Supporting Statement B
Supporting Statement B.docx
Integrating Community Pharmacists and Clinical Sites for Patient-Centered HIV Care
OMB: 0920-1019
Integrating Community Pharmacists and Clinical Sites for Patient-Centered HIV Care
OMB No. 0920-1019
Supporting Statement B
Kathy Byrd, MD, MPH, Project Officer
Centers of Disease Control and Prevention
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
Division
of HIV/ AIDS Prevention- Surveillance and Epidemiology
HIV
Epidemiology Branch
1600 Clifton Rd., MS E-45
Atlanta, GA 30333
Phone: 404-639-3083
Fax: 404-639-6127
Email: gdn8@cdc.gov
TABLE OF CONTENTS
B. COLLECTIONS OF INFORMATION EMPLOYING STATISTICAL METHODS
1. Respondent Universe and Sampling Methods
2. Procedures for the Collection of Information
3. Methods to Maximize Response Rates and Deal with Nonresponse
4. Tests of Procedures or Methods to be Undertaken
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data
B. Collections of Information employing statistical methods
1. Respondent Universe and Sampling Methods
Our sample will be a non-probability based convenience sample. The respondent universe is HIV-infected persons, from targeted minority populations, receiving HIV medical care at one of the 10 project sites. Targeted minority populations include Black, Latino and American Indian/Alaska Native populations. A project site will contain one or more Walgreens pharmacy and one or more medical clinic. Each project site will enroll 100 patients for a total of 1000 patients.
The sample size is based on the maximum number of patients to which Walgreens Company is willing to provide in-kind services. Because project clinics must be chosen in concert with Walgreens project pharmacies (who will ultimately decide which pharmacies will participate), no sampling methods will be employed to choose project sites. Both the project sites and project participants will represent convenience samples.
Participant eligibility includes HIV patients 18 years of age and older who receive medical care at one of the project clinics. CDC will work with Walgreens and the University of North Texas Health Science Center to define additional eligibility criteria. Participants will be enrolled into the project on a rolling basis until each project site has enrolled the targeted number of participants.
2. Procedures for the Collection of Information
Data collection methods
Project clinics and pharmacies are the sites for the data collection. Most data will be abstracted from the clinics’ and pharmacies’ archived patient medical and pharmacy records by project clinic and pharmacy staff (Attachment 10a). Data collected from participants’ medical and pharmacy records are routinely collected and stored information used by participants’ providers for routine medical care. Medical record data will be abstracted at the baseline of the study and quarterly thereafter. Pharmacy record data will be abstracted quarterly. In addition, baseline descriptive data on the characteristics of the project sites will be collected at the beginning of the project by project staff and annually thereafter (Attachment 3, Attachment 4).
Overview of the data collection system
The patient-centered HIV care model project information collection has six primary components: 1) description of project clinics and pharmacies 2) description of non-participant patients 3) medical record abstraction 4) pharmacy record abstraction 5) key informant (project staff) interviews and staff questionnaire 6) time and cost documentation. All information collected is for the purpose of program performance monitoring, adjustment of the project model, as needed, and for determination of program outcomes within the project cohort cost and program costs. Project clinic and pharmacy staff will complete the descriptions of each respective project clinic and pharmacy. Medical and pharmacy record abstraction will be conducted by project clinic and pharmacy staff for all participants of the pilot program. Most data collected from the medical and pharmacy record abstraction are routinely collected information used by medical clinics and pharmacies for normal patient care. Key project staff will participate in key informant interviews and project staff will complete a staff communication questionnaire and collect the time spent on and the cost of program activities.
Project clinic and pharmacy characteristics: Project site clinic (Attachments 3) and pharmacy (Attachments 4) characteristics will be collected retrospectively for two years prior to project site enrollment and annually throughout the project period. Project clinic and pharmacy staff at each respective project site will collect the information. Project sites will send the data to the project grantee who will investigate and resolve data discrepancies. Data will then be sent to CDC through the CDC Secure Data Network.
Description of non-participant patients: Patients who choose not to participate in the project will be given an opportunity to allow their basic demographic information to be collected (Attachment 5). This will allow the project team to understand if the people in the project are similar or different to the people who are not in the project.
