Exposure Investigation Protocol Blood Lead Levels - Iola, KS
Att8 IOLA KS EI Protocl 08 nov 2016 FINALclean OMB comments 11-21 atsdr final.docx
ATSDR Exposure Investigations (EIs)
Exposure Investigation Protocol Blood Lead Levels - Iola, KS
OMB: 0923-0048

Exposure Investigation
Protocol
Blood Lead Levels in Iola, Kansas
Former United Zinc and Associated Smelters Site,
Iola, Allen County,
Kansas
Cost Recovery No: 7A8Q
Prepared by:
Karen Scruton, MS
Lourdes (Luly) Rosales-Guevara, M.D.
LCDR Cory M. Kokko, MPH
Agency for Toxic Substances and Disease Registry (ATSDR)
Division of Community Health Investigations (DCHI)
Science Support Branch (SSB) and
ATSDR Western Branch (Region 7)
November XX, 2016
Table of Contents
Winter 2016/2017 Testing Design 5
Permission/Consent/Assent Forms 7
Description of Risks and Benefits 11
Procedures for Parental Permission/Consent/Assent 11
Protection of Confidentiality 12
Feasibility and Limitations 12
Handling Unexpected or Adverse Events 13
Dissemination, Notification, and Reporting of Results 13
Appendix A: Site Maps and Demographics 16
Appendix B: Fact Sheet and Invitation to Participate 18
Appendix C: Parental Permission/Consent/Assent Forms 24
Appendix E: Data Management Plan 44
Appendix G: Templates for Results Letters to Participants 55
ACRONYMS
ABLES Adult Blood Lead Epidemiology and Surveillance
ATSDR Agency for Toxic Substances and Disease Registry
BLL blood lead levels
CDC Centers for Disease Control and Prevention
DCHI Division of Community Health Investigations
DLS Division of Laboratory Sciences
DMP Data Management Plan
EDTA ethylenediamine tetra-acetic acid
EI exposure investigation
EPA (U.S.) Environmental Protection Agency
HUD Housing and Urban Development.
KDHE Kansas Department of Health and Environment
KS Kansas
µg/dL microgram per deciliter
mg/kg milligram per kilogram
NCEH National Center for Environmental Health
NIOSH National Institute of Occupational Safety and Health
NPL National Priority List
OMB Office of Management and Budget
OSHA Occupational Safety and Health Administration
OU operable unit
PEHSU Pediatric Environmental Health Specialty Unit
SEKMCHD South East Kansas Multi-County Health Department
SSB Science Support Branch
Project Overview
The goals for the Blood Lead Levels in Iola,
KS EI:
Evaluate
Blood Lead Levels (BLLs) for susceptible populations
children
younger than 6 years women
who are pregnant or of childbearing years
Recommend
case management for participants with BLL ≥5µg/dL
Recommend
follow-up with a Pediatrician/Obstetrician Recommend
ways to lower exposure to contaminated soil Recommend
ways to lower exposure to dust in houses built before 1978. Provide
information on nutrition that will help to decrease the absorption
of lead into the body. Coordinate
with EPA to use BLL results to prioritize site cleanup (i.e.,
participants with BLL ≥10 ug/dL and yard soil levels ≥400
mg/kg will be given priority)
Iola is home to the Former United Zinc and Associated Smelters site (called the site), which is a National Priority List (NPL) site (Superfund site). The site resulted from historical smelting operations in Iola from 1902 to 1925. Residential and non-residential properties were heavily contaminated with elevated levels of lead in soil due to these smelting operations.
The U.S. Environmental Protection Agency (EPA) performed a Time-Critical Removal Action at the site in 2005-2006 and the site was placed on the NPL in 2013. A Remedial Investigation/Feasibility Study (RI/FS) was finalized in July, 2016 and a proposed plan for cleanup of the site is available for comment through September, 2017.
The Agency for Toxic Substances and Disease Registry (ATSDR), Division of Community Health Investigation (DCHI) proposes to conduct an Exposure Investigation (EI) in the city of Iola, Kansas (KS) to determine if any children or pregnant women are at risk from elevated blood lead levels.
Based on the 2010 census, the community may be comprised of approximately 500 children aged younger than 6 years, an undetermined number of pregnant women, and approximately 1,100 women of childbearing age (15 to 44 years of age) (Appendix A).
Participants identified as having elevated lead levels (at or above 5 micrograms per deciliter (µg/dL), explained below) will be notified and provided recommendations for clinical follow up. The EPA will prioritize properties for removal of lead contaminated soil based on elevated BLLs identified in the EI.
This EI will be conducted in two stages: one sampling round in winter 2016/2017 and one in summer 2017. Eligible participants for the EI will be chosen based on the targeted population and their potential for exposure to lead. Participants will be recruited from the city of Iola. We will recruit approximately 200 people during the winter event (150 children younger than 6 years old and 50 women who are pregnant or of childbearing age) and 300 people during the summer event (225 children and 75 women) who live in properties where EPA testing documents lead levels in soil of > 800 mg/kg .
ATSDR will partner with the Kansas Department of Health and Environment (KDHE), the Pediatric Environmental Health Specialty Unit (PEHSU) located in Kansas City, the South East Kansas Multi-County Health Department (SEKMCHD) and EPA Region 7.
EI Design
This EI will be implemented in two parts which will allow ATSDR to assess BLL during the winter and summer seasons. Blood lead testing during the winter will 1) identify children and pregnant women with elevated blood levels for appropriate follow-up and 2) provide results that can used by EPA to prioritize the spring 2017 soil clean-up efforts. Blood lead testing during the summer takes into account maximum outdoor activity for children resulting in higher exposure to lead in soil and potentially higher BLL.
Winter 2016/2017 Testing Design
The design for the winter testing will target members of the community believed to be most highly exposed to lead in soil. The strategy for recruitment for the first phase of the EI will be:
ATSDR will recruit residents from homes with greater than 800 mg/kg lead in their soil, as determined by the EPA testing, that have not been remediated (OU-00) (approximately 350 locations).
A recruitment letter and fact sheet (Appendix B) will be sent to these homes two weeks prior to testing asking them to participate in the EI during the winter
One week prior to the testing, ATSDR personnel will implement a door-to-door recruitment of these homes to recruit participants.
Participants to be targeted include:
Children younger than 6 years old
Women that are pregnant or of childbearing age (15 to 44 years old)
Siblings of children included in the EI who are older than 6 years and less than 20 years of age, at the request of the parent.
If other children younger than 6 or pregnant women/women of childbearing age become aware of the testing and desire to be included, they will be tested.
If the recruitment results in fewer than 150 participants (both children and women), recruitment will be made available to any home with lead in their soil above 400 mg/kg.
Collection Day:
Provide each participant with a permission form/assent/consent for sampling and administer a questionnaire.
Obtain a venous blood sample
Submit samples to the lab for lead analysis
Post Collection Day
Notify participants of the results of blood lead sampling
Recommend/coordinate follow-up for children/pregnant women whose BLL results are greater than or equal to 5 µg/dL
Notify EPA of homes with BLL greater than 10 µg/dL.
Summer 2017 Testing Design
The design for the summer testing will target members of the community tested in the winter as well as additional community members. The strategy for recruitment for the second phase of the EI will be:
ATSDR will recruit residents of homes that were sampled during the winter testing (and homes identified as above 800 mg/kg lead but did participant in winter testing).
During the winter testing, the participants will be asked to participate in the summer 2017 testing event.
A recruitment letter and fact sheet (Appendix B) will be sent to these homes three weeks prior to testing asking them to participate in the EI testing in the summer
Two weeks prior to the testing, ATSDR personnel will implement a door-to-door recruitment of these homes to recruit participants.
ATSDR will target daycares/preschools/elementary schools in the area:
A recruitment letter and fact sheet (Appendix B) will be sent to these locations three weeks prior to testing asking them if they would allow us to recruit families at their facility.
Two weeks prior to the testing, ATSDR personnel will visit the locations to answer questions and recruit parents of children at the facilities.
ATSDR will also target homes in OU-00 that were recently remediated and homes in OU-01 (homes with soil lead between 400 and 800 mg/kg) that have not been remediated.
A recruitment letter and fact sheet (Appendix B) will be sent to these homes three weeks prior to testing asking them to participate in the EI testing in the summer
Two weeks prior to the testing, ATSDR personnel will implement a door-to-door recruitment of these homes to recruit participants.
Participants to be targeted include:
Children younger than 6 years old,
Women that are pregnant or of childbearing age (15 to 44 years old),
Any child younger than 6 years old that was not recruited but requests to be tested will be included, and
Siblings of children included in the EI who are older than 6 years old, at the request of the parent.
