Supporting Statement A final
Supporting Statement A final.docx
The Green Housing Study
OMB: 0920-0906
Extension
Information Collection Request
for
The Green Housing Study
OMB Number 0920-0906
(Expiration Date: November 30, 2014)
Supporting Statement
(Part A)
July 30, 2014
Project Official:
Ginger L. Chew, ScD
Principal Investigator
National Center for Environmental Health
U.S. Centers for Disease Control and Prevention (CDC)
4770 Buford Hwy., N.E., MS-F60
Atlanta, GA 30341
Tel: (770) 488-3992
Fax: (770) 488-3635
Table of Contents
A.1. Circumstances Making the Collection of Information Necessary 4
A.1.1. Privacy Impact Assessment 7
A.2. Purpose and Use of Information Collection 14
A.2.1. Privacy Impact Assessment 16
A.3. Use of Improved Information Technology and Burden Reduction 17
A.4. Efforts to Identify Duplication and Use of Similar Information 17
A.5. Impact on Small Businesses or Other Small Entities 18
A.6. Consequences of Collecting the Information Less Frequently 18
A.7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5 18
A.9. Explanation of Any Payment or Gift to Respondents 20
A.10. Assurance of Confidentiality Provided to Respondents 21
A.10.1. Privacy Impact Assessment Information 21
A.11. Justification for Sensitive Questions 23
A.12. Estimates of Annualized Burden Hours and Costs 23
A.13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers 27
A.14. Annualized Cost to the Government 27
A.15. Explanation for Program Changes or Adjustments 27
A.16. Plans for Tabulation and Publication and Project Time Schedule 27
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate 28
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions 28
List of Appendices
APPENDIX A Authorizing Legislation
APPENDIX B 60-Day Federal Register Notice (FRN)
APPENDIX C The Green Housing Study Protocol
APPENDIX D Questionnaires
APPENDIX D1 Screening Questionnaire
APPENDIX D2 Baseline Questionnaire (Home Characteristics)
APPENDIX D3 Baseline (Part 2) Questionnaire (Home Characteristics)
APPENDIX D4 Baseline Questionnaire (Demographics)
APPENDIX D5 Baseline Questionnaire (Children 7-12 with Asthma)
APPENDIX D6 Text Messages (Children 7-12 with Asthma)
APPENDIX D7 3 and 9-month Follow-up Questionnaire (Children 7-12 with Asthma)
APPENDIX D8 6 and 12-month Follow-up Questionnaire (Environment)
APPENDIX D9 6 and 12-month Follow-up Questionnaire (Children 7-12 with Asthma)
APPENDIX D10 Time/Activity Questionnaire (Children with Asthma 7-12 years)
APPENDIX D11 Time/Activity Questionnaire (Mothers/Primary Caregiver)
APPENDIX D12 Illness Checklist
APPENDIX E Recruitment Flyer (prototype)
APPENDIX F Consent Form
APPENDIX G Assent Form
APPENDIX H IRB Approval Letter
APPENDIX I Agency (CDC) consultants on study design and laboratory methodology
A. JUSTIFICATION
A.1. Circumstances Making the Collection of Information Necessary
The Centers for Disease Control and Prevention (CDC) is requesting a three-year extension for an existing information collection request (ICR) titled “The Green Housing Study” (OMB No. 0920-0906; expiration date: 11/30/2014). This ICR is authorized by Section 301 of the Public Health Service Act (42 U.S.C. 241) (Appendix A). The 60-day Federal Register Notice was published on March 21, 2014 (Appendix B), and is further discussed in Section A.8.
This investigation is consistent with CDC’s health protection research agenda, which calls for research to identify the major environmental causes of disease and disability and related risk factors. In addition, this study directly supports several of the United States (US) Health and Human Services’ (HHS) Healthy People 2020 objectives (available at http://healthypeople.gov/2020/topicsobjectives2020/default.aspx).
Per the terms of the original approval of “The Green Housing Study” (OMB No. 0920-0906; expiration date: 11/30/2014), if at any time there are proposed changes to the ICR, then CDC will submit a non-substantive change request to OMB via ROCIS (i.e. the consolidated information system for the Regulatory Information Service Center and Office of Information and Regulatory Affairs) before fielding translated instruments or any site-specific variations on the protocol, consent forms, questionnaires, or recruitment materials developed locally. One such change request has been submitted in the past three years and was approved by OMB on 12/20/2011.
Background
The Green Housing Study began in 2011 to gain a better understanding of the extent to which green-built, low-income housing actually reduces exposures to allergens and toxic substances when compared to standard-built, low-income housing. The study was designed to investigate if changes in such exposures are associated with changes in asthma morbidity among children. This study may provide insight into how specific green building practices may influence levels of substances in the home in different parts of the US. The results of this study are providing data that will allow CDC and the US Department of Housing and Urban Development (HUD) to identify housing factors that are not only energy-efficient, but have the potential to improve the health outcomes of one of the most sensitive populations, low-income children with asthma. The CDC Institutional Review Board (IRB) approved protocol (Appendix C) is summarized in the Supporting Statement Parts A and B.
This study is being undertaken in ongoing building renovation programs including, but not limited to, public housing and the “Mark-to-Market” (M2M) program, sponsored by HUD, which subsidizes both publicly- and privately-owned housing across the country, notably in urban areas. HUD requires that these subsidized properties be rehabilitated to maintain a certain level of habitability. Briefly, the M2M program is a nationwide initiative that encourages landlords of multi-family properties to use green building principles. In partnership with HUD, CDC is leveraging this opportunity to collect survey and biomarker data from residents and to take environmental measurements in their homes. CDC will study rehabilitated properties in 13 study locations (large US metropolitan areas that are located in different climactic regions). We are continuing to recruit study sites to reach our goal of 13 sites (via cooperative agreement). Specifically, the addition of more study sites around the country will enable assessment of green housing effects on exposures and outcomes in different climactic regions, housing stock, and among different household ethnicities.
Since 2011, two study sites (Boston and Cincinnati) have collected data from 101 households. Preliminary data from the first two study sites were presented at national and international meetings and conferences (e.g., the 2012 California Asthma Summit, and the 2012 and 2013 International Society of Exposure Science, the 2013 Chicago Asthma Consortium’s Asthma and Housing, 2014 American Academy of Allergy, Asthma, and Immunology, the 2014 Epidemic Intelligence Surveillance, and the 2014 American Thoracic Society conferences). Some of the very preliminary results included the following:
In the homes of 95 asthmatic children in both Cincinnati and Boston, multivariate regression models showed that indoor particulate matter (PM2.5) and formaldehyde levels were associated with increased lower airway inflammation in asthmatic children not using asthma controller medications. However, this association was not observed for those children who had used asthma controller medications.