Medical record abstraction: Medical record abstraction will be conducted by project clinic staff at each respective project clinic. De-identified client-level data will be collected. Project clinics will send the data to the project grantee who will investigate and resolve data discrepancies. Data will then be sent to CDC through the CDC Secure Data Network. All identifiers will be removed before data are reported to CDC; each program participant will be assigned a unique program ID. The grantee and CDC will store and access data by the assigned participant ID. A one-time retrospective medical record abstraction will occur at the beginning of the project in order to document participants’ baseline characteristics and history (Attachment 6a and 6b). After program implementation, project staff will collect data on a quarterly basis (Attachment 7a and 7b). The grantee will report data to CDC on a quarterly basis.
Pharmacy record abstraction: Pharmacy record abstraction will be conducted by project pharmacy staff at each respective project pharmacy (Attachment 8). De-identified client-level data will be collected. Project pharmacies will send the data to the project grantee who will investigate and resolve data discrepancies. Data will then be sent to CDC through the CDC Secure Data Network. All identifiers will be removed before data are reported to CDC; each program participant will be assigned a unique program ID. The grantee and CDC will store and access data by an assigned participant ID. Project staff will collect data on a quarterly basis. The grantee will report data to CDC on a quarterly basis.
Key informant interviews: Each project site will choose six project staff, who have in-depth knowledge of the project processes, to participate in key informant interviews. It is anticipated that the key informants will be made up of three pharmacists, two physicians and one nurse although the make-up of the key informants may be different per site depending on staff knowledge of the project. Interviews will be conducted twice during the project period: the first interviews will take place approximately 3-6 months after the sites have begun enrollment and the second interviews will take place approximately 9-12 months after enrollment. Each interview is estimated to last approximately 30 minutes and will focus on changes to clinic and pharmacy work systems, processes and outcomes in relation to the model project (Attachment 10a and 10b).
Staff communication questionnaire: Project staff from each project site will complete a project staff questionnaire. It is anticipated that three pharmacists, two physicians and two nurses, at each project site, will complete the questionnaires, although the make-up of the respondents may be different per site depending on current project site staffing. The questionnaire will be administered twice during the project period: at approximately 3-6 months after the sites have begun enrollment and then again approximately 9-12 months after enrollment. The questionnaire is estimated to take 15 minutes to complete and focuses on communication between project pharmacists and medical providers (Attachment 11).
Time and costs associated with project activities: Each project clinic (Attachment 12) and pharmacy (Attachment 13) will document the time spent on project activities and detail associated costs. Each site will collect time and cost information for a one month period near the beginning of the project and for a one month period toward the end of the project period.
Items of Information to be Collected
Data collection |
Attachment number |
Project Clinic Characteristics. An annual collection of project clinics’ characteristics including city and state, type of clinic, total number of patients, demographics of patients, clinic patient volume, number and type of medical providers employed at clinic. |
3 |
Project Pharmacy Characteristics. An annual collection of project pharmacies’ characteristics including city and state, type of pharmacy, length of time as an HIV Center of Excellence, number of HIV clients filling prescriptions, number and average number of clients filling prescriptions, prescription filling volume, insurance status of clients, preventive services offered, number and type of providers employed at pharmacy |
4 |
Patient demographic information form. A one-time collection of basic demographics of project clinic patients who choose not to participate in the project but who agree to have their basic demographic characteristics recorded. Variables include month and year of birth, sex, race/ethnicity, education level, household income, housing, employment and insurance status |
5 |
Initial Patient Information form. A one-time retrospective medical record abstraction will be conducted at the beginning of the project. Variables include patient demographics, date and stage at diagnosis, laboratory test results, antiretroviral and other prescribed medications, opportunistic illnesses, other clinical diagnoses, immunizations, history of tobacco, drug and alcohol use and adherence to clinic appointments. |
6a |
Quarterly Patient Information form. Medical record abstraction of variables routinely collected by clinics, as part of routine patient care, will be conducted quarterly after implementation of the pilot project. Variables include laboratory test results, changes in medication therapy and medical conditions, immunizations, drug and alcohol use and adherence to clinic appointments. |
7a |
Interim Pharmacy form. Pharmacy record abstraction will be conducted quarterly after implementation of the pilot project. The pharmacy record abstraction will collect data from pharmacy records on pharmacy services received, the nature of pharmacy services received, pharmacists’ recommendations and interventions, pharmacists’ consultation with partnered clinics and prescription refills. |
8 |
Interviewer data collection worksheet. The Key informant interviews will be conducted twice with project staff during the project. The interviews will focus on project sites’ work systems, processes and outcomes in relation to the model program. |
10a |
Staff communication questionnaire. A questionnaire will be administered to project site staff twice during the project. The interviews will focus on communication between the project sites’ pharmacists and clinic staff. |
11 |
Clinic cost form. Project clinics will document the time spent on project activities and associated costs for two one month periods during the project. |
12 |
Pharmacy cost form. Project pharmacies will document the time spent on project activities and associated costs for two one month periods during the project. |
13 |
Data Transmittal
The project clinics and pharmacies will send data to the grantee who will clean the data, resolve data discrepancies and then transmit the data to CDC. Data will be electronically transmitted to CDC through the CDC Secure Data Network (SDN). All data transmissions are automatically encrypted by the software that generates the transfer files. Security certificates are used to control access to the SDN.
