Tobacco Product Establishment Registration
Tobacco Product Establishment Registration and Submission of Certain Health Information
Technical Working Instructions_clean_11_03_09
Tobacco Product Establishment Registration
OMB: 0910-0650

How to Create Your eSubmitter Submissions for the Center for Tobacco Products
Last Updated November 3, 2009
Prepared by
Office of Information Management (OIM)
Table of Contents
3. How to Obtain a D-U-N-S® Number
4. How to Create a Digital Signature
5. How to Import Tobacco Product Information using Excel
Center for Tobacco Products eSubmitter Overview
The Center for Tobacco Products eSubmitter application was designed to assist industry with the electronic submission of registration and product listing, ingredient listing and health data information. These instructions are intended to address technical aspects of completing your electronic submission.
Users may electronically submit the following information through the eSubmitter application:
Tobacco Establishment Registration, per section 905(b) of the Federal Food, Drug and Cosmetic Act (the act).
Tobacco Product Listing, per sections 905(i)(1) and 905(i)(3) of the act.
Tobacco Product Ingredient Submission, per sections 904(a)(1) and 904(c) of the act.
Tobacco Product Data Collection, per section 904(a)(4) of the act.
These instructions are meant to accompany but not to substitute for any published guidance documents on these topics. Published guidance documents include:
Registration and Product Listing for Owners and Operators of Domestic Tobacco Product Establishments.
Submission Checklist
To electronically submit information using eSubmitter, users should use the checklist below to facilitate timely and complete submissions:
⃞ 1. Did you compile your submission using the eSubmitter software?
Download the eSubmitter tool at: http://www.fda.gov/ForIndustry/FDAeSubmitter/ucm108165.htm
For help with the compilation of your submission, refer to the eSubmitter Quick Guide and the full-length eSubmitter User Manual.
For technical support using eSubmitter, contact eSubmitter@fda.hhs.gov.
For questions regarding the Draft Guidance or the Tobacco Act, contact http://www.regulations.gov.
⃞ 2. Did you obtain and setup your Electronic Submission Gateway Account?
The Food and Drug Administration (FDA) Electronic Submissions Gateway (ESG) enables secure submission of information to the Center for Tobacco Products. The ESG is used across FDA as a tool for accepting electronic regulatory submissions.
If you are submitting your Registration and Product Listing, Ingredients Listing and Health Data submission electronically, you are encouraged to submit through the ESG. To obtain an ESG account, see the following checklist:
http://www.fda.gov/ForIndustry/ElectronicSubmissionsGateway/ucm114831.htm
For policy questions and to request a WebTrader account, contact esgprep@fda.hhs.gov.
For assistance with the registration or testing process, contact esgreg@gnsi.com.
⃞ 3. Did you obtain a Dun & Bradstreet Data Universal Numbering System (D-U-N-S®) Number for each establishment being registered?
The D-U-N-S® Number is recommended, but not required for electronic submission of registration information. For more information, see below.
⃞ 4. Did you create a self-signed digital signature for use in eSubmitter?
To package your eSubmitter submissions, you must create a personal self-signed digital signature. For help with digital signatures in Adobe Acrobat, refer to the “Creating Digital Signatures Quick Guide” and “eSubmitter Quick Guide: Packaging with Digital Signature” documents located within the “Manual” folder in the eSubmitter directory.
⃞ 5. Did you package the submission using the eSubmitter software, generating a submission .ZIP file?
For help with packaging your submission, refer to the eSubmitter Quick Guide.
How to Obtain a D-U-N-S® Number
A Data Universal Numbering System (D-U-N-S®) Number is a unique nine-digit sequence provided by Dun & Bradstreet. The D-U-N-S® Number is site specific. Therefore, each distinct physical location of an entity (such as branches, divisions, and headquarters) may be assigned a D-U-N-S® Number. The site-specific D-U-N-S® Number is a widely recognized business identification tool, and serves as a useful resource for FDA in identifying and verifying certain business information submitted by a user. It is recommended, but not required, for submission of information to FDA.
If the D-U-N-S® Number for a location has not been assigned, a business may obtain one for no cost directly from Dun & Bradstreet. You may obtain a new number, or verify an existing number, by phone or online. A new D-U-N-S® Number requires approximately 30 business days to be processed, and is assigned by email. A business may receive a D-U-N-S® Number in approximately 10 business days for an expedited service fee of $14.95 per D-U-N-S® Number. Please note that a business entity may not request or apply for a new D-U-N-S® Number on behalf of another business entity due to the verification procedures used by Dun & Bradstreet.
