cooking, showering, laundry, etc.)_______________________________________________________________________________
Do you have any comments regarding the quality and taste of the bulk water? ______________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Do you know of any natural gas extraction activities currently occurring near your home?

Yes

No

Don’t know
If yes, please explain __________________________________________________________________________________________________________________________________________________________________________________________
Have you ever had your home tested for radon gas? If so, what was the result? ___________________________

Yes
 No
No
 Don’t
know
Don’t
know
General Information:
First name (please spell): _______________________________
Last name (please spell): _______________________________
Middle initial: __________________
How long have you lived at this address?

< 1 year

1-10 years

>10 years

Don’t know

Refused
Demographic Information:
Gender ______
 Age
at time of survey _____ Refused
Age
at time of survey _____ Refused
Race :

American Indian or Alaska Native

Asian

Black or African American

Native Hawaiian or Other Pacific Islander

White

Refused
Ethnicity:

Hispanic or Latino

Not Hispanic or Latino
How many people live here fulltime? ________ (if more than 1 person complete General Information question 4 and Demographic Information for each resident)
NOTE: Surveyor/Sampling team will also consult with home owner to diagram the location of a water supply well, septic system, home heating oil tank, and natural gas well pads at the residence of in the immediate vicinity of the property.
Appendix E
Sampling and Analysis Plan
Appendix E.1: Sampling and Analysis Plan (SAP)
Appendix E.2: Quality Assurance Project Plan (QAPP)
Appendix E.1
Sampling and Analysis Plan for
Support to ATSDR in Drinking Water Exposure Investigation in Dimock, PA
Prepared for:
Agency for Toxic Substances and Disease Registry
Division of Community Health Investigations
ATSDR Eastern Branch (Region 3) and
Science Support Branch (SSB)
Prepared by:
Eastern Research Group, Inc.
110 Hartwell Avenue
Lexington, MA 02421
PG Environmental
1113 Washington Avenue, Suite 200
Golden, CO 80401
May 3, 2017
1.1 Background, Objective, and Scope 1
2.1 Sampling Point Selection 2
2.3 Sample Fraction, Bottle Sets, and Sample Collection 6
2.3.1 Quality Assessment Samples 7
2.3.3 Instrument Calibration 8
2.4 Preservation, Shipping, and Analysis 8
2.7 Quality Assurance / Quality Control 9
3.3 Field Sampling Activities 9
3.4.2 Analytical Laboratories 10
Table 1. Analytes and Analytical Methods 4
Table 2. Field Sampling Methodology and Equipment 6
Attachment 1 Dimock EI: Indoor Air Sampling
1.1 Background, Objective, and Scope
The Agency for Toxic Substances and Disease Registry has tasked Eastern Research Group (ERG) with supporting them as part of an upcoming Exposure Investigation (EI) in the Dimock, Pennsylvania area. PG Environmental (PG), a sister company to ERG, will be supporting ATSDR in the following items:
Testing private well water (at the tap and raw groundwater) as well as supplied bulk water sources in the Dimock area for analytes of potential concern for health effects (inorganics, organic compounds, oil and grease, total petroleum hydrocarbon, radionuclides), physical hazard (methane) and general water quality (taste, smell, pH, bacteria, etc.) identified in the Health Consultation (HC) (2016).
Testing indoor air for radon levels.
This document represents PG’s project-specific sampling and analysis plan (SAP) and provides sampling procedures and methods that PG will follow when conducting sampling activities at the participating private homes in the Dimock, PA area. Sampling will be performed by two teams of two PG staff and analyses performed by sub-contracted laboratories. This document, in combination with the Quality Assurance Project Plan (QAPP) developed for this exposure investigation, is intended to serve as a guide to the PG sampling teams and to provide background information to ERG and ATSDR as a review mechanism.
This section provides a discussion of planned sampling procedures, methods, and logistics for conducting sampling activities while at the selected private residences.
Due to the variable voluntary participation of homeowners in the EI, as well as the different residence characteristics (e.g., layout, age, differing water treatment technologies that may be in use, and the overall condition), the specific sampling points have not been specified in advance. The selection of the sampling point, overall site and sample point condition, and the deciding factors for the use of that sampling point will be carefully noted in the field, and described in the post-sampling report. PG is prepared to sample at up to 20 private residences during the proposed sampling at the end of June 2017. Water samples will be collected from multiple locations at each residence, including (1) a sample at the primary drinking water location, e.g, kitchen tap, (2) a sample of raw water, purged and taken before any treatment system, to represent conditions in the underlying groundwater, and (3) a sample of supplied bulk water.
The drinking water sample collected from each residence will be a “first draw” sample collected from the primary drinking water tap (e.g., kitchen sink or refrigerator tap). The raw water sample will be collected following a purge of the plumbing system in order to access raw groundwater (as determined by field measurements of pH, temperature, conductivity, etc.). A supplied bulk water sample will be taken, if available.
In order to determine when a well has been adequately purged, the PG team will monitor the temperature, pH, and specific conductance of the groundwater removed during purging. Stable water quality parameter (temperature, pH and specific conductance) measurements indicate representative sampling is obtainable. Water quality is considered stable if for three consecutive readings:
Temperature range is no more than +1C;
pH varies by no more than 0.2 pH units;
Specific conductance readings are within 10% of the average.
The water in which water quality parameter measurements were taken will not be used to fill sample bottles.
In some situations, even with slow purge rates, a well may be pumped or bailed dry (evacuated). In these situations, this generally constitutes an adequate purge and the well can be sampled following sufficient recovery (enough volume to allow filling of all sample containers). The pH, specific conductance, and temperature should be measured and recorded, during collection of the sample from the recovered volume, as the measurements of record for the sampling event. For wells with slow recovery, attempts should be made to avoid purging them to dryness. This can be accomplished, for example, by slowing the purge rate. As water enters a well that has been purged to dryness, it may cascade down the sand pack and/or the well screen, stripping volatile organic constituents that may be present and/or introducing soil fines into the water column. It is particularly important that wells be sampled as soon as possible after purging. If adequate volume is available immediately upon completion of purging, the well must be sampled immediately. If not, sampling should occur as soon as adequate volume has recovered. Water samples will be analyzed for the constituents listed in Table 2, below.
Indoor air monitoring (grab samples) for total volatile organic compounds (VOCs), using a photoionization detector/flame ionization detector (PID/FID), as well as lower explosive limit (LEL) monitoring will be conducted at locations within the residence, including (1) the point where groundwater plumbing enters the home, and (2) bathrooms, kitchen, and laundry rooms where vapors released from water use may accumulate. Indoor air monitoring for radon will be conducted simultaneously in the lowest floor (i.e., basement or ground floor) and in a primary occupied space, such as the living room or kitchen. In addition, “short-term” (72-hour) indoor testing for radon will be conducted using activated charcoal adsorption devices, placed in up to two locations within each EI participant’s home, one in the basement and one in a living area potentially impacted by water vapor emissions. See Attachment 1 for the sampling approach for indoor air monitoring associated with this EI.
While PG does not anticipate any sampling difficulties, site-specific conditions may result in modifications to planned sampling methodology. For instance, access to a sampling location may not be possible, resulting in the inability to collect a sample. All deviations from the intended sampling schedule will be approved by the on-site ATSDR representative accompanying the PG sampling teams. Any deviations to the sampling plan will be documented PG’s field notes and then included in the final report generated for each sampling location.
The following provides a general description of anticipated sample collection points and techniques to be followed at each private residence. Specific sampling points and locations, and the sampling methodologies for each site will be carefully noted in the field and included in the final sampling report.
Some potential sampling points typical for drinking water supplies are as follows, along with considerations for each:
Accessible Water Taps: Aerators, if present, should be removed; aeration removes VOCs from the sample. The flow of water should be maintained until the water temperature is constant, and then the sample container held under the discharge at an angle to allow the sample to flow down the inside wall of the sample container, which minimizes aeration. Sample container(s) should be filled to the line, if present, or to the top of the container lip.
Supplied Bulk Water: The flow of water should be maintained until the water temperature is constant, and then the sample container held under the discharge at an angle to allow the sample to flow down the inside wall of the sample container, which minimizes aeration. Sample container(s) should be filled to the line, if present, or to the top of the container lip.
Analytes to be collected at each private residence have been pre-selected by ATSDR and are detailed in Table 1, along with the corresponding analytical methods that will be used.
Table 1. Analytes and Analytical Methods |
|
Sample |
Method |
Water Quality: pH, temperature, conductivity, dissolved oxygen, total dissolved solids (TDS) |
Immediate field analysis via hand-held multimeter |
VOCs and combustible gases, including methane (Attachment 1) |
Immediate field analysis via FID/PID and LEL |
Radon (indoor air) (Attachment 1) |
PicoCan 275 open face activated charcoal canister |
Total Coliform (presence/absence only) |
SM 9223 B |
Fecal Coliform |
SM 9223 B |
Inorganic Ions: Bromide, Chloride, Fluoride, Sulfate as SO4 |
EPA 300.0 |
Trace Elements: Aluminum, Antimony, Arsenic, Barium, Beryllium, Boron, Cadmium, Calcium, Chromium, Cobalt, Copper, Iron, Lead, Lithium, Magnesium, Manganese, Nickel, Potassium, Selenium, Silver, Sodium, Strontium, Thallium, Tin, Titanium, Uranium, Vanadium, Zinc |
EPA 200.7 & 200.8 |
Oil and Grease |
EPA 1664B |
Dissolved Gases: methane, ethane, ethene, propane, butane |
RSK-175 |
Total Petroleum Hydrocarbons (TPH) DRO/GRO, Alcohols, Glycols |
EPA 8015C (SW-846) |
Semivolitile Organic Compounds (SVOCs) |
|
1,1-Biphenyl, 1,2,4,5-Tetrachlorobenzene, 1-Methylnaphthalene, 2,3,4,6-Tetrachlorophenol, 2,4,5-Trichlorophenol, 2,4,6-Trichlorophenol, 2,4-Dichlorophenol, 2,4-Dimethylphenol, 2,4-Dinitrophenol, 2,4, Dinitrotoluene, 2,6-Dinitrotoluene, 2-Chloronaphthalene, 2-Chlorophenol, 2-Methoxyethanol, 2-Methylnaphthalene, 2-Methylphenol, 2-Nitroaniline, 2-Nitrophenol, 3,3´-Dichlorobenzidine, 3-Nitroaniline, 4,6-Dinitro-2-methylphenol, 4-Bromophenyl phenyl ether, 4-Chloro-3-methylphenol, 4-Chloroaniline, 4-Chlorophenyl phenyl ether, 4-Methylphenol, 4-Nitroaniline, 4-Nitrophenol, Acenaphthene, Acenaphthylene, Acetophenone, Anthracene, Atrazine, Benzaldehyde, Benzo(a)anthracene, Benzo(a)pyrene, Benzo(b)fluoranthene, Benzo(ghi)perylene, Benzo(k)fluoranthene, Bis(2-chloroethoxy)methane, Bis(2-chloroethyl)ether, Bis(2-chloroisopropyl)ether, Bis(2-ethylhexyl)phthalate, Butyl benzyl phthalate, Caprolactam, Carbazole, Chrysene, Dibenz(a,h)anthracene, Dibenzofuran, Diethyl phthalate, Dimethyl phthalate, Di-n-butyl phthalate, Di-n-octyl phthalate, Fluoranthene, Fluorene, Hexachlorobenzene, Hexachlorobutadiene, Hexachlorocyclopentadiene, Hexachloroethane, Indeno(1,2,3-cd)pyrene, Isophorone, Naphthalene, Nitrobenzene, N-Nitrosodimethylamine, N-Nitroso-di-n-propylamine, N-Nitrosodiphenylamine, Pentachlorophenol, Phenanthrene, Phenol, Pyrene |
EPA 8270 |
Volatile Organic Compounds (VOCs) |
|
1,1,1,2-Tetrachloroethane, 1,1,1-Trichloroethane, 1,1,2,2-Tetrachloroethane, 1,1,2-Trichloroethane, 1,1-Dichloroethane, 1,1-Dichloroethene, 1,1-Dichloropropene, 1,2,3-Trichlorobenzene, 1,2,3-Trichloropropane, 1,2,4-Trichlorobenzene, 1,2,4-Trimethylbenzene, 1,2-Dibromo-3-chloropropane, 1,2-Dibromoethane (EDB), 1,2-Dichlorobenzene, 1,2-Dichloroethane, 1,2-Dichloropropane, 1,3,5-Trimethylbenzene, 1,3-Dichlorobenzene, 1,3-Dichloropropane, 1,4-Dichlorobenzene, 2,2-Dichloropropane, 2-Butanone, 2-Chloroethylvinyl ether, 2-Chlorotoluene 2-Hexanone, 4-Chlorotoluene, 4-Methyl-2-pentanone, Acetone, Acrylonitrile, Benzene, Bromobenzene, Bromochloromethane, Bromodichloromethane, Bromoform, Bromomethane, Carbon disulfide, Carbon Tetrachloride, Chlorobenzene, Chlorodibromomethane, Chloroethane, Chloroform, Chloromethane, cis-1,2-Dichloroethene, cis-1,3-Dichloropropene, Cyclohexane, Dibromomethane, Dichlorodifluoromethane, Ethylbenzene, Freon 113, Hexachlorobutadiene, Isopropylbenzene, Methyl Acetate, Methylcyclohexane, Methylene Chloride, Methyl-tert-butyl ether, m-Xylene/p, Xylene, Naphthalene, n-Butylbenzene, n-Propylbenzene, o-Xylene, p-Isopropyltoluene sec-Butylbenzene, Styrene, tert-Butylbenzene, Tetrachloroethene, Toluene, trans-1,2-Dichloroethene, trans-1,3-Dichloropropene, Trichloroethene, Trichlorofluoromethane, Vinyl acetate, Vinyl chloride |
EPA 8260
|
Radionuclides |
|
Gamma spectroscopy |
EPA 901.1 |
Gross alpha |
EPA 00-02 |
Isotopic Thorium & Isotopic Uranium |
A-01-R |
Radium 226 and 228 |
EPA 903.0 and EPA 904.0 |
Radon |
EPA 913 |
2.3 Sample Fraction, Bottle Sets, and Sample Collection
Samples that require the same preservation or that are in the same pollutant class will be collected in the same sample set, unless otherwise specified by the contract laboratory that will perform the analyses. In general, most parameters will be collected in individual sample bottles/cannisters. Table 2 presents a summary of the parameters, bottle types, sample volume, and preservation requirements.
Table 2. Field Sampling Methodology and Equipment |
|
Analyte |
Field Sampling Method and Equipment |
Water Quality (pH, temperature, conductivity, dissolved oxygen, TDS) |
Immediate field analysis (YSI ProPlus Multiparameter Probe, Hach SL1000 Portable Meter, or similar multiparameter probe) |
Volatile Organic Compounds (VOCs) and combustible gases, including methane |