For the home visits conducted 1-month post-renovation, there was no significant difference in average mold levels for green vs. comparison homes at either of the two study sites. However, by the final home visit (12-months post-renovation) in Boston, green homes had lower concentrations than comparison homes. A similar decrease was not found in Cincinnati homes.
The very preliminary mold results (i.e., contrasting results between the Boston and Cincinnati study sites) above justifies the need for why more study sites are required to assess differences between green and comparison homes. In other words, cities (i.e., study sties) can vary in climate and housing stock which could influence indoor environmental exposures. Also, the preliminary airway inflammation results justifies the need for seeking a larger sample size of children, namely so we can conduct subgroup analyses adjusting for differences in medication usage which might affect our results.
During presentations at the conferences, researchers from the US and abroad have exchanged information on some novel findings. For example, some unanticipated household behaviors (e.g. cultural differences in cooking styles) strongly influence indoor chemical exposures above and beyond the potentially beneficial effects of the green housing renovations. Thus, a careful assessment of time/activity patterns in the home is important to consider in future studies of green buildings. We will continue using the same standardized protocol and methodology (without changes) to enable comparisons across study sites.
Study aims and hypotheses
This is the first multi-site study of how green housing factors are associated with health effects such as asthma. The main goals of this study are: 1) to compare levels of certain environmental chemical and biological agents in green vs. comparison multi-family low-income housing; and 2) to ascertain differences in the health of the residents in these homes (Appendix C).
CDC conducted an extensive literature review to identify data gaps to be addressed by this study (Appendix C). In summary, this study aims to better understand whether environmental exposures and health are affected by HUD guidelines for green renovation projects in a number of ways, including:
Indoor air quality (IAQ);
Integrated pest management (IPM) and pesticide exposures;
Exposure to dust mite allergens;
Growth or reduction of the burden of indoor fungi;
Volatile organic compounds (VOCs); and
Sources of outdoor air pollution.
The Green Housing Study addresses several of the research gaps identified above. The study participants are children with asthma (age 7-12 years) living in green-renovated vs. comparison housing (see inclusion criteria in Table 1).
The hypotheses of this study are as follows, and found in Appendix C:
Green housing utilizes different strategies to reduce environmental contaminants. We hypothesize that these strategies will lead to 1) lower levels of environmental contaminants compared with those of comparison housing, and 2) lower levels of related biomarkers in the residents of green vs. comparison housing.
IPM is a method to reduce pests such as cockroaches and mice by eliminating entry points in the home and harborage areas.
We hypothesize IPM will result in lower cockroach and mouse allergen levels while at the same time lowering the concentrations and array of pesticides in the green vs. comparison homes.
We hypothesize concentrations of pesticide metabolites in urine of children living in green housing will be lower than those living in comparison homes.
The use of low VOC paints, carpeting, and other building materials contain lower concentrations of aldehydes, ketones, and alcohols.
We hypothesize the levels of VOCs will be lower at baseline in green-renovated vs. comparison homes.
We hypothesize concentrations of VOCs in urine of children with asthma (ages 7-12 years) living in green housing will be lower than those living in comparison homes.
Insulation can reduce sources of moisture, specifically condensation.
We hypothesize green housing will have more and possibly better insulation (e.g., higher R-value) than comparison housing.
We hypothesize insulation (e.g., dual-paned windows, insulated cold water pipes, and rigid insulation above concrete floors and in exterior walls) will result in lower concentrations of dust mite (and therefore their allergens) and fungi.
Another aspect of green housing is improved ventilation which can reduce moisture and decrease indoor concentration of VOCs. For example, improved exterior wall insulation can reduce condensation and a properly-sized and maintained central heating, ventilating, and air-conditioning unit (HVAC) can help buildings keep dry and at the same time, exhaust environmental contaminants to the outside. We hypothesize green housing will have a higher percentage of units with the recommended air exchange rates than comparison housing.
We hypothesize green housing units will have lower VOCs than comparison homes.
We hypothesize green housing units will have lower levels of fungi and dust mite allergen than comparison homes.
If irritants and allergens are lower in green vs. comparison housing, residents of green housing should experience decreased asthma morbidity.
Specifically, we hypothesize children with asthma (ages 7-12 years) in green housing will have lower asthma morbidity, adjusting for environmental tobacco smoke (ETS) exposure.
A.1.1. Privacy Impact Assessment
Below, we discuss two aspects of the privacy impact assessment: (i) an overview of the data collection system, and (ii) the items of information to be collected.
Overview of the Data Collection System
The selection criteria for the 13 study sites (i.e., city) are detailed in the protocol (Appendix C. Methods, page 12-15) and described in Supporting Statement Part B. From each of these geographically-stratified study sites, 32 green intervention homes and 32 comparison homes (total = 832) will be included. Within each study site, both the green-renovated and comparison homes will be from the same housing development or neighborhoods to ensure homogeneity with regard to housing type and other socioeconomic factors. Changes in environmental measurements [pesticides, VOCs, particulate matter (i.e., PM2.5 and PM1.0), indoor allergens, and fungi] over the 1-year follow-up in both types of housing (green intervention and comparison) will be compared. In addition, each home’s follow-up measurements will be compared with its own baseline exposure level. At this time, all 13 study sites have not been determined. Sites will be added as funding allows. The data collection partners include: 1) CDC, 2) HUD, and 3) research institutions.
In Figure 1, we describe a scenario of how measurements collected in green-renovated homes are compared with: 1) their own baselines, and 2) those of homes without any renovation at all. Residents participate for 1 month prior to rehabilitation, the time required for rehabilitation of their home (usually just a few days), and 12 months after completion of the rehabilitation. The duration of the participation for the residents of comparison homes is the same except no renovation will occur. More details of the study design are provided in the protocol (Appendix C) and referenced in Part B of this ICR. We will continue using the same standardized protocol and methodology (without changes) to enable comparisons across study sites.
Figure 1. Diagram of renovation schedule (green intervention vs. comparison)
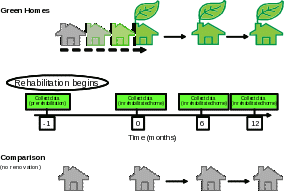
The recruitment process is as follows (Appendix C):
Residents, interested in the study, contact the site project coordinator by telephone or e-mail. Questions that a potential study participant might have about the study can vary, so they are answered on a case-by-case basis. Thus, no formal script is used.
Trained staff schedule a home visit with the residents.
During this home visit, the staff assesses each child’s eligibility based on responses from the mother/primary caregiver (Appendix D1 – Screening Questionnaire).
Eligibility is limited to children with doctor-diagnosed asthma (ages 7-12 years) (Table 1).
When a child is deemed eligible:
The study is explained to the mother/primary caregiver and the child..
If they agree to participate, individual participant consent is obtained from the mother/primary caregiver and child assent is obtained (Appendices F & G).
The children assent to provide blood and urine samples for the study.
Their mothers/primary caregivers consent to respond to survey questions.
