NHANES 2011-2012 Ext Sup St A
NHANES 2011-2012 Ext Sup St A .doc
National Health and Nutrition Examination Survey (NHANES)
OMB: 0920-0237
Supporting Statement A for Request for Clearance
National Health And Nutrition Examination Survey
Extension
OMB No. 0920-0237
(expires 11/30/2012)
Contact Information:
Vicki Burt
National Center for Health Statistics (CDC)
Chief, Planning Branch
National Health and Nutrition Examination Survey
3311 Toledo Road, Room 4211
Hyattsville, MD 20782
Email: vburt@cdc.gov
Phone: 301 458-4127
FAX: 301-458-4028
August 22, 2012
Section A TABLE OF CONTENTS
Sections |
pages |
A. Justification.................................................................................................................................................. |
5 |
1. Circumstances Making The Collection Of Information Necessary............................................................... |
5 |
2. Purpose And Use Of The Information Collection......................................................................................... |
9 |
NHANES Examination Component............................................................................................................... |
10 |
a. Cardiovascular Health........................................................................................................................... |
10 |
b. Diabetes Mellitus................................................................................................................................... |
10 |
c. Dietary Assessment............................................................................................................................... |
10 |
d. Obesity and Growth and Development......................................................................... |
11 |
.......................................................................................................................................... |
11 11 |
e. Oral Health............................................................................................................................................ |
12 |
f. Respiratory Function.............................................................................................................................. |
12 |
Spirometry Measurement.......................................................................................................................... |
12 |
Exhaled Nitric Oxide (ENO) Measurement................................................................................................ |
12 |
g. Sensory Performance............................................................................................................................ |
13 |
Audiometry (Hearing)................................................................................................................................ Chemosensory Variation and Impairment (Taste and Smell)……….…………………............................... h. Physical Activity Monitor (PAM)……..................................................................................................... i. Muscle Strength ………......................................................................................................................... j. Sagittal Abdominal Diameter (SAD) ………...........................................................................................
|
13 13 13 13 14
|
NHANES Laboratory Assessments.............................................................................................................. |
14 |
a. Urine Assessments............................................................................................................................... |
14 |
Urine Osmolality........................................................................................................................................ |
14 |
Urine Flow Rate......................................................................................................................................... |
15 |
b. Environmental Chemical Exposures..................................................................................................... |
15 |
c. Infectious Disease and Immunization Status Assessments................................................................. |
20 |
d. Nutritional Biochemistries, Hematologies And Other Nutrition Related Laboratory Measures.............. |
21 |
e. Biologic Specimen Banking................................................................................................................... |
21 |
f. Other laboratory...................................................................................................................................... |
22 |
The NHANES Interviews............................................................................................................................... |
22 |
a. Text Messaging ……….......................................................................................................................... |
25 |
b. Food Security And Nutrition Program Participation............................................................................... |
25 |
c. Dietary Supplement (DS) Use.............................................................................................................. |
26 |
d. Prescription Drug Use............................................................................................................................ |
26 |
e. Mental Health (Depression)................................................................................................................... f. Cognitive Functioning ……..................................................................................................................... |
26 26 |
g. Weight History, Weight Self Image and Weight Related Behavior...................................................... |
27 |
h. Oral Health........................................................................................................................................... i. Aspirin Use …….................................................................................................................................... j. Muscles Pain and Injury.......................................................................................................................... k. Urologic Health...................................................................................................................................... |
27 27 27 28 |
l. Telephone Interview of Hepatitis C Positive Participants...................................................................... m. Pubertal Maturation Self-Assessment ……......................................................................................... n. Other Interview Information................................................................................................................. |
28 28 29 |
Responding to Emerging Public Health Issues, New Technology and Future Survey Options.................... Privacy Impact Assessment Information...................................................................................................... |
30 31 |
3. Use Of Information Technology And Burden Reduction............................................................................. |
31 |
4. Efforts To Identify Duplication And Use Of Similar Information................................................................... |
32 |
5. Impact On Small Businesses Or Other Small Entities................................................................................ |
32 |
6. Consequences Of Collecting The Information Less Frequently ................................................................. |
32 |
7. Special Circumstances Relating To The Guidelines For 5CFR1320.5........................................................ |
33 |
8. Comments In Response To The Federal Register Notice And Efforts To Consult Outside The Agency.... |
33 |
a. Federal Register Notice............................................................................................................................... |
33 |
b. Outside Consultation.................................................................................................................................... |
33 |
9. Explanation Of Any Payment Or Gifts To Respondents............................................................................. |
34 |
10. Assurance Of Confidentiality Provided To Respondents.......................................................................... |
37 |
11. Justifications For Sensitive Questions...................................................................................................... |
38 |
a. Social Security Number............................................................................................................................ |
38 |
b. CMS Health Insurance Claim Number..................................................................................................... |
39 |
c. Residency Status..................................................................................................................................... |
39 |
d. Other Content........................................................................................................................................... |
39 |
12. Estimates of Annualized Burden Hours and Costs.................................................................................... |
41 |
a. Time Estimates......................................................................................................................................... |
41 |
b. Cost to Respondents................................................................................................................................ |
42 |
13. Estimate Of Other Total Annual Cost Burden To Respondents Or Record Keepers............................... |
42 |
14. Annualized Cost To The Federal Government........................................................................................... |
42 |
15. Explanation For Program Changes Or Adjustments.................................................................................. |
43 |
16. Plans For Tabulation And Publication And Project Time Schedule.......................................................... |
43 |
17. Reason(S) Display Of OMB Expiration Date Is Inappropriate.................................................................. |
45 |
18. Exceptions to Certification for Paperwork Reduction Act Submissions.................................................... |
45 |
|
|
This request is for an extension to complete the 2011-2012 National Health and Nutrition Examination Survey (NHANES) (OMB # 0920-0237 Expires November 30, 2012).
The NHANES has, to date, been authorized as a generic clearance; however, a change in accounting practice requires a shift to a newly-assigned clearance number for future full cycles of the survey. The request for a new approval, beginning with 2013 data collection, is in process.
This extension requests generic clearance for activities needed to successfully complete the 2011-2012 NHANES survey cycle, which ends in early 2013. This includes new and previously approved selected NHANES pilot tests and the completion of the 2012 National Youth Fitness Survey (NYFS).
A nine month clearance is requested.
The NHANES is a major ongoing source of information on the health and nutritional status of the civilian, noninstitutionalized population of the United States. It is conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC).
The activities discussed below have been previously approved for collection in 2011-2012. The major updates are to the burden table to reflect that data will only be collected for a few months instead of two years of data. All of the activities described in this request were approved by OMB on November 3, 2010 or on September 15, 2011.
Brief summary of approved changes for the 2011-2012 NHANES
In 2008 the NHANES program received a letter in response to our 60 day Federal Register notice from the Asian and Pacific Islander American Health Forum. We met with them and investigated the options to sample more Asians in the NHANES survey. We decided this was something we would be able to do. So the 2011-2014 NHANES survey includes an oversample of the Asian population. This is an example of how we at the National Center for Health Statistics try to be responsive to the input we receive from the community and outside organizations.
This request includes the continuation of the NHANES Sample Person Household and Family Interviews, Mobile Examination Center (MEC) interviews and dietary interviews, MEC examination components, Laboratory assessments and Telephone follow-up interviews for 2011-12.