Collection Day:
a. Provide each participant with a permission form/assent/consent for sampling and administer a questionnaire.
b. Obtain a venous blood sample
c. Submit samples to the lab for lead analysis
Post Collection Day
Notify participants of the results of blood lead sampling
Recommend/coordinate follow-up for children/pregnant women whose BLL results are greater than or equal to 5 µg/dL
Notify EPA of homes with BLL greater than 10 µg/dL.
The results of the winter and summer sampling events will be evaluated in an Exposure Investigation report to determine whether a public health hazard exists in the community.
The findings and recommendations of the EI will be presented to the community in a public availability session after completion of the EI report.
Recruitment
Media outlets will be contacted in advance with a request to publicize the upcoming recruitment and implementation for the EI in the city of Iola.
Community members will be targeted for inclusion using the following methods;
Three weeks before the sampling, an invitation to participate and a Fact Sheet, will be mailed to community members as specific above for the winter and summer samplings events (Appendix B). The invitation will provide information on when the EI will occur and how to sign up. The information will indicate that blood lead sampling will be provided free of charge.
As outlined above, a recruitment letter and fact sheet will be provided to facilities where young children are anticipated to be present, such as daycare centers and preschools in the area. The fact sheet will be provided to the directors of the facilities with a request for the director to disseminate the information to families at the daycare and preschools.
Two weeks prior to the sampling, members of the EI team will canvas neighborhoods and recruit community members to take part in the EI. The team will go door to door to discuss the EI with the community. In addition, the team will be at a central location several evenings during the recruitment period to provide information to the community and schedule appointments.
Permission/Consent/Assent Forms
Participants of the winter and summer testing will be provided with a Parental Permission/Consent/Assent form as appropriate prior to the sampling as follows:
Parental Permission for all children younger than 18 years of age
Assent form for children aged 7 to 17 years old
Consent form for pregnant/women of child-bearing age
Participants will have to read and sign the Parental Permission/Consent/Assent form(s) while ATSDR personnel are present (Appendix C). After the participants sign the forms, the ATSDR EI personnel will sign as a witness and collect one of the signed forms. ATSDR EI personnel will answer any questions about the EI including questions about risks and benefits from participating in the EI the community member may have. The participants will be provided with a copy of the parental Permission/Consent/Assent form.
The Parental Permission/Consent/Assent form and all other EI documents used to communicate with participants have been evaluated for reading level using Flesh-Kincaid Grade Level tool in Microsoft Word. The reading level is 8th grade or below. Appendix C includes copies of the Parental Permission/Consent/Assent forms.
Questionnaire
Participants will be asked questions from an Office of Management and Budget (OMB) approved questionnaire about daily activities (Appendix D). The responses will be used in the evaluation of the blood results.
Prior to use, the questionnaire will be approved through the Office of Management and Budget (OMB) using the existing OMB generic package for EIs (OMB number 0923-0048, expires 3/31/2019). The protocol, including the questionnaire, will be submitted to OMB as a GenIC package for the site.
Blood Sampling
Blood lead sampling is the most reliable method for measuring recent and ongoing exposure to lead. Blood will be collected by a certified phlebotomist using appropriate blood drawing protocols. Testing results will identify exposures from all sources. Specific exposure sources will not be identified by a blood lead result.
A phlebotomist will collect 3 milliliters (ml) of blood from a vein from each participants who provides consent. The tubes for the collection have been selected and provided by the NCEH/DLS to ensure there is not contamination. Blood for lead analysis will be collected in 3 ml ethylenediamine tetra-acetic acid (EDTA) coated tubes provided by the laboratory.
The blood samples will be maintained at an appropriate refrigerator temperature (4º C) after collection. Samples will be shipped on ice packs by FedEx overnight delivery to CDC/DLS. The DLS will analyze blood samples for lead concentration using DLS method 30916.8-02 in whole blood by Inductively Couple Plasma Dynamic Reaction Cell Mass Spectrometry (ICP-DRC-MS). Reference: NHANES method 2009-2010:
http://www.cdc.gov/nchs/nhanes/nhanes20092010/lab_methods_09_10.htm
A Data Management Plan (Appendix E) manages the data collected during the EI. To maintain privacy, the samples will be labeled with unique coded identification numbers. The Principal Investigator will maintain control of the coded identifications numbers.
EI personnel will adhere to the Health and Safety Plan provided in Appendix F when handling and shipping blood samples.
EI Time Line
Week 1 and 2 (Winter testing):
Contact local media in advance requesting announcements of ATSDR‘s recruitment process.
Mail recruitment fact sheet and invitation to resident’s homes and to local businesses that deal with children younger than 6 years old (e.g., daycare center, preschools, and elementary schools). The invitation will include an ATSDR-dedicated toll free 800 phone line in Atlanta to allow prospective participants to ask questions about the EI and make appointments for blood draw.
Week 3 in Iola (Winter testing):
Complete a door to door canvas of the community to answer questions resulting from the invitation provided to the community and to recruit participants.
Schedule phlebotomists based on number and schedules of participants.
Continue to communicate with media outlets in Iola about the EI Recruitment process.
Week 4 in a Central Location in Iola (Winter testing):
Call participants the day before their appointment as a reminder.
Register every participant upon arrival to the central location testing place
Provide the parental permission/consent/assent to participants to read and sign and will explain the venous blood testing process. All potential participants have the right to refuse participation without penalty.
Administer a questionnaire to adults 18 years of age and above. The head of household will answer the questions for their children.
Collect blood samples after the parental permission/consent/assent and questionnaire are completed.
Send the blood samples to the CDC/NCEH/DLS for analysis.
Participation will depend on the individual’s capacity to give informed consent and respond to the questionnaire. For minors, a parent or guardian will sign the consent and answer questions for their minor child/ward.
Week 5 to 7: Analyze the blood samples in the CDC/NCEH/DLS (Winter testing):
Analyze blood samples at DLS. Note: Additional 2 weeks will be added to this frame if week 5 coincides with end of year holidays.
Week 6 to 8: Review and interpret results (Winter testing):
Interpret the blood lead results received from the laboratory. The questionnaire answers will help in the interpretation of the results.
Week 9 to 11: Notify the participants of their test results (Winter testing):
Notify the participants of individual tests results, interpretation, and recommendations. Sample letters are included in Appendix G.
Provide/Coordinate follow-up for children/pregnant women with BLL above the CDC reference level.
Follow-up with a personal phone call from the Principal Investigator to the head of households to discuss any questions they may have.
Week 20 to 24 (Summer testing):
Resample the participants sampled in the winter testing, if possible, and identify additional participants, as needed
Collect and analyze blood samples as described for the winter stage of the EI.
Week 25 to 27: Analyze the blood samples in the CDC/NCEH/DLS (Summer testing):
Analyze blood samples at DLS.
Week 28 to 32: Review and interpret results (Summer testing):
Gather and begin to analyze the BLL results in relation to the residential soil data collected by EPA in the past as well as other environmental data and questionnaire information.
Week 33 to 36: Notify the participants of their test results (Summer testing):
Mail letters via the US Post Office to participants with their individual tests results, interpretation, and recommendations. Sample letters are included in Appendix F.
Provide/Coordinate follow-up for children/pregnant women with BLL above the CDC reference level.
Follow-up with a personal phone call from the Principal Investigator to the head of households to discuss any questions they may have.
Week 37 to 52: Prepare EI Report (Winter and Summer testing):
Complete writing the EI Report for publication. Analyze BLL data (ATSDR EI team and branch staff) in relation to the environmental data and questionnaire information.
Submit the EI Report for review and release
Make the report available in the repository at Iola Public Library, located at 218 E Madison Ave, Iola, KS 66749, Phone: (620) 365-3262, Website: iola.mykansaslibrary.org and on the ATSDR website http://www.atsdr.cdc.gov/
Human Subjects
Children (younger than 6 years) and adults (pregnant women/women of childbearing age) living in the target area, with no history of occupational exposure to lead, will be eligible to participate. If a participant is found to have occupational exposure after testing, the results will be provided to the participant but will not be included in the EI report.
ATSDR will target the following number of participants:
Winter testing
150 children younger than 6 years old
50 women who are pregnant or of childbearing age
Summer testing
225 children younger than 6 years old
75 women who are pregnant or of childbearing age
For both rounds of testing, the following participants will be included upon request:
Children younger than 6 years old or pregnant/women of childbearing age that were not specifically recruited
Siblings of children enrolled in the EI, at the request of the parent
Description of Risks and Benefits
Risk: We anticipate the risks to be very low, but cannot entirely exclude them. There may be pain and/or bruising at the site where the blood was collected. There is the slight risk of feeling light headed during blood collection.
Benefits: Participants will be informed if they have recent and ongoing exposure to lead. If we find elevated levels of lead in blood, we will provide recommendations to reduce exposure. There is no monetary incentive for participating in this EI, however, the EI participation, blood lead testing and test interpretation are free of charge.
