Research Plan
Att 7- Research Plan.docx
Formative Research and Tool Development
Research Plan
OMB: 0920-0840
-
UCSD Human Research Protections Program
New Biomedical Application
RESEARCH PLAN
Instructions for completing the Research Plan are available on the HRPP website.
The headings on this set of instructions correspond to the headings of the Research Plan.
General Instructions: Enter a response for all topic headings.
Enter “Not Applicable” rather than leaving an item blank if the item does not apply to this project.
Version date: 9/30/2013
PROJECT TITLE
171555; Addressing the Rise of Congenital Syphilis: Working toward Setting-specific Solutions among High-Risk Pregnant Women
2. PRINCIPAL INVESTIGATOR
Jennifer A. Wagman, PhD, MHS
Assistant Professor
Division of Infectious Disease and Global Public Health
Department of Medicine
University of California, San Diego
9500 Gilman Drive
La Jolla, CA 92093-0507
Tel: (858) 534-9619 | Email: jwagman@ucsd.edu
3. FACILITIES
The proposed 12-moth project will be conducted in collaboration with the Division of Infectious Disease and Global Public Health at the University of California, San Diego (UCSD) School of Medicine and the Department of Epidemiology at the Tulane University School of Public Health and Tropical Medicine. Both Principal Investigators will have access to all university facilities, including administrative and technical support and password-protected/ secured servers. This study is being funded by a grant from the March of Dimes Foundation to UCSD. A sub-award will be made from UCSD to Tulane University, contingent upon IRB approval from UCSD HRPP. IRB approvals will also be sought from the Tulane University HRPP. We will submit the final approval notice to the UCSD HRPP in the form of an amendment and we will not begin work until we receive final local approvals.
A multiple Principal Investigator (PI) mechanism will be used. Dr. Jennifer Wagman (Assistant Professor, UCSD) and Dr. Emily Harville (Associate Professor, Tulane University) will share the authority and responsibility for leading and directing the project, intellectually and logistically. See Section 21. (Privileges/Certifications/Licenses and Research Team Responsibilities) for more details.
The 12-month (August 2017 – August 2018) project will employ a qualitative approach to assess the knowledge, attitudes, factors that influence decision-making and behavioral practices STDs in general, and congenital syphilis in particular, among high-risk pregnant women and prenatal care providers in two of the most hard-hit, high-morbidity counties in the United States (U.S.): Kern County, California (CA) and East Baton Rouge Parish (county), Louisiana (LA).
4. ESTIMATED DURATION OF THE STUDY
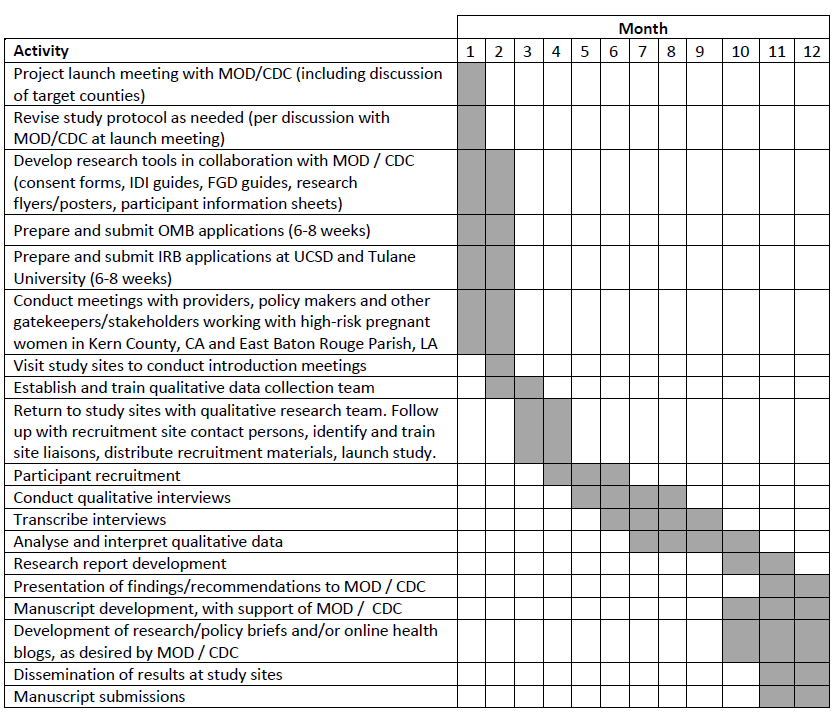
This is 12 month qualitative study with 5 research aims. Below is the proposed timeline for all project milestones.
Aims 1 and 2 involve the conduct (i.e., original data collection) and analysis of qualitative interviews and focus groups to assess prenatal care provider knowledge, attitudes, and practices around congenital syphilis and its prevention and how prenatal care providers and high-risk pregnant women learn about congenital syphilis and where they seek information. Completion of the qualitative research will take between 5-8 months. QUALITATIVE (INTERVIEW AND FOCUS GROUP) GUIDES FOR PRENATAL CARE PROVIDERS WILL BE PROVIDED TO THE IRB AFTER THEY ARE FINALIZED.
Aim 3 involves the conduct (i.e., original data collection) and analysis of qualitative interviews and focus groups to evaluate high-risk pregnant women’s knowledge of STDs and syphilis; patient decision-making around whether to seek prenatal care (focused examination of barriers like substance use) and patient use of prevention behaviors during pregnancy. Completion of the qualitative research will take between 5-8 months. QUALITATIVE (INTERVIEW AND FOCUS GROUP) GUIDES FOR HIGH-RISK PREGNANT WOMEN WILL BE PROVIDED TO THE IRB AFTER THEY ARE FINALIZED.
Aims 4 and 5 involve the conduct and analysis of findings from the Aims 1-3 to seek recommendations for improving outreach to this population and to generate ideas for a culturally appropriate way to engage high-risk pregnant women in the use of prenatal care services. A sequential, empirically-informed approach will be used to synthesize findings to develop recommendations to better inform congenital syphilis outreach and prevention efforts. The entire process will take between 7-12 months. The timeline shown below provides activities to be accomplished during this one-year grant.
5. LAY LANGUAGE SUMMARY OR SYNOPSIS (no more than one paragraph)
The spike in congenital syphilis cases in the United States between 2012 and 2015 represents an urgent public health problem. The proposed study will expand on what is already known about the epidemiology of the incidence in distinct settings and population groups in the United States, and lessen the existing gap in knowledge on how to effectively communicate with patients at highest risk for syphilis infection/transmission and the health care providers who can offer prevention and treatment services. We have a unique opportunity to inform and improve existing syphilis prevention efforts by engaging with pregnant women (including those most hard to reach) and prenatal providers in two of the highest-morbidity regions in the country. Our investigative teams at UCSD and Tulane are ideally positioned to partner with the local departments of health, community organizations and expert researchers in Kern and East Baton Rouge Counties. The results from this study will not only inform prevention efforts in Kern and East Baton Rouge - we believe they will be generalizable to Fresno County, CA, and other high-morbidity jurisdictions through the United States.