New and approved for 2011-12
Adding an oversample of the Asian population to the sample design
Adding additional household questions on cell phone access and text messaging NHANES reminders, dental pain and disability, aspirin use, and chemosenses (taste and smell)
Adding MEC interview questions on cognitive functioning and pubertal maturation self-assessment
Adding MEC examination components for, sagittal abdominal diameter, chemosensory variation and impairment (taste and smell), physical activity monitor (PAM) and muscle strength
Adding laboratory assessments on cytomegalovirus and testosterone
Conducting the NHANES National Youth Fitness Survey, approved 9/16/2011
Modified and approved for 2011-12
Multiple household questionnaire sections (see page 23 for details)
MEC examination components for oral health, dual x-ray absorptiometry and audiometry
Laboratory assessments on the urine metals panel, blood metals panel
Cycled Out for 2011-12
Household questions on inflammatory back pain
MEC interview questions on bowel health and the dietary screener module
MEC examination component on arthritis body measures
Blood pressure methodology study
Laboratory assessments on prostate specific antigen (PSA), vitamin trans fatty acids, acrylamide, glycidamide, iron parameters and perchlorate and Volatile Organic Compounds (VOCs) in the home water supply
Telephone follow-up on flexible consumer spending
Request for continued permission to conduct pilot or methodological testing for future NHANES through a GenIC request.
Justification
1. Circumstances Making the Collection of Information Necessary
The National Center for Health Statistics (NCHS), Division of Health and Nutrition Examination Surveys (DHANES), Centers for Disease Control and Prevention (CDC) is requesting an extension to the National Health and Nutrition Examination Survey (NHANES) (OMB # 0920-0237 Expires November 30, 2012)), generic clearance for activities needed to successfully complete the 2011-2012 NHANES survey cycle, which ends in early 2013. It also covers selected NHANES pilot tests and special studies. A nine month clearance is requested.
Background
NCHS has conducted a series of health and nutrition surveys since the early 1960s. The surveys are unique in that physical examination data are obtained from national samples of the U.S. population. The examination component is conducted in mobile examination centers (MECs) that travel to fifteen survey locations per year. NHANES data have been the cornerstone for numerous national health and nutrition policy and surveillance activities.
The NHANES were conducted on a periodic basis from 1971 to 1994. NHANES became a continuous, annual survey program in 1999. Each year, a nationally representative sample of the civilian, non-institutionalized U.S. population, all ages, is interviewed and examined. The response rates, for participants both interviewed and examined, for 2009 and 2010 to date are 78% and 74% respectively. Innovative recruitment methods and remuneration have contributed to the high response rates over the years. However, it is increasingly difficult to maintain high response rates. While the response rate in 2009 was higher than the 75% response rate in 2007-08, it has declined in 2010 to date.
NHANES data are released in two-year cycles. One-year estimates may be produced if there is a compelling public health need and if one year of data can provide a reliable estimate. Data from NHANES are posted on the NCHS website at http://www.cdc.gov/nchs/nhanes.htm.
The continuous data collection requires that pilot tests of new or revised survey material be conducted during the ongoing data collection. NHANES will continue to request permission to conduct pilot and and other methodologic studies through the use of GenICs.A major advantage of continuous NHANES data collection is the ability to address emerging public health issues and provide objective data on additional health conditions and issues.
NHANES continues to report on major public health topics in a timely and efficient manner. Examples of contributions from NHANES data may be found on the NCHS/NHANES website.
The continuous survey design also makes early availability of the data possible. The first release of NHANES 2007-2008 data occurred in September, 2009. In planning for 2011-2012 we have tried to take maximum advantage of the abilities of all software used in data collection to reduce data review and editing required after data collection. We hope to continue to meet our NCHS/NHANES data release date goals and have the greatest proportion possible of NHANES data released within a year of ending the data collection.
Authorization
Four public laws authorize or necessitate the collection of information about the health of the American people. Excerpts of these laws are in Attachment 1.
a) Section 306 of the Public Health Service Act (42 U.S.C. 242k) directs the National Center for Health Statistics to collect statistics on subjects such as: the extent and nature of illness and disability of the population; environmental, social and other health hazards; and determinants of health.
b) Section 4403 (Joint Nutrition Monitoring And Related Research Activities) of the Food, Conservation, and Energy Act of 2008 (P.L. 110-234) specifies that the Secretary and the Secretary of Health and Human Services shall continue to provide jointly for national nutrition monitoring and related research activities carried out as of the date of enactment of this Act.
c) The Food Quality Protection Act of 1996 (P.L. 104-170) requires the implementation of surveys to collect data on food consumption patterns of infants and children and data on dietary exposure to pesticides among infants and children.
d) Title 21 – Food and Drugs, Chapter 9 of the Federal Food, Drug, and Cosmetic Act
(21 USC 393) authorizes the collection of information to support the Food and Drug Administration’s objective to obtain current, timely, and policy-relevant consumer information to carry out its statutory functions.
The NHANES program, within NCHS, contributes to the mission of CDC by collecting objective data that are used to promote health by preventing and controlling disease and disability. CDC works with partners throughout the nation and the world to monitor public health, formulate and implement prevention strategies, develop health policies, promote healthy behaviors, and foster safe and healthful environments. In addition to the groups within the CDC, NCHS collaborates with over two dozen federal agencies to plan and fund the NHANES. The survey partners include numerous institutes of the National Institutes of Health, several programs within the U.S. Department of Agriculture, the Food and Drug Administration, and the U.S. Environmental Protection Agency. NHANES data are used to assess environmental exposures; evaluate nutrition program and policy impacts; and estimate prevalences of health risk factors, chronic conditions, and infectious diseases.
Privacy Impact Assessment
Overview of the Data Collection System
Westat, Inc. carries out the data collection under contract. Westat’s responsibilities are to select Primary Sampling Units and other units of the sample design, list the segments selected, make advance arrangements for each location, provide input on NCHS’s publicity/outreach methods and materials, set up and maintain field offices, set up and maintain the MECs, translate all questionnaires into Spanish, hire the field staff, create manuals and training programs for all field procedures (including training in NCHS confidentiality guidelines and regulations), train the field staff members, list the households to be sampled, select the sample, conduct screenings and extended interviews in the households, perform all interview and examination procedures in the examination centers, design and carry out quality control procedures, and transmit interview and examination data to NCHS. A complete blood count (CBC) and pregnancy test is conducted in the MEC laboratory and biological specimens are shipped to several laboratories in the United States for analysis.
After the listing procedure, which identifies households to be potentially included in NHANES, a pre-Advance Letter postcard and an Advance Letter (Attachment 4) are sent to each sampled address informing the occupant(s) that they may be visited by an interviewer. When the interviewer arrives at the home, he or she shows official identification and briefly explains the purpose of the survey. If the person answering the screener questions has not seen the Advance Letter, a copy is given to him/her. The interviewer then administers the Household Screener Questionnaire Module 1 (Attachment 9), solely to determine eligibility. The interviewer next explains the household questionnaires to all eligible participants who are at least 16 years old and informs them of their rights and confidentiality (the same information as appears in the Advance Letter, in case they haven't seen it). For persons under 16 who are eligible, the household questionnaire interview is conducted with a proxy, usually the parent or guardian of the survey participant. If there is no one living in the household who is over 16, the teenage participant can be interviewed him/herself. If emancipated minors are prohibited by state law to participate in research they will be sampled but not asked to participate (non responders). If convenient for the participant, the household questionnaire is administered at first contact. Otherwise, an appointment is made to return to conduct the household interviews (Attachment 9) After informing the potential respondent about the interview(s), the respondent is asked to read and sign the Interview Informed Consent Form (Attachment 5), agreeing to participate in the household interview portion of the survey. For participants who are 7-17years of age, a parent or guardian consents and the child assents.
The household interview questions appear in Attachment 9 . The Family Relationship Questionnaire is administered first, followed by the Sample Participant (SP) and Family questionnaires. The Sample Participant and Family Questionnaires are occasionally tape recorded for quality control purposes. If the interview is selected for taping a box is checked on the interview consent form indicating signed consent. Additionally, verbal consent is recorded on the audio-tape at the beginning of the tape. At the end of the interview the participant is also offered the option to be given the tape to dispose of. This offer and response are also on the audiotape.