Procedures for Parental Permission/Consent/Assent
Participants will have time to read the permission/consent/assent form (Appendix C) while ATSDR EI personnel are present. ATSDR EI personnel will answer any questions about the EI, including risks and benefits. After the participant signs the permission/consent/assent form, ATSDR EI personnel will sign as witness and collect one of the two signed forms (the participant will keep one of the signed forms).
Parents/guardians will provide permission to participate for children <18 years of age. An assent form is also provided for participants aged 7 to 17 years. Permission/consent/assent forms, as well as all other EI instruments of communication with participants, have been evaluated for readability using Flesh-Kincaid Grade Level tool in Microsoft Word.
Protection of Confidentiality
Participant’s confidentiality and personal information will be protected by ATSDR to the fullest extent possible by law. Individual tests results will not be made available to the public. Confidential Information will be kept in locked cabinets and/or password protected computers. Answers to the questionnaire will be entered into an Access database using a secured password. At the conclusion of the EI, ATSDR will prepare and publish an EI Report summarizing the findings, but will not reveal personal identifiers (e.g., name or address). Reports produced after the EI implementation will give only group information and will not identify specific individuals or residences.
All blood samples that are sent to the laboratory for analysis will be labeled with a unique identifying number. Results from the laboratory will be entered into an electronic database and sent to the ATSDR Principal Investigator.
Feasibility and Limitations
ATSDR staff has experience in this type of investigation and has previously worked on lead exposure investigations. ATSDR is well positioned to address lead issues and to respond to community concerns that may be voiced during this EI.
Potential limitations include:
We may not know the source of a participant’s exposure.
The blood lead concentrations cannot be used to predict the future occurrence of disease nor be attributed as the cause of current health problems.
The number of participants recruited may not give ATSDR a complete understanding of the extent of exposure.
The results of this EI will be applicable only to the individuals tested and cannot be generalized to other populations.
Handling Unexpected or Adverse Events
There is a small chance of unexpected or adverse events occurring during the course of this EI.
The most likely adverse event is a participant feeling lightheaded or fainting during blood collection. The medical officer of the EI group in Iola and the phlebotomist collecting blood are trained in responding to such situations.
If any adverse event should occur, the Principal Investigator will notify local emergency services and ATSDR in Atlanta.
Data Handling
All data will be managed in accordance with the Former United Zinc Exposure Investigation Data Management Plan (DMP), included as Appendix E. All Parental Permission/Consent/Assent forms and questionnaires will be stored in a secure location while in Iola and then returned to ATSDR in Atlanta where they will be kept in a locked cabinet and/or password protected computer. Blood lead results (with unique identifying number), will be stored in a separate secure location on site and then kept secured by CDC/DLS. All other confidential information will be kept in locked cabinets and/or password protected computers by the ATSDR Principal Investigator. ATSDR will share data with EPA and state partners under a CDC/ATSDR data sharing agreement signed by the respective parties.
Data Analysis
ATSDR
staff will ship the venous blood samples via FedEx overnight express
mail to CDC/DLS in Atlanta for analysis. The laboratory will follow
their standard procedures for analyzing blood for lead (see Biologic
Sampling under Procedures/Methods). The analysis results from the
CDC/DLS will be transmitted to the ATSDR Principal Investigator in an
electronic spreadsheet format. No personal identifiers will be
provided to the laboratory.
Appropriate Data Quality Assurance and Quality Control (QA/QC) will be performed by the CDC/DLS and will meet DLS accuracy and precision standards (Caudill et al., 2008). After all the blood samples are analyzed, the results will be provided to the ATSDR Principal Investigator for interpretation.
Age groups in the EI Report (e.g., children younger than age 6, pregnant women, women of childbearing age) will be grouped to correspond to those found in the updated tables of the National Health and Nutrition Examination Survey (NHANES) http://www.cdc.gov/exposurereport/pdf/fourthreport.pdf.
Dissemination, Notification, and Reporting of Results
Participants will be informed of their tests results by letter that will be mailed via the US Postal Service. The texts of the draft letters will be addressed to parents of children under 18 years of age, pregnant women, and women of childbearing age. Sample letters that will be used to inform participants of their results are included in Appendix G.
An Exposure Investigation report will be written and made available by ATSDR to participants in a repository at Iola Public Library, located at 218 E Madison Ave, Iola, KS 66749, Phone: (620) 365-3262, Website: iola.mykansaslibrary.org
A public meeting and/or an availability session will take place in Iola after the EI Report is released. Speakers from ATSDR will be available to answer question the community may have. A formal power point presentation about the EI will also be given to the community.
References:
Agency for Toxic Substances and Disease Registry (ATSDR). 2007. Toxicological profile for Lead. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
Brink LL, Talbot EO, Sharma RK, March GM, Wu WC, Rager JR, Strosnider HM (2013). Do U.S. Ambient Air Lead Levels Have a Significant Impact on Childhood Blood Lead Levels: Result of a National Study. J. Environ and Public Health. Available at: http://www.hindawi.com/journals/jeph/2013/278042/.
Caudill SP, Schleicher RL, Pirkle JL, 2008. Multi-rule quality control for the age related eye disease study. Stat. Med. 27, 4094-4106.
CDC 2005. Preventing Lead Exposure in Young Children, 2005. http://www.cdc.gov/nceh/lead/publications/prevleadpoisoning.pdf
CDC 2009. The Fourth National Report on Human Exposure to Environmental Chemicals, 2009. Available at: http://www.cdc.gov/exposurereport/pdf/FourthReport.pdf
CDC, 2010. CDC Guidelines for the Identification and Management of Lead Exposure in Pregnant and Lactating Women, November 2010. U.S. DHHS, Atlanta, GA. http://www.cdc.gov/nceh/lead/publications/leadandpregnancy2010.pdf
CDC 2012. Low Level Lead Exposure Harms Children: A call for Primary Prevention. Report on the Advisory Committee on Childhood Lead Poison Prevention (ACCLPP) Of the Centers for Diseases Control and Prevention, 2012; U.S.DHHS, 2012. http://www.cdc.gov/nceh/lead/ACCLPP/Final_Document_030712.pdf
DHHS 2010. Atlanta, Georgia. Guidelines for the Identification and Management of Lead Exposure in Pregnant and Lactating Women. November 2010. U.S. Department of Health and Human Services.
Appendices
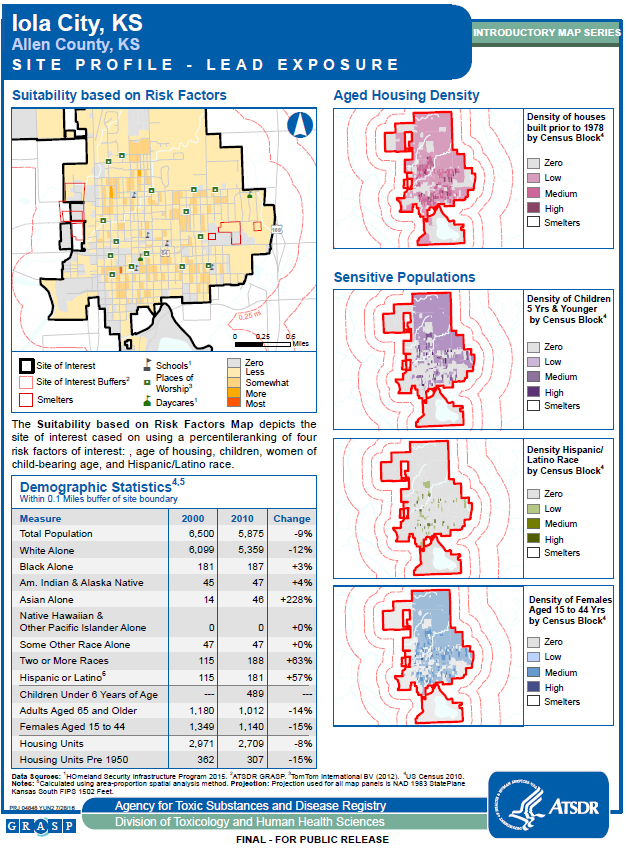
Appendix A: Site Maps and Demographics
Appendix B: Fact Sheet and Invitation to Participate
B1: Fact Sheet for EI
B2: Invitation to Participate for Residents
B3: Invitation to Participate for Daycares/Preschools
Appendix C: Parental Permission/Consent/Assent Forms
C1: Parental Permission form for children younger than 18 years
C2: Consent form for pregnant women and women of childbearing age
C3: Assent form for children between 7 and 18 years
Appendix D: Participant Questionnaire
Appendix E: Data Management Plan
Appendix F: Health and Safety Plan
Appendix G: Sample Results Letters to Participants
Appendix A: Site Maps and Demographics
Appendix B: Fact Sheet and Invitation to Participate
B1: Fact Sheet for EI
B2: Invitation to Participate for Residents
B3: Invitation to Participate for Daycares/Preschools
Appendix B1: Fact Sheet for EI
Flesch-Kincaid Reading level – 7.9
Blood Lead Levels in Iola, Kansas
Agency for Toxic Substances and Disease Registry (ATSDR)
Who are we? |
|
Our Partners |
|
Why are we here? |
|
What are we doing here? |
|
How are we going to do this? |
|
When and where are we going to do the sampling? |
|
Benefits of participating in the EI |
|
Risks of participating in the EI |
|
What happens after the sampling? |
|
Contact Information |
|
Appendix B2: Invitation to Participate for Residents
Flesch-Kincaid Reading level – 11.1
[Address]
Subject: Blood Lead Testing in Iola
Dear Resident:
Former industries in Iola resulted in increased amounts of lead in the soil in the area. The Environmental Protection Agency (EPA) has been testing and removing soils containing lead in the area over the past ten years.