6. SPECIFIC AIMS
As noted, the proposed project has 5 research aims. The 12-month (August 2017 – August 2018) project will employ a qualitative approach to assess the knowledge, attitudes, factors that influence decision-making and behavioral practices STDs in general, and congenital syphilis in particular, among high-risk pregnant women and prenatal care providers in two of the most hard-hit, high-morbidity counties in the United States (U.S.): Kern County, California (CA) and East Baton Rouge Parish (county), Louisiana (LA). In-depth interviews and focus group discussions will be used to accomplish five research aims:
Aim 1 is to assess prenatal care provider knowledge, attitudes, and practices around congenital syphilis and its prevention.
Aim 2 is to assess how prenatal care providers and high-risk pregnant women learn about congenital syphilis and where they seek information. Methods for AIMS 1 and 2 will include up to 20 in-depth interviews (IDIs) with prenatal care providers working in Kern County, California and Baton Rouge County, Louisiana (up to 10 IDIs per county). Interviews will focus on understanding provider knowledge, awareness, and comfort in talking to women about congenital syphilis; attitudes and practices regarding testing; and information-seeking behaviors.
Aim 3 is to evaluate high-risk pregnant women’s knowledge of STDs and syphilis; patient decision-making around whether to seek prenatal care (focused examination of barriers like substance use) and patient use of prevention behaviors during pregnancy. We propose to conduct 4 focus groups with high-risk pregnant women (6-10 women per focus group, up to 4 focus groups per county). Focus groups will assess general attitudes and practices regarding prenatal care; knowledge and awareness about congenital syphilis; information-seeking behaviors; and types of health-messaging that resonate with them.
Aim 4 is to seek recommendations for improving outreach to this population.
Aim 5 is to generate ideas for a culturally appropriate way to engage high-risk pregnant women in the use of prenatal care services. Recommendations will be developed to specifically address culturally appropriate and acceptable ways to provide outreach to high-risk pregnant women and effectively engaging them in the use of needed congenital syphilis -related prenatal care services. Specific steps will be followed. Step 1: Use qualitative data from interviews and focus groups to develop a summary of findings. Step 2: Work with all partners (at UC San Diego and Tulane University) to synthesize qualitative findings to develop a set of recommendations for effective outreach and engagement. Step 3: Present key findings and recommendations to the March of Dimes and obtain additional suggestions for refinement. Step 4: With the support of MOD and CDC, develop findings into scientific manuscripts or reports for dissemination to populations of interest.
7. BACKGROUND AND SIGNIFICANCE
Congenital syphilis (also referred to as Mother-to-child transmission (MTCT) of syphilis) is completely preventable through effective antenatal screening, and treatment of infected pregnant women. A National Plan to Eliminate Syphilis was launched for the United States (U.S.) in 1999 by the Centers for Disease Control and Prevention (CDC) and reframed in 2006. One of the Plan’s goals was to reduce rates of congenital syphilis to fewer than 3.9 per 100,000 live births by 2010 (2). Although this goal was not fully achieved by 2010, a decrease did occur in the overall rate of reported cases between 2008 and 2012, from 10.5 to 8.4 cases per 100,000 live births (1). However, a dramatic subsequent increase was documented between 2012 and 2014 in all regions of the U.S. (1). As noted in the recent March of Dimes (MOD) Request for Proposals on congenital syphilis, the number of cases in the U.S. had increased by 46% by 2015 (compared to 2012), disproportionately impacting people living in specific geographic settings and of certain population groups.
These trends in MTCT of syphilis represent an urgent public health problem in the U.S. that is tragically occurring in many global settings as well. The World Health Organization estimates that two million pregnancies around the world are affected by syphilis each year, approximately 25% of which result in infant mortality (i.e., stillbirth or spontaneous abortion) and another 25% result in infant morbidity (i.e., low birth weight or critical infection) (3).
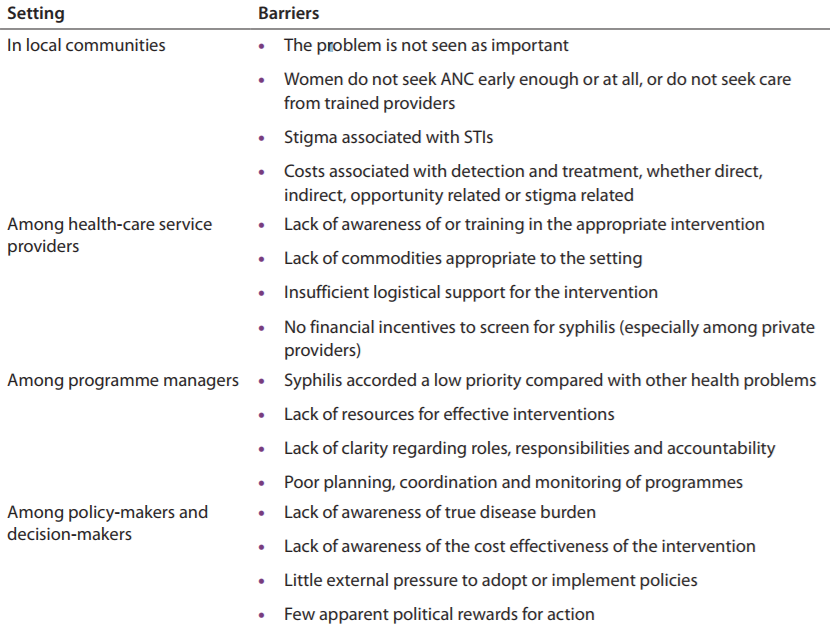
Theoretically, the prevention and detection/treatment of congenital syphilis should be relatively simple and inexpensive. Screening all pregnant women for syphilis during antenatal care is feasible and offers and effective mechanism for detecting the bacterium Treponema pallidum (T. pallidum) which can then be treated and eliminated with proper antibiotics (4). In reality, however, many barriers impede effective prevention and treatment of congenital syphilis. These barriers exist on multiple levels, including the community level, among health care providers and program managers, and also in the realm of policy (5). The Table “Barriers to the prevention of congenital syphilis” was developed by the World Health Organization in 2012 (5) and outlines some of the common barriers to the prevention of congenital syphilis on each of these levels.
In addition to the barriers shown in the Table below, women with limited or no health insurance, and women with substance use issues are at increased risk for receiving inadequate or no prenatal care (6). This is problematic because many cases of congenital syphilis result from lack of prenatal care or screening (during prenatal care) or maternal syphilis detection/treatment offered too late for prevention of MTCT. For instance, 22% of the congenital syphilis cases documented in 2014 occurred in mothers who received no prenatal care and 15% had accessed care but were not tested by their health provider (1, 6).