When the interview is completed, the interviewer reviews with the participant the examination informed consent brochure (Attachment 5), which contains detailed information about the examination. Each person selected in the household is asked to make an appointment for the examination at the MEC. Those who agree to participate are asked to read and sign consent forms for the examination and the storage of specimens. The interviewer then telephones the field office to make the examination appointments. The interviewer informs the participants that they will receive remuneration as well as reimbursement for transportation expenses and childcare, if necessary.
Participants arrive at the MEC, where the Coordinator (receptionist) greets them and verifies identifying information. Next, the participant is given a pair of disposable pajamas, slippers, and a urine cup before starting their examination. In addition to the Coordinator, the survey team at each center consists of a physician, two dietary interviewers, three certified medical technologists, four health technicians (two of whom are radiological technicians), one phlebotomist, two interviewers, a dental hygienist and a facility equipment specialist.
The examination centers are open five days each week, with closed days changing on a rotating basis so that appointments will be available on any day of the week. This rotating schedule also allows collection of dietary recall data across all days of the week, since eating patterns are known to vary for workdays, school days, holidays and weekends.
There are two examination sessions at the MEC each day, held morning, afternoon, or evening for the convenience of participants. At any given time during the survey, examinations are conducted at two survey locations simultaneously, for eleven months of the year, with breaks of about two weeks at New Years and about two weeks in the summer. This requires field office and household interviewing staff to support two complete examination teams throughout NHANES.
Items of Information to be Collected
This clearance request is for continuing the NHANES, which is conducted by the National Center for Health Statistics. NHANES consists of the examination, conducted in the Mobile Examination Center (MEC), laboratory analytes, the household interview and follow-up activities, which take place after the MEC exam. Details concerning each of these elements are outlined below:
NHANES Examination Component
Cardiovascular Health
Diabetes Mellitus
Dietary Assessment
Oral Health
Respiratory Function
Sensory Performance
Physical Activity
Muscle Strength
Sagittal Abdominal Diameter
NHANES Laboratory Assessments
Urine Assessments
Environmental Chemical Exposures
Infectious Disease and Immunization Status Assessments
Nutritional Biochemistries, Hematologies And Other Nutrition Related Laboratory Measures
Biologic Specimen Banking
The NHANES Interviews
Text Messaging
Food Security And Nutrition Program Participation
Dietary Supplement (DS) Use
Prescription Drug Use
Mental Health
Cognitive Functioning
Weight History, Weight Self Image and Weight Related Behavior
Muscles Pain and Injury
Urologic Health
Telephone Interview of Hepatitis C Positive Participants
Alcohol Use
Cigarette and Tobacco Use
Reproductive Health and History
Pubertal Maturation
Information in Identifiable Form (IIF)
Information in identifiable form (IIF) is collected for linkage with other federal sources of data, to allow future recontact of participants and to notify participants of health test results. All of these items have been routinely approved and collected in the past. The identifiable information includes:
Name
Date of Birth
Social Security Number (SSN)
Medicare Beneficiary Number
Biometric Identifiers
Mother’s Maiden Name
Mailing Address
Phone Numbers
Medical Information and Notes
Biological Specimens
Employment Status
Contact information for two people close to the respondent
These items have been approved and collected for more than a decade. Some of these are described in detailed in A.11 Justifications for Sensitive Questions.
Identification of Website(s) and Website Content Directed at Children Under 13 Years of Age
There is no web-based data collection in NHANES. Our pre-advance letter postcard states: “For more information visit the NHANES web site at http://www.cdc.gov/nhanes.” There is no website content directed at children under 13 years of age.
2. Purpose and Use of the Information Collection
The major objectives of NHANES are:
To estimate the number and percentage of persons in the U.S. population and designated subgroups with selected diseases and risk factors,
To monitor trends in the prevalence, awareness, treatment and control of selected diseases,
To monitor trends in risk behaviors and environmental exposures,
To analyze risk factors for selected diseases,
To study the relationship between diet, nutrition and health,
To explore emerging public health issues and new technologies,
To establish a national probability sample of genetic material for future genetic research, and
To establish and maintain a national probability sample of baseline information on health and nutritional status.
There are 3 major components: the NHANES examination, laboratory analytes and the NHANES interviews. Sections that are new or modified are marked at the beginning. The purposes and uses of each survey component are detailed below.
NHANES Examination Component
Mobile Examination Center changes for 2011-12 include:
Adding MEC examination components for sagittal abdominal diameter (SAD), chemosensory variation and impairment (taste and smell), physical activity monitoring (PAM) and muscle strength
Modifying MEC examination components for oral health, dual x-ray absorptiometry and audiometry (hearing)
Cycling out the MEC examination components on inflammatory arthritis and the blood pressure methodology study
a. Cardiovascular Health
The primary objectives of this component are to monitor the prevalence and trends in major cardiovascular conditions and risk factors in the U.S, and to evaluate prevention and treatment programs targeting cardiovascular disease in the U.S. The main elements of the cardiovascular disease component in NHANES are measurement of blood pressure and blood total cholesterol, HDL-cholesterol, LDL-cholesterol, Triglyceride, and Apo (B) levels. See NHANES Laboratory Assessments summary on page 15). Information about treatment for hypertension and hyperlipidemia, including information about primary prevention, will be collected by questionnaire. Other related risk factors such as obesity, tobacco use and exposure, physical activity and diet will be major components of NHANES. The data will be used to monitor the status of hypertension prevalence, awareness, treatment and control, and the success of the National High Blood Pressure Education Program. Laboratory results will be used to monitor the prevalence of hyperlipidemia and the effectiveness of the National Cholesterol Education Program (NCEP).
b. Diabetes Mellitus
Approximately one-third of diabetes is undiagnosed, based on data from NHANES. An additional segment of the population is at high risk for diabetes because they have pre-diabetes. Clinical trials have shown that diabetes can be delayed or prevented in persons with pre-diabetes. The fasting and two-hour blood glucose assessments will allow surveillance of the trends in the prevalence of diabetes and impaired glucose tolerance. Fasting insulin will identify the population at risk for developing diabetes and be a component in assessing insulin resistance and the prevalence of the metabolic syndrome. Measurement of glycohemoglobin (HbA1c) will assess the level of blood glucose control in the diabetic population.
The household questionnaires will include questions about diabetes awareness and treatment. This information, along with the laboratory tests, will be used to assess progress towards the goals of the National Diabetes Education Program.
c. Dietary Assessment
Dietary information has been collected in NHANES since the 1970s. Policy makers and researchers use NHANES dietary data to assess the quality and adequacy of the U.S. diet in relation to health parameters; to evaluate the impact of programs such as welfare reform, food fortification policy, and child nutrition programs; and to identify target groups for public health education and awareness programs.
All NHANES examinees are eligible for two dietary recall (DR) interviews. The first DR will be conducted in-person in the MEC dietary interview room by trained dietary interviewers. The second will be conducted by trained telephone dietary interviewers, during a follow-up phone interview. Additionally, a 24-hour intake of dietary supplements is asked after both the in-person and telephone DR. The 24-hour DR data plus the corresponding dietary supplement intake data are used to estimate total intake of foods and nutrients for the population. Additional questions related to diet are asked in the Dietary Behavior and Nutrition questionnaire section (DBQ), and Consumer Behavior questionnaire section (CBQ) of the household interviews.
d. Obesity and Growth and Development
Overweight and obesity are important nutrition-related public health problems. Overweight is associated with several chronic diseases, including cardiovascular disease, type II diabetes, hypertension, stroke, osteoarthritis, some cancers and decreased quality of life. The increase in overweight prevalence among all sex, age, and racial-ethnic groups has been called an epidemic. NHANES is unique in collecting nationally representative measured to assess obesity. Data from NHANES are used to provide reference data on overweight and obesity, set health objectives, develop public education and intervention programs and monitor trends.