Table 1. The Green Housing Study’s inclusion and exclusion criteria
Inclusion Criteria |
Exclusion Criteria |
- Mother/primary caregiver reports that child has ever been diagnosed with asthma by a physician and child has experienced asthma-related symptoms (wheezing, slow play or night awakening) during the past 6 months. 2. Mothers/primary caregivers of the children listed above. - No clinical markers will be collected, but we will ask questions regarding their home environment that might be related to child’s health outcomes of interest. 3. Green homes will be renovated using low VOC materials and integrated pest management (IPM) principles.
|
|
The general data collection procedures are as follows:
After consent and assent is obtained, the technicians/interviewers collect the study baseline information during the initial visit. The methods of data collection include written survey data collected through in-person, telephone, and text messaging interviews of enrolled mothers/primary caregivers.
Trained staff visit each participating child’s home four times (including the initial visit to obtain consent and baseline measurements) during a 1-year period to administer a battery of questionnaires.
Each of the surveys is administered in-person to the participant’s mother/primary caregiver in the study by bilingual (English and Spanish or English and Chinese) interviewers.
In addition, brief text messages to inquire about respiratory infections are sent at the end of months 1, 2, 4, 5, 7, 8, 10, and 11.
The participant’s mother/ primary caregiver is contacted by phone at two time points during the same 1-year period to update contact information and inquire about respiratory morbidity.
Participating children (ages 7-12 years) are interviewed; however, their mothers/primary caregivers provide information about their children’s exposures and health outcomes.
During their time in the study, each participant and mother/primary caregiver pair responds to a total of 27 questionnaires in an average of 2.5 hours (148 minutes). All forms and estimated time burdens are listed in Section A.12. The forms are found in Appendices D2-D12.
Health and Environmental Assessments (Appendix C):
We will continue using the same standardized protocol and methodology (without changes) to enable comparisons across study sites.
For Intervention Homes:
The baseline measurement occurs up to one (1) month prior to commencement of rehabilitation activities.
Baseline (Part 2) is collected in the home one (1) week after completion of rehabilitation activities (Appendix D3).
Total time of study participation is approximately one (1) year, although the exact time can vary depending upon the rehabilitation scenario. Residents participate for 1 month prior to rehabilitation, the time required for rehabilitation of their home, and 12 months after completion of the rehabilitation.
Estimated time for rehabilitation activities (e.g., new paint, carpeting, Energy Star appliances, IPM) should be only a few days.
For Comparison Homes:
The baseline measurement occurs within one (1) week either before or after the baseline measurements are taken from the matched intervention home.
Baseline (Part 2) is collected in the comparison home within one (1) week either before or after the Baseline (Part 2) measurements are taken from the matched intervention home.
Total time of study participation is approximately 1 year, although the exact time can vary depending upon the rehabilitation scenario. Residents participate for the same amount of time as the matched group of intervention homes.
Summaries of the clinical and environmental measurements are shown in Tables 2 and 3.
Table 2. Summary of clinical measurements
-
Factor
Child with asthma
(Age 7-12)
Blood
Baseline
Urine
Baseline
Baseline (Part 2) occurs after renovation is completed)
6-mo. follow-up
12-mo. follow-up
Pulmonary Function Test
Baseline
Baseline (Part 2) occurs after renovation is completed)
6-mo. follow-up
12-mo. follow-up
Exhaled Nitric Oxide
Baseline
Baseline (Part 2) occurs after renovation is completed)
6-mo. follow-up
12-mo. follow-up
Respiratory Symptoms Questionnaire
Baseline
Baseline (Part 2) occurs after renovation is completed)
6-mo. follow-up
12-mo. follow-up
*Blood is used for assessment of allergy status (IgE)
**Urine is used for assessment of cotinine (marker of ETS exposure), pesticides, and VOC metabolites
Table 3. Summary of environmental measurements in homes*
Type of assessment |
Baseline |
Baseline (Part 2) (after renovation is completed) |
6-Month follow-up |
12-Month follow-up |
Allergens |
|
|
|
|
Fungi |
|
|
|
|
Pesticides |
|
|
|
|
VOCs |
|
|
|
|
Particulate Matter (PM2.5) |
|
|
|
|
Temperature |
|
|
|
|
Relative Humidity |
|
|
|
|
Air Exchange Rate |
|
|
|
|
* The mother/primary caregiver’s home is the same as that of the child. Dust sampling occurs in kitchens and on the children’s beds as well as the bed of the mother/primary caregiver. The mother/primary caregiver bed is sampled because it serves as a proxy of exposure to several of the indoor allergens. This proxy can help with characterization of the indoor environment especially in cases where limited dust is available from the child’s bed. Except for the pesticide measurements in the kitchen, all other measurements are limited to the child’s bedroom.
Assessments for children:
The study baseline information includes: a home characteristics questionnaire, a health questionnaire, and an environmental exposure assessment.
We also collect the participant’s urine samples, a blood sample, nasal and throat swabs for assessment of acute respiratory illness (ARI), exhaled nitric oxide (eNO), and conduct pulmonary function testing by spirometry.
These assessments are summarized below, and are detailed in Appendix C (Health and Environmental Assessments).
Questionnaires: Provenance of the questions is described in Part B. The forms are found in Appendices D2-D12.
The home characteristics questionnaires inquires about the type of building, heating and cooling of the home, furnishings, cleaning regimens, the presence of pets and pests, environmental smoke, and reports of dampness.
Health information is collected on the frequency and duration of asthma-related symptoms, healthcare utilization, school and work absences, and medication use (Appendix C).
Environmental Exposure Assessment: The following exposure assessments are conducted for the Green Housing Study, and the methods, and supporting citations, are described in detail in the protocol (Appendix C).
Temperature and relative humidity measurements: Temperature and relative humidity measurements for each home are obtained during each home visit.
Dust sampling: Sampling for allergens and fungi are carried out by trained technicians using a standardized protocol for sample collection and handling.
Indoor allergen analysis: Frozen dust samples are transported to the laboratory at CDC. Samples are analyzed for dust mite, cockroach, cat, dog, rat, and mouse allergens.
Fungi analysis: Dust samples from the beds are analyzed for a total biomass marker of fungi, ergosterol.
Volatile organic chemicals (VOCs): Continuous air monitoring is conducted using passive diffusion dosimeters for VOCs (solvents and aldehydes).
Pesticides: Dust samples are collected by wiping a measured 12-inch square section of the floor along the baseboard in the kitchens. Samples are gathered on gauze squares and are analyzed in the laboratory. A detailed table listing specific pesticides of interest (organochlorines, pyrethroids, and pyrethrins) is provided in Appendix C.
Air Exchange Rates (AER): The method employed in this study uses non-toxic tracer gases. In brief, the method is accomplished by placing a sponge with a nontoxic tracer gas inside the home and allowing the gas to reach steady state, an air sample is collected and then analyzed for the tracer gas.