The Agency for Toxic Substances and Disease Registry (ATSDR) will be testing participants venous blood for lead levels, the testing will be free of charge, in:
Children younger than 6 years old
Women that are pregnant or of childbearing age (15 – 45 years)
ATSDR will be in Iola on XX at XX for the blood testing. If you or anyone in your family wants to be tested, please contact me at my direct office number: 770-488-0744 or toll-free at 1-888-892-1320.
Sincerely,
Lourdes (Luly) Rosales-Guevara, MD
Medical Officer
ATSDR Science Support Branch
The Agency for Toxic Substances and Disease Registry (ATSDR) is a branch of the Centers for Disease Control and Prevention (CDC), located in Atlanta, GA. ATSDR looks at contaminants in the environment, such as soil and water, to determine if the amount may be a concern to the community. ATSDR also has a local office in Kansas City.
Appendix B3: Invitation to Participate for Daycares/Preschools
Flesch-Kincaid Reading level – 10.8
[Address]
Subject: Venous Blood Lead Testing in Iola:
Dear Principal of Daycare/Preschool/Elementary School:
Former industries in Iola resulted in increased amounts of lead in the soil in the area. The Environmental Protection Agency (EPA) has been testing and removing soils containing lead in the area over the past ten years.
The Agency for Toxic Substances and Disease Registry (ATSDR) will be testing participants blood for lead levels, the testing will be free of charge, in:
Children younger than 6 years old
Women that are pregnant or of childbearing age
ATSDR will be in Iola on XX at XX for the testing. We would appreciate it if you would provide the attached fact sheet to the parents of the children at your facility. If you have any questions about the investigation, please contact me at 770-488-0744 or toll-free at 1-888-892-1320.
Sincerely,
Lourdes (Luly) Rosales-Guevara, MD
Medical Officer
ATSDR Science Support Branch
The Agency for Toxic Substances and Disease Registry (ATSDR) is a branch of the Centers for Disease Control and Prevention (CDC), located in Atlanta, GA. ATSDR looks at contaminants in the environment, such as soil and water, to determine if the amount may be a concern to the community. ATSDR also has a local office in Kansas City.
Appendix C: Parental Permission/Consent/Assent Forms
C1: Parental Permission form for children younger than 18 years
C2: Assent form for children between 7 and 17 years
C3: Consent form for pregnant women and women of childbearing age
Appendix C1: Parental Permission Form for Children less than 18 Years of Age
Flesch-Kincaid Reading level – 5.5
Parental Permission Form for Venous Blood Sampling for Lead
& Questionnaire
Children less than 18 years of age
ATSDR Exposure Investigation (EI)
Iola, Kansas
Who are we?
We are from a federal public health agency, the Agency for Toxic Substances and Disease Registry (ATSDR)
Who are we working with?
Region 7 Environmental Protection Agency (EPA)
Kansas Department of Health and the Environmental (KDHE)
South Eastern Kansas Multi County Health Department (SEKMCHD) and
Region 7 Pediatric Environmental Health Specialty Unit (PEHSU)
Why we are doing this Exposure Investigation (EI)?
We are doing this EI to find out if children living in Iola, Kansas, have high levels of lead in their blood.
What do we want you to do?
Your child is invited to have his/her venous blood tested for lead.
There is NO COST to you for the testing of your child.
The blood collection will take place at _____________________.
What is included in my child’s participation?
There are two parts to your child’s participation.
Venous Blood Collection and Testing for Lead
We will collect less than 1 teaspoon (3 milliliters) of blood from a vein of your child’s arm.
This will take 5 minutes or less.
We will send your child’s blood to a lab to test it for lead.
Answer a Short Questionnaire:
We will ask you some questions about how your child might be exposed to lead.
This should take about 20 minutes.
What will happen to any leftover blood after testing is finished?
The blood will not be used or tested for anything else.
The lab will throw out any leftover blood.
When will you get the test results?
You will get your child’s test results by mail about 12 weeks after testing.
What are the benefits of being in this EI?
You will know if your child has a high level of lead in blood.
If your child has a blood lead level greater than or equal to 5 µg/dL, ATSDR and Region 7 PEHSU can provide you with information that will help you reduce your child’s contact with lead and recommend follow-up with a doctor.
If your child has a blood lead level that is greater than or equal to 10 µg/dL, it will be reported to the KDHE, as required by law. Also, EPA will cleanup your yard faster if they know about high lead levels in your soil and in your child’s blood.
What are the risks of this EI?
Your child might cry because the needle hurts.
Your child’s arm may be bruised where the blood is taken from.
Your child may feel dizzy or lightheaded.
How will we protect your privacy?
We will protect your child’s privacy as much as the law allows.
Kansas law requires that we report blood lead levels to KDHE if the result is greater than or equal to 10 µg/dL.
Kansas law requires that information given to the state may be made public if someone asks them for the information, but your child’s name and address will not be released.
We will share the results with other agencies only with your permission. We will require our government partners to treat your information as private.
We will give your child an identification (ID) number.
Your child’s ID number, not his/her name, will go on the tube of blood.
We will keep a record, under lock-and-key, of your child’s name, address and ID number. We will use this information to link your child’s results with his/her name so we can send you your child’s test results.
We will not use your or your child’s name in any report we write. Only group information that does not include individual names will be reported.
When can you ask questions about the testing?
If you have any questions about this testing, you can ask us now.
If you have questions later, you can call:
Dr. Luly Rosales-Guevara at (770) 488-0744 or
The Iola Exposure Investigation toll free number (888) 892-1320
Parental/Guardian Voluntary Permission
I agree to have my child tested.
I agree to answer questions about my child.
I was given the chance to ask questions on behalf of my child. I feel my questions have been answered.
I know that having these tests done is my choice.
I know that even though we agreed to this testing, my child may leave at any time without penalty.
Regardless of the results, may we share the test result with other federal, state, and local health and environmental agencies? YES / NO (please circle one)
If the results are 5 and up to 10 µg/dL, can we provide your information to the Pediatric Environmental Health Specialty Unit (PEHSU), and may they contact you for follow-up? YES / NO (please circle one)
If the results are greater than or equal to 10 µg/dL, we are required to report it to KDHE. Can we also provide your child’s results to PEHSU for follow-up? YES / NO (please circle one)
Signature
I give permission for my child to be tested and agree to answer questions about my child.
______________________________________
______ ___________
Printed name of child Age Sex of child
___________________________________ __________________
Signature of parent/guardian Date
___________________________________
Printed name of parent/guardian
Address of Child _____________________________ Telephone __________________
______________________________
______________________________
Lab ID Number____________________
Certification of Permission Form Administrator:
I read the permission form to the person named above. He/she had the opportunity to ask questions about the Exposure Investigation and had the questions answered.
_______________________________________
Signature of person administering permission
Appendix C2: Assent Form for Children and Youth 7 to 17 Years of Age
Flesch-Kincaid Reading level – 4.5
Assent Form for Blood Lead and Questionnaire
Youth 7 years to 17 years of age
ATSDR Exposure Investigation (EI)
Iola, Kansas
Who are we?
We are from a federal public health agency, the Agency for Toxic Substances and Disease Registry (ATSDR)
Who are we working with?
Region 7 Environmental Protection Agency (EPA)
Kansas Department of Health and the Environmental (KDHE)
South Eastern Kansas Multi County Health Department (SEKMCHD) and
Region 7 Pediatric Environmental Health Specialty Unit (PEHSU)
Why we are doing this Exposure Investigation (EI)?
We are doing this EI to find out if children and youth living in the city of Iola, Kansas have high levels of lead in their blood.
What are we asking you to do?