More information is available at www.dnb.com.
Step-by-step instructions to obtain a D-U-N-S® Number for businesses based either in the United States or abroad are available at: http://www.dnbgov.com/federal_compliance/fda/DUNSrequest/requestGuide.html.
How to Create a Digital Signature
The
eSubmitter software allows users to digitally sign their submissions.
To use this feature, you must create a digital signature to identify
yourself. Follow the instructions below to create a digital
signature in either Adobe Reader and Adobe Acrobat Professional, and
to import your new digital signature into eSubmitter.
Create a Digital ID
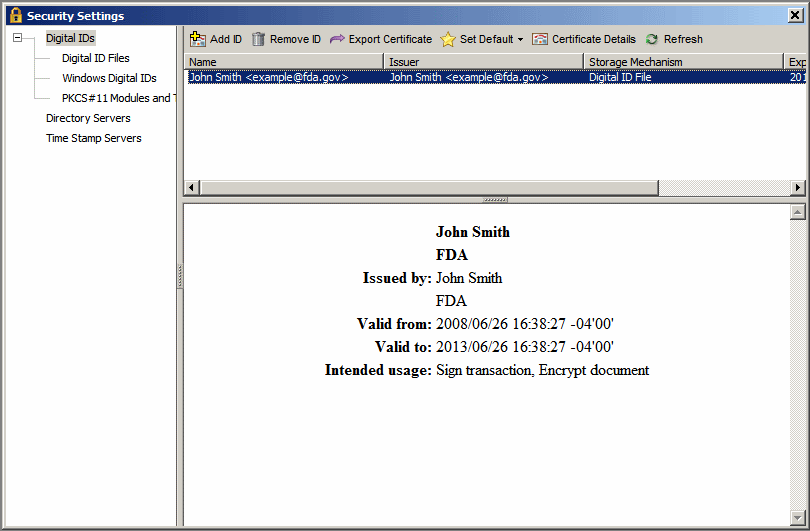
1. Access the Security Settings dialog:
Adobe Reader - Click Document > Security Settings.
Adobe Pro - Click Advanced > Security Settings.
2![]() .
Click
on the button to display the Add
Digital Id
dialog.
.
Click
on the button to display the Add
Digital Id
dialog.
3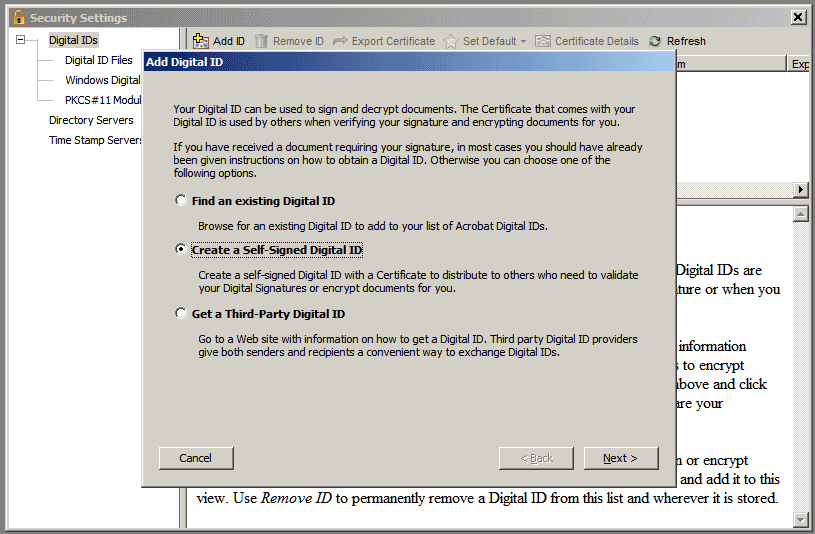 .
Select the Create
a Self-Signed Digital ID radio
button and click Next
>
.