Particulate monitoring: Monitoring for particulate matter ≤ 2.5 µm (PM2.5) is conducted in the child’s bedroom using integrated sampling for a one week period during each home visit to adjust for seasonal variation. As part of collaboration with the National Institute of Environmental Health Sciences (NIEHS), field validation of real-time exposure assessment sensor for VOCs and PM2.5 iss also conducted. The three devices that are used in the Green Housing Study are described in detail and are pictured in Appendix C.
Outdoor air sampling: To obtain an estimate of outdoor PM and VOC exposure for each of the housing developments, we will conduct 1-week air sampling on rooftops under protected cover during winter, spring, summer and fall. These measurements are repeated throughout the entire study period for a given city.
Biomarker Assessment and Respiratory Health Measurements: The following biomarker and respiratory health measurements are conducted for the Green Housing Study; the methods and supporting citations are described in detail in the protocol (Appendix C).
Urine collection: Urine is collected for two main purposes: 1) to assess recent ETS exposure via cotinine measurement); and 2) to assess biomarkers of pesticides and VOCs listed in Appendix C, page 18. The lists of urinary VOC metabolites and urinary pesticide metabolites analyzed by the CDC labs are found in Appendix C.
Blood collection: Venous blood is collected to assess allergic sensitization (described below). After the tubes are centrifuged, serum is aliquoted, and then frozen until they are assayed for total and allergen-specific IgE titer.
Allergy testing: Allergen testing is performed once at baseline following enrollment. We use the immunoCAP method to assess total and allergen-specific (dust mite, cockroach, cat, mouse, tree mix, grass mix, and weed mix) IgE antibodies in serum.
Nasal and throat swabs: Mothers/primary caregivers of the participating children with asthma will collect one nasal swab and one throat swab after onset of symptoms of respiratory virus infections. An illness checklist (Appendix D12) for the child is also completed by the mothers/primary caregivers each occasion of a suspected acute respiratory illness (ARI). Trained staff collect another throat and nasal swab from the child to validate the sample collected by the parent. Nasal swab collection procedures are detailed in Appendix C. Specimens are tested by reverse transcription polymerase chain reaction (RT-PCR) for respiratory viruses.
Exhaled Nitric Oxide (eNO): eNO is a known marker of pulmonary inflammation and can provide a non-invasive means of assessing pulmonary inflammation. Measurement of exhaled nitric oxide is obtained prior to lung function, according to the American Thoracic Society Guidelines. Nitric oxide concentrations are measured using a chemiluminescent analyzer.
Pulmonary function testing: Spirometry (pulmonary function testing or PFTs) is performed for all child participants. All PFT studies are performed at each home visit to assess possible seasonal variation. The procedures for pulmonary function testing are detailed in Appendix C.
Assessment for mothers/primary caregivers of children: The only measurement obtained is questionnaire information regarding the impact of demographic characteristics and behaviors on the respiratory health of the participating child. Such behaviors include but are not limited to smoking, cooking, and working in environments that could conceivably result in passive transport of chemicals and allergens.
Items of Information to be Collected
Data collected about the study participants include: contact information (name, date of birth, phone numbers, medical information and notes, biological specimens, e-mail address, employment status, home address), demographics, housing characteristics, environmental exposures, health outcomes, and healthcare utilization as listed in questionnaires (Appendices D1-D12). We further describe the information in identifiable form (IIF) in Table 4.
Identification of Website(s) and Website Content Directed at Children Under 13 Years of Age
There is no website associated with this study. Therefore, there is no website content directed at children less than 13 years of age.
A.2. Purpose and Use of Information Collection
The specific aims of this study are to: 1) conduct an exposure assessment of environmental contaminants (i.e., pesticides, volatile organic compounds (VOCs), fungi, and indoor allergens) in green vs. comparison housing; and 2) examine the relationship between living in green vs. comparison housing and asthma morbidity. Publications of the study results have the potential to be cited frequently by other researchers, and both CDC and HUD can use data from the Green Housing study to guide their Healthy Homes awardee’s activities via annual conferences and funding opportunities. In Table 4, we have justified the data collection in terms of positive needs and the negative consequences of not having the information, and we have emphasized the practical utility of the expected results to federal, state and local governments
Table 4. Justification and practical utility of the data collection.
Type of data collected |
Positive needs for having the information |
Negative consequence of not having the information |
Practical utility to the government of the expected results |
Environmental exposures |
This data will provide a direct measurement of environmental exposures in the homes of this sample of residents. |
Merely having health data will not allow us to know if any meaningful differences in health status were truly associated with differences in chemical/biological exposures that were related to green housing factors. One could assume that because health symptoms are improved, that the exposures would have been lower, but this would only be an assumption. |
This study will help CDC and HUD programs to advise their healthy homes, asthma, and child health grantees on which green criteria (if any) are positively associated with lower exposures. Subsequently, this will help awardees inform residents about which green housing practices and materials (if any) to implement in their homes not only for energy efficiency, but for lower exposures in their home, a place where people spend a significant proportion of their time. |
Health status |
This data will provide a direct measurement of health effects in this sample of residents. |
Merely having exposure data will not allow us to know if any meaningful improvements in health status will occur with green housing factors. One could assume that because exposures are lower, that the health would be better, but this would only be an assumption. |
This study will help CDC and HUD programs to advise their healthy homes and asthma, awardees on which green criteria (if any) are positively associated with health outcomes (e.g., asthma outcomes). Subsequently, this will help awardees inform residents in their communities on which green housing practices and materials (if any) to implement in their low-income urban multi-family homes not only for energy efficiency, but for improved health e.g., asthma outcomes). |
Healthcare utilization |
This data will provide a direct measurement of healthcare utilization by this sample of residents which enables us to more fully capture the burden of adverse health asthma outcomes.
|
If we did not collect data on healthcare utilization, then we would not be able to fully capture the burden of adverse health outcomes. |
This will help CDC identify possible alternatives to pharmaceuticals to decrease healthcare costs among low-income urban populations. It will inform Center for Medicare and Medicaid Services policies related to re-imbursement for preventative measures. |
Home address |
We need to geocode the address so that we can use it to adjust for influence of outdoor air pollution. EPA currently has outdoor air pollution monitors in cities across the US. By knowing the exact location of our study participants’ homes, we can use EPA’s regional measurements in our statistical models of exposure and health outcomes. |
There is the possibility that even the greenest of homes could be located in a highly-polluted area which could overwhelm any potential health benefits of green housing factors.