FID/PID (Thermo Scientific TVA 2020 Toxic Vapor Analyzer) and LEL Monitor (Honeywell Gas Alert XL or similar) |
Radon |
PicoCan 275 open face activated charcoal canister |
Total Coliform (presence/absence only) |
Grab sample 125mL sterilized, plastic bottle Sodium thiosulfate preservative |
Fecal Coliform (only if Total Coliform present) |
Grab sample 125mL sterilized, plastic bottle Sodium thiosulfate preservative |
Inorganic Ions |
Grab sample 1L plastic sample bottle (No preservative needed) |
Trace Elements |
Grab sample 1L plastic sample bottle Nitric acid |
Oil and Grease |
Grab sample 1L glass sample bottle Hydrochloric Acid or Sulfuric Acid |
Dissolved Gases |
Grab sample 2-40-mL VOA vials Hydrochloric Acid |
TPH DRO/GRO |
Grab sample 3-40-mL VOA vials Hydrochloric Acid |
Alcohols and Glycols |
Grab sample 3-40 mL VOA glass Hydrochloric Acid or Unpreserved |
SVOCs |
Grab sample 250mL glass or plastic bottle acid preservative |
VOCs |
Grab sample 120mL glass vials powdered dechlorinating agent, if needed (ascorbic acid) 1: 1 hydrochloric acid solution for each 20mL of sample volume |
Radionuclides |
Grab sample 1 gallon plastic sample container Hydrochloric Acid or Nitric Acid |
Sample containers and bottles will be pre-cleaned and certified and will not require rinsing with sample. Sample collection methods and the type of sampling point may vary depending on the age, condition, and technology implemented at each private residence.
2.3.1 Quality Assessment Samples
Trip blanks will be used to evaluate possible contamination arising during shipment and handling of samples. The contract laboratories will provide trip blanks, typically an analyte-free matrix like high performance liquid chromatography (HPLC) water, for each of the samples that require it, such as VOCs. One set of trip blanks per sampling site will be used. The sampling technician will preserve the trip samples and transport them unopened to the contract laboratories. The laboratories typically analyze these samples only for volatile organic compounds.
PG will collect a full suite of duplicate samples at ten percent of the total number of private residences sampled as part of the EI. Duplicate samples are collected simultaneously with a standard sample from the same source under identical conditions into separate sample containers. Field duplicates will consist of a homogenized sample divided in two or else a co-located sample. Each duplicate portion will be assigned its own sample number so that it will be blind to the laboratory. A duplicate sample is treated independently of its counterpart in order to assess laboratory performance through comparison of the results.
Hand-held field multimeters used to collect in situ pH, conductivity, dissolved oxygen, temperature, and total dissolved solids measurements will be calibrated on a daily basis following the calibration instructions in the user manual. A calibration precision test will be completed before the FID/PID is placed into service. The calibration standard operating procedures for each instrument are elaborated upon in PG’s QAPP as well as in Attachment A.
2.4 Preservation, Shipping, and Analysis
All samples will be maintained on ice immediately upon collection. Chemical preservatives will be added on site upon sample collection, or pre-preserved bottles will be used according to method-specified protocols. Table 2 lists the analytes, sample containers, sample volumes, and the preservation methods. The samples will be packed in ice chests with a sufficient quantity of wet ice to maintain a temperature of 4°C (+/- 2°C). In order to maintain holding times, all samples will be hand-delivered by the sampling technician or a courier to the contract laboratories.
Each sample will be carefully labeled at the time of collection. Typically, labels are pre-printed, although some parts may also be completed by hand in the field. Each self-adhesive label is completed in permanent ink and contains information on the site, date, sample number, sampling point/description, analysis to be performed, sample bottle type, and preservation, if required.
If any of the pre-printed information is incorrect, it will be revised using indelible ink. In particular, if a preservative is not used, it will be marked out. The specifics of preservation and other sample details will be noted on the Chain-of-Custody sample sheet. Once applied to the sample container, the label will be covered with clear tape to prevent tampering, abrasion, smearing, or loss during transit.
To maintain a record of sample collection, transfer between personnel, shipment, and receipt by the laboratory, a Chain-of-Custody (COC) log is completed for each sample set at each sampling location. These forms are used to document sample custody transfer from the field to the laboratory. The COC forms are completed for all samples sent to all laboratories. At the time of sample delivery, the COC is submitted with the samples to the analytical laboratory.
In addition to the transfer of custody, the COC provides information to the contract analytical laboratory on the sample number, type of sample, sample description, if the sample is preserved, the sample collection date and time, and the analyses requested.
The comment section is used to provide special notes or instructions to the laboratory. As well, deviations from standard sampling protocols (e.g., a sample could not be acid preserved) are noted in the comments section.
When samples are received by the contract analytical laboratory, the laboratory sends a copy of the COC to PG, to acknowledge receipt and the condition of the samples.
2.7 Quality Assurance / Quality Control
Quality assurance/quality control (QA/QC) procedures applicable to this project are outlined in the “Quality Assurance Project Plan for Support to ATSDR Dimock, Pennsylvania Exposure Investigation” (1).
The QA/QC program for sample collection at private residences will include the following:
Sampling according to standard EPA-accepted methods for drinking water (e.g., Sampling Guidance for Unknown Analytes in Drinking Water (2008), EPA/817/R-08/003; EPA’s Interactive Sampling Guide for Drinking Water System Operators (2006), EPA/816/F/03/016)
Documentation for samples through laboratory Chain-of-Custody Records;
Use of trip blank(s) for VOCs analyses
This section of the SAP details specifics of the sampling team, site visit preparation, and
sampling activities.
PG intends on using two teams of two PG staff to conduct the sampling. The PG teams will be responsible for all sample collection, preservation, documentation, and for hand-delivering the samples to the contract laboratory or courier. The PG teams are also responsible for collating the analytical results from each laboratory after completion of the sampling trip. The analytical results will be summarized and transmitted to ATSDR in a trip report.
Prior to the sampling trip, the PG teams will review the planned sampling activities with the ERG and ATSDR EI team members. The PG teams will also review PG’s relevant Health and Safety requirements and procedures for both site visits and sampling activities, as well as the private residence homeowner names and contacts (if available), and the names and addresses of analytical laboratories. Any site-specific health and safety requirements will be identified ahead of time. PG’s Work Assignment Manager (WAM) will coordinate the procurement and shipment of all necessary sampling and Health and Safety equipment that the sampling teams will require.
At each private residence, the PG sampling team working with the ATSDR representative will meet with the homeowner to determine whether samples can be safely collected at representative sampling point(s). Upon making the decision to collect samples, the PG team will note the descriptions of the proposed sampling points in the field notes, and document conditions at the private residence and sampling point.
Field notes will include:
A sample point description and collection procedure followed for each sample point;
A list of the samples collected at each point;
Water and air quality measurements;
General conditions of the sampling point; and
Notes regarding photographs or other documentation collected on-site pertaining to the sample point(s).
Samples will be collected, labeled, sealed, and placed in coolers for delivery to the laboratory. The COC forms will be completed and maintained with the coolers. The coolers will then be hand-delivered to the selected contract laboratory or to the courier employed by the contract laboratory.
The PG WAM will follow up with the contract laboratory the same or following day to ensure that samples were received and to obtain a copy of the COC forms.
This section of the SAP summarizes analytical laboratory contacts and addresses, and sampling team personnel and support functions.
Robert Helverson, MS
ATSDR Region 3 (Philadelphia), Eastern Branch
215-814-3139
Karen Scruton, MS
ATSDR, Science Support Branch
Atlanta, Georgia
770-488-1325
Lora Werner
ATSDR Region 3 (Philadelphia), Eastern Branch
215-814-3141
Microbac Laboratory
1620 North Main Avenue
Scranton, PA 18508
570-348-0775
TestAmerica - Edison
777 New Durham Road
Edison, NJ 08817
732-549-3900
Pace Analytical – Somerset Service Center
12 World's Fair Drive
Somerset, NJ 08873
732-652-6443
AccuStar Labs
929 Mount Zion Road
Lebanon , PA 17046
800-523-4964
717-274-8310
Naida Gavrelis
110 Hartwell Avenue
Lexington, MA 02421
781-674-7318
Kort Kirkeby
1113 Washington Avenue, Suite 200
Golden, CO 80401
720-789-8047
Danny O’Connell
1113 Washington Avenue, Suite 200
Golden, CO 80401
720-789-8032
Zak Maurer-Erickson
1113 Washington Avenue, Suite 200
Golden, CO 80401
720-789-8045
Stephen Clark
1113 Washington Avenue, Suite 200
Golden, CO 80401
720-789-8046
All samples from each private residence will be packed according to the following guidelines and then hand-delivered by a member of the PG team or a courier.
Prior to pouring/collecting samples, label each sample bottle. Cover the label with clear tape to protect this information.
Sample containers will be filled sufficiently to provide at least the minimum volume required for each analysis. In the case of VOC analysis, sample containers will be filled so that there is no airspace present in the container.
Tighten the lid on each filled sample bottle, being careful not to overtighten the lid. If bottle threads are dirty to where the lid is impeded from closing, clean the threads on the bottle being careful to not introduce contamination into the sample. Clean the sample bottle with a cloth rag or paper towel.
Place each sample bottle requiring 4°C preservation into an ice chest with wet ice prior to packaging to cool.
When each sample requiring 4°C preservation has reached the desired temperature, wrap each glass sample bottle with two layers of “bubble wrap” (or place bottle in two “bubble bags”). The bubble wrap must fit snugly and completely cover the sample bottle. Each “bubble-wrapped” container and plastic container must then be enclosed in an individual sealable plastic freezer bag. VOC/SVOC vials may be placed in VOC/SVOC bricks and placed in a freezer bag.
Place two garbage bags inside each other in the cooler. Place sample bottles in garbage bags in the cooler with proper end up, close the interior garbage bag by tying, or with a twist-tie.
Add ice to cooler to keep samples cold during shipment. Arrange blue ice or sealed plastic freezer bags filled with wet ice on top of the sample bottles (if samples require 4°C preservation). If using wet ice, place the ice inside two one-gallon sealable freezer bags. Put at least 4 × ½ gallons of ice (4 × 2.5 lbs of ice) in each large cooler and 2 × ½ gallons of ice (2 × 2.5 lbs of ice) in each small cooler. More ice should be used when ambient temperatures are very high. The ice should be placed inside the second garbage bag. Close the second garbage bag with a twist-tie.
Fill in around the bottles and any free space with additional cushioning material. Sufficient packing material should be used so that the sample containers will not shift during shipment.
Seal the COC form in a plastic zip-lock bag and tape securely to the inside of the cooler lid.
Place a "Return to ..." label on the inside of the cooler lid.
Close cooler.
Make several wraps with tape around the cooler perpendicular to the seal to ensure that the lid will remain closed if the latch is accidentally released or damaged.
Tape the cooler drain plug so it will not open.
Place a completed address label on the lid of the cooler including name, address, and telephone number of the receiving laboratory.
PG Environmental (May 2017). Quality Assurance Project Plan for Support to ATSDR Dimock, Pennsylvania Exposure Investigation. PG Environmental, Golden, CO.
U.S. Environmental Protection Agency (November 2008). Sampling Guidance for Unknown Contaminants in Drinking Water. EPA, Office of Environmental Information, Washington, DC, EPA/817/R-08/003.
U.S. Environmental Protection Agency (March 2006). EPA’s Interactive Sampling Guide for Drinking Water System Operators. EPA, Office of Environmental Information, Washington, DC, EPA/816/F/03/016.
U.S. Environmental Protection Agency (August 2012). Sampling and Analysis Plan Guidance and Template Version 3, Brownfields Assessment Projects. R9QA/008.1.
Attachment 1. Dimock EI: Indoor Air Sampling
Field Measurements of Toxic Vapors and Explosive Gases
Indoor air quality will be measured during drinking water sampling activities using a portable flame ionization detector and photoionization detector (equipped to measure total hydrocarbons and other volatile organic compounds [VOCs]). Sampling points will include, at a minimum, (1) points at which well water plumbing enters the home, and (2) bathrooms, kitchen, and laundry rooms where vapors released from water use may accumulate. In addition, a handheld LEL safety monitor will be used to screen for possible explosive atmospheres within the homes tested. Measurement records will be maintained in a dedicated field logbook or something similar.
TVA 2020
The Thermo Scientific TVA 2020 Toxic Vapor Analyzer is a dual detector device equipped with a flame ionization detector (FID) and a photoionization detector (PID). The device can operate both detectors simultaneously or one or the other. The FID requires hydrogen for the flame and an oxygen atmosphere above 16% to support the flame. The FID is highly sensitive to hydrocarbon vapors, including methane. The PID is sensitive to aromatic and chlorinated compounds. Instrument use requires initial training and strict adherence to standard operating procedures.
A calibration precision test will be completed before the analyzer is placed into service, and will be tested by making and recording a total of three measurements by alternately using zero gas and the specified calibration gas. After calculating the average algebraic difference between the instrument readings and the known value, this average will be divided by the known calibration value and multiplying by 100 to express the resulting calibration precision as a percentage. Calibration precision must be equal to or less than 10 percent of the calibration gas value.

