933 2015 933 FDA Email Enterprise Survey
E-Government Website Customer Satisfaction Surveys
2015 933 FDA Email Enterprise Survey.xlsx
2015 931 DOS Blogs - 2015 933 FDA Email Enterprise Survey
OMB: 1090-0008
⚠️ Notice: This form may be outdated. More recent filings and information on OMB 1090-0008 can be found here:
Document [xlsx]
Download: xlsx | pdf
Model Qsts
Current CQs
Non-Model FDA Email Enterprise
Overview
Welcome and Thank You TextModel Qsts
Current CQs
Non-Model FDA Email Enterprise
Sheet 1: Welcome and Thank You Text

|
||||||
| Welcome and Thank You Text | ||||||
| Welcome Text | Thank You Text | |||||
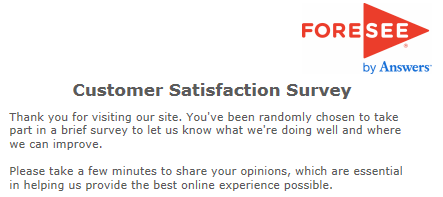
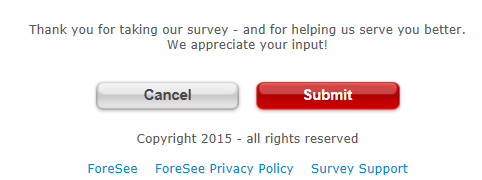
| Thank you for participating in this brief survey. Your feedback is essential in helping us provide the best email experience possible. | Thank you for taking the survey and for helping us to serve you better. Please note you will not receive a response from us based on your survey comments. |
|||||
|
|
|||||
Sheet 2: Model Qsts
| Model Name | FDA Email Enterprise | 
|
||||||
| Model ID | TBD | Underlined & Italicized: Re-order | ||||||
| Partitioned | No | Pink: Addition | ||||||
| Date | 8/1/2015 | Blue: Reword | ||||||
| Label | Element Questions | Label | Satisfaction Questions | Label | Future Behaviors | |||
| Look and Feel (1=Poor, 10=Excellent, Don't Know) | Satisfaction | Visit Website (1=Very Unlikely, 10=Very Likely) | ||||||
| Please rate the visual appeal of this email. | What is your overall satisfaction with this email? (1=Very Dissatisfied, 10=Very Satisfied) |
How likely are you to visit the FDA website as a result of receiving this email? | ||||||
| Please rate the balance of graphics and text in this email. | How well does this email meet your expectations? (1=Falls Short, 10=Exceeds) |
Primary Resource (1=Very Unlikely, 10=Very Likely) | ||||||
| Please rate the readability of this email. | How does this email compare to your idea of an ideal email? (1=Not Very Close, 10=Very Close) |
How likely are you to use the FDA website as a primary resource for information related to this email? | ||||||
| Email Content (1=Poor, 10=Excellent, Don't Know) | Forward Email (1=Very Unlikely, 10=Very Likely) | |||||||
| Please rate the credibility of information in this email. | How likely are you to forward this email to someone else? | |||||||
| Please rate the timeliness of the information in this email. | Future Email Behavior (1=Very Unlikely, 10=Very Likely) | |||||||
| Please rate the degree to which the information provided in this email addresses your interests. | How likely are you to open future emails from FDA? | |||||||
Sheet 3: Current CQs
| Model Name | FDA Email Enterprise | 
|
|||||||
| Model ID | TBD | Underlined & Italicized: Re-order | |||||||
| Partitioned | No | Pink: Addition | |||||||
| Date | 8/1/2015 | Blue: Reword | |||||||
| QID | QUESTION META TAG | Skip From | Question Text | Answer Choices | Skip To | Required Y/N |
Type | Special Instructions | CQ Label |
| Is the frequency at which you receive this email notification appropriate? | Yes, the frequency is fine | Y | Radio button, one-up vertical | Frequency | |||||
| No, I would like to receive it more often. | |||||||||
| No, I would like to receive it less often. | |||||||||
| When would you prefer to receive emails from FDA? | During business hours only | Y | Radio button, one-up vertical | Time Preference | |||||
| As soon as information is made available | |||||||||
| No preference | |||||||||
| Which of the following roles best describes you? | Regulated industry | Y | Radio button, one-up vertical | Skip Logic Group* | Role | ||||
| If you are a consultant or attorney, please select the role of the individual or organization that you represent. | Consumer | ||||||||
| Patient or a patient’s caregiver, family member or friend | |||||||||
| Healthcare provider (includes physician, nurse, physician’s assistant, nurse practitioner, or pharmacist) | |||||||||
| Public health professional | |||||||||
| Scientist, researcher | |||||||||
| Educator, professor, teacher, or student | |||||||||
| Other | A | ||||||||
| A | Please describe your role in visiting the site today: | N | Text area, no char limit | Skip Logic Group* | OE_Role | ||||
| Please select your level of agreement with the following statements about FDA email notifications. | Y | Drop down, select one | Multiple Lists Group* | ||||||
| Subject lines are relevant and easy to understand | Agree | A/D:SubjectLines | |||||||
| Somewhat Agree | |||||||||
| Somewhat Disagree | |||||||||
| Disagree | |||||||||
| The length of the email is appropriate | Agree | A/D:Length | |||||||
| Somewhat Agree | |||||||||
| Somewhat Disagree | |||||||||
| Disagree | |||||||||
| There is enough information provided for me to take action (if necessary) | Agree | A/D:Action | |||||||
| Somewhat Agree | |||||||||
| Somewhat Disagree | |||||||||
| Disagree | |||||||||
| The supplemental link(s) provided in the email are useful | Agree | A/D:UsefulLinks | |||||||
| Somewhat Agree | |||||||||
| Somewhat Disagree | |||||||||
| Disagree | |||||||||