You are invited to have your blood tested for lead.
There is NO COST to you or your parents for the testing.
The blood collection will take place at _______________________.
What is included in my participation?
There are two parts to your participation.
1. Blood Collection and Testing for Lead
We will take less than 1 teaspoon (3 milliliters) of blood from your arm.
This will take 5 minutes or less.
We will send your blood to a lab to test it for lead.
2. Answer Some Questions
During the appointment we will ask you some questions.
This will take about 20 minutes.
Your parents can help you with the questions, if you want.
What will happen to any leftover blood?
It will not be tested or used for anything else.
The lab will throw out any leftover blood.
When will you get the test results?
Your parents will get your test results by mail about 12 weeks after testing.
What are the benefits from being in this EI?
Your parents and you will know if you have a high level of lead in your blood.
If you have a blood lead level that is 5 µg/dL or higher, ATSDR and Region 7 PEHSU can provide you and your parents with information that can help you reduce your contact with lead.
What are the risks of this EI?
The needle stick might hurt a little.
Your arm may get a black and blue mark where the blood is taken.
You may feel a little dizzy for a short time.
How will we protect your privacy?
We will protect your privacy as much as the law allows. Kansas Department of Health and the Environment (KDHE).
Kansas law requires that information given to the state may be made public if someone asks them for the information but your name and address will not be released.
We will give you an identification (ID) number.
We will use your ID number on the tube of blood.
We will keep a record, under lock-and-key, of your name, address and ID number so we can send the test results to your parents.
We will not use your name in any report we write. Only group information that does not include your name will be reported.
When can you ask questions about the testing?
If you have any questions about this testing, you can ask us now.
If you have questions later, you can call:
Dr. Luly Rosales-Guevara at (770) 488-0744 or
The Iola Exposure Investigation toll free number (888) 892-1320
Child Assent
Your parent/guardian said it is all right for you to have the blood test.
Your parent/guardian said it is all right for you to answer some questions.
You don’t have to have this test to answer questions if you don’t want to.
Voluntary Assent
I agree to be tested.
I agree to answer questions.
I was given the chance to ask questions and feel my questions were answered.
I know that having this test done is my choice.
I know that even though I have agreed to this testing, I may leave at any time without penalty.
Signature
I agree to be tested and to answer questions.
_________________________________________ ___________ ______________
Printed name of child Age of child Sex of child
______________________________________________ __________________
Signature or written name of child in child’s handwriting Date
__________________________
Printed name of parent/guardian
Address of child ______________________________ Telephone __________________
______________________________
______________________________
Lab ID Number____________________
Certification of Assent Form Administrator:
I read the assent form to the person named above. He/she had the opportunity to ask questions about the Exposure Investigation and had the questions answered.
_______________________________________
Signature of person administering the assent
Appendix C3: Consent Form for Women Aged 18 Years and Over
Flesch-Kincaid Reading level – 5.2
Consent Form for Venous Blood Lead Testing and Questionnaire
Pregnant Women & Women of Child Bearing Age
ATSDR Exposure Investigation (EI)
Iola, Kansas
Who are we?
We are from a federal public health agency, the Agency for Toxic Substances and Disease Registry (ATSDR)
Who are we working with?
Region 7 Environmental Protection Agency (EPA)
Kansas Department of Health and the Environmental (KDHE) South Eastern Kansas Multi County Health Department (SEKMCHD) and
Region 7 Pediatric Environmental Health Specialty Unit (PEHSU)
Why we are doing this Exposure Investigation (EI)?
We are doing this EI to find out if pregnant women and women of childbearing age living in Iola, Kansas have high levels of lead in their blood.
What are we asking you to do?
You are invited to have your blood tested for lead.
There is NO COST to you for the testing.
The blood collection will take place at _________________________
What
is included in my participation?
There are two parts to
your participation.
1. Blood Collection and Testing for Lead
We will collect less than 1 teaspoon (3 milliliters) from a vein in your arm.
This will take 5 minutes or less.
We will send your blood to a lab to test it for lead.
2. Answer a Short Questionnaire
We will ask you some questions about how you might be exposed for lead.
This should take about 20 minutes.
What will happen to any leftover blood after testing is finished?
The blood will not be used for anything else.
The lab will throw out any leftover blood.
When will you get the test results?
You will get your test results by mail about 12 weeks after testing.
What are the benefits of being in this EI?
You will know if you have high level of lead in your blood.
If you have a high blood lead level, ATSDR and Region 7 PEHSU can provide you with information that will help you reduce your contact with lead.
If your blood lead level is 10 µg/dL or higher, EPA will cleanup your yard faster if there is high lead levels in your soil.
What are the risks of this EI?
The needle stick might hurt a little.
Some bruising may happen where the blood is taken.
You may feel a little lightheaded for a short time.
If you are pregnant there is no risk to the pregnancy from the blood collection.
How will we protect your privacy?
We will protect your privacy as much as the law allows.
Kansas law requires that we report blood lead levels to KDHE if the result is greater than 25 µg/dL.
Kansas law requires that information given to the state may be made public if someone asks them for the information but your name and address will not be released.
We will share the results with other agencies only with your permission. We will require our government partners to treat your information as private.
We will give you an identification (ID) number.
Your ID number, not your name, will go on the tube of blood.
We will keep a record, under lock-and-key, of your name, address, and ID number. The information will be used by ATSDR to link the results to each person and send your blood test results to you.
We will not use your name in any report we write. Only group information that does not include individual names will be reported.
When can you ask questions about the testing?
If you have any questions about this testing, you can ask us now.
If you have questions later, you can call:
Dr. Luly Rosales-Guevara at (770) 488-0744 or
The Iola Exposure Investigation toll free number (888) 892-1320
Voluntary Consent
I agree to be tested.
I agree to answer questions.
I was given the chance to ask questions and I feel my questions were answered.
I know that having the test done is my choice.
I know that even though I have agreed to this testing, I may leave at any time without penalty.
Signature
I give my permission to be tested and agree to answer questions.
May we share the test results with other federal, state, and local health and environmental agencies? YES / NO (please circle one)
___________________________________ __________________ ______
Signature of Person Giving Consent Date Age
Address _____________________________ Telephone __________________
______________________________
______________________________
Lab ID Number____________________
Certification of Consent Form Administrator:
I read the consent form to the person named above. He/she had the opportunity to ask questions about the Exposure Investigation and had the questions answered.
_______________________________________
Signature of person administering the consent
Appendix D: Questionnaire
Iola KS Exposure Investigation Questionnaire
Introduction - Hello my name is {SAY NAME}. We are doing an Exposure Investigation for the Agency for Toxic Substances and Disease Registry, or ATSDR. ATSDR is a sister agency to the Centers for Disease Control and Prevention (CDC). As part of the investigation, we will be asking you some common questions like your name and address. We will also ask questions on your contact with lead. We are asking these questions to better understand all the data we collect.
The questions should take about 20 minutes. After that, we will be offering free blood testing for participants in this exposure investigation. Once we are done with this investigation, you will be given a copy and details of the testing results for you and your children (if you have them). Generally, we are able to get results to you within 12 weeks.
Cost Recovery Number: 7A8Q
Person Administering Questionnaire_______________________________________
Date Questionnaire Administered_________________________________________
Participant last name___________________________________________________
Participants first name__________________________________________________
Address:_____________________________________________________________
Mailing address if different from home address: _____________________________
Laboratory ID________________________________________________________
Public reporting burden of this collection of information is estimated to average 20 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Information Collection Review Office, 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA (0923-0048).
Now I want to ask you questions about how I can contact you. I will also be asking how long you have lived at or visited certain places. This is needed to find out how long you may have had contact with lead and how long it may have lasted. We will also ask your age, address, race, and about how you spend your time (e.g, child at daycare, how often they play outside, your jobs and hobbies). This is useful to help us better understand your test results.
Is the person being interviewed a minor (if NO, skip to question 21)?
Yes No
Name of person answering questions for minor child:
Relationship to child:
Mother
Father
Grandparent
Guardian
Has your child ever had their blood tested for lead (if NO, skip to question 13)?
If yes, when, where and what was the result?
Does your child go to daycare or school during the day (if NO, skip to question 15)?
If yes, how long is your child out of the house during the day and how many times per week do they go?
How many hours per day does your child typically play in your yard?
Does your child wash their hands before eating?
Always
Sometimes
Never
Does the child put their hands or toys in their mouth (if NO, skip to question 19)?
If yes, what and how often?
Have you noticed the child eating dirt while playing outside (if NO, skip to question 21)?
If yes, how often?
How long have you lived at this address?
Less than 6 months
6 months to less than 2 years
2 to 5 years
6 to 10 years
More than 10 years
How long have you lived in Iola, KS?