Barriers to the prevention of congenital syphilis [SOURCE: WHO, 2012]

SIGNIFICANCE
The proposed 12 month qualitative study will assess the knowledge, attitudes, factors that influence decision-making and behavioral practices surrounding sexually transmitted diseases (STDs) in general, and congenital syphilis in particular, among high-risk pregnant women and prenatal care providers in two of the most hard-hit, high-morbidity counties in the United States (U.S.): Kern County, California (CA) and East Baton Rouge Parish (county), Louisiana (LA). All activities will be conducted collaboratively by researchers from the University of California San Diego (UCSD) School of Medicine and Tulane University School of Public Health and Tropical Medicine, in close partnership with officials from the local departments of health (in Kern and East Baton Rouge Counties) and the March of Dimes’ (MOD) Manager of Special Projects. A multiple Principal Investigator (PI) approach will be used, allowing for shared institutional leadership and collaboration among a team of highly experienced scientists in California and Louisiana, two of the states with the current highest rates of syphilis among newborns (1).
Value/Significance of proposed project
Given that MTCT of syphilis is fully preventable, the recent spike in its incidence suggests that unknown and/or more nuanced, complex determinants of its occurrence remain uncovered or insufficiently addressed. Urgent action is needed to increase our understanding of the reason(s) behind the recent surge in syphilis cases so effective solutions can be put in place. There exists a substantial body of practical knowledge and experiential theory on why many opportunities are missed along each step of a continuum of prevention, detection and treatment for syphilis among pregnant women. Concrete, empirical evidence is critically needed to confirm where gaps exist in knowledge, attitudes, decision-making practices and behaviors contributing to the resurgence in syphilis transmission. This evidence is needed to improve/modify congenital syphilis prevention efforts it can be mitigated and eradicated.
8. PROGRESS REPORT
N/A (Initial submission)
9. RESEARCH DESIGN AND METHODS
Overview of Research Design: Two-site qualitative study
The proposed two-site study will involve the design, implementation, and analysis of two qualitative assessment components in Kern County, CA and East Baton Rouge Parish, LA. Through in-depth interviews (10 per site) with a variety of prenatal care providers and focus group discussions (4 per site) with high-risk pregnant women we will collect rich qualitative data reflecting the complexities and setting-specific details on local providers’ and women’s levels of knowledge, attitudes, and practices surrounding congenital syphilis and its prevention. We have drafted interview and focus group screening tools and guides (FINALIZED QUALITATIVE (INTERVIEW AND FOCUS GROUP) GUIDES FOR PRENATAL CARE PROVIDERS AND HIGH-RISK PREGNANT WOMEN WILL BE PROVIDED TO THE IRB AFTER THEY ARE DEVELOPED) that will be refined and finalized through close collaboration (i.e., regular meetings and conference calls) and input from the MOD Manager of Special Projects, colleagues at the Centers for Disease Control and Prevention and our local partners in Kern and East Baton Rouge Counties.
Selection of two diverse, high-risk sites: The multiple Principal Investigator approach we have proposed will allow for shared institutional leadership and collaboration among a team of highly experienced scientists in California and Louisiana, two of the states with the current highest rates of syphilis among newborns (1). Despite that both Kern County and East Baton Rouge County are dealing with critical congenital syphilis outbreaks, their demographics and risk factors are distinct. By collecting rich information on knowledge, attitudes, practices and socioecological determinants from two specific locations, we will be able to generate diverse data that can be more broadly applied toward prevention efforts throughout the U.S.
Specific Aims
Aim 1: Assess prenatal care provider knowledge, attitudes, and practices around congenital syphilis and its prevention
Aim 2 Assess how prenatal care providers and high-risk pregnant women learn about congenital syphilis and where they seek information
Aim 3 Evaluate high-risk pregnant women’s knowledge of STDs and syphilis; patient decision-making around whether to seek prenatal care (focused examination of barriers like substance use) and patient use of prevention behaviors during pregnancy
Aim 4 Seek recommendations for improving outreach to this population
Aim 5 Generate ideas for a culturally appropriate way to engage high-risk pregnant women in the use of prenatal care services.
Qualitative Research Methods
Methods will include up to 20 in-depth interviews (IDIs) with prenatal care providers working in Kern County, California and Baton Rouge County, Louisiana (up to 10 IDIs per county). Interviews will focus on understanding provider knowledge, awareness, and comfort in talking to women about congenital syphilis; attitudes and practices regarding testing; and information-seeking behaviors. Additionally, we propose to conduct 8 focus groups with high-risk pregnant women (6-10 women per focus group, up to 4 focus groups per county). Focus groups will assess general attitudes and practices regarding prenatal care; knowledge and awareness about congenital syphilis; information-seeking behaviors; and types of health-messaging that resonate with them.
In-depth interviews (IDIs) will be conducted among prenatal care providers working in Kern County, CA and Baton Rouge County, LA. Final sites for recruitment in Kern County will be decided in partnership with our local partners/colleagues at the Kern County Public Health Services Department. Through preliminary conversation and site selection discussion we believe that settings such as the Women’s Services Center of Dignity Health Mercy Hospitals would provide an ideal location for recruitment.
In Baton Rouge County, providers will be recruited from the Women’s Hospital, one of the largest women’s hospitals in the United States. The Women’s Hospital provides comprehensive prenatal and postnatal care services to women in East Baton Rouge, and Drs. Buekens and Harville have worked extensively with prenatal care providers at this location. IDIs will be conducted among individuals with knowledge of the local setting and dynamics, and the challenges faced by high-risk pregnant women regarding congenital syphilis and use of health services. In order to understand the perspectives of different specialized providers, interview participants may include providers across various departments, including gynecology, maternal-fetal medicine, infectious disease, and family practice. IDIs will be conducted by experience qualitative researchers, all of whom are trained (and will have supplemental training) in qualitative research, safe and ethical research with pregnant women, and research ethics, and all have a deep understanding of the sensitive nature of research related to vulnerable populations. All interviews will be conducted in private and will last 60-90 minutes.
Focus group discussions (FGDs) will be conducted to complement IDIs and will benefit from group discourse surrounding the exploration of key concepts related to congenital syphilis, prenatal care, and hardships faced by high-risk women related to pre- and post-natal care. FGDs will allow for discussion of general themes, including awareness of sexually transmitted infections, challenges in accessing healthcare while pregnant and how these might be overcome, and ideas for ways that health messaging can best resonate with them. Focus groups will be led by a moderator and notes will be taken by an assistant who will tape each session (with all participants’ consent). All discussions will be structured by use of a guide and will last 1-2 hours.
Data Analysis
Interview and focus group notes will be transcribed and imported into QSR NVivo V10 (7) or analyzed through identification of recurrent themes following Crabtree and Miller’s 5 step “interpretive process” (8). Transcripts will be read to identify common themes, codes will be developed and ~10% of data will be double coded and inter-rater reliability assessed. Coded text will be extracted and organized (7) and read to identify emergent themes. A sequential, empirically-informed approach will be used to synthesize findings to develop recommendations to better inform congenital syphilis outreach and prevention efforts. Recommendations will be developed to specifically address culturally appropriate and acceptable ways to provide outreach to high-risk pregnant women and effectively engaging them in the use of needed congenital syphilis -related prenatal care services. Specific steps will be followed. Step 1: Use qualitative data from interviews and focus groups to develop a summary of findings. Step 2: Work with all partners (at UC San Diego and Tulane University) to synthesize qualitative findings to develop a set of recommendations for effective outreach and engagement. Step 3: Present key findings and recommendations to the March of Dimes and obtain additional suggestions for refinement. Step 4: With the support of MOD and CDC, develop findings into scientific manuscripts or reports for dissemination to populations of interest.
