Final Supporting Statement A NCS Data Archive_02-04-2016_MARKED
Final Supporting Statement A NCS Data Archive_02-04-2016_MARKED.doc
National Children's Study (NCS) Vanguard Data and Sample Archive and Access System (NICHD)
OMB: 0925-0730
REQUEST FOR OMB CLEARANCE
Generic Clearance Request
National Children’s Study (NCS) Vanguard Data and Sample Archive and Access System (NICHD)
Supporting Statement A
February 3, 2016
Dr. Jack Moye
The National Children's Study
Eunice Kennedy Shriver National Institute of Child Health and Human Development
6100 Executive Boulevard, Room 3A01
Bethesda, MD 20892-7510
Phone: 301-594-9147
Email: moyej@mail.nih.gov
Table of contents
A.1 Justification 4
A.5 Impact on Small Businesses or Other Small Entities 7
A.6 Consequences of Collecting the Information Less Frequently 7
A.7 Special Circumstances Relating to the Guidelines of 5 CFR 1320.5 7
A.9 Explanation of Any Payment or Gift to Respondents 7
A.10 Assurance of Confidentiality Provided to Respondents 8
A.11 Justification for Sensitive Questions 8
A.12-1 Estimated Annualized Burden Hours 8
A.12-2 Annualized Cost to Respondents 9
A.14 Annualized Cost to the Federal Government 9
A.15 Explanation for Program Changes or Adjustments 10
A.16 Plans for Tabulation and Publication and Project Time Schedule 10
A.17 Reason(s) Display of OMB Expiration Date is Inappropriate 10
A.18 Exceptions to Certification for Paperwork Reduction Act Submissions 10
List of Attachments
Attachment A.1 Vanguard Downloadable Data Access Form
Attachment A.2 Vanguard Data Request Form
Attachment A.3 Vanguard Specimen and Data Request Form
Attachment A.4 Research Materials Distribution Agreement
Attachment A.5 Example Screenshot to Show NCS Branding on Data Forms
Attachment A.6 NCS Data User Training
Attachment A.7 Comment in Response to Federal Register Notice
Attachment A.8 NIH Privacy Act Officer Memo
A.1 Justification
This document contains information supporting a request for the Office of Management and Budget (OMB) to approve a generic clearance for registration procedures associated with the National Children’s Study (NCS) Data and Sample Archive of the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health.
The NCS Vanguard Study (OMB #0925-0593; expiration date 6/30/2017) undertook data collection at 40 study locations from 2009-2014. During the NCS Vanguard Study data collection period, data was collected from approximately 6,000 U.S. families. Data collection ceased in December, 2014 after an expert review committee advised the NIH Director that moving forward with the larger Main Study would not be the best way to add to the understanding of how environmental and genetic factors influence child health and development. To date, the NCS has restricted data use to Study contractors, former Study contractors, and to a lesser extent, investigators of adjunct studies1. All entities signed a data use agreement. At this time, the NCS seeks OMB approval to collect information from researchers who seek access to NCS Vanguard data. Data will be housed securely in the NCS Vanguard Data and Sample Archive and Access System (referred to hereinafter as the “NCS Archive”), and will allow researchers access to the data through a secure data enclave. There is significant potential public benefit to sharing NCS Vanguard Study data with a broader audience.
The information requested from investigators seeking to access data and/or samples in the NCS Archuive, as part of the data request submission process for this repository, may be made public in part or in whole for tracking and reporting purposes. That information will be posted within the NCS Archive web site to describe approved research projects and will be limited to principal investigator and recipient institution names, project titles, and brief summaries of the research. In addition, citations of publications resulting from the use of NCS Archive research materials may be posted on the NCS Archive website.
Each Data Access Request provides a Privacy Act Notification pursuant to Public Law 93-579, Privacy Act of 1974, 5 U.S.C. Section 552a. Authority for the collection of the information requested from the submitting investigators comes from the authorities regarding the establishment of the National Institutes of Health, its general authority to conduct and fund research and to provide training assistance, and its general authority to maintain records in connection with these and its other functions (42 U.S.C. 203, 241, 289l-1 and 44 U.S.C. 3101), and Section 301 and 493 of the Public Health Service Act. These records will be maintained in accordance with the Privacy Act System of Record Notice 09-25-0200 (http://oma.od.nih.gov/public/ms/privacy/pafiles/0200.htm) covering “Clinical, Basic and Population-based Research Studies of the National Institutes of Health (NIH), HHS/NIH/OD.”
This is a request for a generic OMB approval to allow for the collection of information from potential archive users.