Sample Size Justification
This
project has a fixed maximum sample size of m
= 10 sites and 100 participants per site, giving a total of
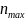 = 1000 participants. We account for the group structure of this
non-randomized trial by assuming a standard intraclass
correlation
of
= 1000 participants. We account for the group structure of this
non-randomized trial by assuming a standard intraclass
correlation
of
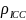 =
5%. We also account for the potential loss of retention by assuming
that
=
5%. We also account for the potential loss of retention by assuming
that
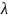 = 20% of
participants will be lost to follow-up over the course of the
project. We
thus obtain an effective sample size of
= 20% of
participants will be lost to follow-up over the course of the
project. We
thus obtain an effective sample size of
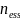 = 441. See Table 1 for the effective sample sizes (ESSs) for
various intraclass correlations (
= 441. See Table 1 for the effective sample sizes (ESSs) for
various intraclass correlations (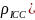 and
proportions of loss-to-follow-up (
and
proportions of loss-to-follow-up (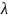 ).
).
The
primary outcomes are the binomial proportions of participants with a
HIV diagnosis who (a) are retained in care (b) are virally suppressed
(c) are adherent to HIV medication therapy. Retention in care will
be defined as the percentage of patients who had at least one medical
visit in each 6-month period of the measurement period with a minimum
of 60 days between medical visits. A medical visit is any visit at
the project clinic with a physician, nurse practitioner and/or a
physician assistant. HIV viral load suppression will be defined as
the proportion of participants with HIV viral loads < 200
copies/ml at the end of the project period. The Proportion
of Days Covered
(PDC) will be used to calculate adherence to HIV medication therapy.
The PDC is defined as the total number of days a patient was in
possession of a medication divided by the number of days between
the patient’s last fill date and last fill date plus the days’
supply of the last fill. These outcomes will be measured for
all participants both at baseline (BL) and 24 months follow-up (FU).
Thus, the data will be paired on each participant. Improvements
(changes) in these outcomes will be tested using McNemar’s test
with a two-sided significance level of
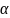 = 5%.
= 5%.
We
assume that the BL proportions for each of these three outcomes are
(a)
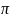 = 45%, [1] (b)
= 45%, [1] (b)
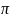 = 75% [2] and (c)
= 75% [2] and (c)
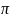 = 69% [3] respectively. For the above effective sample size of
= 69% [3] respectively. For the above effective sample size of
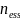 = 441, we
have 80% power to reject the null hypothesis of no change when the
absolute increases in these proportions at FU for each of these three
outcomes are at least (a)
= 441, we
have 80% power to reject the null hypothesis of no change when the
absolute increases in these proportions at FU for each of these three
outcomes are at least (a)
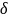 = 10%,
(b)
= 10%,
(b)
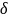 = 8%, and (c)
= 8%, and (c)
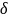 = 9%, respectively. For
the above effective sample size of
= 9%, respectively. For
the above effective sample size of
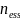 = 441, we
have 90% power to reject the null hypothesis of no change when the