NHANES will collect body measures of height, weight, circumferences and skinfold thicknesses (anthropometry) on all participants. Anthropometry data have been collected with comparable methods since the first National Health Examination Survey (1960-62). Body composition (lean and fat mass), will be measured by dual-energy X-ray absorptiometry (DXA). The DXA techniques will provide national reference data on body composition. Self-reported information on height and weight history, for participants 16 years and older, will be collected to supplement the measured data. The self-reported information will allow evaluation of height loss with aging and the pattern of weight status (stable, cyclical, maintained weight loss).
These data will be used to: 1) monitor secular trends in the prevalence of overweight and obesity; 2) examine the relationship between overweight and obesity and other examination measures, including blood pressure, glucose intolerance, and a battery of indicators for cardiovascular disease; and 3) identify risk factors and diet and physical activity patterns associated with overweight.
e. Oral Health (modified and approved for 2011-2012)
The target age group for the Oral Health component is participants ages 2 and older. The specific assessment a participant receives is dependent on their age. NHANES will collect information on tooth retention and loss, tooth decay, dental restorations, dental fluorosis, dental sealants, and periodontal health status. NHANES will be evaluating tooth status, dental caries experience, sealants and fluorosis using protocols similar to that used during 1999-2004. NHANES will be continuing an evaluation for periodontitis using a clinical gold standard examination, which was used in 2009-2010. Oral health data will be collected using a visual-tactile examination. In 2011-12 NHANES will return to this component being conducted by dentists. Data will be recorded by a separate dental recorder.
Additionally, a rinsed specimen will be obtained to test for oral Human Papilloma Virus (HPV) infection. Molecular evidence supports a role for HPV, particularly HPV-16, in the pathogenesis of a subgroup of squamous-cell carcinoma of the head and neck. Epidemiologic evidence of the role of HPV in squamous-cell carcinomas of the head and neck is less rigorous. Studies are underway to evaluate the natural history of oral HPV infection and its potential health consequences. NHANES will provide information on the prevalence of oral HPV infection in the general population.
f. Respiratory Function
Spirometry Measurement
Spirometry is the measurement of exhaled lung volumes and expiratory airflow rates. It is a standard lung function test in medical practice and is used to define cases of clinical lung disease such as asthma and Chronic Obstructive Pulmonary Disease (COPD). Spirometric measurements of lung function, especially the Forced Vital Capacity (FVC), Forced Expiratory Volume in one second (FEV1), and their ratio (the FEV1/FVC %) are important for characterization of asthma and obstructive airway disease both for clinical as well as epidemiological purposes.
Spirometry will be performed the same way as in the 2009-2010 NHANES. The eligible age range is 6-79 years. Children and adults with airway obstruction detected by baseline pulmonary function testing undergo repeat spirometry after inhalation of a short-acting β2-adrenergic bronchodilator. Bronchodilator reversibility spirometry testing is a standard clinical procedure and has been employed in many population-based surveys of asthma and COPD both in adults and children. Bronchodilator reversibility testing is used to distinguish more precisely between asthma and COPD and other causes of fixed obstructive lung disease, and is used to determine asthma severity and the degree of asthma treatment control. The NHANES spirometric testing protocol for baseline spirometry and bronchodilator testing meets current American Thoracic Society (ATS) Guidelines for quality control.
Exhaled Nitric Oxide (ENO) Measurement
Airway inflammation, a precursor of asthma symptoms, is important to the investigation of underlying respiratory disease. ENO, measured in exhaled breath samples, is a noninvasive marker of airway inflammation. Nitric Oxide (NO) is normally produced and detected in the exhaled breath from the respiratory tract where it plays important regulatory functions. NO concentrations increase following exposures to allergens. There appear to be large differences in ENO concentrations between asthmatics and healthy controls, and ENO may allow detection of cases of mild or incipient asthma which are usually symptom-free. NHANES is therefore conducting ENO testing data to complement its spirometric studies.
g. Sensory Performance
Audiometry (Hearing) (modified and approved for 2011-2012)
In 2011-2012 NHANES cycled out hearing examinations for participants 12-19 years and add hearing examination for adults 20-69 years. Hearing examinations will continue to be done for participants 70 and older. The rationale for returning to testing adults (20-69 years) is to provide updated, estimates of the degree of hearing impairment in the U.S. The last time adults (20-69 years) were tested in NHANES was in 1999-2004. The hearing loss data for older adults is also essential for estimating functional impairments and disability in this subpopulation. These data are central to developing and implementing national hearing loss programs. Because audiometry alone may not be sensitive enough to detect middle ear disease, tympanometry is also conducted to provide an estimate of tympanic membrane compliance.
Chemosensory Variation and Impairment (Taste and Smell) (new and approved for 2011-2012)
In the healthy normal population, both acquired and genetic variation in taste and smell may affect food preferences and hence long term risk of obesity and other medical problems. Also, acquired nutritional deficits may be more prevalent in older persons with olfactory deficits. Age-related changes in flavor perception may contribute to changes in food palatability and nutritional intake. Individuals with chemosensory impairments may also be at increased risk to their safety, from fire and gas leaks or explosions.
Smell testing will be performed using the Brief Smell Identification Test (BSIT- also known as the CC-SIT) which is a well standardized "scratch and sniff" smell identification test. Taste testing will be performed by having the examinee taste a small amount of a test substance (sweet, sour, bitter, salty, etc) to determine if they can correctly identify the taste.
h. Physical Activity Monitor (PAM) (new and approved for 2011-2012)
NHANES had not had a Physical Activity Monitor (PAM) component since its 2003-2006 cycles. Advances in technology allow us to collect improved data that will be a more complete assessment of daily physical activity. In 2003-2006 the PAM device was worn on the waist using a belt and had to be removed for bathing purposes. The new device, introduced for the 2011-2012 cycle, is worn on the wrist. Because it is waterproof, it doesnot need to be removed. In addition to monitoring physical activity, this new device also collects data on patterns of sleep. This component is for participants 6 years and older.
i. Muscle Strength (new and approved for 2011-2012)
The recent Physical Activity Guidelines for Americans (2008) recommends that adults complete muscle strengthening activities in conjunction with general recommendations to obtain 150 minutes or more of physical activity a week. Similar guidelines exist for school age children. Recent studies indicate the relative importance of muscular strength, and its independence from aerobic fitness, to impart health benefits. These benefits include lowered relative risk of all cause and cancer specific mortality and lower relative risk of conditions and putative mechanisms that may underpin the association between muscular strength and all cause mortality.
Upper body muscle strength testing, using a handgrip test, was done in the third Health Examination Survey (HESIII) for adolescents 12-17. Lower body muscle strength testing was done in the NHANES 1999-2002, but only for participants aged 50+. The muscular strength measurements for 2011-12 include two tests, to be done in the MEC exam:
• handgrip dynamometer assessment will be used to assess upper body strength
• lower body (quadriceps/hamstring) strength assessment using a dynamometer placed at the lower shin of the participants.
The 2011-2012 cycle is the first time both these tests have been done for participants 6 years and older, in our survey.
j. Sagittal Abdominal Diameter (SAD) (new and approved for 2011-2012)
The simple description of obesity has traditionally depended on body mass index, a marker of relative weight. However, estimates of trunk fat, especially fat in the visceral (intra-abdominal) deposits are usually better correlated than body mass index with established physiological risk factors for chronic disease. Favorable experience with measurement of waist circumference demonstrates this point. Recent studies, however, suggest that the supine sagittal abdominal diameter (SAD) may offer yet a better method for estimating the aspect of obesity most relevant to the risk of diabetes, cardiovascular disease, and perhaps other conditions.