For daily use, the TVA 2020 battery will be charged, the sample probe connected, and the hydrogen tank filled and installed (this starts the hydrogen flow), at which point the device will be turned on. Field personnel will perform a series of quality control (QC) checks. This will include a daily zero span check and a calibration check before taking any measurements. Calibration for the FID is typically performed with methane gas and the PID with isobutylene gas, and hence the minimum detectable level (MDL) will be expressed in terms of the calibration gas used (FID MDL = 0.5 ppm methane; PID MDL = 0.5 ppm isobutylene). The process involves filling a Tedlar bag with the calibration gas and connecting it to the device probe. For QC purposes, the daily calibration should be performed in the field but away from potential VOC emission sources.
Prior to sampling inside a participant’s home, ATSDR and ERG will determine what rooms and water emission source areas (e.g., pipe surfaces coming from the well, under sinks, in open spaces within the home) will be surveyed. After identifying areas to sampling, sampling staff will enter the home and proceed to the pre-identified locations. Field samples will be collected by placing the probe inlet at or near the predetermined water emission source areas. Sampling at each pre-identified location should be conducted for a minimum of 2 minutes. For example, field personnel will run the probe along pipes or determine ambient conditions by moving the probe in bathrooms or other areas where vapors could potentially accumulate. The TVA2020 probe will then be moved to an area in the room away from the pre-identified location that is believed to be away from potential sources, for a minimum of 2 minutes between sampling. This allows for the proper recovery of the TVA 2020 between measurements. Readings will be recorded by the TVA 2020 and sampling staff should also record general observations in a notebook.
The data can be logged internally on the TVA 2020 and downloaded. The recorded data will be downloaded to a computer using a USB cable. The device also has internal GPS to provide spatial data along with the readings. However, all test locations and readings also will be logged in the field notebook. Settings for data recording and GPS usage will be set each day during the instrument warm up or prior.
The Honeywell Gas Alert XL (or equivalent) is a small handheld/wearable multi-gas meter that is simple to use and runs for up to 18 hours on a charge. It provides real-time measurements for hydrogen sulfide (0-100ppm/1ppm resolution), carbon monoxide (0-500 ppm/1ppm resolution), oxygen (0-30.0%/0.1% resolution), and combustible gases (0-100% LEL/1% resolution). The LEL monitors provide an actual measured value but the TVA2020 will be the primary measurement for VOC emissions.
The primary purpose of the Gas Alert XL will be to alert sampling staff of an unsafe condition. The Gas Alert XL is programmed with several alarm thresholds that are presented in Table 1. One member of each field sampling team will be responsible for donning the meter and alerting the team should the alarm be triggered.
In the event one of the thresholds is exceeded and the Gas Alert XL enters an alarm state, all persons in the area wi be notified and will immediately leave the area. Work will not resume in the area until further sampling has been conducted to show the area is safe for sampling staff to enter.
Table 1. Gas Alert XL Alarm Threshold Settings
|
O2 |
LEL |
H2S |
CO |
%/vol |
%LEL (methane) |
PPM |
PPM |
|
Low |
19.5 |
10 |
10 |
35 |
High |
23.5 |
20 |
15 |
200 |
Time weighted average (TWA) |
NA |
NA |
10 |
35 |
Short-term exposure limit (STEL) |
NA |
NA |
15 |
50 |
Radon in Indoor Air
A “short-term” (2-4 day) field test will be conducted to evaluate average indoor air concentrations of radon in participant homes. Consistent with Pennsylvania and EPA radon short-term measurement protocols (https://www.epa.gov/sites/production/files/2014-08/documents/homes_protocols.pdf), activated charcoal adsorption devices will be used to collect samples. AccuStar Labs (AccuStar), a Pennsylvania-licensed firm, will provide the devices and conduct the analysis. Because radon levels tend to vary from day to day and season to season, a short-term test is less likely than a long-term test to report residents’ year-round average radon level, but provides a screening value.
AccuStar will supply PicoCan 275 open face activated charcoal canister radon sampling devices that consist of a metal canister measuring 2.75 inches in diameter, and 1 inch deep, and contains 25 grams of activated charcoal. The canister is closed with a metal lid and sealed with vinyl tape. AccuStar has developed a complete calibration for each device from devices that have been exposed in a radon chamber operated by Bowser-Morner in Dayton, Ohio, including exposures to different radon concentrations, durations, and relative humidity. Radon test kits will be provided in bulk and will include the device, instructions for use, bulk data chain of custody forms, and note sheets.
Canisters will be placed in up to two locations within each EI participant’s home, one in the basement and one in a living area potentially impacted by water vapor emissions. At the time of canister placement, field personnel will remove the tape and the lid. Field personnel will record and photograph the sample location(s) within each home. Homeowners will be advised not to tamper with or remove the device.
Generally, we will choose locations meeting the following criteria:
Device will not be disturbed
Adequate space for the device exists
Minimal draft potential exists (e.g., away from drafts caused by heating, ventilation and air conditioning vents, doors, fans, and windows)
Away from heat, such as on appliances or in direct sunlight
Avoiding areas of high humidity
At least 3 feet from doors and windows, or other openings to the outdoors
At least 20 inches from the floor, and at least 4 inches from other objects
Do not place in closets, cupboards, sumps, crawl spaces, or other nooks and crannies
For quality assurance, a second (duplicate) device will be placed 4 inches from the originally placed device for 10% of the samples. For example, if 40 locations are selected for radon testing, we will place a duplicate canister at 4 of those locations. In addition, a field blank (device brought to site, but not opened) will be collected, for every 1 in 20 samples.
After 3 days (72 hours), field personnel will collect the canisters, replace the lid on the device, and reseal the edge with vinyl tape. Samples devices must be returned to the lab within 8 days from the date of closing the device. Field personnel will track each device on the chain of custody form, and ship in bulk to AccuStar for laboratory analysis. Laboratory analysis will involve placing the closed device on a gamma detector and counting gamma emissions from the decay of radon adsorbed on the charcoal. The lab will use a calibration factor accounting for exposure time and decay time to calculate the radon concentration, applying a correction factor for any weight gain from moisture absorbed in the charcoal during the exposure period. The average radon concentration over the exposure period will be reported in picoCuries per liter (pCi/L).
Appendix E.2
Quality Assurance Project Plan for
Support to ATSDR in Drinking Water Exposure Investigation in Dimock, PA (Element A.1)
Prepared for:
Agency for Toxic Substances and Disease Registry
Division of Community Health Investigations
ATSDR Eastern Branch (Region 3) and
Science Support Branch (SSB)
Prepared by:
Eastern Research Group
110 Hartwell Avenue
Lexington, MA 02421
PG Environmental
1113 Washington Avenue, Suite 200
Golden, CO 80401
May 3, 2017
Quality Assurance Project Plan for Support to ATSDR Dimock, Pennsylvania Exposure Investigation
Approvals (Element A.1)
Wesley N. Ganter Program Manager PG Environmental, LLC
|
|
Date |
|
Jared Richardson Quality Assurance Officer PG Environmental, LL |
|
Date |
|
|
Naida Gavrelis Program/Task Order Manager Eastern Research Group
|
|
Date |
|
Karen Scruton EI Co-Lead ATSDR Headquarters
|
|
Date |
|
|
Robert Helverson EI Co-Lead ATSDR Region 3
|
|
Date |
|
|
|
|
|
|
Distribution (Element A.3) iii
1.0 Introduction and Background 1
2.0 Project Management Elements 2
2.1 Project/Task Organization (Element A.4) 2
2.1.1 Project Management and Technical Staff 2
2.1.2 Quality Assurance Staff 3
2.2 Problem Definition and Background (Element A.5) 4
2.3 Project/Task Description (Element A.6) 4
2.4 Quality Objectives and Criteria (Element A.7) 4
2.5 Special Training Requirements / Certification (Element A.8) 5
2.6 Documentation and Records (Element A.9) 5
3.0 Data Generation and Acquisition Elements 6
3.1 Sampling Process Design (Element B.1) 6
3.2 Sampling Methods (Element B.2) 7
3.3 Sample Handling and Custody (Element B.3) 9
3.4 Analytical Methods (Element B.4) 9
3.4.1 Analytical Laboratories 10
3.5 Quality Control (Element B.5) 13
3.6 Instrument/Equipment Testing, Inspection, and Maintenance (Element B.6) 14
3.6.1 FID/PID Calibration and Use 14
3.6.2 LEL Monitor Calibration and Use 14
3.6.3 Water Quality Multimeter Calibration and Use 15
3.7 Instrument/Equipment Calibration and Frequency (Element B.7) 17
3.8 Inspection/Acceptance of Supplies and Consumables (Element B.8) 17
3.9 Data Management (Element B.9) 17
3.9.2 Hardware / Software Configuration 18
4.0 Assessment and Oversight Elements 18
4.1 Assessments and Response Actions (Element C.1) 18
4.2 Reports to Management (Element C.2) 19
5.0 Data Review and Validation 19
5.1.2 Site Visits, Sampling, and Analysis 19
6.0 Reconciliation with User Requirements 20
Table 1. QAPP Distribution iii
Figure 1: Project Organization (Element A.4) 3
Attachment.1. Standard Operating Procedure for the Operation and Calibration of the TVA 2020
This Quality Assurance Project Plan (QAPP) will be distributed to staff of the Agency for Toxic Substances and Disease Registry (ATSDR), Eastern Research Group (ERG), PG Environmental (PG) list below in Table 1. A copy of the document will be provided to all PG personnel involved in the project, including those who join the project after the initial distribution of the QAPP.
Table 1. QAPP Distribution |
||
Name Title |
Contact Information |
Mailing Address |
Agency for Toxic Substances and Disease Registry |
||
Robert Helverson, MS EI Co-Lead |
215-814-3139 |
ATSDR US EPA Region 3 1650 Arch Street (3HS00) Philadelphia, PA 19103 |
Karen Scruton, MS EI Co-Lead |
770-488-1325 Isg3@cdc.gov |
ATSDR 4770 Buford Hwy, NE (MS F59) Atlanta, GA 30341 |
Lora Werner Quality Assurance Coordinator |
215-814-3141 Lkw9@cdc.gov |
ATSDR US EPA Region 3 1650 Arch Street (3HS00) Philadelphia, PA 19103 |
Eastern Research Group |
||
Naida Gavrelis Program/Task Order Manager |
781-674-7318 |
Eastern Research Group, Inc.
110 Hartwell
Avenue |
PG Environmental
|
||
Wesley Ganter Program Manager |
720-789-8030 |
PG Environmental, LLC 1113 Washington Avenue, Suite 200 Golden, CO 80401 (303) 279-1793 (fax)
|
Kort Kirkeby Work Assignment Manager |
720-789-8047 |
|
Zak Maurer-Erickson Work Assignment Technical Staff |
720-789-8045 |
|
Danny O’Connell Work Assignment Technical Staff |
720-789-8032 |
|
Stephen Clark Work Assignment Technical Staff |
720-789-8046 |
|
Jared Richardson Quality Assurance Officer |
720-789-8036 |
|
The Agency for Toxic Substances and Disease Registry has tasked Eastern Research Group (ERG) with supporting them as part of an upcoming Exposure Investigation (EI) in the Dimock, Pennsylvania area. PG Environmental (PG), a sister company to ERG, will be supporting ATSDR in the following items:
1. Test private well water (at the tap and raw groundwater) as well as supplied bulk water sources in the Dimock area for analytes of potential concern for health effects (inorganics, organic compounds, oil and grease, total petroleum hydrocarbon, radionuclides), physical hazard (methane) and general water quality (taste, smell, etc.) identified in the Health Consultation (HC) (2016).
2. Test indoor air for radon levels.
This document represents PG’s project-specific QAPP for planning for and collecting primary data. This QAPP is responsive to only the elements in EPA Requirements for Quality Assurance Project Plans QA/R-5 (1) that are applicable to the project, and follows EPA’s Guidance for Quality Assurance Project Plans QA/G-5 (2). It is a project‑specific supplement to PG’s corporate quality management protocols, which detail the responsibilities of PG’s Quality Assurance and Quality Control (QA/QC) personnel and project management team.
PG has organized this document according to the elements outlined in the above-mentioned EPA guidance document. Group A elements are addressed from the title page through Section 2.6 of the document, while applicable Group B elements are addressed in Section 3.0. Group C elements are addressed in Section 4.0, and applicable Group D elements are addressed in Section 5.0. References are provided in Section 7.0.
2.0 Project Management Elements
In this section, PG discusses its project management approach for the assignment. The discussion is organized as follows:
A.1 |
Title and Approval Sheet |
A.2 |
Table of Contents |
A.3 |
Distribution List |
A.4 |
Project/Task Organization |
A.5 |
Problem Definition/Background |
A.6 |
Project/Task Description |
A.7 |
Quality Objectives and Criteria |
A.8 |
Special Training/Certification |
A.9 |
Documents and Records |
Elements A.1 to A.3 were provided earlier in this document. The remaining elements are discussed below.
2.1 Project/Task Organization (Element A.4)
Figure 1 presents the organizational framework under which PG will develop and implement primary data collection. The chart depicts the staff with program management and technical responsibilities and those with QA/QC roles. It shows the relationship and lines of communication among all project participants, including those from ATSDR.
2.1.1 Project Management and Technical Staff
Kort Kirkeby, the PG Work Assignment Manager (WAM), is responsible for all management and administrative aspects of the technical work performed for the project and for ensuring that the quality of work, schedule, and budget meet the requirements of the work assignment. Mr. Kirkeby. He is the principal contact for the EPA PO on project issues, deliverables, and schedule. Mr. Kirkeby will keep Jared Richardson, the PG QA Officer (QAO), informed of any quality issues that arise.
Mr. Kirkeby will lead PG’s efforts and be supported by other PG project staff as needed. PG technical staff will be responsible for collecting, analyzing, and documenting the data collected under this assignment. These staff will adhere to the QA guidelines outlined in this QAPP when providing technical support under the work assignment. In addition, they will maintain complete, legible, permanent, and defensible records documenting all work performed.
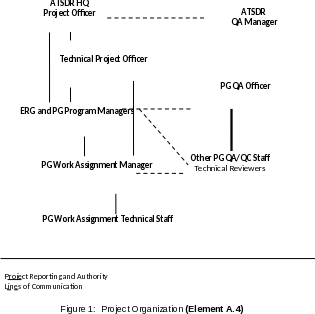
Jared Richardson, PG’s QAO, is responsible for developing and maintaining PG’s QA program based on PG’s quality management protocols. He provides direction and guidance to PG QA/QC staff and, through them, to the technical and administrative staff completing the work. Mr. Richardson, following consultation with PG’s Program Manager (PM), Wesley Ganter, has the authority to suspend the performance of any activities determined to be deviating from the established quality management procedures, work plan, or project-specific QAPP until appropriate corrective actions are instituted. ERG’s Naida Gavrelis, ATSDR contract and task order manager, is ultimately responsible for ensuring that all quality control measures outlined in this QAPP are strictly adhered to throughout the performance of this EI.
2.2 Problem Definition and Background (Element A.5)
The objective of this work assignment is to provide support to ATSDR in their EI of private residences’ water wells in Dimock, PA. PG will provide field sampling assistance for the inspections, which includes the selection of appropriate analytical laboratories, sending field sampling teams to accompany ATSDR staff during their evaluations, measuring drinking water quality and indoor air quality, collecting water samples, and delivering those samples to the laboratories, tracking and receiving the analytical results, and then providing those results in a written report.
This QAPP specifically addresses implementation of the analytical laboratory selection, sample collection, and delivery tasks.
2.3 Project/Task Description (Element A.6)
Task one is the development and approval of the work plan and cost estimates for labor, travel, and laboratory and analysis costs for sample collection. This task is currently underway. Task two is the development of this QAPP and then accompanying ATSDR staff to private residences in Dimock, PA to collect the samples, and delivering the samples to the laboratories. Task three is providing ATSDR staff with a brief summary of the private residence sampling via email the week after the field sampling. Task four is the receipt of analytical results and production of a report, which is to include a brief narrative of the samples that were collected and an overview of the analytical results, a table showing the actual analytical results, GPS data that shows where samples were collected, and then photographs relevant to the field sampling process, such as the location that was sampled and site conditions at that sampling location.
The QA/QC activities associated with Tasks 2 are discussed in Section 3 of this QAPP.
2.4 Quality Objectives and Criteria (Element A.7)
The primary objective of this QAPP is to ensure that the data collected will be of the quality necessary to support ATSDR in their EI of private water wells in the Dimock area. PG will maintain a continuing dialog with ATSDR EI Co-Leads on technical data issues and disclose data quality information provided by the contract laboratories (e.g., limits on use, accuracy estimates).
PG has defined the acceptance criteria (i.e., data specifications) that it will use to evaluate whether the data collected will be suitable for ATSDR’s needs. The acceptance criteria relate to the representativeness, completeness, and comparability of data.
Data representativeness
PG will ensure that field samples collected are representative of the private residence that is being sampled.
Data completeness
PG will ensure that complete samples sufficient to run the requested analyses will be included, and that the necessary quality control trip blanks and laboratory calibration records are provided with the sampling report in order to determine the reliability of the sample data. In any statistical summaries, data gaps will be excluded (i.e., they will not be counted as zero values).
Data comparability
Data sets will be checked for comparability. Comparability is a qualitative measure of the confidence with which data sets can be compared. PG will check that all analytical data received from the contract laboratories use the standard units required by the method to allow data to be compared with other results and national standards. This project will achieve data comparability by following approved, standard analytical procedures and by reporting the results in the standard units of measure required in the methods.