If we do not adjust for outdoor air pollution, then we will not be able to tease out any effects of indoor green housing factors on respiratory symptoms of the study participants. |
Adjusting for outdoor air pollution will allow CDC and HUD to attribute improved respiratory health effects to green housing factors if they indeed exist. Subsequently, CDC and HUD can make informed recommendations about green building materials and practices that are connected to improved health outcomes. These recommendations could vary by city depending upon levels of outdoor air pollution. |
Date of birth |
We need to know the age of participants because age can influence health outcomes such as pulmonary function. |
If we were to ask contracted entities to strip the date of birth and give CDC only age, we believe that some data might come to us in a truncated/rounded form and this would make our statistical models inaccurate. To preclude differences by reporting site, CDC would have better control of modeling this very important variable. |
Accurate modeling of data is paramount to federal agencies defending and promoting their policies and recommendations. |
HUD has committed funds for the Green Housing Study to CDC via interagency agreement (IAA) # I-PHI-01062. This IAA commitment for the next several years also leverages personnel and laboratory resources from CDC.
The proposed study is being conducted in low-income housing primarily in urban environments which is likely to have implications for the generalizability of our findings to suburban and rural residences. Also, it may not be appropriate to generalize our findings to children in families with higher socioeconomic status. However, this study will have the potential to improve the health outcomes of one of the most sensitive populations (low-income children with asthma).
A.2.1. Privacy Impact Assessment
Below, we discuss two aspects of the privacy impact assessment: (i) a description of how the information will be shared and for what purpose, and (ii) a statement detailing the impact the proposed collection will have on the respondent’s privacy. The purpose for collecting IIF during is listed below in Table 5.
Table 5. Information in Identifiable Form (IIF) and intended uses
IIF category |
Collected by awardees but not sent to CDC |
Collected by awardees and sent to Green Housing Study staff at CDC |
Purpose |
Name |
X |
|
Names are required for written informed consent. In addition, names aid both the study participant and the data collector during in-person and telephone questioning. |
Date of birth |
|
X |
To determine eligibility and to also adjust for age in statistical analysis. |
Phone numbers |
X |
|
To administer phone questionnaires. |
Medical information and notes |
|
X |
To assess health outcomes for statistical analysis |
Biological specimens |
|
X |
To assess health-related biomarkers for statistical analysis |
E-mail address |
X |
|
To serve as a secondary means of contacting study participants to administer questionnaires and schedule home visits for sampling |
Employment status |
|
X |
To adjust for possible chemical exposures that could occur in the occupational environment. |
Home address |
|
X |
To enable data collectors to visit homes for sampling and also enable CDC to use geographic information systems (GIS) which can be used for adjusting for factors external to the home which could influence both exposures and health outcomes (e.g., outdoor air pollution). |
All paper copies of consent forms and questionnaires are scanned into electronic files. The paper copies of the data are maintained at each study site’s research institution for a period of 5 years beyond the last peer-reviewed publication of the results. At that time, paper copies will be shredded and then recycled. The electronic files are shared with CDC, and CDC will keep the electronic files in accordance with approved record control schedules. The electronic files contain date of birth, medical information, biological specimens, employment status, and home address, identified by study ID number. While we acknowledge that home address is a unique identifier and the data collectors have the link to names and address, CDC Green Housing Study investigators have taken steps to reduce the amount of individually-identifiable data maintained at CDC.
If there is a breach of confidentiality for any of the above IIF, some effect on the respondent’s privacy could occur. However, the screening form is the only form that contains name, home address, phone number, e-mail address, and study ID together and only the data collectors have this form. The data collectors only use name, phone number, e-mail address, and home address for locating the study participant and ensuring that follow-up questionnaires and clinical and environmental measurements are repeated accordingly.
A.3. Use of Improved Information Technology and Burden Reduction
Approximately 93% of the data collection is via paper forms; however, we are implementing text messaging to aid in monthly assessment of respiratory infections, which is about 7% of data collection efforts.
For the paper forms, the respondents have minimal burden in providing their responses because they do not need to read questions nor write answers; the data collectors record all of their verbal responses. The data collectors then enter the survey data into an electronic database which enables electronic transmission of data to CDC’s Green Housing Study researchers. We chose paper forms for most of the data collection because, at this time, it is the least expensive method (as opposed to transcribing answers from voice recorders or paying for laptop/ notepad computers). The text messages given at months 1, 2, 4, 5, 7, 8, 10, and 11 only take approximately 1 minute to respond to a few brief questions of respiratory infections. The texts can be answered at the respondents’ convenience rather than relying upon direct interaction with the study team. We believe this is an improvement over previous asthma studies that have relied upon a greater time period of recall between assessments.
A.4. Efforts to Identify Duplication and Use of Similar Information
CDC approached this in two ways: 1) we conducted a thorough literature search on green housing and health effects, and 2) we contacted subject matter experts from many different federal government agencies and private research organizations.
In our literature search, we found that many studies had focused on relationships between housing characteristics and asthma, but none had specifically focused on how green housing factors were associated with these outcomes. The results of the extensive literature search, and the citations, are found in Appendix C.
The subject matter experts confirmed that a comprehensive evaluation of green housing factors and these health outcomes would be a novel and innovative approach to filling research gaps. The list of subject matter experts is listed in Table 6 in Section A.8.
A.5. Impact on Small Businesses or Other Small Entities
The collection of this information does not directly impact small businesses or small entities.
A.6. Consequences of Collecting the Information Less Frequently
Some of the environmental and health outcome data are collected repeatedly (e.g., monthly, every 3 months or every 6 months) for several reasons: 1) to address seasonal variation in measurements; 2) to obtain better estimates of average exposure and/ or symptoms; and 3) to minimize recall bias. The technical obstacle to reducing the burden is as follows:
If we do not obtain valid estimates of exposure and health effects, then it will be difficult to accurately attribute any reduction in exposure and improvement in health to specific green practices and/or materials.
There are no legal obstacles to reducing the burden.
A.7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
This request fully complies with the regulation 5 CFR 1320.5.
A.8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A. The text of the Federal Register notice for this information collection, published in Federal Register Volume 79, Number 55, Pages 15748-9 on March 21, 2014, is provided in Appendix B. No public comment was received.
B. During the design phase of the this study, CDC’s NCEH Healthy Homes and Lead Poisoning Prevention Branch reviewed published literature on green housing, and asthma and included consultation with researchers from HUD, EPA, and academic institutions. We have discussed availability of data and frequency of collection issues with subject matter experts (Table 6).