10. HUMAN SUBJECTS
In total this study will enroll up to 100 participants to accomplish research aims. The qualitative methods to be used include (a) in-depth interviews (IDIs) with prenatal care providers working in East Baton Rouge Parish and Kern County; and (b) focus group discussions (FGDs) with high-risk pregnant women (age 18+) living in the counties of interest. We propose to conduct 20 IDIs (10 IDIs per county) and 8 FGDs, each with 6-10 participants (4 FGDs and up to 40 participants per county).
Total number of human subjects
Kern County
Baton Rouge County
Total
Prenatal Care Providers*
High-Risk Pregnant Women
Prenatal Care Providers*
High-Risk Pregnant Women
IDIs
10
--
10
--
20 participants
FGDs
--
4
(6-10 women/FGD)
--
4
(6-10 women/FGD)
64 - 80 participants
*We will select from a variety of different types of prenatal care providers, not just obstetricians.
11. RECRUITMENT AND PROCEDURES PREPARATORY TO RESEARCH
Participants will be recruited in Kern County, CA and East Baton Rouge Parish, LA. Prenatal care providers and high-risk pregnant women will be recruited from local hospitals or clinics specializing in prenatal and infant care (such as the Women’s Hospital in East Baton Rouge Parish or the Women’s Services Department of the Dignity Health system in Kern County). Written informed consent will be obtained for all participants involved in the proposed qualitative research. Consent forms will be written in simple language at a fourth-grade reading level. Details of study participation will be described in the consent form and explained verbally. If a potential participant does not wish to participate, his/her decision will be honored regardless of how well the study information is comprehended. A copy of the consent form, which includes a description of the study, will be provided to all participants and includes telephone numbers of the PIs.
PART A: Overview of Participants to be recruited in Kern County (CA) and East Baton Rouge Parish (LA)
The proposed qualitative study will involve up to 20 in-depth interviews (IDIs) and up to 8 focus group discussions (FGDs).
IN-DEPTH INTERVIEWS
IDI participants: IDIs will be conducted with up to 20 prenatal care providers, 10 providers at each site. Different types of prenatal providers will be recruited, including:
a. Obstetricians/gynecologists (OB/GYN)
b. Family practice doctors (family physicians)
c. Maternal-fetal medicine (MFM) specialists
d. Certified nurse-midwives (CNMs)
e. Family nurse practitioners (FNPs) / women’s health nurse practitioner (WHNPs)
Eligibility criteria for IDI participants includes: (1) Prenatal care provider (including any type described above) that has worked in either Kern County, CA or East Baton Rouge Parish, LA for at least 6 months; (2) Currently working directly with high-risk pregnant women (at least half of their patients); (3) Having a phone or some other way of being contacted; and (4) Consenting to involvement in the study.
FOCUS GROUP DISCUSSIONS
Up to 8 FGDs will be conducted in total, with 4 FGDs being done at each site. Each FGD will include 6-10 participants, thus a total of up to approximately 80 FGD participants will be enrolled, ~40 per site.
FGD participants: Focus groups will involve pregnant women at “high risk” for being infected with syphilis and thus at high risk for mother-to-child transmission of syphilis. Kern County, California and East Baton Rouge Parish, Louisiana are two of the most hard-hit, high-morbidity syphilis counties in the United States. Thus, pregnant women living in these two areas are considered to be high-risk (for both acquisition and transmission of syphilis). Additionally, for the proposed study we consider high-risk pregnant women to include those with any of the following characteristics:
a. A history of syphilis infection
b. A history of incarceration
c. Current or past drug use
d. Multiple or concurrent sex partners
Ideally we will recruit women with some of the above “high risk” characteristics. However, only the minimum eligibility/inclusion criteria will be required, as detailed next.
Minimum eligibility criteria for FGD participants includes: (1) Adult women (18 years and older) living in either Kern County, CA or Baton Rouge Parish/County, LA for at least 6 months; (2) Currently pregnant; (3) Having a phone or some other way of being contacted; (4) Consenting to involvement in study; and (5) English or Spanish speaking. ALL SPANISH TRANSLATED MATERIALS WILL BE SUBMITTED TO THE IRB FOR APPROVAL BEFORE USE.
PART B: Recruitment Plan for Kern County, California
RECRUITMENT SITES IN KERN COUNTY
Four distinct recruitment sites: Prenatal care providers and high-risk pregnant women will be drawn from four sites in Kern County, CA. These sites have been chosen to represent the major types of organizations that provide services to high-risk pregnant women in Kern County, including not-for-profit, public-benefit corporate hospital systems; private medical centers; community-based organizations that provide health services; and government-run organizations, including those that provide substance use services. We have selected the following four sites to obtain a wide variety of participant perspectives, including from individuals who are low income, and those who have had a history of alcohol or substance use: Dignity Health Memorial Hospital, Kern Medical, Planned Parenthood - Bakersfield Health Center, and Kern County Behavioral Health and Recovery Services. If alternate sites are selected, we will inform HRPP of the change, in the form of an amendment.
All recruitment (and data collection) activities will be conducted in Kern County by the locally appointed research team which will consist of two skilled and experienced qualitative researchers. A critical component of the proposed study is to engage with the study points of contact and other gatekeepers/stakeholders at Dignity Health Memorial Hospital, Kern Medical, Planned Parenthood and Kern County Behavioral Health and Recovery Services. To gain access to potential prenatal care provider participants as well as high-risk pregnant woman participants, the UC San Diego Principal Investigator will organize informational/introductory meetings to be conducted at each site to discuss the proposed study.
Each meeting will be conducted at the recruitment site from where participants will be drawn. Each will be facilitated (in person). The time and exact location of the meeting will be decided by the investigators and the study contact person at Dignity Health Memorial Hospital / Kern Medical / Planned Parenthood / Kern County Behavioral Health and Recovery Services. At each meeting, a brief background on the problem of congenital syphilis will be presented, followed by a summary of the research aims. A participant information sheet will be distributed at each meeting, outlining the purpose and details of the study, the inclusion criteria for prenatal care provider participants, the inclusion criteria for pregnant woman participants, incentives and contact details for enrolling in the study.
RECRUITMENT PROCEDURES FOR PRENATAL CARE PROVIDERS IN KERN COUNTY
During the introductory meetings (detailed above) to be conducted at each site, the investigators will identify (through direct inquiry and collaboration/communication with the site contact person at each location) obstetrical providers whose clinics care for a high volume of high risk women. Providers from each of the four sites will then be asked to recommend at least one antenatal provider from their respective clinics to participate in an in-depth interview. A comprehensive list of names and contact information (including cell phone number, work number, email address) for potential prenatal care provider participants will be generated. Study recruiters will use this information to follow-up with each individual through a phone call and/or email to re-invite the potential participant to answer any questions about the study and invite him/her to enroll. If he/she expresses interest in participation, the study recruiter will set up a specific interview appointment time and location.