2.5 Special Training Requirements / Certification (Element A.8)
PG personnel have experience in analytical laboratory selection and contracting, field sampling, data collection and compilation, and report writing. The PG personnel that will perform technical support roles on this assignment have served in similar capacities on other projects.
2.6 Documentation and Records (Element A.9)
PG has developed and instituted document control mechanisms for reviewing, revising, and distributing QAPPs. Each QAPP has a signed approval form, title page, and table of contents. During the course of the project, PG will circulate any revisions to the QAPP to technical and QA/QC project staff at ERG, PG, and ATSDR.
PG will adhere to its standard practices for managing project-related data, documents, and records. All technical data collected, generated, and analyzed on the project will be handled and stored in a centralized file in PG’s Golden, Colorado office. The centralized file will contain all data collected or documents produced in the course of this project.
PG describes how it will manage primary data collected in Section 3.10 of this QAPP under Element B.9, Data Management. Computer files are maintained by PG employees and on PG’s servers. All individual employee documents on workstation hard drives are on an automatic back-up system through a company network.
All quality system documents and records will be maintained for the life of the contract. Quality system records are filed according to guidance provided by the PG Program Manager and QAO.
Disposition of quality system documents and records is determined in accordance with contract requirements.
3.0 Data Generation and Acquisition Elements
The project involves the selection of appropriate analytical laboratories to perform analysis of physical collected samples from the field, and delivery of those samples to the laboratories. PG has identified ten elements that are relevant to this task. PG’s approach to each of these elements is described in the subsections that follow.
Element B.1, Sampling Process Design
Element B.2, Sampling Methods
Element B.3, Sample Handling and Custody
Element B.4, Analytical Methods
Element B.5, Quality Control
Element B.6, Instrument/Equipment Testing, Inspection, and Maintenance
Element B.7, Instrument/Equipment Calibration and Frequency
Element B.8, Inspection/Acceptance of Supplies and Consumables
Element B.9, Data Management
Due to the variable voluntary participation of homeowners in the EI, as well as the different layout, age, differing technologies that may be in use, and the overall condition of the multiple private residences that will be visited, the specific sampling points have not been specified in advance. The selection of the sampling point, overall site and sample point condition, and the deciding factors for the use of that sampling point will be carefully noted in the field, and described in the post-sampling report. PG is prepared to sample up to 20 private residences during the proposed sampling week at the end of June 2017. Water samples will be collected from multiple locations at each residence, including (1) a sample at the primary drinking water location, e.g, kitchen tap, (2) a sample of raw water, purged and taken before any treatment system, to represent conditions in the underlying groundwater and (3) at a tap where supplied bulk water is accessible.
The drinking water sample collected from each residence will be a “first draw” sample collected from the primary drinking water tap (e.g., kitchen sink or refrigerator tap). The raw water sample will be collected following a purge of the plumbing system in order to access raw groundwater (as determined by field measurements of pH, temperature, conductivity, etc.). A supplied bulk water sample will be taken at the bulk water tap, if available.
Water samples will be analyzed for the constituents listed in Table 2, below. PG intends to use Microbac Laboratory in Scranton, PA for the total and fecal coliform analysis. This is due to the short (30 hour) hold time and close proximity of the lab to the Dimock, PA area. PG intends to use either TestAmerica in Edison, NJ or Pace Analytical in Somerset, NJ to complete the remainder of the drinking water sample analysis.
Indoor air monitoring for total volatile organic compounds (VOCs), utilizing a photoionization detector/flame ionization detector (PID/FID), and lower explosive limit (LEL) monitoring will be conducted at multiple locations within the residence, including (1) the point where groundwater plumbing enters the home, and (2) bathrooms, kitchen, and laundry rooms where vapors released from water use may accumulate. In addition, “short-term” (72-hour) indoor air testing for radon will be conducted using activated charcoal adsorption devices, placed in up to two locations within each EI participant’s home, one in the basement and one in a living area potentially impacted by water vapor emissions.
The following provides a general description of anticipated sample collection points and techniques to be followed at each private residence. Specific sampling points and locations, and the sampling methodologies for each site will be carefully noted in the field and included in the final sampling report.
The specific sampling methods and procedures followed at each private residence will be directed by ATSDR staff accompanying the PG sampling team. ATSDR staff will assess the conditions at each private residence and, based on the availability of sampling points, will direct the sampling team to use the most appropriate sampling point and method available.
In an ideal sampling situation, the sampling points would be:
A grab sample at a sampling spigot or tap used as the primary source of drinking water (e.g., kitchen sink);
A grab sample at a pre-treatment sampling spigot or tap that will be purged to allow testing of the raw water in the underlying groundwater; and
A grab sample at a sampling spigot or tap at a location where supplied bulk water is accessible.
At a private residence without such an ideal sampling point, a grab sample would be obtained from the nearest available service point. As a third option, in the absence of an accessible spigot or tap is obtaining a grab sample through the use of a polyethylene bailer.
Table 2 lists the specific field sampling methodology, equipment, and sample preservation for each analyte in the sampling plan.
Table 2. Field Sampling Methodology and Equipment |
|
Analyte |
Field Sampling Method and Equipment |
Water Testing |
|
Water Quality (pH, temperature, conductivity, dissolved oxygen, TDS) |
Immediate field analysis using YSI ProPlus Multiparameter Probe, Hach SL1000 Portable Meter, or similar multiparameter probe |
Total Coliform (presence/absence only) |
Grab sample 125mL sterilized, plastic bottle Sodium thiosulfate preservative |
Fecal Coliform (only if Total Coliform present) |
Grab sample 125mL sterilized, plastic bottle Sodium thiosulfate preservative |
Inorganic Ions |
Grab sample 1L plastic sample bottle (No preservative needed)
|
Trace Elements |
Grab sample 1L plastic sample bottle Nitric acid |
Oil and Grease |
Grab sample 1L glass sample bottle Hydrochloric Acid or Sulfuric Acid |
Dissolved Gases |
Grab sample 2-40-mL VOA vials Hydrochloric Acid |
TPH DRO/GRO |
Grab sample 3-40-mL VOA vials Hydrochloric Acid |
Alcohols and Glycols |
Grab sample 3-40 mL VOA glass Hydrochloric Acid or Unpreserved |
SOCs |
Grab sample 250mL glass or plastic bottle acid preservative |
VOCs |
Grab sample 120mL glass vials powdered dechlorinating agent, if needed (ascorbic acid) 1: 1 hydrochloric acid solution for each 20mL of sample volume |
Radionuclides |
Grab sample 1 gallon plastic sample container Hydrochloric Acid or Nitric Acid |
Indoor Air Testing |
|
Volatile Organic Compounds (VOCs) and combustible gases, including methane |
FID/PID (Thermo Scientific TVA 2020 Toxic Vapor Analyzer) and LEL Monitor (Honeywell Gas Alert XL or similar) |
Radon |
PicoCan 275 open face activated charcoal canister |
Pre-sampling training will be provided to PG’s sampling teams. That training will include:
Health and Safety refresher training, including hazard recognition and avoidance, with a focus on potential hazards that are typically encountered during sampling.
Sampling procedure training, including:
Sampling preparation;
Appropriate drinking water sample collection locations and unique considerations (e.g., the need to purge sample points, removal of aeration devices or screens, flaming of outdoor sample points);
Correct grab sample methods for non-tap locations;
Preservation (e.g., chemicals, ice);
Indoor air monitoring device use;
Water quality multimeter use and calibration; and
Shipping and packing, with attention to preparing bottles, labeling, packing coolers, and proper Chain-of-Custody (COC) use.
Conditions at the sampling locations, such as unusual operating conditions and odors or visual appearance, will be recorded in the sampling teams’ field notes for inclusion in the final report. Sampling staff will also take photographs of the sampling locations for inclusion in the report.
PG’s sampling teams will conduct all sampling activities in a manner to minimize potential contamination and cross-contamination of samples. Sampling staff will wear new nitrile gloves at each sampling point in order to avoid exposure to pollutants and other chemical, physical, and biological hazards, and to prevent cross-contamination of samples. Sampling staff will use a new pair of gloves at each sampling point each time a grab sample is collected. Sampling staff will take care not to touch the insides of bottles or lids and caps during sampling.
Typical field sampling equipment used for sampling at each private residence includes pre-cleaned sample bottles for chemical analyses and sampling collection devices, if needed, such as a polyethylene bailer. PG will use information obtained from the contract laboratory engaged to run the samples in order to determine the proper size and type of bottle to use for each sample, as well as appropriate preservatives. Excess sampling equipment will be ordered and brought to each sampling location as a contingency against breakage, malfunction, or loss.
In order to maintain scientifically defensible sampling data, sample integrity must be maintained at all times from sample collection through analysis. Correct and detailed sample labeling will be used and appropriate Chain of Custody (COC) forms will be maintained. In order to stay within appropriate holding times and ensure safe delivery, the samples that are collected are to be hand-delivered to the laboratories by the sampling technician, or provided to a courier who will hand-deliver the samples. Coolers and ice will be used to transport the samples and maintain holding temperatures. The COC will be correctly signed during the transfer of these samples and at the time of delivery to the lab, and those records will be provided to ATSDR in the final sampling report.
Table 3 lists the analytical methods that will be used for collected samples. These analytical methods are publicly available and details of the methodology are not included in this document.
All sample will be run by certified contract laboratories, who will perform any required sub-sampling, extraction methods, decontamination procedures, and dispose of measured samples as required. Failures in the analytical system will be the responsibility of the contract laboratories, as well as sample analysis anomalies or irregularities, and would be documented and reported in the final sampling report. When possible, sufficient sample volume will be sampled in the field such that in the event of an analytical failure, the laboratory would have the opportunity to run an additional sample.
Microbac Laboratory
1620 North Main Avenue
Scranton, PA 18508
570-348-0775
TestAmerica - Edison
777 New Durham Road
Edison, NJ 08817
732-549-3900
Pace Analytical – Somerset Service Center
12 World's Fair Drive
Somerset, NJ 08873
732-652-6443
AccuStar Labs (Radon)
929 Mount Zion Road
Lebanon , PA 17046
800-523-4964
717-274-8310
Table 3. Analytes and Analytical Methods |
|
Sample |
Method |
Water Sampling |
|
Water Quality: pH, temperature, conductivity, dissolved oxygen, total dissolved solids (TDS) |
Immediate field analysis via hand-held multimeter |
Total Coliform (presence/absence only) |
SM 9223 B |
Fecal Coliform |
SM 9223 B |
Inorganic Ions: Bromide, Chloride, Fluoride, Sulfate as SO4 |
EPA 300.0 |
Trace Elements: Aluminum, Antimony, Arsenic, Barium, Beryllium, Boron, Cadmium, Calcium, Chromium, Cobalt, Copper, Iron, Lead, Lithium, Magnesium, Manganese, Nickel, Potassium, Selenium, Silver, Sodium, Strontium, Thallium, Tin, Titanium, Uranium, Vanadium, Zinc |
EPA 200.7 & 200.8 |
Oil and Grease |
EPA 1664B |
Dissolved Gases: methane, ethane, ethene, propane, butane |
RSK-175 |
Total Petroleum Hydrocarbons (TPH) DRO/GRO, Alcohols, Glycols |
EPA 8015C (SW-846) |
Semi-volatile Organic Compounds (SVOCs) |
|
1,1-Biphenyl, 1,2,4,5-Tetrachlorobenzene, 1-Methylnaphthalene, 2,3,4,6-Tetrachlorophenol, 2,4,5-Trichlorophenol, 2,4,6-Trichlorophenol, 2,4-Dichlorophenol, 2,4-Dimethylphenol, 2,4-Dinitrophenol, 2,4, Dinitrotoluene, 2,6-Dinitrotoluene, 2-Chloronaphthalene, 2-Chlorophenol, 2-Methoxyethanol, 2-Methylnaphthalene, 2-Methylphenol, 2-Nitroaniline, 2-Nitrophenol, 3,3´-Dichlorobenzidine, 3-Nitroaniline, 4,6-Dinitro-2-methylphenol, 4-Bromophenyl phenyl ether, 4-Chloro-3-methylphenol, 4-Chloroaniline, 4-Chlorophenyl phenyl ether, 4-Methylphenol, 4-Nitroaniline, 4-Nitrophenol, Acenaphthene, Acenaphthylene, Acetophenone, Anthracene, Atrazine, Benzaldehyde, Benzo(a)anthracene, Benzo(a)pyrene, Benzo(b)fluoranthene, Benzo(ghi)perylene, Benzo(k)fluoranthene, Bis(2-chloroethoxy)methane, Bis(2-chloroethyl)ether, Bis(2-chloroisopropyl)ether, Bis(2-ethylhexyl)phthalate, Butyl benzyl phthalate, Caprolactam, Carbazole, Chrysene, Dibenz(a,h)anthracene, Dibenzofuran, Diethyl phthalate, Dimethyl phthalate, Di-n-butyl phthalate, Di-n-octyl phthalate, Fluoranthene, Fluorene, Hexachlorobenzene, Hexachlorobutadiene, Hexachlorocyclopentadiene, Hexachloroethane, Indeno(1,2,3-cd)pyrene, Isophorone, Naphthalene, Nitrobenzene, N-Nitrosodimethylamine, N-Nitroso-di-n-propylamine, N-Nitrosodiphenylamine, Pentachlorophenol, Phenanthrene, Phenol, Pyrene |
EPA 8270 |
Volatile Organic Compounds (VOCs) |
|
1,1,1,2-Tetrachloroethane, 1,1,1-Trichloroethane, 1,1,2,2-Tetrachloroethane, 1,1,2-Trichloroethane, 1,1-Dichloroethane, 1,1-Dichloroethene, 1,1-Dichloropropene, 1,2,3-Trichlorobenzene, 1,2,3-Trichloropropane, 1,2,4-Trichlorobenzene, 1,2,4-Trimethylbenzene, 1,2-Dibromo-3-chloropropane, 1,2-Dibromoethane (EDB), 1,2-Dichlorobenzene, 1,2-Dichloroethane, 1,2-Dichloropropane, 1,3,5-Trimethylbenzene, 1,3-Dichlorobenzene, 1,3-Dichloropropane, 1,4-Dichlorobenzene, 2,2-Dichloropropane, 2-Butanone, 2-Chloroethylvinyl ether, 2-Chlorotoluene 2-Hexanone, 4-Chlorotoluene, 4-Methyl-2-pentanone, Acetone, Acrylonitrile, Benzene, Bromobenzene, Bromochloromethane, Bromodichloromethane, Bromoform, Bromomethane, Carbon disulfide, Carbon Tetrachloride, Chlorobenzene, Chlorodibromomethane, Chloroethane, Chloroform, Chloromethane, cis-1,2-Dichloroethene, cis-1,3-Dichloropropene, Cyclohexane, Dibromomethane, Dichlorodifluoromethane, Ethylbenzene, Freon 113, Hexachlorobutadiene, Isopropylbenzene, Methyl Acetate, Methylcyclohexane, Methylene Chloride, Methyl-tert-butyl ether, m-Xylene/p, Xylene, Naphthalene, n-Butylbenzene, n-Propylbenzene, o-Xylene, p-Isopropyltoluene sec-Butylbenzene, Styrene, tert-Butylbenzene, Tetrachloroethene, Toluene, trans-1,2-Dichloroethene, trans-1,3-Dichloropropene, Trichloroethene, Trichlorofluoromethane, Vinyl acetate, Vinyl Chloride |
EPA 8260
|
Radionuclides |
|
Gamma spectroscopy |
EPA 901.1 |
Gross alpha |
EPA 00-02 |
Isotopic Thorium & Isotopic Uranium |
A-01-R |
Radium 226 and 228 |
EPA 903.0 and EPA 904.0 |
Radon |
EPA 913 |
Indoor Air Sampling |
|
VOCs and combustible gases, including methane |
Immediate field analysis via FID/PID and LEL |
Radon (indoor air) |
PicoCan 275 open face activated charcoal canister |
The sampling program will include the use of both field and laboratory quality control samples. These controls are part of the overall review of sampling and process data. The review considers detected and nondetected pollutants, pollutant concentrations, laboratory quality control issues, and site conditions present during field sampling that may have affected analysis results.
The primary field quality control method for this project will be the use of trip blanks and duplicate samples. The primary lab quality control method will be the verification of laboratory control samples.
The contract laboratories will provide trip blanks, typically an analyte-free matrix like high performance liquid chromatography (HPLC) water, for each of the samples that require it, such as volatile organic compounds (VOCs). One set of trip blanks per sampling site will be used. The sampling technician will preserve the trip samples and transport them unopened to the contract laboratories. The laboratories typically analyze these samples only for volatile organic compounds;. The purpose of this field quality control method is to monitor organic contamination (volatiles)of samples during transport, field handling, and storage.
PG will collect a full suite of duplicate samples at 10 percent of the total number of private residences sampled as part of the EI. Duplicate samples are collected simultaneously with a standard sample from the same source under identical conditions into separate sample containers. Field duplicates will consist of a homogenized sample divided in two or else a co-located sample. Each duplicate portion will be assigned its own sample number so that it will be blind to the laboratory. A duplicate sample is treated independently of its counterpart in order to assess laboratory performance through comparison of the results.
Certified contract laboratories that maintain, test, and regularly inspect their equipment have been chosen for this sampling project. Water quality multimeter calibration and air monitoring device calibration and use are elaborated upon in the following sections.
3.6.1 FID/PID Calibration and Use
The Thermo Scientific TVA 2020 Toxic Vapor Analyzer is a dual detector device equipped with a flame ionization detector (FID) and a photoionization detector (PID). The device can operate both detectors simultaneously or one or the other. The FID requires hydrogen for the flame and an oxygen atmosphere above 16 percent to support the flame. The FID is highly sensitive to hydrocarbon vapors, including methane. The PID is sensitive to aromatic and chlorinated compounds. Instrument use requires initial training and strict adherence to standard operating procedures.
A calibration precision test will be completed before the analyzer is placed into service, and will be tested by making and recording a total of three measurements by alternately using zero gas and the specified calibration gas. After calculating the average algebraic difference between the instrument readings and the known value, this average will be divided by the known calibration value and multiplying by 100 to express the resulting calibration precision as a percentage. Calibration precision must be equal to or less than 10 percent of the calibration gas value.