Epidemiologic and clinical applications of the SAD measurement are hampered by a lack of reference data based on a representative US population. Using a low-cost, sliding-beam caliper, NHANES can accumulate population-based reference data. The target group for this measurement includes males and females, ages 2 years and older.
NHANES Laboratory Assessments
Laboratory Assessment changes for 2011-2012 include:
Adding laboratory assessments on cytomegalovirus, testosterone and adding a serum metals panel
Modifying laboratory assessments on the urine metals panel, the blood metals panel and modifying tobacco biomarkers to include urinary Volatile Organic Compounds (VOCs)
Cycling out laboratory assessments on prostate specific antigen (PSA), vitamin B6, trans fatty acids, acrylamide, glycidamide, iron parameters and perchlorate and Volatile Organic Compounds (VOCs) from the home water supply
The following summarizes the laboratory tests/classes of analytes for 2011-12. (See Attachment 8 for additional details about laboratory tests.) Within some categories of chemicals, specific analytes have changed.
a. Urine assessments.
Urine Osmolality
Urine osmolality measures the amount of solute particles contained in urine. It can indicate if the urine is overly diluted or concentrated due to hydration status or impaired renal function. Urine osmolality will be measured on participants ages 6+ years. The concentration of urine analytes (such as environmental chemicals) can fluctuate in spot (single determination) urine specimens depending on whether the urine is too diluted or concentrated. To compensate, the urine analyte concentration is divided by the urine osmolality to “standardize” the spot urine analyte concentration. Urine osmolality will be measured by freezing point osmometers in the Mobile Exam Center.
Urine Flow Rate
Urine analyte concentrations from single determinations (spot urines) are used to determine the exposure to environmental chemicals, however they can vary depending on the water content of the urine. The urine excretion rate of an analyte is a more accurate measure of the exposure to environmental chemicals. The urine excretion rate (mg/min) is the product of the urine flow rate (mL/min) and the urine analyte concentration (mg/mL). Participants ages 6+ will be asked to record their time of last void before coming to the Mobile Examination Center and then asked to void in the Mobile Examination Center where the time of collection and volume of the urine will be recorded and a urine flow rate will be calculated.
b. Environmental Chemical Exposures
The NHANES environmental health component was expanded in 1999 in collaboration with laboratories of the National Center for Environmental Health (NCEH). It now includes more than 200 measures of environmental chemicals or metabolites in blood and urine specimens collected from survey participants. These NHANES data are the cornerstone of the CDC publication, The Fourth National Report on Human Exposure to Environmental Chemicals (URL: http://www.cdc.gov/exposurereport/)
Within classes of chemicals, analytes new in 2011 were added to the protocol because either a method became available to measure the analyte and/or an analyte was added to a panel that was already on the protocol.
Note that selected categories of environmental chemicals are analyzed using pooled specimens. This is done because of the expense of measuring the compounds in hundreds of subjects and because a high proportion of results are below the limit of detection (LOD) for some chemicals.
The environmental analytes include the following classes of chemicals:
• Cotinine
• 4-(Methylnitrosamino)-1-(3-pyridyl)-1-Butanol (NNAL)
• Metals (Full in whole blood, 1/3 sample in urine, 1/3 sample in serum)
• Iodine (urine)
• Phthalates
• Phytoestrogens
• Polycyclic aromatic hydrocarbons (PAHs)
• Organophosphate insecticides: dialky phosphate metabolites
• Organophosphate insecticides: specific metabolites
• Pyrethroid pesticides
• Organochlorine pesticides
• Other pesticides and fungicides
• Herbicides
• Halogenated phenolic compounds
• Perfluorinated compounds
• Polychlorinated and polybrominated dibenzo-p-dioxins and dibenzofurans
• Polychlorinated biphenyls (PCBs)
• Polybrominated diphenyl ether
• Tobacco Biomarkers
• Toxaphenes
• Volatile organic compounds in blood and urine
• Perchlorate and thiocyanate in urine
• Polychlorinated naphthalenes
• Parabens
The uses of the NHANES environmental exposure information by the public health community include the following:
to determine the types of chemicals and concentration levels to which Americans are exposed
for chemicals with a known toxicity level, determination of the prevalence of persons above that toxicity level (e.g., blood lead > 10 µg/dL)
to establish reference ranges that may be used by state and local public health physicians and scientists to determine whether an individual or group has an unusually high exposure
to assess the effectiveness of efforts to reduce exposure to specific chemicals
to determine whether exposure levels are higher among minorities, children, women of childbearing age, and other vulnerable groups
to observe time trends in the levels of exposure within the population
to set priorities for human health effects research
Additional information on the classes of environmental chemicals is described as follows:
Environmental tobacco smoke exposure
Cotinine: Cotinine, a metabolite of nicotine, is measured in the blood as a biochemical marker to substantiate self-report of smoking and to define exposure to environmental tobacco smoke (ETS).
NNAL: NNAL is a tobacco-specific nitrosamine (TSNA) which is a metabolite of NNK (NNK is (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone)) in the body, and which has been detected in the urine of smokers, and in many cases, in nonsmokers exposed to Second Hand Smoke (SHS). NNK is formed in tobacco and in cigarette smoke from nicotine, so it and its NNAL metabolite are as specific for tobacco and cigarette smoke exposure as is cotinine or nicotine itself. Furthermore, both NNK and NNAL are known to be potent pulmonary carcinogens in rodents, and they are believed to be lung carcinogens in people as well. Thus, measuring NNAL in people will help to address the exposures of both smokers and nonsmokers to this potent carcinogen. Total NNAL in urine samples will continue to be analyzed from NHANES 2011-2012. In 2011-12 we plan to test all smokers in the 2/3 of NHANES specimens not currently tested for NNAL as noted in the Tobacco Biomarkers section below.
Tobacco biomarkers (modified and approved for 2011-2012)
On June 22, 2009, the Family Smoking Prevention and Tobacco Control Act which gave the Food and Drug Administration regulatory authority over tobacco products was signed into law. Significant parts of the legislation are focused on setting product standards. To successfully evaluate the impact that product standards might have on the exposure of smokers a system needs to be in place to monitor the exposure of smokers. Currently the NNAL, urinary polycyclic aromatic hydrocarbons, urine metals and Thiocyanate are measured in 1/3 NHANES subsamples. Only 20 percent of the participants are cigarette smokers. To increase the number of smokers we measure these chemicals in the smokers within the other two 1/3 samples. In addition to these chemicals already on the survey urine Volatile Organic Compound (VOC) metabolites will be added to the laboratory protocol (new).
Metals
Whole blood metals (modified and approved for 2011-2012):
The previous NHANES blood metals panel included Lead (Pb),Cadmium (Cd),and Mercury (Hg) (total). It has been proposed to add Manganese (Mn) and Selenium (Se). Manganese is of environmental concern and Selenium is both of nutritional interest as well as toxicological interest. The composition of the 2011-12 panel would then be Pb,Cd,Hg,Mn,Se. These additions would not require any change to sample volume of blood needed. Currently speciated mercury is only inorganic mercury. We have added ethyl mercury (thimersol) and methyl mercury (fish and seafood consumption) to the 2011-12 NHANES.
Urinary Metals (modified and approved for 2011-2012): Trace metals were associated with adverse health effects in occupational studies or laboratory studies, but these substances have not been monitored in general population. Urinary antimony (Sb), arsenic (As), barium (Ba), cadmium (Cd), cesium (Cs), chromium (Cr, cobalt (Co), lead (Pb), molybdenum (Mo), thallium (Tl), tungsten (W), and uranium (U) levels will continue to be measured in a 1/3 sample of urine in NHANES 2011-12 Urinary beryllium (Be) and platinum (Pt) were cycled out and Manganese (Mn), Tin (Sn), and Strontium (Sr) were added to the NHANES 2011-2012 urinary metals. Exposure information will be used to establish population-based reference ranges and to evaluate the need for regulations to reduce levels of exposure.
