2015 ss tb bruc
2015 ss tb bruc.docx
Brucellosis and Bovine Tuberculosis; Update of General Provisions
OMB: 0579-0442
SUPPORTING STATEMENT 0579-XXXX
Brucellosis and Bovine Tuberculosis; Update of General Provisions
December 2015
A. JUSTIFICATION
1. Explain the circumstances that make the collection of information necessary. Identify any legal or administrative requirements that necessitate the collection. Attach a copy of the appropriate section of each statute and regulation mandating or authorizing the collection of information.
The Animal Health Protection Act (AHPA) of 2002 is the primary Federal law governing the protection of animal health. The law gives the Secretary of Agriculture broad authority to detect, control, or eradicate pests or diseases of livestock or poultry. The Secretary may also prohibit or restrict import or export of any animal or related material if necessary to prevent the spread of any livestock or poultry pest or disease.
The AHPA is contained in Title X, Subtitle E, Sections 10401-18 of P.L. 107-171, May 13, 2002, the Farm Security and Rural Investment Act of 2002.
Disease prevention is the most effective method for maintaining a healthy animal population and for enhancing the United States’ ability to compete in the world market of animal and animal product trade.
The agency charged with carrying out this disease prevention mission is the Animal and Plant Health Inspection Service (APHIS). APHIS regulations for preventing the dissemination of animal diseases within the United States are contained in title 9 of the Code of Federal Regulations (9 CFR), Subchapter B: Cooperative Control and Eradication of Livestock or Poultry Diseases. Veterinary Services (VS), a division within APHIS, is responsible for administering these regulations.
In October 2009, USDA published two concept papers in the Federal Register that proposed new directions for two of its longstanding disease eradication programs - bovine tuberculosis (TB) and brucellosis. Several objectives described in the concept papers were common among both programs. USDA is streamlining its rulemaking efforts by merging the needs of both programs into a single rule while ensuring the particular needs of each program are met.
TB is a contagious disease of both animals and humans. It is caused by three specific types of bacteria that are part of the Mycobacterium group: Mycobacterium bovis, M. avium, and M. TB. Bovine TB, caused by M. bovis, can be transmitted from livestock to humans and other animals.
Brucellosis is an infectious disease of animals and humans caused by the bacteria of the genus Brucella. The disease is characterized by abortions and impaired fertility in its principal animal hosts. Brucellosis is mainly a disease of cattle, bison, and swine. Sheep, goats, and horses are also susceptible, but are rarely infected. Brucella abortus is associated with the disease in cattle and bison, Brucella suis with the disease in swine, and Brucella melitensis with the disease in sheep and goats. Brucellosis in horses is caused by Brucella abortus and most commonly manifests as fistulous withers. There is no economically feasible treatment for brucellosis in livestock.
APHIS is asking OMB to approve its use of these information collection activities in connection with the TB-brucellosis program.
2. Indicate how, by whom, how frequently, and for what purpose the information is to be used. Except for a new collection, indicate the actual use the agency has made of the information received from the current collection.
APHIS will use the following information activities to prevent, detect, control, and eradicate bovine TB and brucellosis from the United States.
9 CFR 76.2 - Animal Health Plan
These plans describe a State’s or Tribe’s brucellosis and bovine TB program activities and their compliance with the Federal regulations, policies, and performance standards. APHIS will use the animal health plan to classify States and Tribes with a status for the brucellosis and bovine TB programs. Animal health plans include the following information:
Confirmation that the State or Tribe has a legal and regulatory basis for the activities and measures specified within the animal health plan.
A description of the organization and infrastructure of the animal health and wildlife authorities within the State or Tribe.
The name and contact information for the responsible person that the State or Tribe has designated to oversee implementation, performance, and enforcement of activities and measures carried out under the plan within the State or Tribe, and the name and contact information for the person the State has designated to oversee implementation, performance, and enforcement of wildlife activities and measures carried out under the plan. States may designate a single individual to serve in multiple roles.
A description of program animal demographics within the State or Tribal lands including the approximate number and types of program animal herds within the State or Tribal lands; and the approximate number and geographic distribution of any animal concentration points within the State or Tribal lands.
A description of the surveillance activities for brucellosis or bovine TB in animals within the State or Tribal lands that are being conducted or would be conducted under the animal health plan.