For daily use, the TVA 2020 battery will be charged, the sample probe connected, and the hydrogen tank filled and installed (this starts the hydrogen flow), at which point the device will be turned on. Field personnel will perform a series of quality control (QC) checks. This will include a daily zero span check and a calibration check before taking any measurements. Calibration for the FID is typically performed with methane gas and the PID with isobutylene gas, and hence the minimum detectable level (MDL) will be expressed in terms of the calibration gas used (FID MDL = 0.5 ppm methane; PID MDL = 0.5 ppm isobutylene). The process involves filling a Tedlar bag with the calibration gas and connecting it to the device probe. For QC purposes, the daily calibration should be performed in the field but away from potential VOC emission sources. See Attachment 1 for detailed SOPs for the TVA 2020 analyzer.
3.6.2 LEL Monitor Calibration and Use
The Honeywell Gas Alert XL will be calibrated prior to use by applying a known concentration of methane, hydrogen sulfide, and carbon monoxide separately to the meter’s detector. The calibration involves an adjustment to the zero value calibration factor and the span calibration factors. Each day prior to operation, a “bump” test will conducted. A “bump” test is accomplished by challenging the meter with a known concentration of gas to ensure it is responsive (appropriately triggers the alarm). The calibration procedure will only need to be repeated every 180 days or if a “bump” test fails.
3.6.3 Water Quality Multimeter Calibration and Use
PG staff will collect pH, conductivity, dissolved oxygen, temperature, and total dissolved solids (TDS) measurements at each home utilizing a hand-held water quality meter.
pH Calibration
Calibrations must be conducted every day prior to use of the multimeter probe. Calibrations for pH include a 2-point calibration at the typical extremes of surface water pH, followed by a validation at an intermediate pH value. Each meter's user’s manual will be used for specific instructions on accessing the calibration menus for that meter. The following provides step-by-step instructions for completing the calibration:
Rinse the pH probe and the calibration cup thoroughly with deionized water.
Fill the calibration cup half-way with pH 4.0 buffer, immerse the probes into the buffer within the cup, and then remove the cup and pour the pH 4.0 buffer over the pH probe and other probes to displace any remaining tap water. Carefully shake free any remaining fluid from the calibration cup and the probe.
Fill the calibration cup to the appropriate level with fresh pH 4.0 buffer.
Allow the pH readings on the meter to stabilize (minimum of 60 seconds) before calibrating to the pH 4.0 buffer. Record on the initial calibration reading immediately prior to calibration, whether the reading was calibrated, and the final reading on the meter. The time of meter calibration is also recorded along with the name of the analyst performing the calibration.
Rinse the calibration cup and pH probe (and other affected probes) thoroughly with deionized water.
Fill the calibration cup half-way with pH 10.0 buffer, immerse the probes into the buffer within the cup, and then remove the cup and pour the pH 10.0 buffer over the pH probe and other probes to displace any remaining tap water. Carefully shake free any remaining fluid from the calibration cup and the probe.
Fill the calibration cup to the appropriate level with fresh pH 10.0 buffer.
Allow the pH readings on the meter to stabilize (minimum of 60 seconds) before calibrating to the pH 10.0 buffer. Record the final stabilized reading for this pH 10.0 buffer.
Rinse the calibration cup and pH probe (and other affected probes) thoroughly with deionized water.
Fill the calibration cup half-way with pH 7.0 buffer, immerse the probes into the buffer within the cup, and then remove the cup and pour the pH 7.0 buffer over the pH probe and other probes to displace any remaining tap water. Carefully shake free any remaining fluid from the calibration cup and the probe.
Fill the calibration cup to the appropriate level with fresh pH 7.0 buffer.
Allow the pH readings on the meter to stabilize (minimum of 60 seconds) before reading this intermediate verification value. Record the final stabilized reading for this pH 7.0 buffer.
Rinse the calibration cup and pH probe (and other affected probes) thoroughly with deionized water, and prepare for either further calibrations or for storage and travel.
pH Measurement
Collect a sufficient volume of water in a thoroughly rinsed (using deionized water) glass jar so that the probe is fully submersed. Tum the meter on, record the time, and wait an initial 60 seconds before checking pH readings. Starting 60 seconds following deployment/turning on the electronics, observe the pH readings and record an initial pH reading and the time of measurement at the point when pH values stabilize (e.g., no change more than 0.02 units within 10 seconds). Record a duplicate reading and the time of the measurement at least 60 seconds following the initial reading and up to 5 minutes following that reading. Remove the probe, retain a small volume of either tap water within the travel cup, and finish packing the meter for travel.
Conductivity Calibration
Rinse the electrode with deionized water to remove impurities. Shake or air-dry.
Perform the calibration using standard solution of 1413 μS and record the details
Rinse the electrode thoroughly with deionized water.
Conductivity Measurement
Rinse the electrode with deionized water to remove impurities. Shake or air-dry.
Place probe in water to be measured. The probe must be completely immersed past the temperature sensor. Bubbles can become trapped inside of internal gap, which will result in erroneous values. To avoid this situation, shake probe after immersion to dislodge air bubbles.
Wait until the temperature reading has stabilized.
After the display has stabilized, record the conductivity value and note the units of measurement (μS/cm or mS/cm).
Record the measurement. The data value should be recorded in μS (1 mS = 1000 μS).
Rinse the electrode between samples and after the last measurement with deionized water.
Dissolved Oxygen Calibration
Calibration of the meter should occur prior to initial sample collection. Allow the probe to warm-up and stabilize for 15 to 30 minutes - this will vary depending on the model. Calibration checks should be conducted prior to additional collections or at least every 4 hours. If the calibration check is greater than 5% of the theoretical result, the meter should be re-calibrated. A calibration should be completed at a temperature that is as close as possible to the sample temperature. Results of the calibration check should be recorded on the appropriate field data sheet and/or field notebook.
Dissolved Oxygen Measurement
1. The probe should be submerged completely.
2. Allow sufficient time for the probe to stabilize with the sample temperature. If the probe was calibrated under ambient temperature conditions, this step is not required.
3. Allow a few minutes for the dissolved oxygen to stabilize.
4. Record the result in the field notebook.
5. Rinse electrode and replace in humidity chamber.
6. Leave meter on until the last site is sampled for the day. This will avoid the need for recalibration.
Temperature Calibration
The field collector should select one meter to make electronic measurements and use that meter to make all temperature readings. Preferably, the dissolved oxygen meter should be used for temperature readings. This meter, along with all other meters, should be calibrated against a National Institute of Standards and Technology (NIST) certified thermometer (or to one that is traceable to a NIST thermometer) each quarter.
Temperature Measurement
After placing the probe in water, allow at least one minute for equilibrium to occur; switch meter to temperature mark. Read temperature in degrees Celsius to the nearest 0.01.
TDS Calibration
There is no calibration procedure for TDS. TDS is calculated internally based on the specific conductivity reading (in mS/cm) and a TDS constant (between 0.3 and 1.00).
TDS Measurement
After placing the probe in water, allow at least one minute for equilibrium to occur; switch meter to TDS mark. Read TDS in mg/L.
The PG WAM will request calibration and laboratory control sample analysis records from the certified contract laboratories that are engaged to perform the sample analysis. Hand-held multimeters, used to measure water quality, and indoor air monitoring equipment will be calibrated daily prior to collecting field measurements.
Sampling supplies and consumables for this project include those that come in contact with samples, such as sample bottles, preservation chemicals, blank water, and sampling equipment. The WAM and the sampling staff are responsible for purchasing or requesting required materials from the contract laboratories and inspecting those materials upon receipt to ensure they are usable for this project (e.g., inspecting for breakage, evidence of tampering, or contamination). Where practical, PG will use new sampling equipment materials, such as nitrile gloves and polyethylene bailers, which will be dedicated to a single sampling point/grab and then disposed.
PG will implement the following data management and computer hardware and software configuration to maintain the quality of the data collected.
PG will adhere to the documentation and data reporting procedures described below during field data collection operations. Field documentation tools for this project will include sample labels, field logs, and COC. The sampling technician will review all field documentation and ensure that all records are legible and complete.
3.9.2 Hardware / Software Configuration
The work that PG will perform as part of this project will involve the acquisition, receipt, and processing of data (such as photographs, field notes, analytical results, checklists, and documents) and then the generation of final reports and documents, all of which will require the maintenance of computer resources. PG maintains their computers using in-house specialists for routine maintenance and service.
PG uses surge suppressors to protect the electronic components within their microcomputers from potentially damaging voltage spikes. PG regularly reminds their personnel to maintain files on the network server. Additionally, PG backs up its network servers nightly during the business week. It is company policy to screen files for viruses before they are loaded onto microcomputers. PG has installed virus-screening systems on their workstation computers and network servers; these systems are updated regularly.
This section describes the methods that PG will use to assess the quality of work conducted for the data collection task.
The PG WAM and PG QAO will appoint Technical Reviewers as needed during the project. Technical reviews are critical evaluations of work performed. PG will adhere to its standard procedures for conducting and documenting such reviews, as well as the responsibilities of reviewers. In general, technical reviews are conducted by professionals who are proficient in the work areas of interest, but who were not directly responsible for performance of the work. Technical reviewers document the results of their reviews. For this work assignment, the PG WAM is responsible for ensuring that technical reviews were conducted by the appropriate personnel and documented according to company policies.
PG’s QAO will assess the implementation of QA/QC procedures on this project as follows:
Review this QAPP for completeness and applicability and sign the signature page of the QAPP following successful review(s).
Train all project staff to ensure that all QC procedures in the QAPP are understood prior to initiation of technical support.
Audit project files to verify that project staff is (1) using the QC procedures identified in this QAPP, (2) using appropriate standard operating procedures, and (3) documenting the deliverable review process. If not, the PG QAO will direct the PG WAM to implement corrective action and document that the action was taken. Corrective action may include additional staff instruction or the development and use of additional measures to ensure the quality of data management and review.
4.2 Reports to Management (Element C.2)
The PG WAM will inform ERG and ATSDR of the progress of all work activities throughout the project. PG will also give verbal reports during status meetings and, if urgent issues arise, verbal reports will be provided to ERG and ATSDR immediately.
PG’s Technical Reviewers and the QAO will communicate any quality deficiencies identified as a result of reviews or audits to the PG WAM in writing. If quality objectives and criteria are not met, the PG WAM is responsible for ensuring that the appropriate corrective actions are taken and reported to PG’s QAO.
This section describes data review, verification, and validation. It also discusses how validated data will be evaluated to determine if they adequately answer the stated goals in Section 2.3.
5.1 Data Review, Verification, and Validation; and Verification and Validation Methods (Element D.1 and D.2)
This subsection discusses how PG will check information collected during the sampling program to determine how they can be used.
Data review is an in-house data examination, made to ensure that data have been recorded, transmitted, and processed correctly; for example, by checking for transcription and calculation errors. PG’s data management procedures ensure the quality and completeness of analytical laboratory reports are described in Section 3.10 and subsections therein.
Data verification is the confirmation by examination and review of objective evidence that the data are complete, correct, consistent, and in compliance with technical requirements, established standards, and contractual requirements. Data validation is the confirmation by a person different from the data generator that the particular requirements for a specific intended use are fulfilled.
PG will verify transcription of analytical laboratory data into final reports. PG will use procedures appropriate to the data source and transfer procedures to verify that data obtained from the analytical laboratory reports, both electronic and hard copy, have been transcribed accurately. Specifically, PG will use 100 percent checking of all transcribed data. If any errors are detected, PG will correct it, and document the error if necessary.
5.1.2 Site Visits, Sampling, and Analysis
PG will conduct an overall assessment of the sampling and analytical data. This review will consider the detected and non-detected contaminants, contaminant concentrations, laboratory quality control issues described in the data review narratives, and data variability in relation to drinking water samples collected. PG will report any used measures of quality used to determine the accuracy, precision, and completeness of the site visit and sampling data to ERG and ATSDR.
The primary data collected will be used to provide support to ATSDR in their EI, as detailed in Section 2.2 of this QAPP. The analytical results from the field samples collected will be used in ATSDR EI reports.
PG will concisely and completely document and report all sampling conditions, the samples collected, and analytical results as required for the completion of the project. PG will document all analytical issues or deviations from the original sampling plan, if any, in order to meet ATSDR’s standards of transparency, objectivity, integrity, and utility.
U.S. Environmental Protection Agency (March 2001). EPA Requirements for Quality Assurance Project Plans, EPA QA/R-5. EPA, Office of Environmental Information, Washington, DC, EPA/240/B-01/003.
U.S. Environmental Protection Agency (December 2002). Guidance for Quality Assurance Project Plans, EPA QA/G-5. EPA, Office of Environmental Information, Washington, DC, EPA/240/R-02/009.
U.S. Environmental Protection Agency (November 2008). Sampling Guidance for Unknown Contaminants in Drinking Water. EPA, Office of Environmental Information, Washington, DC, EPA/817/R-08/003.
U.S. Environmental Protection Agency (March 2006). EPA’s Interactive Sampling Guide for Drinking Water System Operators. EPA, Office of Environmental Information, Washington, DC, EPA/816/F/03/016.
Attachment 1
Standard Operating Procedure for the Operation and
Calibration of the TVA 2020
ENGINEERING AND SCIENCE DIVISION
TITLE: Standard Operating Procedure for the operation and calibration of the TVA 2020 |
EFFECTIVE DATE:
|
REFERENCES
|
|
SATELLITE FILES: N/A |
|
REVISIONS: NA |
|
WRITER/EDITOR:
N
|
PROJECT MANAGER:
N
|
||||||||||||||||||
QUALITY ASSURANCE COORDINATOR: NAME/DATE
|
NEXT SCHEDULE REVIEW:
|
1.0 IDENTIFICATION AND PURPOSE
The purpose of this document is to provide a method for the operation and calibration of the TVA 2020.
2.0 MATRIX OR MATRICES
The Thermo Scientific TVA 2020 Toxic Vapor Analyzer is a dual detector device equipped with a flame ionization detector (FID) and a photoionization detector (PID). The device can operate both detectors simultaneously or one or the other. The FID requires hydrogen for the flame and an oxygen atmosphere above 16% to support the flame. The PID is not the detector of choice for measuring high concentrations (range is 0-2000 ppm) of vapors, since is more susceptible to interference from water vapor than the FID. The PID does not require hydrogen or oxygen and is the detector of choice when fuel is limited or unavailable or when ambient oxygen concentrations are low. The PID is sensitive to aromatic and chlorinated compounds, and can even measure some inorganic compounds such as ammonia, carbon disulfide, carbon tetrachloride, chloroform, ethylamine, and hydrogen sulfide to name a few, see operation manual for more compounds. The FID is unaffected by ambient levels of CO, CO2 and water vapor, and is highly sensitive to hydrocarbon vapors, including methane.