Select the Create
a Self-Signed Digital ID radio
button and click Next
>
4. Read the message that is displayed and click Next >
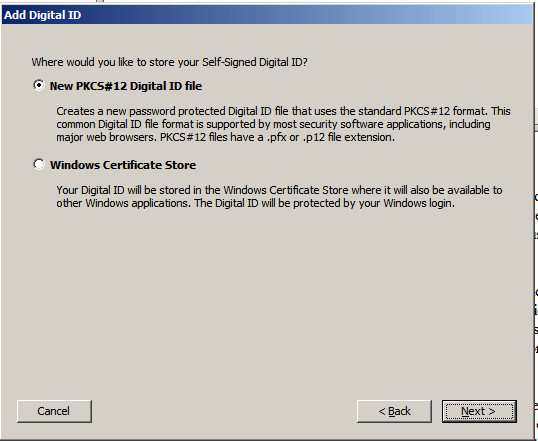
5. In the following dialog you are prompted to select the storage location of your Digital ID. Select the New PKCS#12 Digital ID file radio button and click Next >
6. Add your information in the text fields provided and click Next >
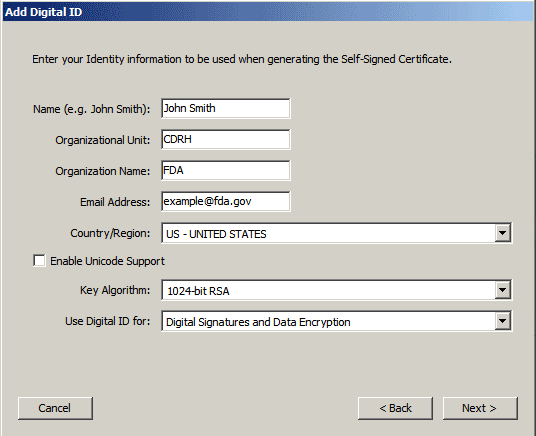
.
Note:
The values you enter for your email
address and
organization
name
will be used to validate your
signature
against FDA records You will need to supply information for the
first five fields only. Be sure to
accept
the default values for the remaining two fields and check the Enable
Unicode Support box.
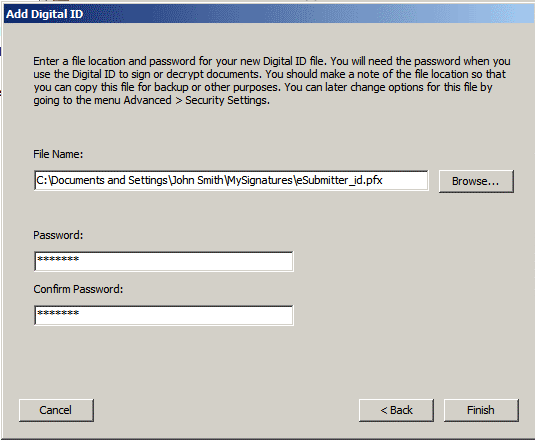
7. Enter the location where you would like your digital id saved, and a password. Confirm the password and click Finish.
8. In the Security Settings dialog, click on the newly created digital id in the upper right-hand pane to view the summary of the id. The summary is shown in the lower right-hand pane.
(Continued
on Next Page)
(Continued
on Next Page)





How to Import Tobacco Product Information using Excel
To submit product information via eSubmitter, each industry user is able to complete the following steps to import pre-populated data to ensure accurate and timely submission. Screens in eSubmitter that are capable of imports will contain the “import data” icon in the upper right quadrant of the page. There are current four (4) screens relating to your Tobacco product that allow data import.