A description of the known sources of brucellosis or bovine tuberculosis that pose a risk of disease introduction into program animals within the State or Tribal lands, and an assessment of the likelihood of transmission of brucellosis or bovine TB from these sources to program animals within the State or Tribal lands. The description must include the approximate number of herds or wildlife populations within the State or Tribal lands that are known sources of brucellosis or bovine TB, and the approximate number of animals in these herds or populations; and the approximate prevalence of brucellosis or bovine TB infection in those populations, the geographic distribution of the populations within the State or Tribal lands, and any other factors that make the populations a potential source of brucellosis or bovine TB transmission to program animals within the State or Tribal lands; and the potential for exposure of program animals within the State or Tribal lands to these known source populations; and the factors, other than mitigation measures that are or would be implemented by the State or Tribe, that may influence this potential for exposure; and an assessment of the likelihood of transmission of brucellosis or bovine TB from known source populations to program animals within the State or Tribal lands.
If the State or Tribe has identified known source populations of brucellosis or bovine TB that pose a risk of disease introduction into program animals within the State or Tribal lands, a description of the measures that the State or Tribe has implemented or would implement to mitigate the risk that program animals within the State or Tribal lands will become infected with brucellosis or bovine TB.
9 CFR 76.4 - Annual Report
State or Tribal animal health authorities must submit an annual report to APHIS certifying that the State (or zone within the State) is complying with the provisions of the Animal Health Plan and describe occurrences of disease such as the numbers of cattle, privately-owned bison, captive elk, and other cervid species that were infected with TB and/or brucellosis. This report must be submitted to APHIS each year between October 1 and November 30. It enables APHIS to carefully monitor State activities with regard to Brucellosis and TB surveillance, containment, and eradication.
In addition to the annual reporting requirements States and Tribes with recognized management areas must submit a separate annual report covering the same information as an annual report for each recognized management area in the State or Tribe with their annual animal health plan report.
These reports must be sufficiently specific to allow APHIS to determine whether the State or Tribe continually meets or exceeds the level of disease detection estimated for the National Surveillance Plans.
9 CFR 76.7 - Initial Epidemiological Report
If a program animal has a non-negative test result for brucellosis or TB, within 15 days of receiving notification of these results, the State in which the animal was detected must initiate an investigation to determine the herd from which the animal originated and all herds in which it has resided.
An initial epidemiological investigation situation report will be completed by the district epidemiologist and submitted to the cattle commodity specialist within 15 days of identification of a herd as affected.
Epidemiological situation reports require the following information:
The date the herd was determined to be affected;
The State, county, town, zip code, and area status of the affected herd;
The type of herd and the number of animals in each age group (0-2 months, 2-6 months, 6-24 months, greater than 24 months);
A list of critical actions to occur in the immediate future and dates (if possible);
A list of critical decisions that need to be made and the responsible parties with deadlines (if possible);
A list of accomplishments;
A list of planned activities;
Whether or not the source of the infection has been identified;
Other information important to the investigation;
Date of testing, number tested, number of suspects, number of reactors, and number necropsied; and
Whether or not wildlife surveillance has been planned or conducted and a description of the surveillance activities performed.
9 CFR 76.7 - Update Epidemiological Report
Updated epidemiological investigation situation reports will be submitted to the cattle commodity specialist at least every 4 weeks following submission of an initial epidemiological situation report. Update reports include the information provided in the initial report as well as new information obtained since the submission of the initial report.
9 CFR 76.7 - Closing Epidemiological Report
Investigation closure (“closing”) reports will be submitted to the cattle commodity specialist within 60 days following conclusion of an epidemiological investigation. Closing reports include the information provided in the initial and update reports as well as new information obtained since the submission of the previous report.
9 CFR 76.17- Lab Approval Request
In order to be considered an official testing laboratory, a Federal, State, or university laboratory, or any other laboratory approved by the National Animal Health Laboratory Network, must submit a written application to the Assistant District Director (ADD) for the State in which the laboratory is located, who will consult with the SAHO or Tribal animal health official regarding the request.
The written application requires the following information:
Name and address of the laboratory;
Name of the legally responsible official and, if different, the name of the laboratory director;
A description of the facilities and equipment the laboratory will use to perform the brucellosis or bovine TB official diagnostic tests;
A list of the types of diagnostic samples that will be tested (blood, milk, etc.);
A list of the specific brucellosis or bovine TB official diagnostic tests for which the laboratory requests approval;
A detailed description of the brucellosis or bovine TB official diagnostic test procedures; and
A list of the individuals performing brucellosis or bovine TB official diagnostic tests and their qualifications.
After reviewing the laboratory's submission package and a conducting the site visit, the ADD, with the concurrence of the SAHO or the Tribal animal health official, may recommend approval of the laboratory. The original submission package and the ADD’s letter of recommendation are sent to the NVSL Director, with copies to the District Director and the Cattle Health Commodity staff.