3.0 METHOD DETECTION LIMIT
The minimum detectable level (MDL) is defined as seven times the standard deviation of the peak-to-peak noise (TVA2020 Instruction Manual). The FID is calibrated in methane gas and the PID in isobutylene gas and hence the MDL is expressed in reference to the calibration gas used.
FID MDL – 0.5 ppm of methane
PID MDL – 0.5 ppm of isobutylene
4.0 SCOPE AND APPLICATION
This SOP covers the operation of the Thermo Scientific TVA 2020 Toxic Vapor Analyzer. The document describes the calibration that has to be performed every day before use, the preparation of the device to be used, how to use the device, and data download.
5.0 METHOD SUMMARY
The TVA 2020 battery is charged, the sample probe is connected, the hydrogen tank is filled and installed (this starts the hydrogen flow) and the device is ready to be turned ON. Ignite the FID flame, turn the FID and PID detectors ON and allow the device to warm up for 30 minutes. The settings for data recording and usage of GPS can be set while the device is warming up. After warm up perform a multiple concentration calibration or calibration span check every day prior use. Calibration for the FID is typically performed with methane gas and the PID with isobutylene gas. The recorded data is downloaded to a computer using the USB cable. Data recording resolution and other specifications are project based and can be entered during warm up or prior.
6.0 DEFINITIONS
TVA 2020 Toxic Vapor Analyzer 2020
FID Flame Ionization Detector
MDL Method Detection Limit
PID Photo Ionization Detector
7.0 INTERFERENCES
The TVA 2020 FID detector requires hydrogen for the flame and an oxygen atmosphere of no less than 16%. Low oxygen concentrations would jeopardize the FID ability to maintain the flame. The PID detector is more susceptible to interference from water vapor.
8.0 SAFETY
The TVA 2020 FID hydrogen gas is filled from a larger cylinder into the 85cc tank. Ensure that all cylinders are secured to the wall or bench stand and check for leaks when the regulator to fill the 85cc tank is securely installed, prior opening and filling the tank. Do not fill the 85cc tank pass 2200 psig. Do not store or transport the TVA with hydrogen in the 85cc tank or with the empty tank installed in the device, bleed the tank using the regulator into a well ventilated space and remove the tank and place it in its normal location in the carrying case. Could take 2 minutes to fill and empty. Use the required personal protective equipment to handle cylinders as specified in ERG’s health and safety manual. Use similar precautions when performing calibrations every day.
For extended hours of operation consider using a backpack to transport the TVA 2020 in place of the shoulder strap. The extended probe displays real-time measurements and can even change some settings in the program.
Each location that the TVA 2020 is used will have their unique safety guidelines, contact the site operators or project managers to find their safety precautions and recommendations.
9.0 EQUIPMENT AND SUPPLIES
Familiarize with the TVA 2020 device components prior use. The TVA 2020 is comprise of the following parts.
Keypad and main unit – this component contains inside the detectors (FID/PID), GPS, memory card, rechargeable battery, pumps and the keypad.
Enhance probe – this probe contains a display that shows real-time measurements that are recorded and displayed in the unit.
85cc hydrogen tank – is filled with hydrogen to sustain the FID flame.
Regulator – is used to fill the hydrogen tank from a large hydrogen supply cylinder.
Power supply and cables – to charge the device’s rechargeable battery.
USB cables and hub – are used to download the recorded data to a computer.
Extra battery – the battery is for emergencies and requires to open the devices’ main unit to install it.
Shoulder strap.
Pelican case – is used to store and transport the TVA 2020 and its components.
Water Trap filters – extras are located in the pelican case and the current filter in use goes in the probe. It filters excess water vapor from going inside the device.
Tedlar bag – for calibrations.
10.0 MATERIALS
Depending on the specific task and measurements, the TVA 2020 can be programmed ahead of time for specific time recording resolution, to record the average/max/min, to record GPS coordinates per measurement or known coordinates (these can be programmed ahead of time and labeled as Tags).
Other expendable parts are:
water trap filters (available in the pelican case), Part No. CR015DK
charcoal filters (replacement needs to be ordered, Part No. 510095-1)
The TVA 2020 contains many parts that can be replace should an error message shows in the display or malfunctioning of the device. Consult the TVA2020 Instruction Manual for more parts and their numbers.
11.0 CHEMICALS, REAGENTS, AND STANDARDS
The TVA 2020 requires the following chemicals for its operation:
Hydrogen – zero grade hydrogen (certified total hydrocarbons as methane < 0.5 ppm recommended)
Methane – 103 liter cylinder of 100 ppm
Isobutylene – 103 liter cylinder of 100 ppm
Zero Air – 103 liter cylinder of zero air
Note: Air, methane, and isobutylene can be purchased as a calibration kit, Part No. CR0124H from ThermoFisher.
12.0 COLLECTION, PRESERVATION, SHIPMENT, AND STORAGE
The response time using a charcoal filter adapter is less than 15 seconds for 90% of final value using 500 ppm of isobutylene for the PID detector and the recovery under the same conditions is 20 seconds to return to 10% of the original value. The FID detector response time is less than 15 seconds for 90% of the final value from 10,000 ppm of methane and it recovers in 20 seconds to 10% of the original value. The instrument can be programmed to record every second, but its ability to recover from abrupt changes should be considered when sampling.
The TVA is stored in a pelican case and can be transported in it, inside a box with padding for extra protection. The hydrogen tank has be stored or transported empty.
13.0 CALIBRATION AND STANDARDIZATION
The TVA 2020 dual detectors can be calibrated simultaneously or individually (recommended). It is recommended to calibrate each detector individually because the detectors are calibrated with different gases at different ranges. However, this can change if the TVA is to be calibrated for a gas different than methane and isobutylene, for the FID or PID respectively. It is recommended to zero both detectors together.
The instrument must be ON and warmed up for approximately 30 minutes. The pump must be ON, the PID lamp must be ON and the FID must be ignited throughout the warm-up period.
13.1 Zero reference point calibration
13.1.1 Use zero air gas if both detectors are to be zeroed simultaneously.
13.1.2 Fill a Tedlar sampling bag with the Zero Air.
13.1.3 From the CALIB MENU display, press 1.
13.1.4 To perform a zero on the FID and PID simultaneously press 1 (BOTH).
13.1.5 Apply the zero gas at sample inlet at ambient pressure and press ENTER.
13.1.6 If the CAL SAVE MODE was set to MANUAL then the display will show the former zero and the new one and will prompt you to accept or redo. If the CAL SAVE MODE was set to AUTO then the display will show the former zero and will accept the new zero without asking you and will delete the former zero.
13.2 Calibration
There are different applications and hence different types of calibration can be used. Almost all published response factors for FIDs and PIDs are based upon methane and isobutylene, respectively. By employing a multipoint calibration for these compounds the accuracy of each detector is improved over the entire dynamic range. Response factors/curves can then be used for correcting the detector’s response to different compounds. However, once the multipoint calibration has been used, any response curve must characterize only the relative response at each concentration, excluding curvature of the calibrated compound. Thus, the use of both multipoint calibration and response curves at the same time is difficult, and not recommended.
If, for example, you want to measure several different compounds over a wide concentration range, it is best to use a single-point calibration and then enter response curves for each specific compound (up to 9 response factors can be entered into the analyzer).
If instead, you want to measure in direct readings (response factor =1) for one specific compound with maximum accuracy over a wide range of concentrations, perform a multipoint calibration with the specific compound. Up to 9 span points (plus zero) can be entered for each detector. The use of a response curve is thus unnecessary as the detector is already reading the direct ppm for that specific compound.
13.2.1 From the MAIN MENU press 2 for SETUP and then press 1 for CALIB. The ZERO is done first, see section 13.1, then the following options provide different alternatives for the calibration:
SPAN (press 2) – to calibrate the reference point(s) using known span gases (methane and isobutylene). Can have as many as nine (9) span gas values.
BACKGROUND (press 3) – takes a background reading to then adjust the SPAN measurements. Not recommended if using clean air.
RF (press 4) – to set instrument response factors if necessary. If using other gases than methane and isobutylene.
FLOW (press 5) – to calibrate the flow sensor. Performed after long term storage and prior a start of a project.
13.2.2 Calibration configuration – Before calibrating the TVA 2020 there are some customization settings that need to be selected. Once these settings are configured they are set for other calibrations unless there is a need to change some setting in the customization.
CAL CONFIG MENU – After selecting SPAN (press 2) in the CALIB MENU, the CAL CONFIG MENU will prompt two options:
Number span pt (number of span points) press 1
Enter the number of span points to be done per detector by choosing BOTH (1), PID (2) or FID (3). Is recommended to do the span for each detector separately unless using the same gas and same concentration, but that is not normally the case.
Back to the CAL CONFIG MENU, press 2 for SPAN CONCS.
Press 2 for PID and enter the span concentration value per point. Then press 3 for FID and enter the span concentration value per point.
The CAL CONFIG MENU contains other options that can be accessed with the up/down arrows in the display keyboard. The BACKGND CORRCT and RF CALC MODE are only used if background correction is going to be used (nor recommended) and new response factors are used. For more details consult the TVA2020 Instruction Manual.
CAL ACCEPT MODE – After configuration of the CAL CONFIG MENU the next menu is the CAL ACCEPT MODE. This menu is to select if the new zero and calibration measurements will be saved automatically or manually. MANUAL (press 1) will prompt you to accept the measurement after displaying the before and new measurements (recommended). The AUTO (press 2) will automatically determine the value to be stored and when to do it.
CAL SAVE MODE – after the CAL ACCEPT MODE menu. This menu is for selecting if the new calibration is to be saved manually or automatically. Recommend MANUAL, press 1.
13.2.3 SPAN calibration – After the calibration configuration settings are selected, span numbers are entered, and the device as warmed-up for 30 minutes is time to perform the actual span calibration.
Fill a Tedlar bag with the calibration gas and connect it to the probe.
In the SPAN calibration menu select the detector. The display will show APPLY SPAN GAS 1 with the provided concentration value. Press ENTER when all is ready.
When the analyzer is done with the measurement it will display the before and new span values to be ACCEPTED if the SAVE MODE is set to MANUAL.
The date and time of the calibration are stored and can be accessed through the INFO menu.
If more span gas points are programmed and selected the SPAN CALIBRATION display returns. Move to the next span point with the up/dwn arrows in the display keyboard and select (ENTER) the point to be done. Repeat the steps in this section for each point.
14.0 PROCEDURE
Charge the battery, fill and install the hydrogen tank, and connect the enhance probe. Warm-up the instrument for approximately 30 minutes. The pump must be ON, the PID lamp must be ON and the FID must be ignited throughout the warm-up period. Operational settings can be entered while warming up the instrument. The TVA 2020 has a RUN MODE that is programmed with the desired setup for easy quick turn on and start measuring. The setup options entail logging resolution, GPS, alarms and more. There are four (4) possible choices in the LOG mode, if the Bluetooth module is active, data automatically streams out continuously from the instrument while the RUN mode is ON.
14.1 Fill hydrogen tank – using the regulator provided in the pelican case, connect it to the hydrogen cylinder and the 80 cc tank. Check for leaks with a soap solution and ensure there are no flames or sources of flame around. If there are no leaks open the hydrogen cylinder and move the regulator valve to the FILL position. From empty it takes about 2 min to fill the 80 cc tank to about 2200 psig. DO NOT fill the 88 cc tank above 2200 psig.
14.2 Warm up – Press CONTROL in the display keyboard and the CONTROL MENU will show five (5) options: (1) pump, (2) FID, (3) Ignt, (4) PID, and (5) More. The word on or off will show on the side of the pump, FID and PID options, this shows what will occur if you press the option number not what is happening at the moment. For example if the pump says on and you press 1 the pump will turn on. To turn on and warm up the instrument, all options should say on when you first turn on the instrument. Press 1 then 2, then 3, wait for the flame to ignite (you will hear a light sound) then press 4. Press 5 and follow the menu to turn on the GPS and Bluetooth. Once you have turned on the GPS you do not have to do it again unless you want it off for the measurements and you go back to the CONTROL MENU option 5. You do have to always go to CONTROL MENU to turn on the pump, FID, PID and the flame. Allow the instrument to warm up for 30 minutes before calibrating or taking measurements. Press EXIT to display MAIN MENU.
14.3 Setup – The setup options are programmed in the memory and kept until manually changed. These settings can be easily changed at any time and will not delete previous data. The data logger only resets and deletes when you manually delete the memory in the MEMORY menu option. The MAIN MENU provides four (4) options: (1) Run, (2) Setup, (3) Info, and (4) Memory. Once the SETUP options are saved to the instrument’s memory you can press 1 (RUN) and the instrument will use the setup choices to make and log measurements. If there is no setup in the memory you must do the SETUP before you can press 1, or if you need to make changes to the existing setup options. Press 2 for SETUP in the MAIN MENU.
14.3.1 The SETUP MENU provides 5 options: (1) Calib, (2) Alarm, (3) Log, (4) passcode, and (5) Other. Option 1 is for calibration, see Section 13 for further options in this menu. If the present work requires an audible alarm to advise the user of a dangerous environment or specific concentration press 2 to setup the alarm options. Follow the menu options and enter the alarm concentrations. To setup the logging options press 3 and to enter a password press 4 and follow the menu options. Furthermore the LOG options provides four (4) more options that are important and specific to each project:
If you do not select an option, when the instrument is in RUN MODE there will be no logging and you can only display readings.
AUTO – you will chose logging time between 1 second and 999 minutes.
VOC or FE – The VOC option prompts you to type a tag identifier. After the tag is entered the instrument stands ready until you initiate the logging mode with the enhance probe or press the ENTER key on the display keyboard. Once the LOG is initiated the instrument counts down and the sample is taken in the count down. The reading can be the average, highest or last reading. The stored values are cleared by pressing ENTER in the display or SELECT in the enhance probe. Under this option each log is done manually and individually under the tag name.
The FE option is a manual means of triggering a sample/log using a pre-configured monitor route file which has been downloaded and stored in the instrument’s memory. Ensure that the file is under the label, ROUTE.TXT always, when you transfer it to the TVA. This choice requires the use of the enhanced probe. Choose sample time between 2 and 30 seconds and choose to log either the highest reading or average (or last) reading achieved. Once configured for FE and the RUN mode is ON, the FE probe menu screen guides you through the monitor route, displaying each pre-configured tag identifier in sequential order. A SEARCH function is available at the probe menu to allow to find tags that are out of order. The probe display prompts to confirm the tag identifier, after which the instrument stands ready until you either select LOG from the probe or press ENTER from the keyboard on the instrument. Once initiated, the instrument begins the count down, sampling over the configured sample time. The reading sampled during the countdown is displayed on both displays and you are given the option to save the reading in memory or to select LOG again. The stored values are cleared by selecting LOG on the probe or pressing ENTER on the instrument’s keyboard. Each FE log is done individually and manually.