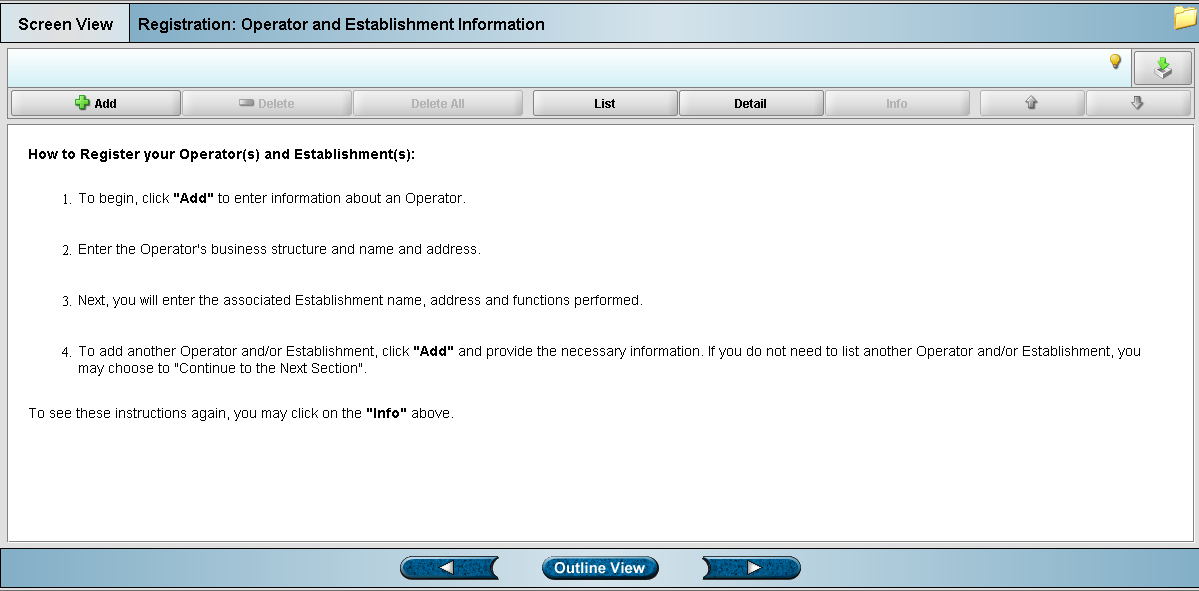
Screen 1: Operator and Establishment Information (905)
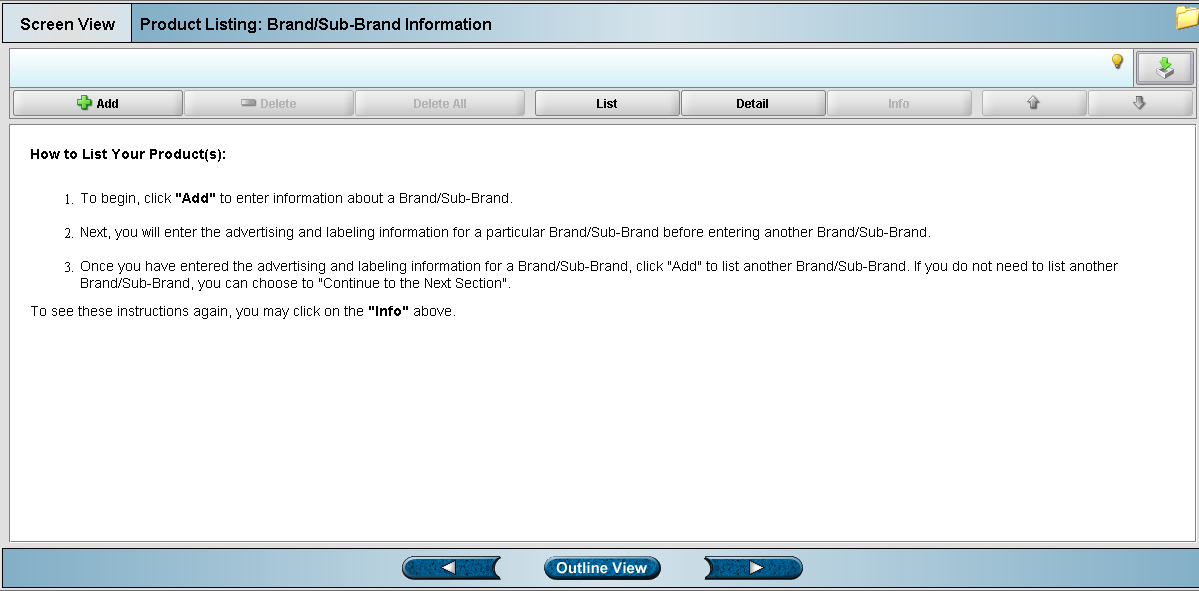
Screen 2: Brand/Sub-Brand Information (905)
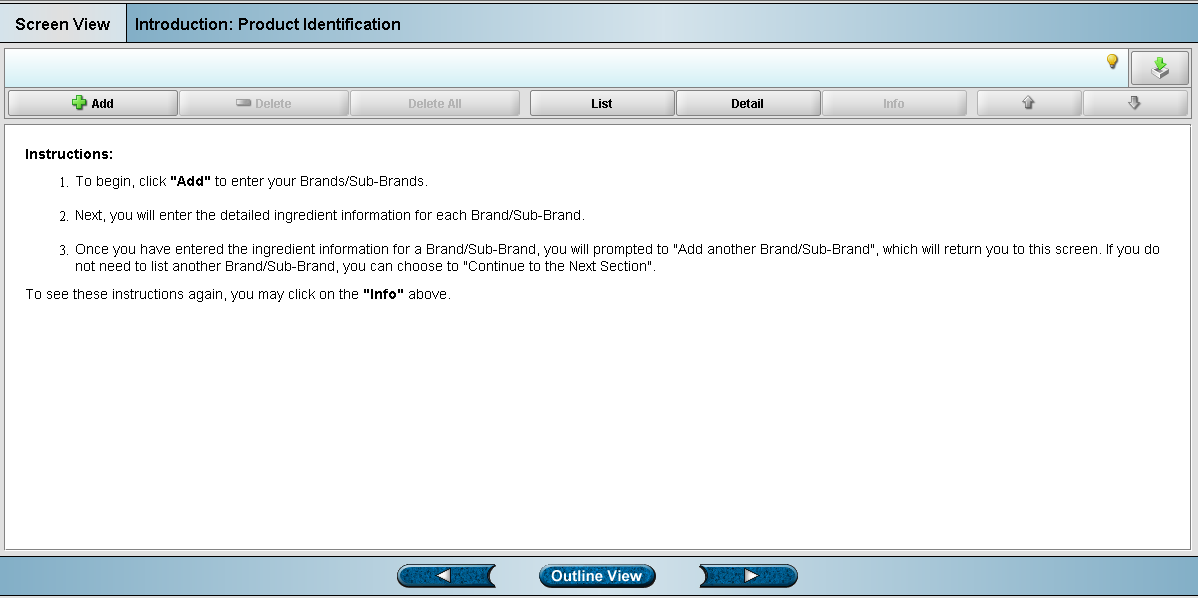
Screen 3: Product Identification (904)
Screen 4: Ingredient Identification (904)