absolute increases in these proportions at FU for each of these three
outcomes are at least (a)
= 441, we
have 90% power to reject the null hypothesis of no change when the
absolute increases in these proportions at FU for each of these three
outcomes are at least (a)
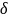 = 11%,
(b)
= 11%,
(b)
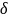 = 9%, and (c)
= 9%, and (c)
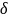 = 10%, respectively. Tables 2 and 3
= 10%, respectively. Tables 2 and 3
Table
1: Effective sample sizes for
various intraclass correlations (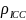 )
and
proportions of loss to follow-up (LTFU,
)
and
proportions of loss to follow-up (LTFU,
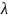 )
)
|
intraclass
correlation ( |
||||||||||
LTFU ( |
0.00 |
0.01 |
0.02 |
0.03 |
0.04 |
0.05 |
0.06 |
0.07 |
0.08 |
0.09 |
0.10 |
0.00 |
1000 |
917 |
847 |
787 |
735 |
689 |
649 |
613 |
581 |
552 |
526 |
0.05 |
902 |
827 |
764 |
710 |
663 |
622 |
586 |
553 |
524 |
498 |
475 |
0.10 |
810 |
743 |
686 |
637 |
595 |
558 |
525 |
496 |
470 |
447 |
426 |
0.15 |
722 |
662 |
612 |
568 |
531 |
498 |
469 |
443 |
420 |
399 |
380 |
0.20 |
640 |
587 |
542 |
503 |
470 |
441 |
415 |
392 |
372 |
353 |
336 |
0.25 |
562 |
516 |
476 |
442 |
413 |
387 |
365 |
345 |
327 |
310 |
296 |
0.30 |
490 |
449 |
415 |
385 |
360 |
337 |
318 |
300 |
284 |
270 |
257 |
0.35 |
422 |
387 |
358 |
332 |
310 |
291 |
274 |
259 |
245 |
233 |
222 |
0.40 |
360 |
330 |
305 |
283 |
264 |
248 |
233 |
220 |
209 |
198 |
189 |
Table
2: Sample sizes required to have 80% power to reject the null
hypothesis of no change from baseline to follow-up using McNemar’s
test with two-sided significance level of
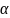 = 0.05.*
= 0.05.*
|
absolute
increase from BL to FU ( |
||||||||
BL
% ( |
0.05 |
0.06 |
0.07 |
0.08 |
0.09 |
0.10 |
0.11 |
0.15 |
0.20 |
0.35 |
1474 |
1029 |
761 |
586 |
465 |
379 |
315 |
173 |
99 |
0.40 |
1537 |
1071 |
789 |
606 |
481 |
391 |
324 |
176 |
100 |
0.45 |
1568 |
1090 |
802 |
615 |
487 |
395 |
326 |
176 |
99 |
0.50 |
1568 |
1088 |
799 |
611 |
483 |
391 |
322 |
173 |
96 |
0.55 |
1537 |
1064 |
780 |
595 |
469 |
379 |
312 |
166 |
91 |
0.60 |
1474 |
1018 |
745 |
567 |
446 |
359 |
295 |
155 |
84 |
0.65 |
1380 |
951 |
693 |
527 |
413 |
332 |
272 |
141 |
76 |
0.70 |
1254 |
862 |
626 |
474 |
370 |
296 |
242 |
124 |
65 |
0.75 |
1097 |
750 |
543 |
409 |
318 |
253 |
206 |
103 |
52 |
0.80 |
909 |
617 |
443 |
332 |
256 |
202 |
163 |
78 |
-- |
0.85 |
689 |
463 |
328 |
242 |
184 |
143 |
114 |
-- |
-- |
*Assuming
a minimal within-participant correlation of
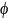 = 0.
= 0.
Table
3: Sample sizes required to have 90% power to reject the null
hypothesis of no change from baseline to follow-up using McNemar’s
test with two-sided significance level of
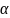 = 0.05.*
= 0.05.*
|
absolute
increase from BL to FU ( |
||||||||
BL
% ( |
0.05 |
0.06 |
0.07 |
0.08 |
0.09 |
0.10 |
0.11 |
0.15 |
0.20 |
0.35 |
1972 |
1377 |
1017 |
783 |
622 |
506 |
420 |
230 |
132 |
0.40 |
2056 |
1432 |
1056 |
811 |
642 |
522 |
432 |
234 |
133 |
0.45 |
2098 |
1459 |
1073 |
822 |
650 |
527 |
436 |
234 |
132 |
0.50 |
2098 |
1456 |
1069 |
817 |
645 |
522 |
431 |
230 |
128 |
0.55 |
2056 |
1424 |
1043 |
796 |
627 |
506 |
417 |
220 |
121 |
0.60 |
1972 |
1362 |
996 |
758 |
596 |
480 |
394 |
206 |
112 |
0.65 |
1846 |
1272 |
927 |
704 |
552 |
443 |
363 |
188 |
100 |
0.70 |
1678 |
1152 |
837 |
633 |
494 |
396 |
323 |
164 |
86 |
0.75 |
1467 |
1003 |
725 |
546 |
424 |
338 |
274 |
136 |
68 |
0.80 |
1215 |
825 |
592 |
443 |
341 |
270 |
217 |
104 |
-- |
0.85 |
921 |
618 |
438 |
323 |
245 |
191 |
151 |
-- |
-- |
*Assuming
a minimal within-participant correlation of
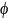 = 0.