Recruitment of prenatal providers will also be done through distribution of the participant information sheets (at the introductory meetings). People who attend these meetings will be asked to share this information with colleagues who were unable to attend. Interested, prospective participants can use the contact details provided on the information sheet to get in touch with the study investigators.
Another method for recruitment will include using posters (placed in the specific locations where services are provided for pregnant women) in each of the four sites. Posters will include details of the study, eligibility criteria for prenatal providers, information about incentives and contact details for the research team. If prospective participants are interested in discussing the study further or participating, they can contact the investigators directly using contact details on the poster.
RECRUITMENT OF HIGH RISK PREGNANT WOMEN IN KERN COUNTY
Recruitment procedures in Kern County will be the same across sites and are outlined below, separated by recruitment method.
Study Posters: The investigators will work with a specified local contact person at each location to compile a list of clinics or departments and/or providers that provide services or engage with high-risk pregnant women in Kern County. These local contacts will distribute study flyers and posters, which will provide information about the study, as well as contact information for the study team for those who are interested in participating. Posters/flyers will be informative but brief, including information about the purpose of the study; format of data collection (i.e., focus groups); eligibility for participation; details on incentives; and contact details for the study team. All posters/flyers will be submitted to the IRBs for approval AFTER THEY ARE DEVELOPED AND TRANSLATED. Potential participants interested in talking to investigators about the study and/or wanting to enroll can call the investigators, as directed on the flyer. Additionally, in order to reach women who may not have been exposed to these advertisements, study coordinators will hang flyers in other public places, such as large grocery stores that allow flyers, the Department of Human Services, and childcare centers (such as the YMCA) throughout Kern County. Recruitment through study posters will be ongoing, until the overall sample size is reached.
Antenatal care providers: Study researchers will ask all willing antenatal care providers at the recruitment sites to mention the study to patients who they believe may be eligible. Specific names of providers will be identified during the first few months of study preparations, to ensure that they can be trained and ready to identify patients by the time recruitment begins. Training for antenatal care providers is expected to be completed during months three and four of the grant, with participant recruitment occurring during months four through six. Providers will be trained on study procedures, identification of potentially eligible patients, and ways to refer them to the study. Investigators will also ensure that all clients receiving services from antenatal care providers are given a copy of the study flyer and a “Participant Information Sheet” during consultations. The information sheet will contain a more detailed explanation of the study, as well as contact information for the UC San Diego research team.
Snowball Sampling: Participants will also be recruited via snowball sampling, whereby participants who are recruited/informed about the study will be asked to pass on study contact details and a participant information sheet or flyer to pregnant women they know who fulfill the inclusion criteria. This way, pregnant women receiving services at any of the recruitment sites can talk about the study with friends and family and participants can be recruited as a result of word of mouth.
On-site Liaisons: At each site, 1-2 staff members will be identified and trained to serve as a liaison between the recruitment site and the study team. These staff members will be informed about the study, its purpose, the type of data collection to be done (FGDs), the characteristics of the participants to be recruited (i.e., eligibility criteria) and the study team contact details. Pregnant women will be informed through flyers and antenatal care providers that an alternative to directly contacting the investigators is to leave their information with a liaison at the clinic, who will forward this information to a research team member, and a researcher will contact the interested, potential participant by phone. Women’s names and numbers obtained at by liaisons will only be used for recruitment and will not be stored or shared with anyone. For confidentiality purposes, liaisons will not ask women to identify themselves by their last name, and will only collect first names. This information will be recorded in a password protected document and will be emailed through an encrypted and secure email service. This information will not be made available to anyone outside the research team. After contacting the participant, their information will be deleted from the document. Liaisons will also share flyers and answer questions from potential participants, as approved by the local contact.
PART C: Updated Recruitment Plan for East Baton Rouge Parish (County), Louisiana
RECRUITMENT SITES IN EAST BATON ROUGE PARISH
Three distinct recruitment sites: Prenatal care providers and high-risk pregnant women will be drawn from three sites in East Baton Rouge Parish, LA. Three distinct types of recruitment sites have been chosen, including a private hospital specializing in care of women and infants, a public-private partnership OB/GYN clinic and a community-based organization that conducts community outreach and case management to engage with pregnant and parenting women and help them improve their health and the health of their babies and families. We have selected distinct types of recruitment sites so as to diversify the kinds of prenatal care providers enrolled in the study, and to engage with high-risk pregnant women from different demographic and socioeconomic backgrounds. Recruitment sites may include: Woman’s Hospital, LSU OB/GYN Clinic at Woman’s Hospital, and Family Road Healthy Start. If alternate sites are selected, we will inform HRPP of the change, in the form of an amendment.
All recruitment and data collection activities will be conducted by the locally appointed qualitative research team.
INTRODUCTION OF STUDY AT EACH EAST BATON ROUGE PARISH SITE
Engaging with the study points of contact and other gatekeepers/stakeholders at Woman’s Hospital, LSU OB/GYN Clinic at Woman’s Hospital and Family Road Healthy Start will be essential to the successful recruitment of participants at each site. To gain access to potential participants (including both prenatal care providers and high-risk pregnant women), the Tulane-based Principal Investigator will organize meetings at each site to discuss the proposed study.
Woman’s Hospital conducts monthly meetings for all obstetrical staff, including those from LSU OB/GYN Clinic. The investigators will arrange to attend this meeting during month two of the proposed study, to address obstetrical providers and introduce the study. A brief background on the problem of congenital syphilis will be presented, followed by a summary of the research aims. A participant information sheet will be distributed which outlines the purpose and details of the study, the inclusion criteria for prenatal care provider participants, the inclusion criteria for pregnant woman participants, incentive details, and contact details for enrolling in the study.
Family Road Healthy Start. The investigators will partner with a contact at this location to arrange a meeting to introduce the study at Healthy Start. Program Directors and prenatal providers will be invited to this meeting. A brief background on the problem of congenital syphilis will be presented, followed by a summary of the research aims. A participant information sheet will be distributed which outlines the purpose and details of the study, the inclusion criteria for prenatal care provider participants, the inclusion criteria for pregnant woman participants, information about incentives, and contact details for enrolling in the study.
RECRUITMENT PROCEDURES FOR PRENATAL CARE PROVIDERS IN EAST BATON ROUGE PARISH
During the introductory meetings (detailed above) to be conducted at each site, the investigators will identify (through direct inquiry and collaboration/communication with the site contact person at each location) obstetrical providers whose clinics care for a high volume of high risk women. Providers from each of the three sites will then be asked to recommend at least one antenatal provider from their respective clinics to participate in an in-depth interview. A comprehensive list of names and contact information (including cell phone number, work number, email address) for potential prenatal care provider participants will be generated. Study recruiters will use this information to follow-up with each individual through a phone call and/or email to re-invite the potential participant to answer any questions about the study and invite him/her to enroll. If he/she expresses interest in participation, the study recruiter will set up a specific interview appointment time and location.