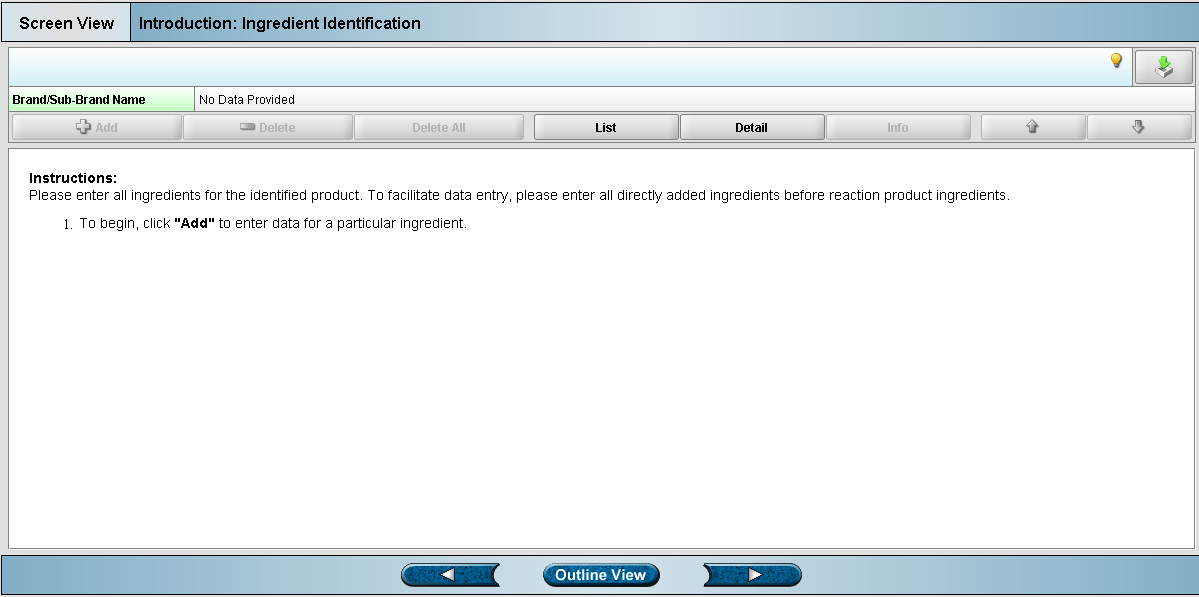
Preparing your excel spreadsheet
The Center for Tobacco Products has supplied spreadsheet templates for proper entering of data. These templates have been developed to properly import the data into the eSubmitter tool. Templates are available for each of the four screens shown above.
