Form 1 2010 HAI & Antimicrobial Use Point Prevalence Survey: Pr
Prevalence Survey of Healthcare Associated Infections (HAIs) and Antimicrobial Use in U.S. Acute Care Hospitals
Attachment C_PrevSurveyForms (2)
EIP Personnel Prevalence Survey #1, Phase 2
OMB: 0920-0852
Form
Approved OMB
No. 0920-XXXX
Exp.
Date xx/xx/20xx
2
Form
Approved OMB
No. 0920-XXXX
Exp.
Date xx/xx/20xx
Form
Approved OMB
No. 0920-XXXX
Exp.
Date xx/xx/20xx
Form
Approved OMB
No. 0920-XXXX
Exp.
Date xx/xx/20xx
PRIMARY TEAM DATA COLLECTION FORM
CDC ID: - Survey date: //
I. Identifiers (for Primary Team and EIP Team use only; Identifiers are not transmitted to CDC)
|
|
Patient name: ___________________________________ (Last, First, MI) |
|
Date of birth: // |
Hospital name: __________________________________ |
Hospital unit name: ______________________________ |
Room number: __________________________________ |
Medical record no.: ______________________________
|
Data collector initials: ____________________________
|
II. Demographics
|
|
Age: _______ years months days
|
Admission date: // |
Gender: M F Unknown |
CDC location code: __________________________
|
III. Risk factors (in place on the survey date) |
||
Urinary catheter: |
No Yes Unknown
|
|
Ventilator: |
No Yes Unknown
|
|
Central line: |
No Yes
Unknown |
If “Yes,” check all that apply:
PICC Femoral line Other central line
|
IV. Antimicrobials |
|
On antimicrobials on the survey date or the calendar day prior to the survey date: |
No Yes Unknown |
**Qualification for hemodialysis and peritoneal dialysis patients ONLY**
On any of the following antimicrobials in the 4 calendar days prior to the survey date: vancomycin, amikacin, gentamicin, tobramycin, streptomycin, kanamycin
|
NA, not a dialysis patient
No Yes Unknown |
F
Public
reporting burden of this collection of information is estimated to
average 5 minutes per response, including the time for reviewing
instructions, searching existing data sources, gathering and
maintaining the data needed, and completing and reviewing the
collection of information. An agency may not conduct or sponsor,
and a person is not required to respond to a collection of
information unless it displays a currently valid OMB Control Number.
Send comments regarding this burden estimate or any other aspect of
this collection of information, including suggestions for reducing
this burden to CDC/ATSDR Reports Clearance Officer, 1600 Clifton
Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA 0920-xxxx.
2
Form
Approved OMB
No. 0920-XXXX
Exp.
Date xx/xx/20xx
EIP TEAM ANTIMICROBIAL USE FORM
Date: // Data collector initials: ________ |
CDC ID: - |
|
|
|
|
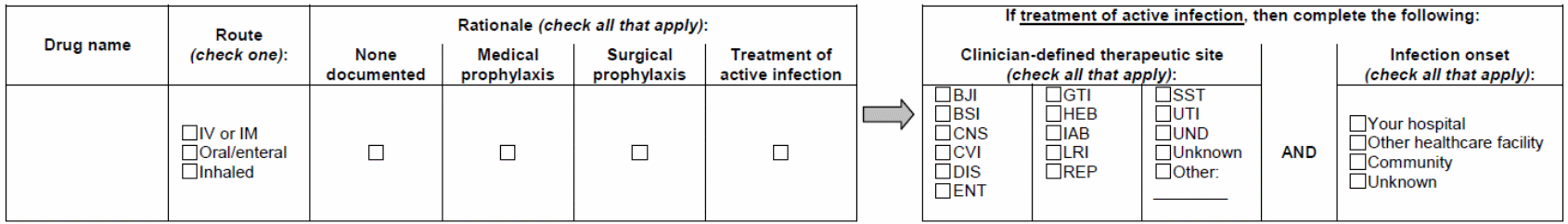
Check here if no antimicrobials administered/scheduled to be administered. Otherwise, fill in table(s) below, for up to 6 antimicrobial agents.
Therapeutic site codes:
BJI = Bone or joint, BSI = Bloodstream infection, CNS = Central nervous system, CVI = Cardiovascular (other than BSI), DIS = Systemic, disseminated infection, ENT = Eyes, ears, nose, throat (includes upper respiratory infection, GTI = Gastrointestinal tract, HEB = hepatic and biliary system infections (including pancreas), IAB = intraabdominal infection other than GTI and HEB (e.g., spleen abscess), LRI = Lower respiratory infection, REP = Reproductive tract infection, SST = Skin or soft tissue infection (includes muscle infection), UTI = Urinary tract infection, UND = Undetermined, Other = specify other site.


C
Public
reporting burden of this collection of information is estimated to
average 15 minutes per response, including the time for reviewing
instructions, searching existing data sources, gathering and
maintaining the data needed, and completing and reviewing the
collection of information. An agency may not conduct or sponsor,
and a person is not required to respond to a collection of
information unless it displays a currently valid OMB Control Number.
Send comments regarding this burden estimate or any other aspect of
this collection of information, including suggestions for reducing
this burden to CDC/ATSDR Reports Clearance Officer, 1600 Clifton
Road NE, MS D-74, Atlanta, Georgia 30333; ATTN: PRA 0920-xxxx.
CDC ID: -
Form
Approved OMB
No. 0920-XXXX
Exp.
Date xx/xx/20xx



If Rationale for ANY drug listed above is “None documented” or “Treatment of active infection” GO TO HAI FORM.
If Rationale for ALL drugs listed above is “Medical prophylaxis” or “Surgical prophylaxis” DON’T fill out HAI Form. Data collection complete.