Table 6. List of experts consulted regarding study design and frequency of data collection
Name |
Title |
Affiliation |
Contact information |
Year of Consultation |
Peter Ashley, DrPH |
Director, Policy and Standards Division |
U.S. Dept. of Housing and Urban Development |
Phone: 202-402-7595 |
2011 |
Karen Bradham, PhD |
Physical Scientist |
U.S. Environmental Protection Agency |
Phone: 919-541-9414 |
2009 |
Daniel Stout, PhD |
Biological Scientist |
U.S. Environmental Protection Agency |
Phone:919-541-5767 |
2009 |
Warren Friedman, PhD |
Senior Advisor to the Director |
U.S. Dept. of Housing and Urban Development |
Phone: 202-549-7868 |
2009 |
David Balshaw, PhD |
Project Scientist |
NIH, NIEHS |
Phone: 919-541-2448 |
2010 |
Sung-Roul Kim |
Research Associate |
Johns Hopkins University |
Phone: 011-82-2-380-7685 |
2009 |
Mark Mendell, PhD |
Staff Scientist |
Lawrence Berkeley National Laboratory |
Phone: 510-486-5762 |
2009 |
Brett Singer, PhD |
Staff Scientist |
Lawrence Berkeley National Laboratory |
Phone: 510-486-4779 |
2009 |
Kim Dietrich, PhD |
Professor |
Univ. of Cincinnati |
Phone: 513-558-0531 |
2009 |
Gary Adamkiewicz, PhD |
Research Scientist |
Harvard School of Public Health |
Phone: 617-384-8852 |
2008 |
Wanda Phipatanakul |
Assistant Professor |
Harvard Medical School |
Wanda.Phipatanakul@childrens.harvard.edu Phone: 617-355-6117 |
2008 |
Robin Whyatt, DrPH |
Professor |
Columbia University |
Phone: 646-459-9609 |
2008 |
Andrew Gelman, PhD |
Professor of Statistics |
Columbia University |
Phone: 212-851-2142 |
2008 |
Elizabeth Matsui, MD |
Associate Professor |
Johns Hopkins University |
Phone: 410-955-5883 |
2010 |
Patrick Breysse, PhD |
Professor |
Johns Hopkins School of Public Health |
Phone: 410-955-3608 |
2010 |
Herman Mitchell, PhD |
Vice President & Senior Research Scientist |
Rho Federal Systems Division |
Phone: 919-408-8000 x 6223 |
2011 |
Tiina Reponen, PhD |
Professor |
University of Cincinnati |
Phone: 513-558-0571 |
2011 |
Doug Brugge, PhD |
Professor |
Tufts University |
Phone: 617.636.0326 |
2011 |
Pat Ryan, PhD |
Assistant Professor |
University of Cincinnati |
Phone: 513-803-4704 |
2011 |
Dave Turcotte, ScD |
Research Professor |
University of Massachusetts Lowell |
Phone: 978-934-4682 |
2011 |
Steve Chillrud |
Research Professor |
Columbia University |
Phone: 845 365 8893 |
2011 |
A.9. Explanation of Any Payment or Gift to Respondents
As previously approved by OMB, study participants (mothers/primary caregivers of children enrolled in study) receive monetary tokens of appreciation (Table 7) for their participation in the study and to increase response rates. Many of the low-income families in the proposed cohort use “pay-as-you-go” cell phones. The Green Housing Study team researched several calling card providers and found that they range in costs. For example, one company offers pre-paid plans at 25 cents a minute and another for 60 minutes at $19.99. For this reason, compensation for the text messaging and phone calls are provided to help defray the costs to the participants.
Table 7. Monetary tokens of appreciation for study participants
Type of activity |
Time point |
Description of activities/ information/samples collected |
Time |
Amount |
Home visit
|
- Baseline
|
Explanation of the study (includes informed consent process), blood sample, urine sample, lung function test, lung inflammation test, questionnaire, and environmental sampling in home* |
60 minutes |
$50
|
- Baseline (Part 2) |
urine sample, lung function test, lung inflammation test, questionnaire, and environmental sampling in home* |
55 minutes |
$50
|
|
- 6 month follow-up |
urine sample, lung function test, lung inflammation test, questionnaire, and environmental sampling in home* |
55 minutes |
$50 |
|
-12 month follow-up |
urine sample, lung function test, lung inflammation test, questionnaire, and environmental sampling in home* |
55 minutes |
$50 |
|
Phone calls |
- 3 months - 9 months |
Questionnaire |
5 minutes 5 minutes |
$2 $2 |
Text messages |
- 1, 2, 4, 5, 7, 8, 10, and 11 months |
Questionnaire. Each month, a series of 3 1-sentence texts are sent to obtain this information, and the respondents reply with 3 separate texts. |
1 minute for each month |
$2 each time (maximum = $16) |
* This time indicates the amount of time required for setting up the environmental sampling equipment. Some environmental sampling equipment is left in home for 5 days, but it does not require any supervision.
Each study site will likely have certain rules about how money can be disbursed to the participants. We use pre-paid credit cards (e.g., VISA, MasterCard), which enable the following:
One card can be given to each enrollee’s mother/primary caregiver at the beginning of the study.
The mother/primary caregiver will sign one receipt (at the beginning of the study) which acknowledges that the card will be uploaded with funds automatically (via a study site project coordinator) upon completion of each activity.
If the card is lost or stolen, the mothers/ primary caregivers can call the project coordinator who can cancel the card online. However, any funds missing from the lost or stolen card (prior to cancellation) will not be replaced. Only new funds are added upon completion of each of the remaining study activities listed in the incentive table. The mother/primary caregiver will receive the replacement card at the next home visit.
Rather than using checks or cash, this option provides immediate funds after phone call questionnaires, reduces number of receipts, minimizes the danger of study staff carrying large sums of money to home visits, improves accounting, eliminates the need for low-income participants to pay check cashing fees, and ensures that the study participant retains our study phone number (which will be written on back of card).
A.10. Assurance of Confidentiality Provided to Respondents
A.10.1. Privacy Impact Assessment Information
This submission has been reviewed by the CDC Information Collection Request Office (ICRO) which determined that the Privacy Act does apply. The applicable System of Records Notice is 09-20-0136, Epidemiologic Studies and Surveillance of Disease Problems. While full names are not sent to CDC, the data collectors have the capability of maintaining the link between name and study ID number; therefore, the Privacy Act does apply.
The Green Housing study staff (CDC and awardees) make every effort to keep the data secure by a variety of methods. Data from paper questionnaires are entered into a password-protected database (e.g. Microsoft Access). Dates of birth and home addresses are the primary direct identifiers and the contractor’s removal of other direct identifiers (such as name, phone numbers, e-mail addresses) minimizes the potential for disclosure, but does not completely eliminate it. A unique Study ID is assigned as a key identifier for all study forms. The environmental and biological samples and measurements are only identified by study ID. Data collectors maintain their paper files in locked cabinets and their electronic files are stored on secured servers with password protection. Encrypted data files are sent electronically to Green Housing Study investigators at CDC. Data are stored on highly-secured CDC servers in Atlanta, GA. The servers are housed in a secure computer room complete with climate control, emergency power, and an uninterruptible power supply (UPS). Daily back-ups and integrated security are implemented through the CDC computer services infrastructure. All data access is password-protected, and all network communications use encryption. All servers and PCs that are part of the CDC infrastructure are protected by both host-based firewalls and software in order to prevent the undetected installation of "spyware." At CDC, only Green Housing Study investigators are given access to read the encrypted data files.