Within each spreadsheet you can find descriptive information about each field by scrolling over the column names.
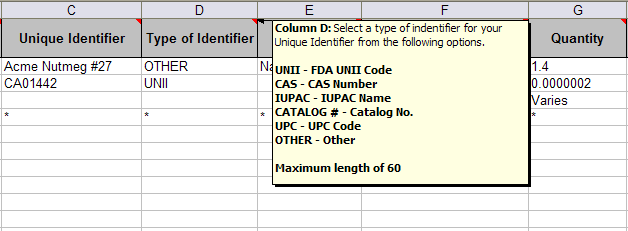
Load your data into the spreadsheet template and save it as an .xls file. You will be required to recall this file location during the import process. Note: eSubmitter is not compatible with .xlsx files at this time.
![]()
On each of the 904 and 905 Tobacco import screens, you can begin by pressing the “Import data” icon which will bring up the “Data Import Wizard.”
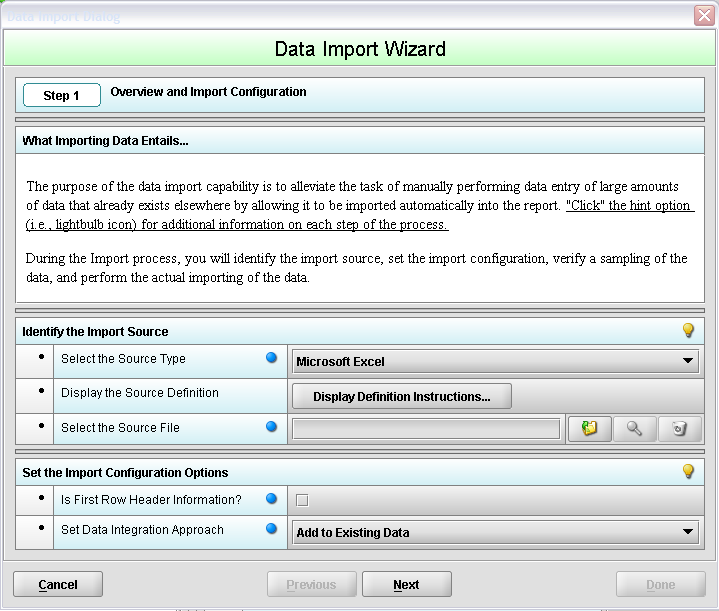
The Data Import Wizard contains the following fields:
Identify the Import Source
Select the Source Type – Only Microsoft Excel files are allowable
Display the Source Definition – The Source Definition will assist in defining your variables and the constraints of your data. Source Definitions for each of the four (4) Tobacco screens are included in the appendices.
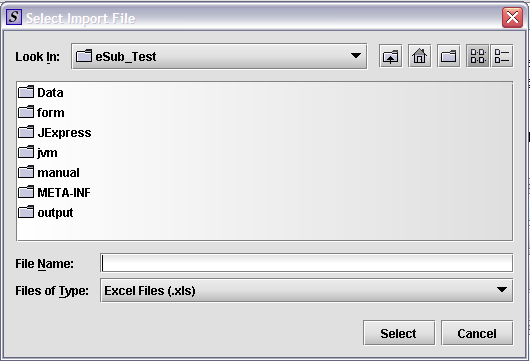
Select the Source File – allows the user to use an existing excel spreadsheet on their computer to pre-populate the data. There are three options aligned to the “Select the Source File” field.
 Select
File – Select one of the four (4) Tobacco Excel spreadsheet
templates provided by the Center for Tobacco Products that you have
input data into
Select
File – Select one of the four (4) Tobacco Excel spreadsheet
templates provided by the Center for Tobacco Products that you have
input data into
 View
File – Preview the spreadsheet that you would like the data
to be imported from
View
File – Preview the spreadsheet that you would like the data
to be imported from Clear
File – To select a different Tobacco product file or stop the
process towards importing data, select the Clear File icon
Clear
File – To select a different Tobacco product file or stop the
process towards importing data, select the Clear File icon
Set the Import Configuration Options
Is First Row Header Information – Always check the box to indicate that the first row of the spreadsheet contains column title information. Each of the provided Tobacco templates includes header rows that describe the type of information that needs to be entered.