Serum metal profile (new and approved for 2011-2012): Testing of Selenium (Se), Copper (Cu), and Zinc (Zn) was added to NHANES 2011-2012. Selenium is a part of several enzymes necessary for the body to properly function. Generally, selenium functions as an antioxidant that works in conjunction with vitamin E. Copper is involved in the absorption, storage and metabolism of iron and the formation of red blood cells. Zinc is important in a number of key activities, ranging from protein and carbohydrate metabolism to the immune system, wound healing, growth and vision.
Iodine
Iodine is a trace element required by the thyroid gland for the production of the thyroid hormones thyroxine (T4) and triiodothyronine (T3), which in turn are necessary for multiple processes related to growth and development.
Pesticides and Other Chemicals
Phthalates: Phthalate acid esters (phthalates) are used extensively as plasticizers in a wide range of applications such as children’s toys, food packaging, and medical supplies. Because some of these compounds are known to be estrogenic and have been associated with a host of health problems in rats, such as cancers and teratogenicity, governments in Europe and Japan have become increasingly concerned about levels in food packaging materials and children’s toys. Biomeasures of phthalates in humans is necessary to evaluate potential human health threats from exposure to these chemicals.
Phytoestrogens: Many different plants produce compounds, called phytoestrogens that mimic or interact with estrogen. The major classes of phytoestrogens are lignans (present in flaxseed, carrots, berries, and grapes) and isoflavones (present in soybeans and other legumes). Biomeasures of phytoestrogens are necessary to establish reference ranges for these compounds and to evaluate their potential effects on human health.
Polycyclic Aromatic Hydrocarbons (PAHs) in Urine. PAHs constitute a group of chemicals which are formed during the incomplete combustion of coal, oil and gas, garbage, and other organic substances. These compounds require metabolic activation prior to their interactions with cellular macromolecules. PAHs are ubiquitous, thus exposure to them is widespread. In general, people are exposed to mixtures of PAHs, the sources of which include vehicle exhausts, asphalt roads, coal, coal tar, wild fires, agricultural burning, charbroiled foods, and hazardous waste sites. Although most of the data regarding the carcinogenicity of these compounds comes from rats and mice, epidemiologic studies have shown increased mortality due to lung and bladder cancer in humans exposed to coke-oven emissions, roofing-tar emissions, and cigarette smoke. PAHs enter the body quickly and easily by all routes of exposure and are readily and predominantly metabolized to hydroxylated metabolites as well as glucuronide metabolites. These metabolites are excellent indicators of exposure to the parent PAHs. While background level ranges of PAHs in air and water are known, the equivalent metabolite background levels in humans are not known.
Non-persistent pesticides (organophosphate insecticides, pyrethroid pesticides, other pesticides and fungicides, and herbicides) (modified and approved for 2011-2012): In the 2011-12 NHANES analysis of many pesticides are measured in plasma as well as in urine. Parent compounds are measured in plasma, whereas metabolites of pesticides are generally measured in urine. Additional pesticide metabolites in urine are added in 2011-2012 because of development of laboratory methods.
Persistent organochlorines (organochlorine pesticides, polychlorinated and polybrominated dibenzo-p-dioxins and dibenzofurans, and polychlorinated biphenyls (PCBs)): Organochlorines are diverse, synthetic chemicals that are persistent in the environment and tend to bioaccumulate. Most of these chemicals are banned in the U.S. Assessment of exposure to persistent organochlorines in a representative sample of the U.S. population is needed to determine current prevalence and level of exposure and the potential for human health threat from exposure to these chemicals.
Perfluorinated compounds: Organic fluorochemicals are used in multiple commercial applications including surfactants, lubricants, paints, polishes, food packaging, and fire-retarding foams. Recent scientific findings suggest that several perfluorinated surfactants, a group of these fluorochemicals, are ubiquitous contaminants found both in humans and animals worldwide. Polytetrafluorethylene (PTFE) has numerous uses in many industrial and consumer products, including coatings on textiles and carpet; uses in the automotive, mechanical, aerospace, chemical, electrical, medical, and building/construction industries; personal care products; and non-stick coatings on cookware. Polyvinylidene fluoride (PVDF) is used primarily in electrical/electronics, building/construction, and chemical processing industrial sectors.
Polybrominated diphenyl ethers (BDEs): Brominated flame retardants (BFRs) are heavily used as additive or reactive chemicals in polymers and textiles. Increasing levels of polybrominated diphenyl ethers (PBDE) have been observed in mothers’ milk from Sweden, Germany and Norway. PBDE concentrations found in North Americans are considerably higher than those found in Europeans. There is an increasing usage of PBDEs worldwide and results of several studies indicating that concentrations in North American populations may be increasing. This suggests that more information is needed.
Toxaphene: Toxaphene is a mixture of chemicals that was one of the most commonly used insecticides in the United States prior to 1982. It consists predominantly of polychlorinated camphenes that are lipophilic (dissolve well in lipids) and persist for years in the environment. EPA banned the use of toxaphene in the U.S. in 1990. In 1993, EPA banned the importation of food that contained toxaphene residues. Toxaphene is considered a probable human carcinogen by EPA and the National Toxicology Program.
Volatile organic compounds (VOC) (blood and urine) (modified and approved for 2011-2012): Additional volatile compounds were added in 2011-2012 because of laboratory method development. Exposure to volatile organic compounds (VOCs) is ubiquitous. Chronic exposure to extremely high levels of VOCs can lead to cancer and neurocognitive dysfunction. VOC exposure assessment will be expanded to include additional analytes of toxicological significance to include chemicals that are on priority toxicant or critical contaminant lists, and thus of toxicological concern. Hexane is a widely used solvent with neurotoxic properties. Acrylonitrile is a probable human carcinogen used widely in the polymer industry. Cis- and trans-1,3-dichloropropenes and 1,2-dibromoethane are widely used as soil fumigants resulting in unknown human exposure. Furan also became a VOC toxicant of interest on May 7, 2004 when FDA released extensive data showing levels of this potential human carcinogen in food products.
In addition to assessing levels of VOCs in blood, VOC levels aare measured in urine specimens provided by NHANES participants as noted in the section above on Tobacco Biomarkers.
Perchlorate and thiocyanate: Perchlorate is a polyatomic anion that can disrupt thyroid function by competitively inhibiting iodide uptake. Widespread use of perchlorate salts has led to widespread environmental contamination. Perchlorate is primarily produced as ammonium perchlorate for use as an oxidant in solid fuel propellants for rockets and missiles. Lesser amounts of perchlorate are used in matches, fireworks, and automotive airbags.
Polychlorinated naphthalenes (PCNs): Polychlorinated naphthalenes (PCNs) have been commercially produced and used mainly in electrical devices, but also for impregnation of wood, paper, and textiles to attain water-proofness, flame resistance, and protection against insects, molds, and fungi. Today, the PCNs are widespread in the environment and are to be regarded as an environmental problem. Generally, the levels are lower compared to polychlorinated biphenyls (PCBs), but high levels have been observed near point sources such as manufactures of chlorine/soda, magnesium, copper and aluminum. PCB products and incineration process are also sources of PCN releases.