Less than 6 months
6 months to less than 2 years
2 to 5 years
6 to 10 years
More than 10 years
How often do you clean your home (e.g., sweep, mop)?
Daily
Several times a week
Weekly
Monthly
Other
Do you speak a language other than English at home? [If NO, skip to next section]
Yes No
If you speak another language in the household do you prefer receiving follow up information in another language? What is this language?
Demographic Questions - Script: The next questions are about qualities of the person who is being tested (you or your child/ward) your or your child’s qualities own qualities and will help us better understand your test results.
What is your or your child/ward’s sex?
Male
Female
What is your or your child/ward’s date of birth?
Are you or your child/ward Hispanic, Latino/a, or Spanish Origin? (one or more categories may be selected)
No, not of Hispanic Latino/a, or Spanish origin
Yes, Mexican, Mexican American, Chicano/a
Yes, Puerto Rican
Yes, Cuban
Yes, Other Hispanic, Latino, or Spanish Origin
What is your or your child/ward’s race? (one or more categories may be selected)
White
Black or African American
American Indian or Alaska Native
Asian Indian
Chinese
Filipino
Japanese
Korean
Vietnamese
Other Asian
Native Hawaiian
Guamanian or Chamorro
Samoan
Other Pacific Islander
Participant declined to answer
Are you pregnant? If yes in what month of pregnancy?
Don't know
No
Yes, 0 to 3 months
Yes, 4 to 6 months
Yes, 7 to 9 months
How much time do you or your child/ward spend outdoors in a typical day?
Never go outside
Less than 1 hour
1 to 3 hours
More than 3 hours
Attributes of the Structure or Home - The following questions are about the qualities and characteristics of your home.
Do you live in a(n):
Apartment
Single Family Home
Townhouse or Condominium
Mobile Home
Other
About when was the building built?
2000—present
1990—1999
1980—1989
1970—1979
1960—1969
1950—1959
1940—1949
1939 or earlier
Don’t know
What is the condition of your home or building?
Good
Fair
Poor
Do the windows (e.g., sills) have peeling paint?
Yes No
Is there peeling paint in other places such as cabinets, interior walls and/or exterior walls?
Yes No Don’t know
Soil Information (Tracking inside home)
Does your home have a yard with grass/dirt?
How often do you or your child/ward remove shoes before entering your home?
Never do this
Seldom do this
Sometimes do this
Always do this
Does anyone in the home work primarily outdoors in a job with frequent soil contact? (construction worker, landscaping, etc.) (if NO, skip to question 39)
Yes No Don’t know
How often do they change clothing when entering the home after work outdoors?
Never do this
Seldom do this
Sometimes do this
Always do this
Have you used any Mexican pottery in the past month?
Yes No Don’t know
Have you used any home remedies in the past month for any illnesses?
Yes No Don’t know
Have you eaten any Mexican candy in the past month?
Yes No Don’t know
44. Is there anything you want us to know about you or your child that we did not ask about?
Appendix E: Data Management Plan
NCEH/ATSDR Data Management Plan Form
This plan describes the anticipated use and release by CDC of the dataset named below. All CDC DMPs are required to be in compliance with the CDC/ATSDR Policy on Releasing and Sharing data, available at http://isp-v-maso-apps.cdc.gov/Policy/Doc/policy385.pdf. This plan is modifiable and does not represent a legal contract between CDC and any other entity. The elements included do not necessarily constitute an exhaustive list of all possible elements for a DMP, so users should add elements as needed.
The DMP is submitted through eClearance for review and approval. Use “TBD” if you cannot determine some of this information at the time of submission. Elements with an asterisk (*) are required data fields for metadata.
Table 1 – Core DMP Elements (should be filled out when project approval is sought)
Label (Definition) |
*Title: Blood Lead Levels in Iola, KS EI, Former United Zinc and Associated Smelters NPL Site, Iola, Allen County, Kansas: Exposure Investigation.
|
*Description (Human-readable description with sufficient detail to enable a user to quickly understand whether the project or data set is of interest. Short clear description is ideal.) This exposure investigation (EI) will sample up to 600 persons to evaluate lead exposure in the city of Iola. Eligible participants for the EI will be chosen based on their potential for exposure to lead and will focus on children younger than 6 years old and pregnant women or women of childbearing age. The participants will be sampled twice to allow for evaluation during two seasons: late 2016/early 2017 and June/July 2017. This EI will be a collaboration between ATSDR headquarters, ATSDR Region 7, the Kansas Department of Health and Environmental (KDHE), the Pediatric Environmental Health Specialty Unit (PEHSU) from Kansas City, and EPA Region 7.3
|
*Last DMP Update: November 2, 2016.
|
Contact Name and Email |
CDC PI or POC Name (last, first): Rosales-Guevara, Lourdes |
CDC PI or POC e-mail address: lir0@cdc.gov |
CDC PI or POC phone number: 770-488-0744 |
Organization
ATSDR/DCHI/SSB
|
*Unique Identifier (A unique identifier for the project as maintained within an Agency catalog or database. For intramural submissions, protocol/S3P number can be used. For extramural submissions, grant/Co Ag/contract number can be used to map to related documents.)
7A8Q
|
Public Access Level (The degree to which the data collected as part of this project could be made publicly available, regardless of whether it has been made available.) |
□ Public release (Data set can be made available without restrictions; data steward no longer controls data.) |
□ Release by request (Data set is available to members of the public by request only; data steward no longer controls data.) |
X Restricted use data sharing (Data set is available to particular parties under certain use restrictions; data not always under CDC custody.) |
□ Restricted access data sharing (Data set is only available in an RDC; data need to remain under CDC custody.) |
□ Summary data or data tables only (Underlying data set cannot be released or shared, but summary data or data tables can.) |
□ No release or data sharing |
Access Rights/Restrictions (Include information regarding access or restrictions based on privacy, security, or other policies of the owner of the data. Include an explanation for the selected “Public Access Level” above.)
Venous blood lead data will be available as aggregated data without identifiers in the ATSDR Exposure Investigation Report.
|
License/Other Agreements (The license or non-license [i.e. Public Domain] status with which the data set will be published. See Open Licenses for more information. May include DTA, MTA, IAA, MOU or other agreements concerning data use and access.)
None |
*Publisher/Owner (The publishing entity and optionally their parent organization(s). This could be the “owner” of the data.)
ATSDR/DCHI/SSB
|
Access URL, If Known (URL providing indirect access to the DMP, data set, data dictionary [variable names and valid values], data collection instrument and other relevant information, including the research protocol if possible.)
None
|
Download URL, If Known (URL providing direct access to a downloadable file of the data set, summary data, or data tables.)
Not applicable.
|
Spatial (The range of spatial applicability of a data set. Could include a geographic region or a named place [city, county, state, region, country].)
Iola City, Kansas
|
Temporal (The range of temporal applicability of project [i.e., a start and end year of applicability for the data]. Include the years for the project or data set.)
November, 2016 to November 2017
|
Table 2 – Additional DMP Elements (should be filled out where possible when project approval is sought; however, many fields can only be filled out later when publication/report is cleared)
Label (Definition) |
*Tags (Keywords to help users discover the data set; include terms that would be used by technical and non-technical users.)
Exposure Investigation Blood Lead Levels Former United Zinc and Associated Smelters NPL Site Iola, Kansas.
|
Project Type (Multiple selections may apply.) |
X Intramural |
□ Extramural (grant, cooperative agreement, contract, IAA, CDC Foundation, other) Specify mechanism: |
□ Surveillance |
□ Research |
□ Ongoing collection |
□ Other |
Project Status Estimated start date (late 2016/early 2017): Estimated date of release (2018, January): |
Data Category (For explanation of D1 to D10 codes, see Table on page 1) |
X D1 □ D2 □ D3 |
□ D4 □ D5 □ D6 □ D7 |
□ D8 □ D9 □ D10 |
Population Represented (e.g., “residents of x,” “inpatients at x,” “users of product x”)
Residents from the City of Iola, KS. Primarily children < 6 years old, pregnant women and women of childbearing years that have an increased potential exposure to lead.
|
Data Collection Protocol (Brief description with reference to document or website that provides detailed information.)
Data collection is described in the Blood Lead Levels in Iola, KS EI Protocol.
Participants will be identified as children younger than 6 years old and women who are pregnant or of child-bearing age that live in the city of Iola, KS. Participants will be recruited and testing will occur in two phases: late 2016/early 2017 and June/July 2017.
|
Data Management Protocol (Brief description with reference to system/sources where data will be housed (internal SQL database, external SQL database, etc.) and to data formats (proprietary vs. open source data formats.)
Data will be housed within the ATSDR/DDCHI Exposure Investigation Database
|
Process for Omitting Identifying Information (Description of what identifiers are in the database, how they will be removed, and by whom.)