Recruitment of prenatal providers will also be done through distribution of the participant information sheets (at the introductory meetings). People who attend these meetings will be asked to share this information with colleagues who were unable to attend. Interested, prospective participants can use the contact details provided on the information sheet to get in touch with the study investigators.
Another method for recruitment will include using posters (placed in the specific locations where services are provided for pregnant women) in each of the three sites. Posters will include details of the study, eligibility criteria for prenatal providers and contact details for the research team. If prospective participants are interested in discussing the study further or participating, they can contact the investigators directly using contact details on the poster.
RECRUITMENT OF HIGH RISK PREGNANT WOMEN IN EAST BATON ROUGE PARISH
Woman’s Hospital: The investigators will work with identified contact persons to obtain/generate a list of antenatal and other medical providers and obstetrical clinics that provide prenatal care services for a high volume of high-risk women at Woman’s Hospital. Study posters/flyers will be distributed and displayed in recommended clinic sites so patients are exposed to these materials during routine prenatal care visits. Posters/flyers will be informative but brief, including information about the purpose of the study; format of data collection (i.e., focus groups); eligibility for participation; details on incentives; and contact details for the study team. All posters/flyers will be submitted to the IRBs for approval before use. Potential participants interested in talking to investigators about the study and/or enroll can call the investigators, as directed on the flyer. Recruitment will also be done on specific days when the investigators/study recruiters will spend time at Woman’s Hospital, giving out flyers and answering questions from potential participants.
LSU OB/GYN Clinic: Per the same protocol described above for Woman’s Hospital, study flyers/posters will be displayed openly throughout the LSU OB/GYN Clinic so patients are highly exposed to the information. Additionally, the investigators will partner with a LSU OB/GYN Clinic’s social worker ensure that all clients receiving services from the Clinic’s social workers are provided a copy of the study flyer during consultations.
Participants will also be recruited via snowball sampling whereby participants who are recruited/informed about the study by their social worker will be asked to pass on study contact details and a participant information sheet or flyer to pregnant women they know who fulfill the inclusion criteria. This way, pregnant women receiving services at LSU OB/GYN Clinic can talk about the study with friends and family and participants can be recruited as a result of word of mouth.
The investigators will also identify 1-2 clinic staff members to liaise between the recruitment site and the investigative team. These staff members will be informed about the study, its purpose, the type of data collection to be done (FGDs), the characteristics of the participants to be recruited (i.e., eligibility criteria) and the study team contact details. Women receiving services at the LSU OB/GYN Clinic will be informed (through flyers and by the social work staff) that an alternative to directly contacting the investigators is to leave their information with a staff member at the clinic and have a researchers contact them by phone. In this case, the potential participant can leave her first name and phone number with the designated staff member, who will relay the information to the researchers. Women’s names and numbers will only be used for recruitment and will not be stored or shared with anyone. This information will be recorded in a password protected document and will be emailed through an encrypted and secure email service. This information will not be made available to anyone outside the research team. After contacting the participant, their information will be deleted from the document. On specific days, investigators/ recruiters will be available to give out flyers and answer questions from potential participants at LSU OB/GYN Clinic locations.
Family Road Healthy Start: Per the same protocol described above for Woman’s Hospital, study flyers/posters will be displayed throughout Healthy Start so patients are exposed to the information. Additionally, case workers who meet individually with pregnant women in the Healthy Start Program will be asked to distribute the study flyers about the study purpose, focus group format, incentives, and contact details of research team. Interested participants can reach out directly to the study team.
Participants will also be recruited via snowball sampling whereby participants recruited/informed about the study by their Healthy Start case worker will be asked to pass on study contact details and a participant information sheet or flyer to pregnant women they know who fulfill the inclusion criteria. This way, pregnant women receiving services at Family Road Healthy Start can talk about the study with friends and family and participants can be recruited as a result of word of mouth.
Pregnant women participating in the Healthy Start Program who are interested in the study can also leave their information with a case worker (who will be trained to serve as a liaison between the recruitment site and the study team). The case worker will share this information with the study team and a researcher will contact the potential participant by phone. Women’s first names and numbers obtained at the Family Road Health Start location will only be used for recruitment and will not be stored or shared with anyone. For confidentiality purposes, liaisons will not ask women to identify themselves by their last name, and will only collect first names. This information will be recorded in a password protected document and will be emailed through an encrypted and secure email service. This information will not be made available to anyone outside the research team. After contacting the participant, their information will be deleted from the document. On specific days, investigators/ recruiters will be available to give out flyers and answer questions from potential participants at Healthy Start, as approved by a study contact person.
12. INFORMED CONSENT
Only individuals 18 years and old will be involved in the proposed study. Written consent will be obtained for the proposed qualitative research. All participants will be asked their consent to audio record interviews or focus groups. Participants will be asked to give their consent to be audio recorded, and will provide oral consent if they agree to be audio recorded. If they do not consent to be audio recorded, they will be informed that they will still be able to participate in the study. Consent forms will be written in simple language at a fourth-grade reading level. Details of study participation will be described in the consent form and explained verbally. If a potential participant decides he/she does not wish to participate, his/her decision will be honored regardless of how well the study information is comprehended. A copy of the consent form, which includes a description of the study, will be provided to all participants and includes telephone numbers of the PI where participants can call with questions or concerns. Only qualified personnel will consent subjects.
13. ALTERNATIVES TO STUDY PARTICIPATION
The alternative is not to participate. There is no prospect of direct benefit to the participant and this is noted in the consent document.
14. POTENTIAL RISKS
The research procedures for the proposed study are minimally invasive and present low risks to the study participants. The possible risks include the possibility that participants will become emotionally distressed during the interview process and the potential loss of confidentiality. We have described these risks in detail (below) as well as procedures for dealing with emotional distress or the risk of loss of confidentiality, if it should arise. This research is NOT a clinical trial and it involves interviewing pregnant women. The Supplement for research involving pregnant women, neonates, and/or human placenta or fetal material is submitted to confirm that this study does not pose greater than minimal risk to participants.
Emotional risks: Some pregnant women may find it distressing to talk about congenital syphilis, prenatal care, and hardships related to pre- and post-natal care. Note, however, that no direct questions related to personal experience will be included in FGD study tools, though it is possible that FGD members may share personal examples in the course of the discussion. In such cases, FGD facilitators will be trained to guide the conversation on general attitudes and practices rather than personal disclosure.
Loss of confidentiality: As a result of participating in this study, it is possible that participants may be identified by others, including healthcare personnel. There is also a slight risk that confidentiality may be breached in the management of data, although multiple safeguards will be implemented to avoid this risk. Participants will be made aware of this risk during the consent procedure. However, multiple safeguards will be implemented to avoid these risks and participants will be made aware of risks during the consent procedure.