Parabens: Parabens (alkyl esters of p-hydroxybenzoic acid) are a group of phenols widely used as antimicrobial preservatives in cosmetic products, pharmaceuticals, and food processing. Some parabens possess weak estrogenicity, although toxic effects of parabens in humans are mostly unknown. Human exposure to parabens may be assessed by measuring the conjugated or free species of these compounds or their metabolites in urine. To understand the extent of exposure to parabens in the general US population, information on concentrations of these phenolic compounds in the non-occupationally exposed population is required.
c. Infectious Disease and Immunization Status Assessments
Collection of the venipuncture specimen in NHANES provides an opportunity to assess previous infection or immunity to vaccine preventable diseases.. Measurements of cytomegalovirus and the QuantFERON Gold in Tube blood test for tuberculosis infection were added to the venipuncture protocol in 2011-12. Additionally, tuberculin skin testing is being done.
Chlamydia. Active infection with Chlamydia trachomatis can be evaluated in a urine specimen using Ligase Chain Reaction assays. Urine from examinees ages 14-39 will be tested for Chlamydia trachomatis using ligase chain reaction assays. NHANES offers an opportunity to assess the prevalence of chlamydial infection in the general population and to monitor trends in prevalence as prevention programs are established and expanded.
Cytomegalovirus (new and approved for 2011-2012): Cytomegalovirus (CMV) usually does not cause significant disease in healthy individuals, but pregnant women can transmit CMV to their unborn babies, who are then at risk. Congenital CMV infection is a significant source of morbidity among children, causing a wide range of clinical outcomes including hearing loss, mental retardation, and even death. During 1988-1994 (NHANES), 36% of 6-11 year old children had evidence of ever being infected with CMV, while currently, 0.7% of infants are born with congenital infection each year. However, the seroprevalence among children who may acquire infection postnatally until the age of 5 has yet to be described at the national level.
Young children with CMV infection shed the virus in high titers and are a major source of transmission to other susceptible children and adults, which is of special concern for pregnant women. For this reason, characterizing infection among children less than 6 years old is essential to expanding our understanding of important transmission exposures, mainly breastfeeding and close contact during childcare, and patterns of primary and recurrent infections. Such information would inform the development of prevention strategies to protect vulnerable populations including pregnant women and their fetuses. Additionally, young children have been identified as a potential target population for CMV vaccine development, recently ranked of highest priority by the Institute of Medicine. Describing the serological profile of children under the age of 6 would improve our understanding of immunity during early childhood and facilitate vaccine development.
Hepatitis: Sera will be tested for hepatitis A (6 years and older); hepatitis B core antibody (6 years and older), hepatitis B surface antibody (an indicator of immunization with HBV vaccine, 2 years of age and older), hepatitis B surface antigen (6 years and older); hepatitis C enzyme immunoassay, with positives confirmed and HCV RNA genotyping of positive specimens (6 years and older), Hepatitis C genotyping(6 years and older), Hepatitis D, and Hepatitis E antibody (6 years and older),. Hepatitis C genotyping is helpful in defining the epidemiology of hepatitis C. The determination of HCV genotypes in NHANES will provide a nationally representative assessment of genotype distribution of circulating HCV genotypes and monitoring changes in this distribution over time will provide insight into epidemiologic patterns of HCV infection in the U.S. In addition, the efficacy of available treatments differs by genotype, and a representative picture of the nationwide distribution of HCV genotypes may provide a sense of what the expected impact of treatment might be.
Herpes: Sera from NHANES subjects ages 14-49 will continue to be tested for antibody to Herpes simplex 1 and 2 (HSV-1/2) to continue to monitor the prevalence of HSV-1/2 infection in the U.S. HSV-2 is an index of sexually transmitted infections. In addition, questions about those sexual behaviors that are risk factors for sexually transmitted infections and that are the focus of major national HIV and sexually transmitted diseases risk reduction efforts are included in the MEC interview. The joint availability of sexually transmitted infection and risk factor data in a national sample on a periodic basis is a unique and invaluable resource for evaluation of
national HIV/STD risk reduction efforts and for risk-based modeling of the frequency and trends of sexually transmitted infections.
HIV: Sera from examinees ages 18-59 will be tested for HIV. The estimated prevalence of human immunodeficiency virus (HIV) infection in the United States population is an important measure of the extent of the medical and financial burden the nation faces due to this virus. NHANES III data (1988-94) and the NHANES 1999-present data on HIV infection will serve as a baseline for monitoring the changes in the epidemic over time in the general population of the United States.
HPV: HPV (Oral HPV): A rinsed specimen will be continue to be obtained to test for oral Human Papilloma Virus (HPV) infection. In addition, current human papilloma virus (HPV) infection will continue to be evaluated via the collection of a vaginal swab to examine for DNA from specific high and low risk sub-types of HPV. Additionally sera from individuals aged 14-59 years will be tested for antibody to HPV-16. Trends in HPV seroprevalence will be compared with data from the previous surveys and with trends in herpes simplex virus type 2 (HSV-2) seroprevalence. HPV vaccine was approved in June 2006 increasing the importance of continuing to monitor the prevalence of this infection.
MMRV : Sera from examinees ages 6-49 will be tested for measles, mumps, rubella, and varicella. The number of doses recommended for varicella, measles, and mumps has changed over the years, largely in response to outbreaks. It remains important to monitor the proportion of the US population susceptible to each of these diseases and to investigate demographic subgroups with higher susceptibility to help guide our national immunization policies. Additionally, most of immunity for measles, mumps and rubella was achieved through vaccination, with very little boosting from wild disease that makes serology monitoring essential. Varicella vaccination policies changed in 2006 from a routine 1-dose to a 2-dose program.
Tuberculosis: To support public health programs in the control, prevention, and eventual elimination of tuberculosis (TB) in the United States, accurate and comprehensive data are needed about the extent of the TB burden. National prevalence was last measured in NHANES 1999–2000. At that time 4.2% of the civilian, non-institutionalized population had latent TB infection (LTBI). Foreign-born participants had a higher LTBI prevalence (18.7%) than US born (1.8%), with 63% of TB infections among people who were foreign-born. In addition, only 19.5% of foreign-born persons had been previously diagnosed and only 11.6 % were prescribed treatment.
To determine the current prevalence of TB infection, NHANES participants 6 years of age and older will receive two tests for M. tuberculosis infection: a skin test and a blood test.
d. Nutritional Biochemistries, Hematologies and other nutrition related laboratory measures
NHANES data will be used to estimate deficiencies and toxicities of specific nutrients in the population and subgroups, to provide population reference data, and to estimate the contribution of diet, supplements, and other factors to serum levels of nutrients.
Complete blood counts, serum folate, RBC folate, standard biochemical profile, lipids, vitamin D, caffeine, and omega-3 fatty acids continue in 2011-12.
e. Biologic Specimen Banking:
Serum, plasma and urine continue to be stored for future research. Collection of a genetic specimen continue in 2011-12.
The availability of stored biologic specimens from a representative sample of the U.S. population provides the scientific research community with a potential resource for the measurement of new and evolving laboratory tests for emerging diseases, risk factors, and environmental exposures. With the present explosion of gene determinations associated with disease, the penetrance of susceptible genes in the population can only be determined from a representative sample such as NHANES. The additional data collected during the survey, both biochemical and questionnaire, provide phenotypic information that can be associated with these genes.
NCHS solicits proposals for use of the stored specimens. A technical panel will review and approve all proposals. Proposals for performing genetic research will be evaluated by the NHANES Genetic Technical Panel. All uses of stored specimens are subject to review and approval by the NCHS Ethics Review Board and the NCHS Confidentiality Officer.
All unused serum from laboratories will be stored for potential additional analyses.
f. Other laboratory
Kidney function: NHANES continues to collect urinary albumin and urinary creatinine to be used, along with the serum creatinine, to estimate the population of persons with chronic kidney disease. A second urine specimen is collected by the participant after the examination to obtain a repeat measure of urinary albumin. The participant will be asked to collect the first void after awakening. Osmolality and urine flow rate calculations will continue.