A.2 Purpose and Use of the Information Collection
The primary use of this information collected from potential data users is to document, track, and monitor the use of the NCS Archive. The Downloadable Data Access Form (Attachment A.1) requests contact information including name, email, institution, address and phone which is required for the registered website. This is the first point where information is requested. The Data Request Form (Attachment A.2) and/or Specimen and Data Request Form (Attachment A.3) requests information about the proposed project, including Requestor Information, Recipient Institution Information, and other details about the type of information or number and type of samples requested. The Research Materials Distribution Agreement (Attachment A.4) outlines the terms and conditions for use of the Research Materials. These attachments are required for the researcher portal level of access. This form requires signature of the requesting investigator and the authorized representative of the recipient institution. This information is necessary to fulfill the requirements of the proposed research projects and in addition will help NIH better understand the use of archived data and samples by the research community.
A.3 Use of Information Technology and Burden Reduction
The NCS Archive will rely on a web-based portal that enables investigators to request data access and obtain data electronically. The NCS Archive will allow for access to electronic data files and biological and environmental samples.
Data users will provide basic contact information and agree to terms and conditions of data and archive use prior to accessing any data.
Data users will start at the public facing website where they can review limited Study information. The researcher may register to access additional Study information, access the downloadable metadata files, and to submit a proposal and request for additional study data/elements. The proposal request may include existing electronic NCS data and/or a request for NCS samples. If the researcher’s request is approved by the NICHD review committee, the researcher will be given access to a secure virtual workstation in the Researcher Portal and a statistical and data support team member will work with the researcher and make available the requested data. The support team member can help bring in laboratory result data or extant data as needed. The researcher will be able to work with NCS data in the secure environment with provided statistical tools. Once the researcher completes his/her work and is ready to publish, a data reviewer will work to help assure the requested data meets disclosure standards.
The NCS Data Archive will have three components, a public website, a NCS Registered Website and a secure Researcher Portal. An example screenshot is provided to show NCS branding for the data request forms (Attachment A.5). The flow diagrams in Figure 1 show the access requirements for the three tiers of access.
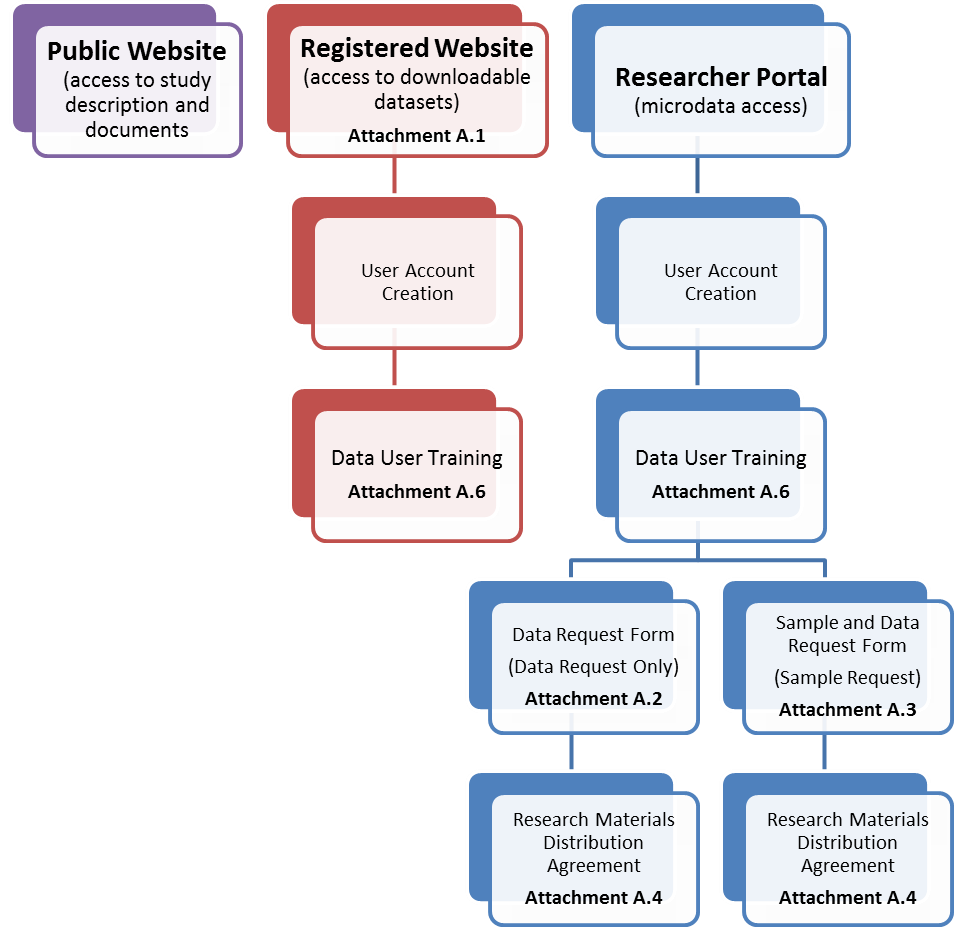
Figure 1. Access requirements for data in the three proposed tiers: Public Website, Registered Website, and Researcher Portal.