2
Form
Approved OMB
No. 0920-XXXX
Exp.
Date xx/xx/20xx
EIP TEAM HAI FORM
Date: // Data collector initials: ________ |
CDC ID: - |
|
|
Does the patient have an HAI? |
|
No data collection complete Yes complete the table below.
|
|
Enter only one HAI on each HAI Form. This is HAI Form # _____ out of _____ total HAI Forms for this patient.
HAI |
Specific Site |
Device and Procedure Information |
Comments |
||
|
UTI |
SUTI ABUTI OUTI |
Catheter-associated? No Yes |
|
|
|
PNEU |
PNU1 PNU2 PNU3 |
Ventilator-associated? No Yes |
|
|
|
BSI
|
LCBI CSEP |
Central line-associated? No Yes |
|
|
|
SSI |
SUP INC DEEP INC ORGAN/SPACE (for ORGAN/SPACE, specify site : ___________ ) |
NHSN operative procedure category code :
OR (if operative procedure but not NHSN) check the following: OTH |
|
|
|
BJ
|
BONE JNT DISC |
|
|
|
|
CNS |
IC MEN SA |
|
|
|
|
CVS |
VASC ENDO |
CARD MED |
|
|
|
EENT |
CONJ EYE EAR |
ORAL SINU UR |
|
|
|
GI |
GE GIT HEP |
IAB NEC |
|
|
|
LRI |
BRON LUNG |
|
|
|
|
REPR |
EMET EPIS |
VCUF OREP |
|
|
|
SST |
SKIN ST DECU BURN |
BRST UMB PUST CIRC |
|
|
|
SYS |
DI |
|
|
|
Was there a Secondary Bloodstream Infection associated with this HAI? No Yes Unknown
Enter up to three pathogen codes for this HAI: 1) ________ 2)________ 3) _________ OR No pathogen identified
Enter the CDC location of attribution for this HAI: _______________ Unknown Not applicable (i.e., SSI)
Date: // Data collector initials: ________ |
Form
Approved OMB
No. 0920-XXXX
Exp.
Date xx/xx/20xx CDC ID: - |
Antimicrobial Susceptibility Testing—Instructions:
Check the appropriate box(es) to indicate which of the pathogen(s) below (if any) caused this HAI. “E. coli”=Escherichia coli; “E. faecium”=Enterococcus faecium; “E. faecalis”=Enterococcus faecalis; “P. aeruginosa”=Pseudomonas aeruginosa; “S. aureus”=Staphylococcus aureus.
Check the appropriate susceptibility test results for the antimicrobial agents listed: S=sensitive/susceptible. I=intermediate, R=resistant, N=not tested.
Antimicrobial agent abbreviations: AMK=amikacin, AMP=ampicillin, AMPSUL=ampicillin/sulbactam,CEFEP=cefepime, CEFOT=cefotetan, CEFTAZ=ceftazidime, CEFTRX=ceftriaxone, CIPRO=ciprofloxacin, CLINDA=clindamycin, DAPTO=daptomycin, DOXY=doxycycline, ERYTH=erythromycin, GENT=gentamicin, IMI=imipenem, LEVO=levofloxacin, LNZ=linezolid, MERO=meropenem, OX=oxacillin, PENG=penicillin G, PIP=piperacillin, PIPTAZ=piperacillin/tazobactam, QUIDAL=quinupristin/dalfopristin, RIF=rifampin, TETRA=tetracycline, TMZ=trimethoprim/sulfamethoxazole, VANC=vancomycin.
Check here if NONE of the organisms below are pathogens for this HAI (data collection is now complete).
Acinetobacter baumannii other |
AMK |
AMPSUL |
CEFEP |
CEFTAZ |
CIPRO
|
COL/PB |
GENT |
IMI |
LEVO |
MERO |
PIPTAZ |
TOBRA |
TIG |
|||||||||||||
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
|
E. coli |
AMK |
AZT |
CEFEP |
CEFOT |
CEFTAZ |
CEFTRX |
CIPRO |
GENT |
IMI |
LEVO |
MERO |
TOBRA |
||||||||||||||
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
|||
Positive test for extended-spectrum beta lactamase (ESBL) production? Yes No Don’t know |
Positive test for carbapenemase production? Yes No Don’t know |
|
||||||||||||||||||||||||
E. faecium |
AMP |
DAPTO |
LNZ |
PENG |
QUIDAL |
VANC |
||||||
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
|
E. faecalis |
AMP |
DAPTO |
LNZ |
PENG |
VANC |
|||||
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
|
Klebsiella pneumoniae oxytoca other |
AMK |
AZT |
CEFEP |
CEFOT |
CEFTAZ |
CEFTRX |
CIPRO |
GENT |
IMI |
LEVO |
MERO |
TOBRA |
|||||||||||||
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
||
Positive test for extended-spectrum beta lactamase (ESBL) production? Yes No Don’t know |
Positive test for carbapenemase production? Yes No Don’t know |
|
|||||||||||||||||||||||
P. aeruginosa |
AMK |
AZT |
CEFEP |
CEFTAZ |
CIPRO |
GENT |
IMI |
LEVO |
MERO |
PIP |
PIPTAZ |
TOBRA |
||||||||||||
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
|
S. aureus |
CLIND |
DAPTO |
DOXY |
ERYTH |
GENT |
LNZ |
OX |
QUIDAL |
RIF |
TETRA |
TMZ |
VANC |
||||||||||||
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
|
FORM IS COMPLETE
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Shelley Magill |
| File Modified | 0000-00-00 |
| File Created | 2021-01-29 |
© 2026 OMB.report | Privacy Policy