= 0.
3. Methods to Maximize Response Rate and Deal with Nonresponse
Most of the data collected for this project is routinely collected and archived by the project clinics and pharmacies and does not involve participant response to any surveys. A data manager at each clinic will collect the data and send the data to the grantee. The clinics will be funded to participate in the project and will be required to submit data as a condition of funding. Submission of data by the clinics is, therefore, expected to be high. The grantee will work with the project sites to address any problems with data collection and to resolve data discrepancies. The grantee will electronically transmit the data to CDC.
4. Tests of Procedures or Methods to be Undertaken
Data collection for this project does not involve participant response to any surveys. Most data will be abstracted from the clinics’ and pharmacies’ archived patient medical and pharmacy records by project clinic and pharmacy staff. The data collection forms have been reviewed by project team members from CDC, Walgreens and the University of North Texas Health Science Center. In addition, input was received from clinicians at one large HIV-care clinic and from staff from the Health Resources and Services Administration and the National Minority AIDS Council.
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data
The following individuals were consulted on the statistical aspects of the project:
Craig Borkowf PhD,
Mathematical Statistician
Quantitative Sciences and Data Management Branch,
Division of HIV/AIDS Prevention
Centers for Disease Control and Prevention
1600 Clifton Rd. NE, MS E48
Atlanta, GA 30333
T: 404.639.5235
Email: uzz3@cdc.gov
Yi Pan PhD,
Mathematical Statistician
Quantitative Sciences and Data Management Branch, Division of HIV/AIDS Prevention
Centers for Disease Control and Prevention
1600 Clifton Rd. NE, MS E48
Atlanta, GA 30333
T: 404.639.0960
Email: jnu5@cdc.gov
The following CDC staff will analyze project data:
Kathy Byrd MD, MPH
Medical Epidemiologist
Epidemiology Branch, Division of HIV/AIDS Prevention
1600 Clifton Rd. NE, MS E45
Atlanta, GA 30333
T: 404.639.3083
Email: gdn8@cdc.gov
Craig Borkowf PhD
Mathematical Statistician
Quantitative Sciences and Data Management Branch,
Division of HIV/AIDS Prevention
Centers for Disease Control and Prevention
1600 Clifton Rd. NE, MS E48
Atlanta, GA 30333
T: 404.639.5235
Email: uzz3@cdc.gov
Yi Pan PhD
Mathematical Statistician
Quantitative Sciences and Data Management Branch,
Division of HIV/AIDS Prevention
Centers for Disease Control and Prevention
1600 Clifton Rd. NE, MS E48
Atlanta, GA 30333
T: 404.639.0960
Email: jnu5@cdc.gov
The following contracted staff will analyze project data:
Nasima Camp, MPH
Public Health Analyst
Epidemiology Branch, Division of HIV/AIDS Prevention
1600 Clifton Rd. NE, MS E45
Atlanta, GA 30333
T: 404.639.8246
Email: yul9@cdc.gov
CDC personnel responsible for receiving and approving contract deliverables:
LaShonda Billingsley
Public Health Analyst
Epidemiology Branch, Division of HIV/AIDS Prevention
1600 Clifton Rd. NE, MS E07
Atlanta, GA 30333
T: 404.639.6047
Email: lqb6@cdc.gov
Data will be collected form the project sites by the University of North Texas Health Science Center through a co-operative agreement.
References
1. Gardner, L, Giordano T, Marks G, Wilson T, Craw J, Drainoni M, Keruly J, Rodriguez A, Malitz F, Moore R, Bradley-Springer L, Holman S, Rose, Girde S, Sullivan M, Moore R, Metsch L, Saag M, Mugavero M. Enhanced personal contact with HIV patients across time improves retention in primary care: a randomized trial in six U.S. HIV clinics (unpublished data, under review in 2014)
2. CDC. Vital Signs: HIV Prevention Through Care and Treatment — United States. MMWR 2011; vol 60 (47): 1618-1623.
3. Murphy P, Cocohoba J, Tang A, Pietrandoni G, Hou J, Guglielmo BJ. Impact of HIV-specialized pharmacies on adherence and persistence with antiretroviral therapy. AIDS Patient Care STDS. 2012; 26(9): 526-31
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | CDC User |
| File Modified | 0000-00-00 |
| File Created | 2021-01-25 |
© 2026 OMB.report | Privacy Policy