Access to the Public Website does not require any information from the user. In order to access the downloadable files a user must create a user account and complete Data User Training (Attachment A.6). To access microdata the user must complete the same user account creation and Data User Training (if not already completed), then complete a Data Request form followed by a Research Materials Distribution Agreement. Specimen requests are similar, starting with a user account, Data User Training then a Sample and Data Request form followed by the Research Materials Distribution Agreement.
A.4 Efforts to Identify Duplication and Use of Similar Information
No similar data are gathered or maintained by the agency or are available from other sources known to the agency.
A.5 Impact on Small Businesses or Other Small Entities
No impact on small businesses since they will not be involved.
A.6 Consequences of Collecting the Information Less Frequently
The information requested in the NCS Archive forms does not ask investigators to generate any new information, because the type of information being requested (see A.2 and A.3) is fundamental to conducting any research study. The data are collected on an as needed basis. Data will be collected only once for each investigator request.
A.7 Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
Not applicable.
A.8.1 Comments in Response to the Federal Register Notice
A Federal Register Notice was published on April 22, 2015, Vol. 80 FR 77. One comment was received in response to the notice (Attachment A.7) and acknowledgment of receipt was sent to commenter.
A.8.2 Efforts to Consult Outside Agency
The NCS consulted experts within and outside the agency on various aspects of the data archive design. The NCS received demonstrations from the Data and Specimen Hub (DaSH, NIH/NICHD), the Biospecimen Repository Access and Data Sharing program (BRAD, NIH/NICHD), the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO, NIH/NCI) and the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC, NIH/NHLBI) in order to determine which existing archive and repository solutions may best serve the dissemination of NCS Vanguard Study data. The NCS reviewed data sharing practices for NHANES (CDC/NCHS), NDAR (NIH/NIMH), and the NICHD Population Dynamics Branch. Some of the criteria evaluated included data specifications, data documentation and discovery tools, remote analysis functionality, and privacy protection measures.
The NCS also consulted the Office of Management and Budget’s Office of Information and Regulatory Affairs on March 16, 2015, who indicated that the NCS must have a generic clearance that is specific to data sharing.
A.9 Explanation of Any Payment or Gift to Respondents
No incentives will be provided to respondents.
A.10 Assurance of Confidentiality Provided to Respondents
The Privacy Act of 1974 ensures that no sensitive or personally identifiable information, located in federal systems of records (e.g., Recipient NIH records), is shared. A system of records is any group of records under the control of a federal agency from which information is retrieved by the name of the individual or by some identifying number, symbol, or other identifying particular assigned to the individual. The NIH and any sites that are provided access to the datasets will have access to the data collected from the Recipient for the purposes described above. In addition, the Act allows, in accordance with the routine uses made of the information stored in the system, the release of some information in the Recipient’s records without his/her permission; for example, for research purposes and if it is required by members of Congress or other authorized individuals. The information requested is voluntary, but necessary for obtaining access to data.
The information requested from the investigator seeking access to the NCS Archive, as part of the NCS Data Use Agreement, Data Request, and RMDA forms, may be made public in part or in whole for tracking and reporting purposes.
A Privacy Act Notification is displayed on the face page of the Data User Agreement, Data Request, and RMDA forms pursuant to Public Law 93-579, Privacy Act of 1974, 5 U.S.C. Section 552a. Authority for the collection of the information requested comes from the authorities regarding the establishment of the National Institutes of Health, its general authority to conduct and fund research and to provide training assistance, and its general authority to maintain records in connection with these and its other functions (42 U.S.C. 203, 241, 289l-1 and 44 U.S.C. 3101), and Section 301 and 493 of the Public Health Service Act. These records will be maintained in accordance with the Privacy Act System of Record Notice 09-25-0200 (https://oma.od.nih.gov/forms/Privacy%20Documents/PAfiles/0200.htm) covering “Clinical, Basic and Population-based Research Studies of the National Institutes of Health (NIH), HHS/NIH/OD.” The NIH System of Record Notice was previously published in the Federal register on September 26, 2002, Volume 67, No 187, page 60742. Although the NCS Vanguard Study data will be coded (de-identified) and the NIH will not hold direct identifiers to individuals within the NCS Archive, the agency recognizes the personal and potentially sensitive nature of NCS Vanguard Study data. All investigators who request access to NCS Vanguard Study data must be authorized by the NCS and the Federal government to receive and store study information and agree to protect participant privacy by reporting aggregate results only. As noted in A.2, investigators must complete a RMDA and abide by the RMDA terms.