Based on recommendations from some housing tenant’s organization members and property managers, flyers (Appendix E – Flyer Prototype) are used to describe the study. Residents who are interested in the study can contact the site projector coordinator by telephone or e-mail. The opportunity to consent to participate in the Green Housing Study is discussed in the protocol (Appendix C. Recruitment and Eligibility Criteria). Copies of the consent and/or assent forms are provided to the study participants (Appendices F & G). Data collectors are required to have human subjects training in accordance with their institution’s Institutional Review Board (IRB) and/or the CDC’s IRB. A component of human subjects training addresses data security measures.
During the consent process, CDC-trained interviewers explain to the residents that participation in the study is voluntary and they may withdraw from the study at any time without negative consequences. The interviewers also explain the intended uses of the data (i.e., to study how green housing affects respiratory outcomes), with whom information will be shared (i.e., Green Housing Study researchers), and the legal authority for the data collection (i.e., through the Public Health Service Act).
This study was originally approved by the CDC’s IRB (Protocol #5587) on March 30, 2009 and has received annual continuations (Appendix H).
Data are treated in a secure manner and are not disclosed, unless otherwise compelled by law.
A.11. Justification for Sensitive Questions
Several questions in the questionnaires ask for information that could be considered sensitive by at least a segment of the general population (Table 8), but variables such as smoking and presence of cockroaches, mice, and rats are specifically geared toward factors that could be related to respiratory health. These items are necessary to assess the relationship between the presence of environmental exposures and the residents’ health (Chew et al., 1998 ). A copy of the questionnaires can be found in Appendices D1-D12. The interviewers are given detailed instructions within each of the questionnaires on how to collect the information, including skip patterns and when to probe for certain questions (e.g., types of inhaled corticosteroid medications typically used by the child with asthma). Interviewers are trained to be sensitive to any questions likely to cause discomfort, and the respondent is informed of her right to refuse to answer any interview question.
Table 8. Questions of a possibly sensitive nature
Questions (possibly sensitive) |
Specific uses of information |
|
|
Which one or more of the following would you say is your race? |
To adjust for race in statistical models. |
What is the highest level of school that you have completed or the highest degree that you have received? |
To adjust for socioeconomic status in statistical models. |
Which category represents the total combined income of all members of this family during the past 12 months? |
To adjust for socioeconomic status in statistical models. |
Do you smoke cigarettes? |
To adjust for smoking exposure in statistical models. Smoking could affect our environmental and clinical measurements. |
During the past 6 months, how often have you seen cockroaches in your household? |
To assess cockroach exposures pre- and post- interventions. |
During the past 6 months, how often have you seen mice in your household? |
To assess mouse exposures pre- and post- interventions. |
During the past 6 months, how often have you seen rats in your household? |
To assess rat exposures pre- and post- interventions. |
This explanation is given to respondents: “These questions are needed for this study and some of them have been shown to be associated with environmental exposures and health outcomes, so we need to take them into account.”
A.12. Estimates of Annualized Burden Hours and Costs
Approximately 1000 adults will complete the screening forms. Housing researchers obtained a screening percentage of 73% in their New York City Housing Authority intervention study (Kass et al., 2009). We estimate that after screening, 20% of households will not be eligible.
Two large-scale housing intervention studies in low-income neighborhoods that had a 1-year follow-up have reported response rates of 92-93% (Morgan et al., 2004; Persky et al., 2009). With an anticipated loss to follow-up in our study of 20%, we will recruit 832 households with asthmatic children to end up with 650 enrolled children with asthma (ages 7-12 years). All health and environmental exposure information about children will be provided by their mothers/ primary caregivers (i.e., no children will fill out questionnaires). For the purposes of assessing potential burden, we are using the maximum of 832 mothers/ primary caregivers who could conceivably fill out the forms. The burden hours for each type of respondent are listed below in Table A
Data collected from the first two study sites indicated that the burden hours for each of the questionnaires was similar to original estimates from the pilot study. Originally, each of the questionnaires was pilot-tested at CDC among nine predominantly college-educated CDC employee-volunteers. The pilot tests were administered by two Green Housing Study researchers. Based upon pilot testing, the questionnaires were revised to increase ease of understanding and speed of response. We conservatively estimated of the response times for our study participants (low-income mothers/ primary caregivers living in multifamily, urban housing) based on the average response times recorded during our pilot tests.
Table A. Estimated Annualized Burden Hours
Type of Respondents |
Form Name |
No. of Respondents |
No. of Responses per Respondent |
Average
Burden per Response |
Total
|
Mothers/Primary
caregivers |
Screening Questionnaire |
1000 |
1 |
10/60 |
167 |
Mothers/Primary
caregivers |
Baseline Questionnaire (Home Characteristics) |
832 |
1 |
15/60 |
208 |
Mothers/Primary
caregivers |
Baseline (Part 2) Questionnaire (Home Characteristics) |
832 |
1 |
5/60 |
69 |
Mothers/Primary
caregivers |
Baseline Questionnaire (Demographics) |
832 |
1 |
5/60 |
69 |
Mothers/Primary
caregivers |
Baseline Questionnaire (Children 7-12 with Asthma) |
832 |
1 |
15/60 |
208 |
Mothers/Primary
caregivers |
Text Messages (Children 7-12 with Asthma) |
832 |
8 |
1/60 |
111 |
Mothers/Primary
caregivers |
3 and 9-month Follow-up Questionnaire (Children 7-12 with Asthma) |
832 |
2 |
5/60 |
139 |
Mothers/Primary
caregivers |
6 and 12-month Follow-up Questionnaire (Environment) |
832 |
2 |
10/60 |
277 |
Mothers/Primary
caregivers |
6 and 12-month Follow-up Questionnaire (Children 7-12 with Asthma) |
832 |
2 |
10/60 |
277 |
Mothers/Primary
caregivers |
Time/Activity Questionnaire (Children with Asthma 7-12 years) |
832 |
4 |
5/60 |
277 |
Mothers/Primary
caregivers |
Time/Activity Questionnaire (Mother/Primary Caregiver) |
832 |
4 |
5/60 |
277 |
Mothers/Primary
caregivers |
Illness Checklist |
832 |
4 |
5/60 |
277 |
Total |
2,356 |
||||
We assume earning potential for participants in our study (low-income mothers/primary caregivers living in multifamily, urban housing) is minimum wage based on HUD data regarding income of public housing residents (https://hudapps.hud.gov/public/picj2ee/Mtcsrcr?category=rcr_income&download=false&count=0&sorttable=table1). From March 1, 2013 through June 30, 2014, the average income of residents living in public housing was $13,967 and 69% of the residents reported an income of $15,000 or less. For our study, we selected a conservative estimate of annualized burden cost (i.e., $7.25 per hour for one year of employment = $15,080). As of July 2014, the Federal minimum wage remains $7.25 per hour (http://www.dol.gov/whd/minimumwage.htm). Therefore, the true annualized burden could be lower than the estimates in Table B.