9 CFR 76.7- Request for Alternate Protocol
While the standard protocols and procedures set forth in regulations and standards are grounded in generally accepted best practices for conducting epidemiological investigations, we recognize that, in certain instances, a State may exercise due diligence in conducting such investigations, yet either not be able to determine all potentially affected herds, or not be able to do so within the timeframe specified within the regulations. In such instances, States could submit an alternate protocol for conducting an epidemiological investigation to APHIS to the address provided in the Program Standards document.
Alternate protocols must include:
Plans and protocols for identifying potential sources and spread of disease;
Methods applied to restrict the spread of disease from known affected herds and animals
Methodology for eliminating disease from known affected herds and animals.
9 CFR 93.439 - Trust Fund Agreement
Operators of privately-owned quarantine facilities must sign this agreement to allow USDA personnel to inspect and monitor animals imported to the facility, and to pay USDA for these services. APHIS inspects and monitors the animals to ensure they are healthy and do not pose a disease risk to the U.S. livestock population.
9 CFR 93.439 - Deposit of Funds
To reserve and use space at USDA-operated quarantine facilities, the importer or agent gives a financial instrument (letter of credit, cashier’s check, certified check, or money order) to APHIS in person, through the mail, or by courier.
3. Describe whether, and to what extent, the collection of information involves the use of automated, electronic, mechanical, or other technological collection techniques or other forms of information technology, e.g., permitting electronic submission of responses, and the basis for the decision for adopting this means of collection. Also describe any consideration of using information technology to reduce burden.
APHIS is considering a fillable template annual reports and lab approval requests in which States and tribes could electronically submit via email.
TB and brucellosis test charts (epidemiological reports) can be generated via the mobile information management (MIM) system. Data files can be uploaded to the MIM data repository and then can be directed to other databases, i.e. SCS, by State/VS personnel. APHIS is currently developing a MIM compatible system in which electronic data can be entered by the testing veterinarian through a Web site, a test chart printed, and the data automatically uploaded to the desired database.
4. Describe efforts to identify duplication. Show specifically why any similar information already available cannot be used or modified for use for the purpose described in item 2 above.
The information that APHIS collects is not available from any other source. APHIS is the only Federal agency responsible for preventing, detecting, controlling, and eradicating TB and brucellosis from the United States.
5. If the collection of information impacts small businesses or other small entities, describe any methods used to minimize burden.
The information APHIS collects in connection with this program is the absolute minimum needed to prevent, detect, control, and eradicate TB and brucellosis from the United States. APHIS estimates that 5 of the 6 business respondents are small entities.
6. Describe the consequence to Federal program or policy activities if the collection is not conducted or is conducted less frequently, as well as any technical or legal obstacles to reducing burden.
Failure to collect this information would make it much more difficult for APHIS to prevent, detect, control, and eradicate TB and brucellosis from the United States.
7. Explain any special circumstances that require the collection to be conducted in a manner inconsistent with the general information collection guidelines in 5 CFR 1320.5.
requiring respondents to report information to the agency more often than quarterly;
requiring respondents to prepare a written response to a collection of information in fewer than 30 days after receipt of it;
requiring respondents to submit more than an original and two copies of any document;
requiring respondents to retain records, other than health, medical, government contract, grant-in-aid, or tax records for more than three years;
in connection with a statistical survey, that is not designed to produce valid and reliable results that can be generalized to the universe of study;
requiring the use of a statistical data classification that has not been reviewed and approved by OMB;
that includes a pledge of confidentiality that is not supported by authority established in statute or regulation, that is not supported by disclosure and data security policies that are consistent with the pledge, or which unnecessarily impedes sharing of data with other agencies for compatible confidential use; or
requiring respondents to submit proprietary trade secret, or other confidential information unless the agency can demonstrate that it has instituted procedures to protect the information's confidentiality to the extent permitted by law.
No special circumstances exist that would require this information collection to be in a manner inconsistent with the guidelines established in 5 CFR 1320.5.
8. Describe efforts to consult with persons outside the Agency to obtain their views on the availability of data, frequency of collection, the clarity of instructions and recordkeeping, disclosure, or reporting form, and on the data elements to be recorded, disclosed, or reported. If applicable, provide a copy and identify the date and page number of publication in the Federal Register of the agency's notice, soliciting comments on the information collection prior to submission to OMB.