Custom – this option allows you to choose all logging options:
Log data type: None, auto, VOC and FE.
Log sample time – between 1 second and 999 minutes.
Log sample type – last reading, maximum or average.
Log unit lock – decide whether to enable auto-ranging or lock the TVA 2020 units to either ppm or %.
Log auto repeat – determine if the instrument will automatically cycle through logging events (auto repeat ON) or pause for a user command before logging (auto repeat OFF).
Log save accept – determine whether the TVA will prompt you to save the data manually (MANUAL) or automatically saves the reading without a user prompt (AUTO).
Tag active – to assign tags to logged readings. For tags choose ON.
Route active – pre-load route list of tags and choose ON.
Once the SETUP options are selected and the instrument has warmed-up return to the MAIN MENU and press 1 (RUN) this will start measurements according to the SETUP options.
14.4 Data download
From the MAIN MENU press 4 (MEMORY). The instrument will display the following message: “Creating Files Please Wait”. Once the display shows, “USB MODE” connect the USB cables to the TVA and the computer USB ports. Browse the computer’s drivers and USB ports to find the TVA 2020, probably as Removable Disk. From that point the TVA data is like any other data storage hardware. Double click and search for the data file in the TVA and copy/paste the text (.txt) file to the computer. The file in the TVA will be under the label, LOG.txt.
If the memory of the TVA has not been deleted the data file will contain all of the data in the memory in the format that was programmed to be logged. To delete the TVA data files, press 4 in the MAIN MENU for MEMORY and then press 2 for CLEAR LOG MEM.
15.0 CALCULATIONS
Although the FID and PID are calibrated with span gases of known concentration, methane and isobutylene, respectively, both detectors respond to many different compounds with differing levels of sensitivity. In order to adjust the analyzer reading from ppm of methane or ppm of isobutylene to ppm of the compound of interest, a correction factor must be applied to the reading. This correction factor is also known as the response factor. The TVA 2020 can have up to nine (9) user-defined response factors or use the default response factor of 1.00.
15.1 Response factor multiplier – When using a response factor multiplier to correct a TVA 2020 reading, the instrument multiplies the reading by the response factor and displays the corrected measurement.
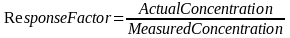
15.2 Response Curve – it changes as concentration changes. See the TVA2020 Instruction Manual page 4-11 for details.
16.0 QUALITY CONTROL
The TVA 2020 requires a 30 minute calibration period prior operating and to be calibrated prior taking measurements every day. Each project will define the unique acceptance criteria for the data and data quality objectives.
17.0 PREVENTION
NA.
18.0 CORRECTIVE ACTION
The TVA 2020 instrument displays an error message if there is a malfunction. Refer to the TVA2020 Instruction Manual for specific error messages and troubleshooting.
19.0 WASTE MANAGEMENT
NA.
20.0 MAINTENANCE
Chapter 5 of the TVA2020 Instruction Manual provides steps to take to perform preventive maintenance in more detail. Following is a summary of the preventive maintenance that is perform regularly. Regularly can be, once a year if is not in use or after more than 6 months of no use. Note that not all of the steps below need to be done every time, but they need checking for damage.
Hydrogen gas tank – never store the tank with hydrogen in it, and in places were flames, excessive heat or sparks may occur. Bleed the tank using the regulator in a well ventilated location.
FID checks – if something looks damage and follow the Manual’s instructions
Cleaning an FID cartridge
Cleaning the FID detector cap
Cleaning the FID detector cavities
Replacing water trap probe filter and O-rings
Cleaning or replacing a sintered metal filter cup
Replacing charcoal filter
21.0 SHORTHAND PROCEDURE
Before starting the instrument:
Charge battery.
Connect sample probe.
Fill/install hydrogen tank.
To start the instrument:
Press ON.
Press CONTROL.
Turn pump ON.
Turn FID ON.
Ignite FID.
Turn PID ON.
Allow the instrument to warm up for 30 minutes prior use.
Press 2 for SETUP.
Press 1, CALIBRATE.
Press 6, CONFIGURE.
Press 2, SPAN CONCENTRATION.
Enter Span concentration(s) and press ENTER.
Press 1, ZERO.
Press 1 for BOTH.
Connect the Zero Air Tedlar bag to the probe.
Press ENTER, start.
Wait to stabilize.
Press ENTER to accept value.
Press 2 for SPAN.
For PID press 2.
Connect the isobutylene Tedlar bag to the probe.
Press ENTER, start.
Accept value with ENTER.
Press 2 for SPAN.
For FID press 3.
Connect methane Tedlar bag to the probe.
Press ENTER, start.
Accept value with ENTER.
If changes to the Run program are needed, go the SETUP MENU and make the changes, if not changes are needed, press 1 for RUN. The instrument is now surveying.
22.0 DOCUMENTATION AND DOCUMENT CONTROL
The TVA 2020 logged data can be downloaded to a computer via USB. The data is logged in text (.txt) files and is stored in the TVA under the label, LOG.txt. This file can be downloaded to the computer and given a unique name. The TVA does not delete the data in the memory until is manually deleted, see Section 14.4. The TVA will contain other text files for the configuration, route, calibration, and others, DO NOT DELETE them from the computer.
23.0 REFERENCE
Thermo Scientific. TVA 2020 Instruction Manual, Toxic Vapor Analyzer. December 15, 2014.
24.0 TABLES, DIAGRAMS, FLOWCHARTS, VALIDATION DATA
NA.
Appendix F
Chemical Analytes, Laboratory Reporting Limits, and Health-Based Comparison Values
Table F.1 Chemical Analytes, Laboratory Reporting Limits, and Health-Based Comparison Values
Table F.2 Chemical Analytes, Laboratory Reporting Limits, and Health-Based Comparison Values: Radionuclides
Table F.1 Chemical Analytes, Laboratory Reporting Limits, and Health-Based Comparison Values
Analyte |
Method |
Analyte
|
Units |
RL |
MDL |
HCV |
HCV source |
Bromide |
EPA 300.0 |
Inorganic ions |
µg/L |
250 |
53 |
NA |
NA |
Chloride |
EPA 300.0 |
Inorganic ions |
µg/L |
200 |
20 |
2,500 |
SMCL |
Fluoride |
EPA 300.0 |
Inorganic ions |
µg/L |
100 |
26 |
350 |
Chronic EMEG |
Sulfate as SO4 |
EPA 300.0 |
Inorganic ions |
µg/L |
500 |
50 |
250,000 |
SMCL |
Aluminum (Al) |
EPA 200.8 |
Metals – Total |
µg/L |
50 |
20 |
7,000 |
cEMEG ch |
Antimony (Sb) |
EPA 200.8 |
Metals – Total |
µg/L |
5 |
2 |
2.8 |
RMEG ch |
Arsenic (Ar) |
EPA 200.8 |
Metals – Total |
µg/L |
10 |
4 |
0.02 |
CREG |
Barium (Ba) |
EPA 200.8 |
Metals – Total |
µg/L |
2 |
0.9 |
1,400 |
cEMEG ch |
Beryllium (Be) |
EPA 200.8 |
Metals – Total |
µg/L |
0.5 |
0.2 |
14 |
cEMEG ch |
Boron (B) |
EPA 200.8 |
Metals – Total |
µg/L |
100 |
45 |
1,400 |
iEMEG ch |
Cadmium (Cd) |
EPA 200.8 |
Metals – Total |
µg/L |
0.5 |
0.2 |
0.7 |
cEMEG ch |
Calcium (Ca) |
EPA 200.8 |
Metals – Total |
µg/L |
100 |
45 |
NA |
NA |
Chromium (Cr) |
EPA 200.8 |
Metals – Total |
µg/L |
10 |
4 |
6.3 |
cEMEG ch |
Cobalt (Co) |
EPA 200.8 |
Metals – Total |
µg/L |
2 |
0.9 |
70 |
iEMEG ch |
Copper (Cu) |
EPA 200.8 |
Metals – Total |
µg/L |
1 |
0.4 |
70 |
iEMEG ch |
Iron (Fe) |
EPA 200.8 |
Metals – Total |
µg/L |
50 |
20 |
300 |
SMCL |
Lead (Pb) |
EPA 200.8 |
Metals – Total |
µg/L |
3 |
1 |
15 |
Action level |
Lithium (Li) |
EPA 200.8 |
Metals – Total |
µg/L |
5 |
2 |
40 |
RSL |
Magnesium (Mg) |
EPA 200.8 |
Metals – Total |
µg/L |
50 |
20 |
NA |
NA |
Manganese (Mn) |
EPA 200.8 |
Metals – Total |
µg/L |
2 |
0.9 |
350 |
RMEG ch |
Nickel (Ni) |
EPA 200.8 |
Metals – Total |
µg/L |
5 |
2 |
140 |
RMEG ch |
Potassium (K) |
EPA 200.8 |
Metals – Total |
µg/L |
100 |
45 |
NA |
NA |
Selenium (Se) |
EPA 200.8 |
Metals – Total |
µg/L |
5 |
2 |
35 |
cEMEG ch |
Silver (Ag) |
EPA 200.8 |
Metals – Total |
µg/L |
2 |
0.9 |
35 |
RMEG ch |
Sodium (Na) |
EPA 200.8 |
Metals – Total |
µg/L |
50 |
20 |
20,000 |
EPA DWL |
Strontium (Sr) |
EPA 200.8 |
Metals – Total |
µg/L |
5 |
0.5 |
4,200 |
RMEG ch |
Thallium (Tl) |
EPA 200.8 |
Metals – Total |
µg/L |
2 |
0.9 |
2 |
MCL |
Tin (Sn) |
EPA 200.8 |
Metals – Total |
µg/L |
2 |
1.2 |
2,100 |
iEMEG ch |
Titanium (Ti) |
EPA 200.8 |
Metals – Total |
µg/L |
5 |
2 |
NA |
NA |
Uranium (U) |
EPA 200.8 |
Metals – Total |
µg/L |
1 |
0.4 |
1.4 |
iEMEG ch |
Vanadium (V) |
EPA 200.8 |
Metals – Total |
µg/L |
10 |
4 |
70 |
iEMEG ch |
Zinc (Zn) |
EPA 200.8 |
Metals – Total |
µg/L |
20 |
7.5 |
2,100 |
cEMEG ch |
1,1,1,2-Tetrachloroethane |
524.2 |
VOCs |
µg/L |
0.5 |
0.24 |
0.93 |
CREG |
1,1,1-Trichloroethane |
524.2 |
VOCs |
µg/L |
0.5 |
0.15 |
14,000 |
RMEG ch |
1,1,2,2-Tetrachloroethane |
524.2 |
VOCs |
µg/L |
0.5 |
0.13 |
0.12 |
CREG |
1,1,2-Trichloroethane |
524.2 |
VOCs |
µg/L |
0.5 |
0.16 |
0.43 |
CREG |
1,1-Dichloroethane |
524.2 |
VOCs |
µg/L |
0.5 |
0.078 |
2.8 |
RSL |
1,1-Dichloroethene |
524.2 |
VOCs |
µg/L |
0.5 |
0.15 |
63 |
cEMEG ch |
1,1-Dichloropropene |
524.2 |
VOCs |
µg/L |
0.5 |
0.095 |
NA |
NA |
1,2,3-Trichlorobenzene |
524.2 |
VOCs |
µg/L |
0.5 |
0.14 |
7 |
RSL |
1,2,3-Trichloropropane |
524.2 |
VOCs |
µg/L |
0.5 |
0.17 |
0.0004 |
CREG |
1,2,4-Trichlorobenzene |
524.2 |
VOCs |
µg/L |
0.5 |
0.12 |
70 |
RMEG ch |
1,2,4-Trimethylbenzene |
524.2 |
VOCs |
µg/L |
0.5 |
0.17 |
70 |
RMEG ch |
1,2-Dibromo-3-chloropropane |
524.2 |
VOCs |
µg/L |
0.5 |
0.3 |
14 |
iEMEG ch |
1,2-Dibromoethane (EDB) |
524.2 |
VOCs |
µg/L |
0.5 |
0.2 |
0.012 |
CREG |
1,2-Dichlorobenzene |
524.2 |
VOCs |
µg/L |
0.5 |
0.16 |
630 |
RMEG ch |
1,2-Dichloroethane |
524.2 |
VOCs |
µg/L |
0.5 |
0.086 |
0.27 |
CREG |
1,2-Dichloropropane |
524.2 |
VOCs |
µg/L |
0.5 |
0.096 |
490 |
iEMEG ch |
1,3,5-Trimethylbenzene |
524.2 |
VOCs |
µg/L |
0.5 |
0.16 |
70 |
RMEG ch |
1,3-Dichlorobenzene |
524.2 |
VOCs |
µg/L |
0.5 |
0.11 |
140 |
iEMEG ch |
1,3-Dichloropropane |
524.2 |
VOCs |
µg/L |
0.5 |
0.1 |
370 |
RSL |
1,4-Dichlorobenzene |
524.2 |
VOCs |
µg/L |
0.5 |
0.13 |
490 |
cEMEG ch |
2,2-Dichloropropane |
524.2 |
VOCs |
µg/L |
0.5 |
0.2 |
NA |
NA |
2-Butanone |
524.2 |
VOCs |
µg/L |
10 |
5 |
4,200 |
RMEG ch |
2-Chloroethylvinyl ether |
524.2 |
VOCs |
µg/L |
0.5 |
|
NA |
NA |
2-Chlorotoluene |
524.2 |
VOCs |
µg/L |
0.5 |
0.11 |
140 |
RMEG ch |
2-Hexanone |
524.2 |
VOCs |
µg/L |
10 |
5 |
35 |
RMEG ch |
4-Chlorotoluene |
524.2 |
VOCs |
µg/L |
0.5 |
0.13 |
250 |
RSL |
4-Methyl-2-pentanone |
524.2 |
VOCs |
µg/L |
10 |
5 |
6,300 |
RSL |
Acetone |
524.2 |
VOCs |
µg/L |
10 |
5 |
6,300 |
RMEG ch |
Acrylonitrile |
524.2 |
VOCs |
µg/L |
0.5 |
|
0.045 |
CREG |
Benzene |
524.2 |
VOCs |
µg/L |
0.5 |
0.082 |
0.44 |
CREG |
Bromobenzene |
524.2 |
VOCs |
µg/L |
0.5 |
0.091 |
56 |
RMEG ch |
Bromochloromethane |
524.2 |
VOCs |
µg/L |
0.5 |
0.3 |
83 |
RSL |
Bromodichloromethane |
524.2 |
VOCs |
µg/L |
0.5 |
|
0.39 |
CREG |
Bromoform |
524.2 |
VOCs |
µg/L |
0.5 |
0.079 |
3.1 |
CREG |
Bromomethane |
524.2 |
VOCs |
µg/L |
1.0 |
0.2 |
9.8 |
RMEG ch |
Carbon disulfide |
524.2 |
VOCs |
µg/L |
0.5 |
|
700 |
RMEG ch |
Carbon Tetrachloride |
524.2 |
VOCs |
µg/L |
0.5 |
0.11 |
0.35 |
CREG |
Chlorobenzene |
524.2 |
VOCs |
µg/L |
0.5 |
0.14 |
140 |
RMEG ch |
Chlorodibromomethane |
524.2 |
VOCs |
µg/L |
0.5 |
0.13 |
0.29 |
CREG |
Chloroethane |
524.2 |
VOCs |
µg/L |
1.0 |
0.22 |
21,000 |
RSL |
Chloroform |
524.2 |
VOCs |
µg/L |
0.5 |
0.2 |
70 |
cEMEG ch/ RMEG ch |
Chloromethane |
524.2 |
VOCs |
µg/L |
0.5 |
0.15 |
190 |
RSL |
cis-1,2-Dichloroethene |
524.2 |
VOCs |
µg/L |
0.5 |
0.09 |
14 |
RMEG ch |
cis-1,3-Dichloropropene |
524.2 |
VOCs |
µg/L |
0.5 |
0.081 |
0.47 |
RSL |
Cyclohexane |
524.2 |
VOCs |
µg/L |
0.5 |
|
13,000 |
RSL |
Dibromomethane |
524.2 |
VOCs |
µg/L |
0.5 |
0.16 |
NA |
NA |
Dichlorodifluoromethane |
524.2 |
VOCs |
µg/L |
0.5 |
0.34 |
1,400 |
RMEG ch |
Ethylbenzene |
524.2 |
VOCs |
µg/L |
0.5 |
0.099 |
700 |
RMEG ch |
Freon 113 |
524.2 |
VOCs |
µg/L |
0.5 |
0.15 |
210,000 |
RMEG ch |
Hexachlorobutadiene |
524.2 |
VOCs |
µg/L |
0.5 |
0.26 |
0.31 |
CREG |
Isopropylbenzene |
524.2 |
VOCs |
µg/L |
0.5 |
0.15 |
700 |
RMEG ch |
Methyl Acetate |
524.2 |
VOCs |
µg/L |
1 |
|
20,000 |
RSL |
Methylcyclohexane |
524.2 |
VOCs |
µg/L |
0.5 |
|
NA |
NA |
Methylene Chloride |
524.2 |
VOCs |
µg/L |
0.5 |
0.2 |
6.1 |
CREG |
Methyl-tert-butyl ether |
524.2 |
VOCs |
µg/L |
0.5 |
0.093 |
2,100 |
iEMEG ch |
m-Xylene/p-Xylene |
524.2 |
VOCs |
µg/L |
1 |
0.15 |
1,400 |
cEMEG ch /RMEG ch |
Naphthalene |
524.2 |
VOCs |
µg/L |
1 |
0.43 |
140 |
RMEG ch |
n-Butylbenzene |
524.2 |
VOCs |
µg/L |
0.5 |
0.17 |
1,000 |
RSL |
n-Propylbenzene |
524.2 |
VOCs |
µg/L |
0.5 |
0.17 |
660 |
RSL |
o-Xylene |
524.2 |
VOCs |
µg/L |
0.5 |
0.086 |
1,400 |
cEMEG ch /RMEG ch |
p-Isopropyltoluene |
524.2 |
VOCs |
µg/L |
0.5 |
0.21 |
NA |
NA |
sec-Butylbenzene |
524.2 |
VOCs |
µg/L |
0.5 |
0.14 |
2,000 |
RSL |
Styrene |
524.2 |
VOCs |
µg/L |
0.5 |
0.089 |
100 |
MCL |
tert-Butylbenzene |
524.2 |
VOCs |
µg/L |
0.5 |
0.14 |
690 |
RSL |
Tetrachloroethene |
524.2 |
VOCs |
µg/L |
0.5 |
0.18 |
12 |
CREG |
Toluene |
524.2 |
VOCs |
µg/L |
0.5 |
0.086 |
560 |
RMEG ch |
trans-1,2-Dichloroethene |
524.2 |
VOCs |
µg/L |
0.5 |
0.09 |
140 |
RMEG ch |
trans-1,3-Dichloropropene |
524.2 |
VOCs |
µg/L |
0.5 |
0.11 |
0.24 |
CREG |
Trichloroethene |
524.2 |
VOCs |
µg/L |
0.5 |
0.13 |
0.43 |
CREG |
Trichlorofluoromethane |
524.2 |
VOCs |
µg/L |
0.5 |
0.23 |
2,100 |
RMEG ch |
Vinyl acetate |
524.2 |
VOCs |
µg/L |
0.5 |
|
410 |
RSL |
Vinyl chloride |
524.2 |
VOCs |
µg/L |
0.5 |
0.16 |
0.017 |
CREG |
Diesel Range Organic-DRO |
SW846 8015 |
TPH |
mg/L |
125 |
100 |
NA |
NA |
Gas Range Organics-GRO |
SW846 8015 |
TPH |
µg/L |
100 |
47 |
NA |
NA |
1-Butanol |
8015B_DAI |
Alcohols |
mg/L |
10 |
1 |
700 |
RMEG ch |
1-Propanol |
8015B_DAI |
Alcohols |
mg/L |
5 |
1 |
NA |
NA |
2-Butanol |
8015B_DAI |
Alcohols |
mg/L |
5 |
1 |
2,000 |
RSL |
Ethanol |
8015B_DAI |
Alcohols |
mg/L |
5 |
1 |
NA |
NA |
Methanol |
8015B_DAI |
Alcohols |
mg/L |
1 |
0.17 |
14,000 |
RMEG ch |
2-Butoxyethanol |
8015B_DAI |
Glycols |
µg/L |
5 |
1 |
490 |
iEMEG ch |
2-Methoxyethanol |
8015B_DAI |
Glycols |
µg/L |
5 |
1 |
29 |
RSL |
Di ethylene glycol |
8015B_DAI |
Glycols |
µg/L |
25 |
20 |
8,000 |
ATSDR/Dmk? |
Ethylene Glycol |
8015B_DAI |
Glycols |
µg/L |
25 |
12 |
5,600 |
iEMEG ch |
Tetra ethylene glycol |
8015B_DAI |
Glycols |
µg/L |
25 |
|
8,000 |
ATSDR/Dmk? |
Tri ethylene glycol |
8015B_DAI |
Glycols |
µg/L |
25 |
20 |
8,000 |
ATSDR/Dmk? |
1,1-Biphenyl |
8270D_LL |
SVOCs |
µg/L |
1 |
0.1 |
3.0 |
CREG |
1,2,4,5-Tetrachlorobenzene |
8270D_LL |
SVOCs |
µg/L |
1 |
0.1 |
2.1 |
RMEG ch |
1-Methylnaphthalene* |
8270D_LL |
SVOCs |
µg/L |
0.8 |
0.4 |
490 |
cEMEG ch |
2,3,4,6-Tetrachlorophenol |
8270D_LL |
SVOCs |
µg/L |
1 |
0.1 |
210 |
RMEG ch |
2,4,5-Trichlorophenol |
8270D_LL |
SVOCs |
µg/L |
1 |
0.12 |
700 |
RMEG ch |
2,4,6-Trichlorophenol |
8270D_LL |
SVOCs |
µg/L |
1 |
0.17 |
2.2 |
CREG |
2,4-Dichlorophenol |
8270D_LL |
SVOCs |
µg/L |
1 |
0.1 |
21 |
iEMEG ch/ RMEG ch |
2,4-Dimethylphenol |
8270D_LL |
SVOCs |
µg/L |
2 |
0.69 |
140 |
RMEG ch |
2,4-Dinitrophenol |
8270D_LL |
SVOCs |
µg/L |
10 |
1.1 |
14 |
RMEG ch |
2,4-Dinitrotoluene |
8270D_LL |
SVOCs |
µg/L |
1 |
0.12 |
7.0 |
cEMEG ch |
2,6-Dinitrotoluene |
8270D_LL |
SVOCs |
µg/L |
1 |
0.13 |
28 |
iEMEG ch |
2-Chloronaphthalene |
8270D_LL |
SVOCs |
µg/L |
1 |
0.1 |
560 |
RMEG ch |
2-Chlorophenol |
8270D_LL |
SVOCs |
µg/L |
1 |
0.12 |
35 |
RMEG ch |
2-Methoxyethanol* |
OLC03.2/3520C |
SVOCs |
µg/L |
5 |
|
29 |
RSL |
2-Methylnaphthalene |
8270D_LL |
SVOCs |
µg/L |
0.2 |
0.1 |
28 |
RMEG ch |
2-Methylphenol |
8270D_LL |
SVOCs |
µg/L |
1 |
0.74 |
350 |
RMEG ch |
2-Nitroaniline |
8270D_LL |
SVOCs |
µg/L |
1 |
0.16 |
NA |
NA |
2-Nitrophenol |
8270D_LL |
SVOCs |
µg/L |
1 |
0.1 |
NA |
NA |
3,3´-Dichlorobenzidine |
8270D_LL |
SVOCs |
µg/L |
20 |
2 |
0.054 |
CREG |
3&4-Methylphenol |
8270D_LL |
SVOCs |
µg/L |
2 |
0.66 |
350 |
RMEG ch |
3-Nitroaniline |
8270D_LL |
SVOCs |
µg/L |
5 |
0.16 |
NA |
NA |
4,6-Dinitro-2-methylphenol |
8270D_LL |
SVOCs |
µg/L |
5 |
1 |
28 |
iEMEG ch |
4-Bromophenyl phenyl ether |
8270D_LL |
SVOCs |
µg/L |
1 |
0.12 |
NA |
NA |
4-Chloro-3-methylphenol |
8270D_LL |
SVOCs |
µg/L |
1 |
0.12 |
NA |
NA |
4-Chloroaniline |
8270D_LL |
SVOCs |
µg/L |
4 |
2 |
28 |
RMEG ch |
4-Chlorophenyl phenyl ether |
8270D_LL |
SVOCs |
µg/L |
1 |
0.1 |
NA |
NA |
4-Nitroaniline |
8270D_LL |
SVOCs |
µg/L |
5 |
0.5 |
3.8 |
RSL |
4-Nitrophenol |
8270D_LL |
SVOCs |
µg/L |
8 |
4 |
60 |
LTHA |
Acenaphthene |
8270D_LL |
SVOCs |
µg/L |
0.2 |
0.1 |
420 |
RMEG ch |
Acenaphthylene |
8270D_LL |
SVOCs |
µg/L |
0.2 |
0.1 |
530 |
RSL |
Acetophenone |
8270D_LL |
SVOCs |
µg/L |
1 |
0.3 |
700 |
RMEG ch |
Anthracene |
8270D_LL |
SVOCs |
µg/L |
0.2 |
0.1 |
2,100 |
RMEG ch |
Atrazine |
8270D_LL |
SVOCs |
µg/L |
4 |
2 |
21 |
iEMEG ch |
Benzaldehyde |
8270D_LL |
SVOCs |
µg/L |
1 |
0.1 |
700 |
RMEG ch |
Benzo(a)anthracene |
8270D_LL |
SVOCs |
µg/L |
0.2 |
0.1 |
0.012 |
RSL |
Benzo(a)pyrene |
8270D_LL |
SVOCs |
µg/L |
0.2 |
0.1 |
0.012 |
CREG |
Benzo(b)fluoranthene |
8270D_LL |
SVOCs |
µg/L |
0.2 |
0.1 |
0.034 |
RSL |
Benzo(ghi)perylene |
8270D_LL |
SVOCs |
µg/L |
0.2 |
0.1 |
NA |
NA |
Benzo(k)fluoranthene |
8270D_LL |
SVOCs |
µg/L |
0.2 |
0.1 |
0.034 |
RSL |
Bis(2-chloroethoxy)methane |
8270D_LL |