Table B. Estimated Annualized Burden Costs
Type of Respondents |
Form Name |
No. of Respondents |
No. of Responses per Respondent |
Average
Burden per Response |
Total
|
Hourly Wage Rate |
Total Respondent Costs |
Mothers/
Primary
caregivers |
Screening Questionnaire |
1000 |
1 |
10/60 |
167 |
$7.25 |
$1208.33 |
Mothers/
Primary
caregivers |
Baseline Questionnaire (Home Characteristics) |
832 |
1 |
15/60 |
208 |
$7.25 |
$1508.00 |
Mothers/
Primary caregivers |
Baseline (Part 2) Questionnaire (Home Characteristics) |
832 |
1 |
5/60 |
69 |
$7.25 |
$502.67 |
Mothers/
Primary caregivers |
Baseline Questionnaire (Demographics) |
832 |
1 |
5/60 |
69 |
$7.25 |
$502.67 |
Mothers/
Primary caregivers |
Baseline Questionnaire (Children 7-12 with Asthma) |
832 |
1 |
15/60 |
208 |
$7.25 |
$1508.00 |
Mothers/
Primary caregivers |
Text Messages (Children 7-12 with Asthma) |
832 |
8 |
1/60 |
111 |
$7.25 |
$804.27 |
Mothers/
Primary caregivers |
3 and 9-month Follow-up Questionnaire (Children 7-12 with Asthma) |
832 |
2 |
5/60 |
139 |
$7.25 |
$1005.33 |
Mothers/
Primary caregivers |
6 and 12-month Follow-up Questionnaire (Environment) |
832 |
2 |
10/60 |
277 |
$7.25 |
$2010.67 |
Mothers/
Primary caregivers |
6 and 12-month Follow-up Questionnaire (Children 7-12 with Asthma) |
832 |
2 |
10/60 |
277 |
$7.25 |
$2010.67 |
Mothers/
primary caregivers |
Time/Activity Questionnaire (Children with Asthma 7-12 years) |
832 |
4 |
5/60 |
277 |
$7.25 |
$2010.67 |
Mothers/
Primary caregivers |
Time/Activity Questionnaire (Mother/ Primary Caregiver) |
832 |
4 |
5/60 |
277 |
$7.25 |
$2010.67 |
Mothers/
Primary caregivers |
Illness Checklist |
832 |
4 |
5/60 |
277 |
$7.25 |
$2010.67 |
Total |
$17,092.67 |
||||||
A.13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
There is no anticipated cost burden to respondents resulting from the collection of information, except the costs associated with the respondents’ time. Respondents are not be required to incur (a) capital or start-up costs; or (b) operation and maintenance and purchase of services costs. Respondents are not asked or required to keep any records.
A.14. Annualized Cost to the Government
The Green Housing Study is conducted by CDC and its awardees. The estimated cost for CDC personnel, study coordination, laboratory analysis, data analysis and oversight of the awardees’ work is $1,429,000 over a 3-year period. Table 9 shows the annualized costs.
Table 9. Annualized Cost Estimate of Proposed Study
Category |
Annual Costs (dollars) |
CDC, including -three staff (GS-13) at 75% effort - travel for site visits |
Total = $231,000 $225,000 $6,000 |
Awardees, including all staff, travel, interviewing, supplies, sample collection, laboratory analyses, data analysis, and reporting. |
$200000 |
Laboratory analysis |
$45,333 |
Total costs |
$476,333 |
A.15. Explanation for Program Changes or Adjustments
The burden has not changed from the burden shown in the current inventory.
A.16. Plans for Tabulation and Publication and Project Time Schedule
Appendix I lists CDC staff who have provided subject matter expertise on the Green Housing Study. Reports associated with the study will include reports for respiratory outcomes. In addition to those reports, CDC will prepare at least three peer-reviewed journal articles of respiratory outcomes. CDC will also provide technical information and recommendations to various housing programs based on the findings of this study.
The research program will be conducted over a period of 3 more years. Table 10 shows the projected schedule of accomplishments and milestones for the study
Table 10. Project Time Schedule
Activity |
Months after OMB approval |
Select one new study site |
*4 months prior |
Train study staff from each site to collect environmental, survey, and clinical data |
*2 months prior |
Data collection |
1 |
Subcontract with laboratories to assay environmental samples and biomarkers collected during the study.
|
2 |
Summary of laboratory results from subcontracted institutions |
6, 12, 24, 36 |
Summary of survey results from study sites |
6, 12, 24, 36 |
Conduct statistical analysis |
6, 12, 18, 24, 30, 36 |
Forms used for reporting study results back to participants and community |
6, 12 |
Submit articles for peer review in journals |
12, 24, 36 |
* Asterisked items are included here for completeness since much of the data analysis and dissemination of study findings will occur before the 3-year OMB review and approval timeframe.
The analysis plan includes the following: 1) descriptive statistics to show prevalence of environmental exposures and health outcomes (i.e., asthma morbidity) and 2) logistic and linear regressions to examine associations between environmental exposures such as indoor allergens, mold, pesticides, and VOCs and health outcomes. Detailed statistical analyses are described in Part B.
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate
The selection of study sites across the country will occur on a rolling basis over the course of the study. At each study site, data will be collected using CDC’s OMB-approved questionnaires. It is conceivable that data collection at one or more study sites will start or be continued from one OMB approval period to the next. As an extension ICR, the data collection forms will remain unchanged. To make the most efficient use of stockpiled forms, CDC previously requested, and OMB approved, that the expiration date not be printed on the form. The CDC again is seeking OMB approval for this request.
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification.
REFERENCES
Chew, G. L., H. B. Burge, et al. (1998). "Limitations of a home characteristics questionnaire as a predictor of indoor allergen levels." Am. J. Respir. Crit. Care Med. 157: 1536-1541.
Kass, D., W. McKelvey, et al. (2009). "Effectiveness of an integrated pest management intervention in controlling cockroaches, mice, and allergens in New York City public housing." Environ Health Perspect 117(8): 1219-25.
Morgan, W. J., E. F. Crain, et al. (2004). "Results of a home-based environmental intervention among urban children with asthma." N. Engl.J. Med. 351(11): 1068-1080.
Persky, V., J. Piorkowski, et al. (2009). "The effect of low-cost modification of the home environment on the development of respiratory symptoms in the first year of life." Ann Allergy Asthma Immunol 103(6): 480-7.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | INFORMATION COLLECTION REQUEST FOR OMB REVIEW |
| Subject | AHHS draft ICR |
| Author | Chew, Ginger L. (CDC/ONDIEH/NCEH) |
| File Modified | 0000-00-00 |
| File Created | 2021-01-27 |
© 2026 OMB.report | Privacy Policy