| In general, how do you use the information found in this email notification? | For my own health, my family's health or for a friend | Y | Radio button, one-up vertical | Skip Logic Group* | Info Usage | ||||
| For a physician's office/hospital | |||||||||
| For a patient or client | |||||||||
| For a public health agency | |||||||||
| For a research institution | |||||||||
| For a business / workplace | |||||||||
| For an educational institution or teaching purposes | |||||||||
| For a news report or article | |||||||||
| Other | B | ||||||||
| B | Please describe how you use the email information you receive. | N | Radio button, one-up vertical | Skip Logic Group* | OE_InfoUsage | ||||
| On average, how many individuals do you share the information with? | Zero | Y | Radio button, one-up vertical | Share Info | |||||
| 1-10 people | |||||||||
| 11-20 people | |||||||||
| 21-50 people | |||||||||
| 51-100 people | |||||||||
| 101-500 people | |||||||||
| More than 500 people | |||||||||
| On what device do you typically read FDA email notifications? | Desktop/laptop computer | Y | Radio button, one-up vertical | Device | |||||
| Tablet (iPad, etc.) | |||||||||
| Smartphone (iPhone, Android, Blackberry, etc.) | |||||||||
| Other | |||||||||
| Where are you when you typically read FDA email notifications? | Home | Y | Radio button, one-up vertical | Location | |||||
| Work | |||||||||
| On the go | |||||||||
| When you receive an FDA email, do you most often: | Read the email the same day you receive it | Y | Radio button, one-up vertical | Timing | |||||
| Flag the email to read at a later date | |||||||||
| File the email with the possibility of needing to reference it in the future | |||||||||
| Other | |||||||||
| Do you receive other email notifications from the FDA? | Yes | A, B | Y | Radio button, one-up vertical | Skip Logic Group* | Other:Emails | |||
| No | |||||||||
| A | How many email notifications have you subscribed to receive from the FDA (including this one)? | 1-5 | Y | Radio button, one-up vertical | Skip Logic Group* | Other:Number | |||
| 6-10 | |||||||||
| More than 10 | |||||||||
| B | Is the presentation of information consistent across the emails you receive from FDA? | Yes | Y | Radio button, one-up vertical | Skip Logic Group* | Other:Consistency | |||
| No | |||||||||
| If you could suggest one improvement for FDA email notifications, what would it be? | N | Text area, no char limit | OE_OneImprovement | ||||||
Sheet 4: Non-Model FDA Email Enterprise
| Model Name | FDA Email Enterprise | 
|
|||||||
| Model ID | TBD | Underlined & Italicized: Re-order | |||||||
| Partitioned | No | Pink: Addition | |||||||
| Date | 8/1/2015 | Blue: Reword | |||||||
| QID | QUESTION META TAG | Skip From | Question Text | Answer Choices | Skip To | Required Y/N |
Type | Special Instructions | CQ Label |
| Why have you subscribed to receive FDA email notifications? | N | Text area, no char limit | OE_Why Subscribed | ||||||
| Do the emails you receive generally meet your needs? | Yes | Y | Radio button, one-up vertical | Skip Logic Group* | Meet Needs | ||||
| No | A | ||||||||
| A | How can FDA emails be improved to meet your needs? | N | Text area, no char limit | Skip Logic Group* | OE_Needs | ||||
| How are you using the information provided within the FDA emails? | N | Text area, no char limit | OE_Why Subscribed | ||||||
| Which of the following roles best describes you? | Regulated industry | Y | Radio button, one-up vertical | Skip Logic Group* | Role | ||||
| If you are a consultant or attorney, please select the role of the individual or organization that you represent. | Consumer | ||||||||
| Patient or a patient’s caregiver, family member or friend | |||||||||
| Healthcare provider (includes physician, nurse, physician’s assistant, nurse practitioner, or pharmacist) | |||||||||
| Public health professional | |||||||||
| Scientist, researcher | |||||||||
| Educator, professor, teacher, or student | |||||||||
| Other | A | ||||||||
| A | Please describe your role in visiting the site today: | N | Text area, no char limit | Skip Logic Group* | OE_Role | ||||
| On what device do you typically read FDA email notifications? | Desktop/laptop computer | Y | Radio button, one-up vertical | Device | |||||
| Tablet (iPad, etc.) | |||||||||
| Smartphone (iPhone, Android, Blackberry, etc.) | |||||||||
| Other | |||||||||
| Where are you when you typically read FDA email notifications? | Home | Y | Radio button, one-up vertical | Location | |||||
| Work | |||||||||
| On the go | |||||||||
| When you receive an FDA email, do you most often: | Read the email the same day you receive it | Y | Radio button, one-up vertical | Timing | |||||
| Flag the email to read at a later date | |||||||||
| File the email with the possibility of needing to reference it in the future | |||||||||
| Other | |||||||||
| If you could suggest one improvement for FDA email notifications, what would it be? | N | Text area, no char limit | OE_OneImprovement | ||||||
| File Type | application/vnd.openxmlformats-officedocument.spreadsheetml.sheet |
| File Modified | 0000-00-00 |
| File Created | 0000-00-00 |
© 2026 OMB.report | Privacy Policy