15. RISK MANAGEMENT PROCEDURES AND ADEQUACY OF RESOURCES
Throughout the project great care will be taken to minimize the potential for distress or harm, for example through careful wording of questions to ensure that they are non-judgmental; facilitating IDIs and FGDs in private spaces where discussions are not overheard by others; comprehensive training for all researchers that includes clear protocols regarding how to respond to participants disclosing confidential information. In addition, each potential participant will be offered careful explanation about the purpose of the research, the voluntary nature of their participation, and will have an opportunity to ask questions before starting the FGD and IDI. No data collection activity will begin without first obtaining informed consent from the participant. No research will be carried out with children.
Collaboration with community-based organizations and individuals in Kern and East Baton Rouge Counties will ensure minimizing emotional risks to participants. All planned activities (including selection of research sites, recruitment of participants, informed consent procedures and collection of interview and focus group data) will be done with oversight and guidance from local health departments, and in partnership with local research consultants who are intimately connected with the communities and have long histories of qualitative research in both counties.
As mentioned above, it is possible that participants will become emotionally distressed during data collection sessions and there is potential for loss of confidentiality. Below we detail the procedures we will follow for dealing with these issues, should they arise.
Emotional risks: Dr. Wagman has more 17 years of experience in training staff to conduct confidential and sensitive interviews/counseling sessions regarding these sorts of highly personal data. All research staff will receive / have received human subjects/research ethics training, including the need for complete confidentiality and to approach questions with sensitivity and in a supportive manner and to provide appropriate referrals when needed. All interviews will be conducted in private. Women will be referred to a counselor upon request and provided with a list of resources for additional counseling and any further assistance. Researchers will be/ are trained not to press participants to answer questions that seem to be distressing to them, and sessions are terminated if the participant is too distressed, too fatigued, or too frustrated by the effort. In the event that significant depression symptoms are noted, the staff will be instructed to follow procedures in the safety protocol, which include immediately contacting one of the Principal Investigators, and arranging for referral to appropriate service agencies within the community.
Loss of confidentiality: Multiple safeguards will be implemented to avoid these risks and participants will be made aware of risks during the consent procedure. All paper data collection tools will be stored in secure, locked facilities and only designated staff members have access to these records. Transcription, and analysis of the data will be done only through the secure password-protected server. All personnel involved in this project have received their certification of completion for the CITI Training for Human Subjects Research (or equivalent). The risks posed to participants in the proposed study are reasonable in relation to the anticipated benefits and in relation to the importance of the knowledge that may reasonably be expected to result. Provider responses will not be reported to supervisors or used for evaluations.
16. PRIVACY AND CONFIDENTIALITY CONSIDERATIONS INCLUDING DATA ACCESS AND MANAGEMENT
Consent documents and data collection tools will be retained in locked filing cabinets in the PI’s office at UC San Diego or Tulane University, and will only be accessible to senior investigators or designated staff. All participants will be identified by study ID numbers only. Participants’ names will not be included on study materials. A master list of participant names linked with study IDs will be kept in a password-protected computer file. All computer records will be protected by standard measures that limit data access to authorized personnel, and will be identifiable only by participants’ IDs. Digitally-recorded qualitative data will be stored on a secure password protected server following interview; followed by the deletion of the original audio file. Transcription and data analysis will be done through secure password-protected server. All of these procedures will be adhered to in the proposed study.
17. POTENTIAL BENEFITS
This research holds out the prospect of a direct benefit to pregnant women and fetuses who are at high risk for congenital syphilis as providers can offer prevention and treatment services more effectively by engaging them to the study. Participants might find that talking about their experiences is therapeutic and or beneficial. After the data collection session has been completed (including the interviews and focus group discussions) the participant may like to and will be invited to talk with someone further about the things that were discussed during the interview. This additional session as well as any information shared during this time, referrals made might be of benefit to the participant. The information collected in these interviews/focus group discussions may help researchers understand how to better help women and babies, especially when it comes to protecting them from syphilis infection.
18. RISK/BENEFIT RATIO
The risks posed to participants in the proposed study are reasonable in relation to the anticipated benefits and in relation to the importance of the knowledge that may reasonably be expected to result. First, while the proposed study poses potential emotional risk and loss of confidentiality, we believe these risks are minimal in that the probability they will occur and the magnitude of harm or discomfort anticipated are not greater than encountered in daily life. Second, we believe most participants will experience no harm or discomfort and those that do will recover and/or find it beneficial or therapeutic to talk about their experiences and participate in the proposed project. Finally, findings from the proposed research will be critical to the development, implementation, and evaluation of theoretically-driven, empirically informed STI behavioral interventions that address the multiplicity of risks faced by pregnant women. The recommendations for improving outreach to this population and development of culturally appropriate ways to engage high-risk pregnant women in the use of prenatal care services will ultimately be of significant importance and benefit to the participants in this study and others within the research population. Therefore, we believe the benefits of the proposed study outweigh the risks.
19. EXPENSE TO PARTICIPANT
There is no cost to the participant for taking part in the study.
20. COMPENSATION FOR PARTICIPATION
All research participants will be compensated with a $25 gift card, which has been previously judged appropriate by the IRBs.
21. PRIVILEGES/CERTIFICATIONS/LICENSES AND RESEARCH TEAM RESPONSIBILITIES
Multiple Principal Investigator (PI) Approach
A multiple Principal Investigator (PI) approach will be used, allowing for shared institutional leadership and collaboration among a team of highly experienced scientists in California and Louisiana, two of the states with the current highest rates of syphilis among newborns. The UCSD School of Medicine and Tulane School of Public Health and Tropical Medicine, two distinct academic institutions recognized for their excellence in education and research, are uniquely positioned to reach and engage with the communities in Kern and East Baton Rouge Counties.
This study will be conducted under the joint leadership of Jennifer Wagman, PhD, MHS, (UCSD PI) and Emily Harville, PhD (Tulane PI). Our team’s capacity and promise for delivering the desired outcomes of this study are greatly strengthened by the contributions of our Co-Investigators at UCSD (Davey Smith, MD, MAS) and Tulane (Pierre Buekens, MD, PhD, MPH), both of whom are internationally recognized as leaders in the fields of public health and infectious disease research.
Co-Principal Investigators (PI) at UCSD and Tulane
UCSD PI
Tulane PI
Name
Jennifer A. Wagman, PhD, MHS
Emily Harville, PhD, MSPH
Position
Assistant Professor
Associate Professor
Institution
Division of Global Public Health
Department of Medicine
University of California, San Diego
Department of Epidemiology
Tulane University, School of Public Health and Tropical Medicine
Address
9500 Gilman Drive
La Jolla, CA 92093-0507
1440 Canal Street, Suite 2000
New Orleans, LA 70112
Email | phone
jwagman@ucsd.edu | 858-534-9619
harville@tulane.edu | 504-988-7327
Jennifer Wagman, PhD, MHS, is an Assistant Professor in the Division of Global Public Health (GPH) and Associate Director of the Center on Gender Equity and Health (GEH) in the Department of Medicine at the University of California, San Diego. Dr. Wagman has close to 20 years of HIV prevention and intervention research and experience working in resource poor, high risk populations. She is a social epidemiologist trained in mixed methods and reproductive and perinatal health research. Dr. Wagman is skilled in the use of qualitative and quantitative methods, population-based HIV surveillance research, randomized controlled trials, implementation science and research ethics. She has led qualitative/ethnographic research to understand and improve knowledge, attitudes and practices surrounding mother-to-child transmission of HIV and other sexually transmitted diseases (STDs).