Set Data Integration Approach
Add to existing data - This option allows you to add to the existing list of Tobacco Product values currently in your data set. This does not replace the values already entered.
Replace existing data – This option allows you to overwrite Tobacco Product information that is in your data set and replace using the values in the imported spreadsheet.

Click
![]() to import your Tobacco Product file into eSubmitter
to import your Tobacco Product file into eSubmitter
Appendix A: Ingredient Identification Source Definition
Source Definitions: Ingredient Identification (904)
Question/Column |
Constraints |
Unique Identifier |
Maximum length of 60 |
Response required |
|
|
|
Type of Unique Identifier |
Response required |
An applicable code (shown before the dash) from the following list of options: |
|
UNII - FDA UNII Code |
|
CAS - CAS Number |
|
IUPAC - IUPAC Name |
|
CATALOG # - Catalog No. |
|
UPC - UPC Code |
|
OTHER – Other... |
|
|
|
Type of Unique Identifier, If Other |
Maximum length of 60 |
Response required |
|
|
|
Name of Ingredient Manufacturer |
Maximum length of 60 |
Quantity of Ingredient |
Maximum length of 60 |
Response required |
|
|
|
Unit of Measure |
Response required |
An applicable code (shown before the dash) from the following list of options: |
|
g - grams |
|
mg - milligrams |
|
mcg - micrograms |
|
pg - picograms |
|
|
|
Quantity Reported Per |
An applicable code (shown before the dash) from the following list of options: |
GOP - gram of product |
|
UOU - unit of use |
|
|
|
Amount Description, if Varies |
Maximum length of 1000 |
Response required |
|
|
|
Limit of Detection, If 0 |
Maximum length of 60 |
Response required |
|
|
Question/Column |
Constraints |
Unit of Measure, if Limit of Detection |
Response required |
An applicable code (shown before the dash) from the following list of options: |
|
g - grams |
|
mg - milligrams |
|
mcg - micrograms |
|
pg - picograms |
|
|
|
Type/Grade/Quality |
Maximum length of 100 |
Expected Ingredient Functions |
|
Expected Ingredient Functions, If Other |
Maximum length of 100 |
Ingredient Added to Paper? |
Response required |
An applicable code (shown before the dash) from the following list of options: |
|
Y - Yes |
|
N - No |
|
|
|
Ingredient Added to Filter? |
Response required |
An applicable code (shown before the dash) from the following list of options: |
|
Y - Yes |
|
N - No |
|
|
|
Ingredient Added to Tobacco? |
Response required |
An applicable code (shown before the dash) from the following list of options: |
|
Y - Yes |
|
N - No |
|
|
|
Ingredient Added to, If Other |
Maximum length of 100 |
Is the ingredient Tobacco? |
Response required |
An applicable code (shown before the dash) from the following list of options: |
|
Y - Yes |
|
N - No |
|
|
Question/Column |
Constraints |
Cure Method, If Tobacco |
Response required |
An applicable code (shown before the dash) from the following list of options: |
|
AIR - Air |
|
FIRE - Fire |
|
FLUE - Flue |
|
STEAM - Steam |
|
SUN - Sun |
|
OTHER - Other |
|
|
|
Cure Method to, If Other |
Maximum length of 100 |
Genetic or Transgenic Modification |
Maximum length of 60 |
Response required |
|
|
|
Is this a Reaction Product? |
Response required |
An applicable code (shown before the dash) from the following list of options: |
|
Y - Yes |
|
N - No |
|
|
|
Reactive Ingredients, If Reaction |
|
Custom made to your specifications? |
Response required |
An applicable code (shown before the dash) from the following list of options: |
|
Y - Yes |
|
N - No |
|
|
|
Specified Ingredients, If Custom |
|
Specifications, If Custom |
|
| File Type | application/msword |
| File Title | Technical Guidance/Working Instructions |
| Author | chowb |
| Last Modified By | Jonna Capezzuto |
| File Modified | 2009-11-04 |
| File Created | 2009-11-04 |
© 2026 OMB.report | Privacy Policy