Identifiers (names and addresses) will be flagged and housed in special subdirectory by the Database manager and will not be released unless directed by the CDC Office of General Counsel.
|
Data Quality Protocol (to address issues of confidentiality protection and statistical stability) (Brief description with reference to document or website that provides detailed information. Describe methods for data validation and error resolution, removal or shielding of any proprietary information, removal or shielding of sensitive information [i.e. data with dual use applicability], removal or shielding of any individually identifying information including indirect identification.)
The Blood Lead Levels in Iola, KS EI protocol describes the Data Quality Objectives.
ATSDR will share blood lead testing results with state and Federal partners provided that consent is obtained and the government partner signs a CDC/ATSDR data sharing agreement.
|
Data Retention/Disposal Plan (State when and how the dataset will be archived or destroyed [in accordance with CDC/ATSDR Records Control Schedule: http://isp-v-maso-apps/RecSched/images/RCS.pdf ].)
EI data are considered as a component of the ATSDR “Site Files” (Section 3.6, page 64). These files Maintain in the Records and Information Management Branch. Transfer to an FRC 5 years after publication of final health assessment, consultation, or advisory report. Destroy when 30 years old.
|
Data Analysis Plan (Brief description of planned use of the data. Can include reference to document [e.g. Information Collection Request, Research Protocol, or other] that provides more detailed information.)
The data will be used to determine whether the concentrations of lead in soil in Iola may result in elevated blood lead levels in the Iola community, primarily in young children and pregnant women or women of childbearing age. The blood lead results will be used to determine if a public health risk exists in Iola and to prioritize remedial action by EPA.
|
Publication Plan (Brief description of planned CDC-authored and CDC-coauthored publications, including topic, type of publication, and estimated timeline.)
ATSDR will use the data to publish an ATSDR Exposure Investigation Report |
Access URL (URL providing indirect access to the DMP, data set, data dictionary [variable names and valid values], data collection instrument and other relevant information, including the research protocol if possible.)
Not applicable
|
Download URL (URL providing direct access to a downloadable file of the data set, summary data, or data tables.)
Not applicable
|
Data Set Name
To be determined
|
Data Release Documentation (List documents provided to users, e.g. variable definitions, codebook, metadata file, guidance on data use.)
To be determined
|
Data Release Format (Specify dataset formats, e.g., Excel, SAS, ASCII, etc.; interactive data query website; mixed mode. Also specify data dictionary file format, e.g., JSON, RDF, SAS.)
Excel will be used to store the blood lead level data for the EI
|
Data Release Notification (State how potential users will be informed of dataset availability.)
ATSDR will include a notice in the final Blood Lead Levels in Iola, KS Exposure Investigation Report.
|
Date Form Completed: 10/02/2016 By: Lourdes Rosales-Guevara, M.D.
Name, Title
Date Form Last Revised: _________________ By: _________________________________
Appendix F: Health and Safety Plan
IOLA Site Health and Safety Plan
Blood Lead Levels in Iola, KS
Former United Zinc and Associated Smelters Site,
Iola, Allen County, Kansas
Principle Investigator
Lourdes (Luly) Rosales-Guevara, M.D.
Senior Medical Officer
Division of Community Health Investigations (DCHI)
Agency for Toxic Substances and Disease Registry (ATSDR)
Atlanta, GA
This Site Health and Safety Plan (SHSP) defines applicability and responsibility regarding compliance with the Agency for Toxic Substances and Disease Registry (ATSDR) Health and Safety Program for Hazardous Substance Field Activities.
This SHSP defines site requirements and protocol applicable during all activities. It extends to all ATSDR employees, ATSDR contractors, and site visitors invited by ATSDR.
Site emergency response procedures and any potential fire, explosion, health, or safety hazards of the operation must be communicated to all personnel. Noncompliance with site safety procedures will not be tolerated. Personnel not observing safety procedures could be suspended from participation in site activities.
Development of this plan included consideration of current safety standards and recommendations as defined by the Environmental Protection Agency (EPA), the Occupational Safety and Health Administration (OSHA), the National Institute for Occupational Safety and Health (NIOSH), the American Conference of Governmental Industrial Hygienists (ACGIH), health effects and standards for known contaminants, and procedures designed to account for potential exposure to unknown substances.
Personnel Training Requirements
All site personnel will be trained in accordance with the requirements contained in the CDC/ATSDR Mandatory Training Requirements. At a minimum, all personnel will be trained to recognize on-site hazards, the provisions of this SHSP, and identification of responsible personnel.
All personnel are required to complete the following training courses:
Blood Borne Pathogen Training
Safety Survival Skills Part 1 - General Responsibilities
HAZWOPER (current 8-hour refresher)
Human Research Protections Training
First aid/CPR/Automated External Defibrillator (AED) Training
If you need one or more of those trainings, please go to: http://intranet.cdc.gov/od/hcrmo/CDCU/mandatorytraining.shtml
Anyone entering the site must be fully aware of and protected against potential hazards. The purpose of personal protective equipment (PPE) is to shield or isolate individuals from chemical, physical, and biological hazards that could be encountered at the site.
Personnel working with blood are required to wear Level D PPE to include closed toed shoes, long pants, and gloves. Eye protection and respirators are not required. Gloves should be changed in between handling each participant’s sample. Blood collection materials should be placed in appropriate biohazard containers.
On-site personnel will use the following standard emergency procedures. Notify the principle investigator of any on-site emergencies. The principle investigator is responsible for ensuring that appropriate emergency procedures are followed.
When an injury occurs the principle investigator will assess its nature. A qualified first aid provider should initiate appropriate first aid and continue appropriate emergency medical services. If necessary, injured personnel will be transported to the hospital listed below.
Allen County Regional Hospital
3066 N. Kentucky St.
Iola, KS 6674956-974-2100
Hospital phone: (620) 365-1000
If a fire or explosion occurs on site, the emergency will be announced and all personnel will leave the area through emergency exits (unless directed otherwise). The fire department shall be contacted (911), and all personnel shall be moved a safe distance from the involved area. If it is safe to do so, site personnel can take the following actions:
Use on site fire-fighting equipment to control or extinguish the fire; and
Remove or isolate flammable or other hazardous materials that could contribute to the fire.
The principle investigator has responsibility for safety of ATSDR personnel if natural hazards (e.g., thunderstorms, tornadoes, hurricanes, etc.) occur. The principle investigator will inform personnel of current and impending weather conditions.
If any site worker experiences a protective equipment failure or alteration that affects the protection factor, that person shall immediately wash hands as needed and replace the failed equipment.
If any other on-site equipment fails to operate properly, the principle investigator shall be notified and will then determine the effect of this failure on continuing operations at the site.
Appendix G: Templates for Results Letters to Participants
Appendix G: Iola, Kansas EI Results Letter Templates
Results Letter Templates
Template G1: Sample Results Letter to the Parent of a Participant Younger Than 18 Years of Age |
Template G2: Sample Results Letter to a Pregnant Woman or Woman of Child Bearing Age (15 to 44 Years of Age) |
Results Inserts
Reporting child blood lead level: |
Insert A. Blood Lead Level below Investigation Exposure Level (IEL) |
Insert B. Blood Lead Level equal to or above Investigation Exposure Level (IEL) |
Reporting women blood lead level: |
Insert C. Blood Lead Level below Investigation Exposure Level (IEL) |
Insert D. Blood Lead Level equal to or above Investigation Exposure Level (IEL) |
Factsheets
Factsheet A: Tips for Reducing Exposure to Lead to Include with Results Letter for Children |
Factsheet B: Tips for Reducing Exposure to Lead to Include with Results Letter for Pregnant Women and Women of Childbearing Age |
Template G1 (ATSDR Letterhead)
Flesch-Kincaid Reading level XX
Sample Results Letter to the Parent of a Participant
Younger than 18 years of age
DATE ________________
Name XXXXX
Address XXXX
Dear ____________
Thank you for allowing your child, _______________, to take part in the Agency for Toxic Substances and Disease Registry’s (ATSDR) Exposure Investigation (EI) in the city of Iola, Kansas. The goal of the EI is to determine whether people living in Iola, are being exposed to lead.
ATSDR collected a blood sample from your child on [Month Day, Year]. This letter contains the results of your child’s blood lead test.
Your child's test result is shown below.
Blood Lead Test Results for First name Last name |
||
Test |
Test Result |
Investigation Exposure Level |
Blood Lead |
XXX µg/dL |
5 µg/dL1 |
1 The investigation exposure level for blood lead is 5 µg/dL. This value is the upper reference interval value of the 97.5th percentile of the distribution of the combined 2007-2008 and 2009-2010 cycles of the Centers for Diseases Control and Prevention (CDC) National Health and Nutrition Examination Survey (CDC 2013- http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6213a3.htm). |
||
Reporting child blood lead level: |
Insert A. Blood Lead Level below Investigation Exposure Level (IEL) |
Insert B. Blood Lead Level equal to or above Investigation Exposure Level (IEL) |
ATSDR’s recommendations for reducing exposure to lead are in the enclosed factsheet.