APHIS engaged in productive consultations with the following individuals concerning the information collection activities associated with this program:
Rick Smith
TB Eradication Program
525 West Allegan Street
Lansing, MI 48909
Linda Glaser
Minnesota Board of Animal Health
625 North Robert Street
Saint Paul, MN 55155
Dr. Jim Logan
Wyoming State Veterinarian
1934 Wyott Drive
Cheyenne, WY 82002
APHIS’ proposed rule (APHIS-2011-0044) describes its information gathering requirements, and also provides a 60-day comment period. During this time, interested members of the public will have the opportunity to provide APHIS with their input concerning the usefulness, legitimacy, and merit of the information collection activities APHIS is proposing.
9. Explain any decision to provide any payment or gift to respondents, other than remuneration of contractors or grantees.
This information collection activity involves no payments or gifts to respondents.
10. Describe any assurance of confidentiality provided to respondents and the basis for the assurance in statute, regulation, or agency policy.
No additional assurance of confidentiality is provided with this information collection. However, the confidentiality of information is protected under 5 U.S.C. 552a.
11. Provide additional justification for any questions of a sensitive nature, such as sexual behavior or attitudes, religious beliefs, and other matters that are commonly considered private. This justification should include the reasons why the agency considers the questions necessary, the specific uses to be made of the information, the explanation to be given to persons from whom the information is requested, and any steps to be taken to obtain their consent.
This information collection activity will ask no questions of a personal or sensitive nature.
12. Provide estimates of the hour burden of the collection of information. Indicate the number of respondents, frequency of response, annual hour burden, and an explanation of how the burden was estimated.
•Indicate the number of respondents, frequency of response, annual hour burden, and an explanation of how the burden was estimated. If this request for approval covers more than one form, provide separate hour burden estimates for each form and aggregate the hour burdens in Item 13 of OMB Form 83-I.
See APHIS Form 71. Burden estimates were developed from discussions with States; tribes; foreign federal governments; and producers of cattle, bison, and captive cervids.
•Provide estimates of annualized cost to respondents for the hour burdens for collections of information, identifying and using appropriate wage rate categories.
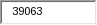
Respondents are the owners of cattle, bison, and captive cervids; State veterinarians; accredited veterinarians; and foreign federal government animal health representatives. APHIS estimates the total annualized cost to these respondents to be $1,416,424. APHIS arrived at this figure by multiplying the hours of estimated response time (39,063 hours) by the estimated average hourly wage of the above respondents ($36.26).
$36.26 is the hourly rate derived from the U.S. Department of Labor, Bureau of Labor Statistics May 2014 Report - Occupational Employment and Wages in the United States. See http://www.bls.gov/news.release/pdf/ocwage.pdf
Owners of cattle, bison, and captive cervids: $20.90 per hour [median]
State and accredited veterinarians: $34.90 per hour [median]
Foreign Federal Government: $52.99 [median]
13. Provide estimates of the total annual cost burden to respondents or record keepers resulting from the collection of information (do not include the cost of any hour burden shown in items 12 and 14). The cost estimates should be split into two components: (a) a total capital and start-up cost component annualized over its expected useful life; and (b) a total operation and maintenance and purchase of services component.
No annual cost burden is associated with capital and startup costs, operation and maintenance expenditures, and purchase of services.
14. Provide estimates of annualized cost to the Federal government. Provide a description of the method used to estimate cost and any other expense that would not have been incurred without this collection of information.
The annualized cost to the Federal Government is estimated at $4,287,473. (See APHIS Form 79.)
15. Explain the reasons for any program changes or adjustments reported in Items 13 or 14 of the OMB Form 83-1.
ICR Summary of Burden:

|
Requested |
Program Change Due to New Statute |
Program Change Due to Agency Discretion |
Change Due to Adjustment in Agency Estimate |
Change Due to Potential Violation of the PRA |
Previously Approved |
Annual Number of Responses |
239 |
0 |
239 |
0 |
0 |
0 |
Annual Time Burden (Hr) |
39,063 |
0 |
39,063 |
0 |
0 |
0 |
Annual Cost Burden ($) |
0 |
0 |
0 |
0 |
0 |
0 |
This is a new information collection resulting 39,063 burden hours.
16. For collections of information whose results are planned to be published, outline plans for tabulation and publication.
APHIS has no plans to publish information it collects in connection with this program.
17. If seeking approval to not display the expiration date for OMB approval of the information collection, explain the reasons that display would be inappropriate.
No forms are associated with this information collection.
18. Explain each exception to the certification statement, "Certification for Paperwork Reduction Act."
APHIS can certify compliance with all provisions of the Act.
B. Collections of Information Employing Statistical Methods
Statistical methods are not employed in this information collection activity.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | SUPPORTING STATEMENT 0579-0165 |
| Author | Kay Brown |
| File Modified | 0000-00-00 |
| File Created | 2021-01-24 |
© 2026 OMB.report | Privacy Policy