A.11 Justification for Sensitive Questions
The NCS Archive will not ask prospective data users any sensitive questions.
A.12-1 Estimated Annualized Burden Hours
A.12-1 Estimated Annualized Burden Hours |
||||
Form |
Number of Respondents |
Frequency of Response |
Average Time Per Response ( in hours) |
Total Annual Burden Hours |
Vanguard Downloadable Data Access Form (Attachment A.1) |
300 |
1 |
10/60 |
50 |
Vanguard Data Request Form (Attachment A.2) |
50 |
1 |
20/60 |
17 |
Vanguard Specimen and Data Request Form (Attachment A.3) |
50 |
1 |
30/60 |
25 |
Research Materials Distribution Agreement (Attachment A.4) |
100 |
1 |
10/60 |
17 |
Total |
|
|
|
109 |
A.12-2 Annualized Cost to Respondents
A.12-2 Annualized Cost to Respondents |
|||
Form |
Estimate Total Annual Burden Hours |
Wage Rate |
Total Costs |
Downloadable Data Access Form (Attachment A.1) |
50 |
$ 91.00 |
$ 4,550 |
Vanguard Data Request Form (Attachment A.2) |
17 |
$ 91.00 |
$ 1,547 |
Vanguard Specimen and Data Request Form (Attachment A.3) |
25 |
$ 91.00 |
$ 2,275 |
Research Materials Distribution Agreement (Attachment A.4) |
17 |
$ 91.00 |
$1,547 |
Total |
|
|
$ 9,919 |
Salary/Wage Source: Office of Personnel Management 2014 General Schedule Locality Salary Table for various GS-levels; Bureau of Labor Statistics/Occupational Employment “Life, Physical, and Social Scientist” http://www.bls.gov/oes/current/oes_13644.htm#19-0000, occupational code 19-2099.
A.13 Estimate of Other Total Annual Cost Burden to Respondents or Record Keepers
There are no additional costs other than the respondents’ cost burden given in A12.
A.14 Annualized Cost to the Federal Government
Staff |
Grade/Step |
Salary |
% of Effort |
Fringe (if applicable) |
Total Cost to Gov’t |
Federal Oversight |
|
|
|
|
|
Program Officer |
GS-14 Step 4 |
$116,887 |
10 |
|
$11,689 |
Branch Chief |
GS-15 Step 4 |
$137,494 |
5 |
|
$6,875 |
Contractor Cost |
|
|
|
|
|
Technical Support |
|
$135,000 |
5 |
$6,750 |
$13,500 |
Total Annualized Cost |
|
|
|
|
$32,064 |
Salary/Wage Source: Office of Personnel Management 2014 General Schedule Locality Salary Table for various GS-levels; Bureau of Labor Statistics/Occupational Employment “Life, Physical, and Social Scientist” http://www.bls.gov/oes/current/oes_13644.htm#19-0000, occupational code 19-2099.
A.15 Explanation for Program Changes or Adjustments
This is a new data collection.
A.16 Plans for Tabulation and Publication and Project Time Schedule
A.16 - 1 Project Time Schedule |
|
Activity |
Time Schedule |
Data archive open to public |
1 week after OMB approval |
Information collection from data users |
Ongoing after data archive opens to public |
There are currently no plans to publish the data collected through requests for data access. The data are for internal monitoring purposes only, to assess the archive resource requirements.
A.17 Reason(s) Display of OMB Expiration Date is Inappropriate
Not applicable.
A.18 Exceptions to Certification for Paperwork Reduction Act Submissions
Not applicable.
1 Adjunct studies are studies with external funding intended to study exposure-response relationships and were intended to be integrated with the NCS Main Study when launched.
| File Type | application/msword |
| File Title | Supporting Statement 'A' Preparation - 04/05/2011 |
| Subject | Supporting Statement 'A' Preparation - 04/05/2011 |
| Author | OD/USER |
| Last Modified By | Currie, Mikia (NIH/OD) [E] |
| File Modified | 2016-02-05 |
| File Created | 2016-02-03 |
© 2026 OMB.report | Privacy Policy