[For winter only]ATSDR will be testing in Iola during the summer of 2017 when children are playing outdoors and may be contacting soil. We recommend that your child get tested again during the summer. ATSDR will notify you when and where the summer testing will take place.
If you have questions concerning this Exposure Investigation or your child’s test results, please contact me at (770) 488-0744, toll-free at (888) 892-1320 or by email at: LRosales-Guevara@cdc.gov
Sincerely,
Luly Rosales-Guevara,MD
Principal Investigator – Iola, Kansas Exposure Investigation
ATSDR Division of Community Health Investigations, Exposure Investigation Team
Enclosure {Factsheet A}
Template G2: (ATSDR Letterhead)
Flesch-Kincaid Reading level – XX
Sample Results Letter to a Pregnant Woman or Woman of Child Bearing Age
(15 to <44 years)
DATE ________________
Name XXXXX
Address XXXX
Dear ____________
Thank you for participating in the Agency for Toxic Substances and Disease Registry’s (ATSDR) Exposure Investigation (EI) in Iola, Kansas. The goal of the EI is to determine whether people living in the city of Iola are being exposed to lead.
ATSDR collected venous blood from you on [Month Day, Year]. This letter contains the results of your blood lead tests.
Your test results are shown below.
Blood Lead Test Results for First name Last name |
||
Test |
Test Result |
Investigation Exposure Level |
Blood Lead |
XXX µg/dL |
5 µg/dL1 |
Reporting women blood lead level: |
Insert G. Blood Lead Level below Investigation Exposure Level (IEL) |
Insert H. Blood Lead Level equal to or above Investigation Exposure Level (IEL) |
ATSDR’s recommendations for reducing exposure to lead are in the enclosed factsheets.
If you have questions concerning this Exposure Investigation or your test results, please contact me at 770-488-0744, toll-free at (888) 892-1320 or by email at LRosales-Guevara@cdc.gov.
Sincerely,
Luly Rosales-Guevara, MD
Lead Investigator – Iola, Kansas Exposure Investigation
ATSDR Division of Community Health Investigations, Exposure Investigation Team
Enclosures {Factsheet B}
Insert A. Blood Lead Level below Investigation Exposure Level (IEL).
Your child’s blood lead level was below the investigation exposure level which indicates that your child’s results are similar to most other children’s results in the United States. Therefore, no additional action is required based on these results.
Although no additional action is required, you should be aware there is no known safe blood lead level for children. ATSDR suggests taking steps to minimize the risk of exposure to lead in the future. |
Insert B. Blood Lead Level equal to or above Investigation Exposure Level (IEL).
Your child’s blood lead level was equal to or above the investigation exposure level for blood lead.
As a result, we recommend that you follow up with your Pediatrician. Your child’s health care provider should evaluate your child and consider appropriate venous retesting and/or interventions. Blood lead levels equal to or above the investigation exposure level does not mean your child will develop health effects. Health effects depend on the blood lead level, length of exposure, your child's age, and present health status. Potential health effects from lead exposure can include: learning problems such as speech and language delay; problems with attention; decreased intelligence quotient (IQ); anemia (fewer red blood cells than normal).
The attached fact sheet provides information on how you can:
You should be aware there is no known safe blood lead level for children. This is the reason why we are recommending steps to reduce your family’s exposure to lead.
You can contact Dr. Luly Rosales-Guevara at her direct office number: (770) 488-0744 or The Iola toll free number: (888) 892-1320.
|
Insert C. Blood Lead Level below Investigation Exposure Level (IEL)
Your blood lead level was below the investigation exposure level in your blood sample which indicates that your results are similar to most other people’s results in the United States. Therefore, no additional action is required based on these results.
Although no additional action is required, you should be aware there is no known safe blood lead level for pregnant women or for women of childbearing age. ATSDR suggests taking steps to minimize the risk of exposure to lead in the future. |
Insert D. Blood Lead Level equal to or above Investigation Exposure Level (IEL)
Your blood lead level was equal to or above the investigation exposure level for blood lead.
As a result, we recommend that you follow up with your Obstetrician. The Centers for Disease Control and Prevention (CDC) has set a reference value of 5 µg/dL for blood lead for children under the age of 6 years. Lead can pass from a mother to her unborn baby and no safe blood lead for children, including infants, has been identified. As a result, blood lead levels in women who are pregnant or may become pregnant should be kept as low as possible and below the level of 5 µg /dL. ATSDR uses 5 µg/dL as the investigation exposure level to identify pregnant women with an elevated blood lead level who should be advised to take steps to reduce their lead exposure.
Your health care provider should evaluate you and consider appropriate venous retesting and/or interventions. Blood lead levels equal to or above the investigation exposure level do not mean that you (or your baby, if you are pregnant) will develop health effects. Health effects depend on the blood lead level, length of exposure, your age, and present health status. Potential health effects for pregnant women include increased risk of miscarriage, low birth weight, or premature birth. Potential health effects for a child from lead exposure can include: learning problems such as speech and language delay; problems with attention; decreased intelligence quotient (IQ); anemia (fewer red blood cells than normal).
The attached fact sheet provides information on how you can:
You can contact Dr. Luly Rosales-Guevara at her direct office number: (770) 488-0744 or The Iola toll free number: (888) 892-1320.
As a woman who is, or may become, pregnant, you should be aware there is no known safe blood lead level for children. This is the reason why we are recommending steps to reduce your family’s exposure to lead. |
Factsheet A: (To be Included with Result Letters to Participants)
Flesch-Kincaid Reading level – XX
Factsheet with Tips for Reducing Exposure to Lead to Include with Results Letter for Children
Things you can do reduce your family’s contact with lead
In Iola, Kansas, the soil in some residential and non-residential locations have high levels of lead. There may also be lead sources in your home.
If you
or your child has a high blood lead level, there are things you can
do to help. Ask
your doctor to re-test you or your child’s venous blood for
lead
Work
together with your doctor to find the best treatment for you or
your child. Ask questions if you don’t understand something.
You
may need to: Go
to your doctor for follow up venous lead testing.
Test
your child for learning and developmental problems. This test is
called a “developmental assessment.” 
Pay attention to bare soil and dusty conditions in your community and try to avoid them.
Find the lead in your home.
Homes built before 1978 may have lead-based- paint. It is important to find and fix lead paint in your home as soon as possible. If you need help, contact the Kansas Department of Health and Environment at _______________ to discuss options for getting a Healthy Homes Inspection of your home.
Don’t remodel or renovate your home yourself until your home has been inspected for lead. If you can’t get a home inspection before doing home repairs, keep children and pets out of the house during the repairs, and wet mop and wet clean all dust you make. Repairs like sanding or scraping paint can make dangerous lead dust. Also protect yourself from the lead dust.
Homes built before 1986 may have lead service pipes, fixtures and solder. Flush your cold-water pipes by running the water until it becomes as cold as it will get.
Clean up dust in your home.
Wet-mop floors and wet-wipe windowsills, window wells, counters, and furniture every weeks. Avoid dry dusting and sweeping because it spreads dust into the air.
Use contact paper or duct tape to cover chipping or peeling paint.
Wash hands and toys often with soap and water. Always wash hands before eating and sleeping.
Wash pets such as dogs and cats, especially if they spend time outdoors, at least every 2-3 weeks.
Prevent dust in the first place by taking off shoes before going into your home.
Give your family healthy foods.
Feed your family healthy foods with calcium, iron, and vitamins D, C, and zinc. These foods may help keep lead out of the body.
Calcium is in milk, yogurt, cheese, and green leafy vegetables like spinach.
Iron is in lean red meats, beans, peanut butter, and cereals.
Vitamin C is in oranges, green and red peppers, and juice.
Vitamin D is in egg yolk, sardines, milk and butter.
Zinc is in oysters, red meat, poultry, seafood and fortified breakfast cereals.
Wash and peel all fruits, vegetables, and root crops (such as potatoes), especially any locally grown or home grown items.
Learn more. Get support.
Factsheet B:
Factsheet with Tips for Reducing Exposure to Lead to Include with Results Letter for Pregnant Women and Women of Childbearing Age
ATSDR plans to use the CDC lead program’s factsheet “Are You Pregnant,” available here: http://www.cdc.gov/nceh/lead/tools/Are_You_Pregnant.pdf.


| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Modified | 0000-00-00 |
| File Created | 2021-01-24 |
© 2026 OMB.report | Privacy Policy