Emily Harville, PhD, MSPH, is an Associate Professor in the Department of Epidemiology at the Tulane University, School of Public Health and Tropical Medicine. Dr. Harville’s research focuses on understanding how pregnancy and reproduction relate to health throughout the life course, including biological mechanisms by which health disparities are created. Her major projects include NIH- and foundation-funded quantitative and qualitative research on the effects of disaster on pregnant and postpartum women; effects of environmental and social risk factors on reproductive-aged women in the aftermath of the Gulf oil spill; and the relationship between cardiovascular and reproductive health in the Bogalusa Heart Study. Dr. Harville has extensive research experience in Baton Rouge.
Co-Investigators at UCSD and Tulane
UCSD Co-I
Tulane Co-I
Name
Davey Smith, MD, MAS
Pierre Buekens, MD, MPH, PhD
Position
Professor of Medicine
Head, Division of Infectious Diseases
Dean and W.H. Watkins Professor,
Department of Epidemiology
Institution
Department of Medicine
University of California, San Diego
Tulane University, School of Public Health and Tropical Medicine
Address
9500 Gilman Drive
La Jolla, CA 92093-8208
1440 Canal Street, Suite 2000
New Orleans, LA 70112
Email
Davey Smith, MD, MAS, is Head of the UCSD School of Medicine’s Division of Infectious Diseases and Director of the San Diego Center for AIDS Research (CFAR) Translational Virology Core. Dr. Smith's primary research involves examining transmission of infectious diseases, including syphilis. He is an active member of the San Diego County Syphilis Eradication Task Force. He also specializes in researching HIV transmission and HIV reservoir dynamics, focusing on understanding the correlates that drive HIV transmission and persistence and finding new ways to interrupt these mechanisms. In 2010, Dr. Smith was named HIV Researcher of the Year by the HIV Medical Association, and in 2015 he was elected as a fellow to the American Society of Clinical Investigation and American College of Physicians.
Pierre Buekens, MD, MPH, PhD is a W.H. Watkins Professor of Epidemiology and Dean of the Tulane University School of Public Health and Tropical Medicine and Clinical Professor of Obstetrics and Gynecology at Tulane University School of Medicine. He is PI on a $6.5 million grant from the Bill & Melinda Gates Foundation to study new methods to implement the prevention of mother-to-child-transmission of syphilis in the Democratic Republic of the Congo and Zambia. The study aims to address barriers to syphilis testing and treatment, including lack of awareness among healthcare providers. It evaluates an intervention to provide point-of-care rapid tests and treatment kits along with reminders, monitoring and feedback to health providers. Dr. Buekens also collaborates with researchers from the UC San Diego School of Pharmacy to study congenital Chagas Disease.
Note: all key personnel have completed the appropriate CITI training.
22. BIBLIOGRAPHY
Bowen V, Su J, Torrone E, Kidd S, Weinstock H. Increase in incidence of congenital syphilis - United States, 2012-2014. MMWR Morb Mortal Wkly Rep. 2015 Nov 13;64(44):1241-5. doi: 10.15585/mmwr.mm6444a3.
Centers for Disease Control and Prevention (CDC). The 2006 National Plan to Eliminate Syphilis from the United States - Executive Summary. 2007. Accessed from: https://www.cdc.gov/stopsyphilis/SEEexec2006.htm
World Health Organization. The Global elimination of congenital syphilis: rationale and strategy for action. 2007. WHO Press. ISBN 978 92 4 159585 8.
Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64 (No. RR-3).
World Health Organization. Investment case for eliminating mother-to-child transmission of syphilis: promoting better maternal and child health and stronger health systems. 2012. WHO Press. ISBN 978 92 4 150434 8
Morbidity and Mortality Weekly Report (MMWR). Dear Colleague Letter - Congenital Syphilis. November 12, 2015. Accessed from: https://www.cdc.gov/std/dstdp/dcl-csmmwr-nov-12-2015.pdf
NVivo qualitative data analysis software; QSR International Pty Ltd. Version 10, 2012.
Benjamin Crabtree & William Miller (Eds.) (1999). Doing Qualitative Research (2nd edition). London: Sage, 406 pages, ISBN 0-7619-1497-8.
Office of Research on Women’s Health, NIH. Enrolling Pregnant Women: Issues in Clinical Research. Accessed from: https://orwh.od.nih.gov/resources/pdf/ORWH-EPW-Report-2010.pdf
23. FUNDING SUPPORT FOR THIS STUDY
This study is funded by a grant from March of Dimes (MOD) and CDC. This project is part of a cooperative agreement between MOD and CDC and thus involves substantial involvement between the CDC, as the Federal awarding agency, and the MOD in carrying out the projects activities. A sub-agreement will be made between UCSD and Tulane University, contingent upon IRB approval from UCSD HRPP.
24. BIOLOGICAL MATERIALS TRANSFER AGREEMENT
The protocol for the proposed study does not involve any human tissue or biological fluids, etc. from the participants. Thus, there will be no transfer of biological materials involved.
25. INVESTIGATIONAL DRUG FACT SHEET AND IND/IDE HOLDER
The proposed study does not involve an FDA-regulated investigation.
26. IMPACT ON STAFF
The proposed study does not involve the nursing staff from UCSD and/or RCHSD staff (nursing, laboratory, pathology, pharmacy, medical records) and therefore no time or staff skills from UCSD/RCHSD will be required.
27. CONFLICT OF INTEREST
Neither the PIs nor any key personnel associated with this study have any financial interests or other “conflicts” related to this study.
28. SUPPLEMENTAL INSTRUCTIONS FOR CANCER-RELATED STUDIES
The proposed study is not a cancer-related study, nor does it qualify under the California Clinical Trial Law (Section 1370.6 of the Health and Safety Code; Section 10145.4 of the Insurance Code; and Sections 14087.11, 14132.98, and 14132.99 of the Welfare and Institutions Code).
29. OTHER APPROVALS/REGULATED MATERIALS
To our knowledge, approval or authorization from other UCSD review committees is not required for the proposed study.
30. PROCEDURES FOR SURROGATE CONSENT AND/OR DECISIONAL CAPACITY ASSESSMENT
Surrogate consent will not be sought on this study.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | UCSD Human Research Protections Program |
| Author | mcaligiuri |
| File Modified | 0000-00-00 |
| File Created | 2021-01-21 |
© 2026 OMB.report | Privacy Policy