SVOCs |
µg/L |
1 |
0.1 |
59 |
RSL |
Bis(2-chloroethyl)ether |
8270D_LL |
SVOCs |
µg/L |
1 |
0.1 |
0.022 |
CREG |
Bis(2-chloroisopropyl)ether |
8270D_LL |
SVOCs |
µg/L |
1 |
0.1 |
280 |
RMEG ch |
Bis(2-ethylhexyl)phthalate |
8270D_LL |
SVOCs |
µg/L |
5 |
2 |
1.7 |
CREG |
Butyl benzyl phthalate |
8270D_LL |
SVOCs |
µg/L |
1 |
0.12 |
1,400 |
RMEG ch |
Caprolactam |
8270D_LL |
SVOCs |
µg/L |
1 |
0.13 |
3,500 |
RMEG ch |
Carbazole |
8270D_LL |
SVOCs |
µg/L |
2 |
0.1 |
NA |
NA |
Chrysene |
8270D_LL |
SVOCs |
µg/L |
0.2 |
0.045 |
2.9 |
cRSL |
Dibenz(a,h)anthracene |
8270D_LL |
SVOCs |
µg/L |
0.2 |
0.1 |
0.0029 |
cRSL |
Dibenzofuran |
8270D_LL |
SVOCs |
µg/L |
1 |
0.1 |
NA |
|
Diethyl phthalate |
8270D_LL |
SVOCs |
µg/L |
1 |
0.11 |
5,600 |
RMEG ch |
Dimethyl phthalate |
8270D_LL |
SVOCs |
µg/L |
1 |
0.1 |
NA |
NA |
Di-n-butyl phthalate |
8270D_LL |
SVOCs |
µg/L |
1 |
0.39 |
700 |
RMEG ch |
Di-n-octyl phthalate |
8270D_LL |
SVOCs |
µg/L |
1 |
0.17 |
2,800 |
iEMEG ch |
Fluoranthene |
8270D_LL |
SVOCs |
µg/L |
0.2 |
0.1 |
280 |
RMEG ch |
Fluorene |
8270D_LL |
SVOCs |
µg/L |
0.2 |
0.1 |
280 |
RMEG ch |
Hexachlorobenzene |
8270D_LL |
SVOCs |
µg/L |
1
|
0.1 |
0.015 |
CREG |
Hexachlorobutadiene |
8270D_LL |
SVOCs |
µg/L |
1 |
0.1 |
0.31 |
CREG |
Hexachlorocyclopentadiene |
8270D_LL |
SVOCs |
µg/L |
2 |
0.5 |
42 |
RMEG ch |
Hexachloroethane |
8270D_LL |
SVOCs |
µg/L |
1 |
0.5 |
0.61 |
CREG |
Indeno(1,2,3-cd)pyrene |
8270D_LL |
SVOCs |
µg/L |
0.2 |
0.1 |
NA |
NA |
Isophorone |
8270D_LL |
SVOCs |
µg/L |
1 |
0.1 |
26 |
CREG |
Naphthalene |
8270D_LL |
SVOCs |
µg/L |
0.2 |
0.1 |
140 |
RMEG ch |
Nitrobenzene |
8270D_LL |
SVOCs |
µg/L |
1 |
0.1 |
14 |
RMEG ch |
N-Nitrosodimethylamine |
8270D_LL |
SVOCs |
µg/L |
1 |
0.25 |
0.00024 |
REG CREG |
N-Nitroso-di-n-propylamine |
8270D_LL |
SVOCs |
µg/L |
1 |
0.13 |
0.0035 |
CREG |
N-Nitrosodiphenylamine# |
8270D_LL |
SVOCs |
µg/L |
# |
# |
5 |
CREG |
N-N-Diphenylamine# |
8270D_LL |
SVOCs |
µg/L |
1 |
0.37 |
180 |
RMEG ch |
Pentachlorophenol |
8270D_LL |
SVOCs |
µg/L |
5 |
0.4 |
0.061 |
CREG |
Phenanthrene |
8270D_LL |
SVOCs |
µg/L |
0.2 |
0.1 |
NA |
NA |
Phenol |
8270D_LL |
SVOCs |
µg/L |
1 |
0.13 |
2,100 |
RMEG ch |
Pyrene |
8270D_LL |
SVOCs |
µg/L |
0.2 |
0.1 |
210 |
RMEG ch |
Fecal Coliform |
SM 9222B |
Bacteria |
cfu/mL |
1 |
|
0## |
MCLG |
Total Coliform |
SM 9222B |
Bacteria |
cfu/mL |
1 |
|
0## |
|
|
|
|
|
|
|
|
|
Notes:
µg/L = micrograms per liter
cEMEG = ATSDR chronic environmental media evaluation guideline
cfu/mL = colony forming units per milliliter
ch = child
cRSL = EPA risk screening level for cancer
DWL = EPA drinking water guidance level
HCV = health-based comparison value
iEMEG = ATSDR intermediate environmental media evaluation guideline
LTHA = EPA lifetime health advisory
MCL = EPA maximum contaminant level
NA = not available
RL = reporting limit
RMEG = remedial media evaluation guideline
RSL = EPA risk screening level
SMCL = secondary maximum contaminant level
SVOC = semi-volatile organic compounds
TPH = total petroleum hydrocarbons
VOC = volatile organic compounds
# N-Nitrosodiphenylamine is broken down to Diphenylamine during analysis and is reported as N,N-Diphenylamine
## - coliform should not be detected in drinking water – if detected it must be addressed
Table F.2 Chemical Analytes, Laboratory Reporting Limits, and Health-Based Comparison Values: Radionuclides
Analyte |
Description of analysis |
Units |
Reporting Level |
MCL used for HCV in all cases‡ |
ATSDR Notes |
MCL (pCi/L) based on 4 millirem/y¶ |
Things to look at |
Th-228 |
Isotopic |
pCi/L |
1 |
15 |
Decay product of Th-232 |
20 |
Compare to Th-232 |
Th-230 |
Isotopic |
pCi/L |
1 |
15 |
Decay product of U-238 |
7 |
Compare to U-238 |
Th-232 |
Isotopic |
pCi/L |
1 |
15 |
Parent of Th-232 chain |
6.5 |
|
U-233/234 |
Isotopic |
pCi/L |
1 |
See U-238 |
Decay product of U-238; presence of U-233 would indicate possible nuclear reactor involvement |
30 |
Compare to U-238 |
U-235/236 |
Isotopic |
pCi/L |
1 |
See U-238 |
Naturally occurring; presence of U-236 would indicate possible nuclear reactor involvement |
32 |
Should be about 1/20th of U-238 |
U-238 |
Isotopic |
pCi/L |
1 |
20§ |
Parent of U-238 chain |
33 |
|
Cs-137 |
Gamma spec |
pCi/L |
20 |
200 |
Fission product |
109 |
|
Gross alpha |
Non-specific identification |
pCi/L |
3 |
15 |
Generic, MCL used for screening only |
No dose can be determined |
|
Gross beta |
Non-specific identification |
pCi/L |
4 |
4 |
Generic, MCL used for screening only |
No dose can be determined |
|
Ra-226 (alpha emitter) |
Ra-226* |
pCi/L |
1 |
5 |
Decay product of U-238 |
5 |
Compare to U-238 |
Ra-228 (beta emitter) |
Ra-228† |
pCi/L |
1 |
5 |
Decay product of Th-232 |
3 |
Compare to Th-232 |
*not necessarily specific for Ra-226 unless the method is 903.1 but can be used as a screen. If value is greater than 5 pCi/L then the amount of Ra-228 must be determined †based on the presence of Actinium-228, the decay product of Ra-228 ‡unless specified in notes, the MCL reference source is 1976 MCL rule updated in 2000 (65 FR 76708). Basically, there was no change between the 1976 rule and the 2000 rule. Dose based MCL is 4 millirem per year §The standard is based on a total uranium concentration of 30 micrograms per liter which is approximately 20 pCi/L. ¶based on Federal Guidance Report 13 (FGR 13) electronic (CD) dose coefficients. Depending on dosimetric system used, the concentrations will vary. FGR 13 is being updated. The International Commission on Radiation Protection (ICRP) Report 119 contains recommended dosimetric values that may be used for the FGR 13 update. |
|||||||
Appendix G: Individual Results Letter
(ATSDR Letterhead)
Date
Dear NAME:
In July 2017 you allowed the Agency for Toxic Substances and Disease Registry (ATSDR) to take samples of your private well water and bulk water (if appropriate) and test them for chemicals. We also sampled indoor air at your home for radon gas. We are providing you with the test results in this letter. We thank you for allowing us to test your well. If you have any questions, please call or e-mail Robert Helverson at 215-814-3139, or by email at gfu6@cdc.gov
Sincerely,
Robert Helverson, MS
Region 3 Representative
Division of Community Health Investigations (DCHI), Eastern Branch
Agency for Toxic Substances and Disease Registry (ATSDR)
Enclosure (WILL BE CHEMICAL-SPECIFIC WRITEUPS FOR EACH CONTAMINANT)
Appendix H: Data Management Plan
NCEH/ATSDR Data Management Plan Form
This plan describes the anticipated use and release by CDC of the dataset named below. All CDC DMPs are required to be in compliance with the CDC/ATSDR Policy on Releasing and Sharing data, available at http://isp-v-maso-apps.cdc.gov/Policy/Doc/policy385.pdf. This plan is modifiable and does not represent a legal contract between CDC and any other entity. The elements included do not necessarily constitute an exhaustive list of all possible elements for a DMP, so users should add elements as needed.
The DMP is submitted through eClearance for review and approval. Use “TBD” if you cannot determine some of this information at the time of submission. Elements with an asterisk (*) are required data fields for metadata.
Table 1 – Core DMP Elements (should be filled out when project approval is sought)
MRID (NCEH/ATSDR metadata repository identifier - for NCEH/ATSDR OD use only.)
|
*Title (Human-readable name of the project. Title should be in plain English and include sufficient detail to facilitate search and discovery.) Drinking Water Investigation in Dimock PA
|
*Description (Human-readable description with sufficient detail to enable a user to quickly understand whether the project or dataset is of interest. A short, clear description is ideal.)
This exposure investigation (EI) will sample up to 20 homes for drinking water and bulk water, as appropriate, as well as levels of radon in indoor air. Eligible participants will be chosen from homes that were previously sampled by EPA in 2012. The participants’ water and indoor air will be sampled in summer 2017. This EI will be a collaboration between ATSDR headquarters, ATSDR Region 3, the Pennsylvania Department of Environmental Protection (PADEP), the Pennsylvania Department of Health (PADOH) and EPA Region 3.
|
*Last DMP Update (Most recent date on which the DMP was changed, updated, or modified.) 5/24/2017
|
*Contact Name and Email CDC PI or POC Name (last, first): Helverson, Robert CDC PI or POC e-mail address: gfu6@cdc.gov CDC PI or POC phone number: 215-814-3139 |
Organization (Use CIO/Division/Branch as locator of where the project is conducted or supported; or use the awardee institution for an extramural project.) ATSDR/DCHI/EB
|
*Unique Identifier and catalog/database name (A unique identifier for the project as maintained within an Agency catalog or database. For intramural submissions, protocol/S3P number can be used. For extramural submissions, grant/cooperative agreement/ contract number can be used to map to related documents.)
3ATA |
*Data Access Level(s) – CHECK ALL APPLY (The degree to which the data collected as part of this project could be made publicly available, regardless of whether it has been made available. Projects can have multiple datasets or different data elements within a single dataset that are approved for different levels of public access.)
PUBLIC Release ☐ Public release – Full dataset (Dataset can be made available without restrictions; data steward no longer controls data. This should be the default selection for all datasets unless justified otherwise.)
☒ Public release – Aggregate data (Underlying dataset cannot be released or shared, but aggregate/summary data can.be made available to public access without restriction) Justification (required if selected):
Participants in the EI will be provided their individual sampling results. Results will be shared with partners including PADOH, PADEP and EPA. Aggregated/summary data, but not individual data, will be provided in the EI report. Individual data will not be released to protect the privacy of EI participants. Individual data will not be released to protect the privacy of EI participants.
☐ Public release - Release by ad-hoc request (Metadata will be released and the dataset is available by ad-hoc request; data requests CANNOT be denied; no data use agreement or restrictions; data steward no longer controls data.) Justification (required if selected):
RESTRICTED Release ☐ Restricted use data sharing (Dataset is available to particular parties under certain use restrictions or use agreement; data not always under CDC custody. The use restriction/agreement (or template) needs to be attached. Justification (required if selected):
☐ Restricted access data sharing (Dataset is only available in an RDC; data need to remain under CDC custody.) Justification (required if selected):
No Data Release/Sharing ☐ No release or data sharing Justification (required if selected):
|
Access Rights/Restrictions (Include information regarding access or restrictions based on privacy, security, or other policies of the owner of the data. Include an explanation for the selected “Public Access Level” above.)
Water and indoor air data will be available as aggregated/summary data without identifiers in the ATSDR Exposure Investigation Report.
|
License/Other Agreements (The license or non-license [i.e., public domain] status with which the dataset will be published. See Open Licenses for more information. May include DTA, MTA, IAA, MOU or other agreements concerning data use and access.)
None
|
*Publisher/Owner (The publishing entity and optionally their parent organization(s). This could be the “owner” of the data.)
ATSDR/DCHI/EB
|
Access URL(s), If Known (URL providing indirect access to the DMP, dataset, data dictionary [variable names and valid values], data collection instrument and other relevant information, including the research protocol if possible.)
None
|
Download URL(s), If Known (URL providing direct access to a downloadable file of the dataset, summary data, or data tables.)
Not applicable
|
*Spatial (The range of spatial applicability of a dataset. Could include a geographic region or a named place [city, county, state, region, country].)
Dimock PA
|
*Temporal (The range of temporal applicability of project) Start date of data collection (month/year): 6/2017 End date of data collection (month/year): 9/2018
|
Table 2 – Additional DMP Elements (should be filled out where possible when project approval is sought; however, many fields can only be filled out later when publication/report is cleared)
*Tags/Keywords (Keywords to help users discover the dataset.)
Exposure Investigation Private well water Radon Testing Dimock PA Hydraulic Fracturing
|
*Intramural or Extramural Project ☒ Intramural ☐ Extramural (grant, cooperative agreement, contract, IAA, CDC Foundation, other) Specify mechanism:
|
Project Type – CHECK ALL APPLY (Multiple selections may apply.) ☐ Research ☐ Emergency ☒ Non-research ☒ Exposure investigation ☐ Surveillance ☐ Ongoing collection ☐ Evaluation ☐ Other |
Dates Estimated date of data release/sharing (month/year): 9/2018 Preservation expiration date (year that the dataset will be available until): 9/2048 |
Data Category (For explanation of D1 to D10 codes, see Table on page 1) ☒ D1 ☐ D2 ☐ D3 ☐ D4 ☐ D5 ☐ D6 ☐ D7 ☐ D8 ☐ D9 ☐ D10 Justification: (provide detailed information about the data category selected above. If D6 is selected, provide quantitative estimates of costs in releasing data and expected volume of use. If D7 is selected, specify the reason that prevents the owner from releasing/sharing the data.)
|
Population Represented (e.g., “residents of x,” “inpatients at x,” “users of product x”)
Residents from Dimock PA whose private wells were tested by EPA in 2012. |
Data Collection Protocol (Brief description with reference to document or website that provides detailed information.)
Data collection is described in the Drinking Water Investigation in Dimock PA EI Protocol.
The Sampling and Analysis Plan (SAP), included in the protocol, describes in detail the sampling methodology and laboratory analyses that will be performed on drinking water, groundwater, and bulk water as well as for indoor air tested for radon. The EI will be conducted in Summer 2017.
|
Data Management Protocol (Brief description with reference to physical location(s) or system(s) where data will be housed (e.g., CDC shared network drive, data host system name, SQL database, etc.) and to data formats. Include the locations of dataset both before data release and after data release.)
Data will be housed within the ATSDR/DDCHI Exposure Investigation Database.
|
Process for Omitting Identifying Information (Description of what identifiers are in the database, how they will be removed, and by whom.)
Identifiers (names and addresses) will be flagged and housed in special subdirectory by the Database manager and will not be released unless directed by the CDC Office of General Counsel.
|
Data Quality Protocol (to address issues of confidentiality protection and statistical stability) (Brief description with reference to document or website that provides detailed information. Describe methods for data validation and error resolution, removal or shielding of any proprietary information, removal or shielding of sensitive information [i.e., data with dual use applicability], removal or shielding of any individually identifying information including indirect identification.)
The Sampling and Analysis Plan (SAP) provided in the EI protocol describes the Data Quality Objectives.
|
Data Retention/Disposal Plan (State when and how the dataset will be archived or destroyed [in accordance with CDC/ATSDR Records Control Schedule: http://isp-v-maso-apps/RecSched/images/RCS.pdf ].)
EI data are considered as a component of the ATSDR “Site Files” (Section 3.6, page 64). These files Maintain in the Records and Information Management Branch. Transfer to an FRC 5 years after publication of final health assessment, consultation, or advisory report. Destroy when 30 years old.
|
Data Analysis Plan (Brief description of planned use of the data. Can include reference to document [e.g., information collection request, research protocol, or other] that provides more detailed information.)
The results of the water and indoor radon testing will be evaluated and used to determine whether the private well water and supplied water sources are safe to use and whether the level of radon in the home may be hazardous.
|
Publication Plan (Brief description of planned CDC-authored and CDC-coauthored publications, including topic, type of publication, and estimated timeline.)
ATSDR will use the data to publish an ATSDR Exposure Investigation Report.
|
Data Release Documentation (List documents provided to users, e.g., variable definitions, codebook, metadata file, guidance on data use.)
To be determined
|
Data Release Format (Recommend to use non-proprietary format when possible, such as CSV, JSON, etc. Also specify data dictionary file format.)
Excel will be used to store the water and indoor air data for the EI.
|
Data Release Notification (State how potential users will be informed of dataset availability.)
ATSDR will include a notice in the final Drinking Water Investigation in Dimock, PA Exposure Investigation Report.
|
Date Form Completed: 5/24/2017 By: Karen Scruton, Environmental Health Scientist
Name, Title
Date Form Last Revised: _________________ By: _________________________________
Name, Title
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Modified | 0000-00-00 |
| File Created | 2021-01-22 |
© 2026 OMB.report | Privacy Policy