Liver function: NHANES’ biochemistry profile provides some indication of liver function to be analyzed in concert with the Hepatitis profile.
Celiac disease: Celiac disease is an intolerance to dietary glutens that has protean manifestations. In population surveys in other countries, it is found in about 0.5 to 1 percent of the population. It may well be as common in the United States, but has not been adequately examined. Advances in diagnostic testing now allow accurate disease prevalence estimates using two step serological testing--antihuman recombinant -Tissue transglutaminase (TTG) and endomysial antibody (EMA).
C reactive protein (CRP):
CRP is used to correct the iron status measures which are affected by inflammation. It can also be used to measure the body's response to inflammation from chronic conditions, such as arthritis, and environmental exposures to agents such as tobacco
Thyroid profile:
The thyroid profile will be used to assess thyroid function.
Testosterone (Sera) (new and approved for 2011-2012):
Measurement of testosterone is highly valuable in assessing disease risk, diagnosing disease, and monitoring treatment, as reflected in may clinical guidelines, recommendations and review articles. However, lack of generally accepted reference ranges for people at all ages, sex, and ethnicities profoundly limits progress in disease research and translating valuable research findings into information useful for patient care and disease management as stated in several editorials and research publications.
The NHANES Interviews
The topics presented in this section are questionnaire data, collected as stand-alone components or to complement one or more NHANES examination or laboratory components. The questions are asked in the home or the MEC. Household interview changes are summarized in the following table. Details of select sections follow. The complete questionnaires are found in Attachment 9. The table of contents lists each questionnaire section by component name and corresponding 3 letter component abbreviation. Page numbers for where each section begins are also provided.
Changes in Questionnaire (Approved for 2011-2012)
Screener Questionnaire 2011-2012
Component Name and (3 Letter ID) |
Descriptions |
Screener Module 1 (SCQ) |
|
Sample Person Questionnaire
Component Name and (3 Letter ID) |
Descriptions |
Immunization (IMQ) |
|
Medical condition (MCQ) |
|
Diabetes (DIQ) |
|
Audiometry (AUQ) |
|
Chemosenses (CSQ) |
|
Oral Health (OHQ) |
|
Physical Activity (PAQ) |
|
Diet behavior& Nutrition (DBQ) |
|
Occupation (OCQ) |
|
Acculturation (ACQ) |
|
Demographics (DMQ) |
|
Dietary supplements and antacids (DSQ) |
|
Arthritis (ARQ) |
|
Dietary Screener Module (ages 2-11) (DTQ) |
|
Family Questionnaire
Component Name and (3 Letter ID) |
Descriptions |
Demographics (DMQ) |
|
MEC Questionnaire - CAPI
Component Name and (3 Letter ID) |
Descriptions |
Alcohol use (ALQ) |
|
Physical activity (PAQ) |
|
Muscle Pain and Injury (CKQ) |
|
Dietary Screener Module (ages 12+) (DTQ) |
|
Bowel Health (BHQ) |
|
MEC Questionnaire - ACASI
Component Name and (3 Letter ID) |
Descriptions |
Smoking and tobacco use (SMQ) |
|
Pubertal Maturation (PMQ) |
|
Follow-up Questionnaire - Telephone
Component Name and (3 Letter ID) |
Descriptions |
Consumer Behavior (CBQ) |
|
a. Text Messaging (new and approved for 2011-2012)
The NHANES program has added questions in the home interview to ask respondents, 12 years and older, if they have a cell phone. If respondents have a cell phone, NHANES asks permission to text them reminders to fast. Text messaging is also used to remind respondents to call for their STD test results. Only participants who agree to receive text messages get these reminders.
b. Food Security and Nutrition Program Participation
The 2011-2012 NHANES continues to include a food security section (FSQ) that contains the 18-item U.S. Household Food Security Survey Module (US FSSM) and individually-referenced food security questions for respondents 12 and older.
Questions on participation in the Supplemental Nutrition Assistance Program (SNAP), formerly known as the Food Stamp Program are also included in the FSQ section. SNAP and Household and Child Special Supplemental Nutrition Program for Women, Infants and Children (WIC) data are collected, for infants and children, in the Family and Sample Person interviews. WIC data for women of childbearing age are collected in the reproductive health section of the MEC interview.
NHANES is the only nationally representative survey that collects information on food security at the household and individual level, as well as food program participation, physical health, and mental health. The data will be used to examine the association of food security with diet and health and the impact of food program participation on food security.
c. Dietary Supplement Use (DS)
NHANES continues to collect dietary supplement use information on all respondents during the household interview. The information collected on DS and antacid use pertains to all DSs and antacids taken in the past 30 days. This includes the name of the specific supplement, duration and frequency of use, and the amount taken. Since 2007-2008 NHANES has collected a 24 hour supplement intake recall after both of the dietary recalls. Collecting dietary supplement data, using the same methodology and time frame as the dietary collection, allows us to continue to combine these data and calculate total nutrient intake.
d. Prescription Drug Use (RXQ)
NHANES continues to collect information on all prescription medications used by participants during the past month. The duration of drug use and reason for use are also collected.
e. Mental Health (Depression) (DPQ)
NHANES continues to administer a depression screener questionnaire to all respondents 12 years and older. One goal of this component will be to understand the co-morbidity of depression and other chronic diseases, including cardiovascular disease, diabetes, and obesity, in adolescents and adults. Depression is being assessed using the Patient Health Questionnaire (“PHQ-9”). This screening instrument has been validated against independent structured diagnostic interviews in both clinical and general population studies, and serves both as a depression severity measure as well as a diagnostic instrument for the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) depressive disorders. The PHQ-9 refers to the previous 2-week interval and consists of 9 items of depression symptoms and question on functional impairment
f. Cognitive Functioning (new and approved for 2011-2012)
The Cognitive Functioning component was proposed by the Healthy Aging Program, Division of Adult and Community Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). Cognitive functioning was assessed in NHANES 1999-2002 during the household interview portion of the survey, and as an examination component in NHANES III (1988-94).
The addition of a cognitive functioning component in the mobile examination center has great public health relevance and would enhance the overall utility of NHANES data. An assessment of cognitive functioning of older adults is useful to include because it is an important risk factor for loss of independence, institutionalization and mortality in this group. Additionally, its inclusion in NHANES provides the ability to investigate prevalence and co-morbidities of declining cognitive functioning with other self-reported and objective physical measures.
Participants ages 60 and older are administered three tests:
The Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Word List Learning Test assesses recall and memory. The examinee is asked to read aloud 10 words, one at a time, displayed on the computer screen. There are three trials that are administered identically, except for the order of the words. Ten words are presented at a rate of 2 seconds between each word. After the words are presented, the examinee is asked to recall as many of the words as possible within 90 seconds. A fourth recall occurs after the other two tests are administered.
The Wechsler Adult Intelligence Scale (WAIS) digit symbol subtest evaluates attention and processing speed. It involves substituting a symbol for a random succession of numbers ranging between 1 and 9. This was previously used in NHANES III and the 1999-2002 NHANES.
The Animal fluency Test is designed to assess categorical verbal fluency, component of executive function. The participant is asked to name as many animals as they can in 60 seconds.
g. Weight History, Weight Self Image and Weight Related Behavior (WHQ)
NHANES continues to collect data for all participants 8 and older related to weight history and weight self-image. Children and adolescents are especially prone to fad diets and eating disorders. Unhealthy methods of weight loss can compromise growth and are not recommended by health care professionals. Questions on the reasons for weight loss and the types of weight loss practices used by children and adolescents ages 8-15 years will be asked in the MEC CAPI interview. This information will be used with socio-demographic and related nutrition and health information to develop public policies and programs to prevent and manage overweight among children and adolescents. The weight history component, for participants 16 years and older, is designed to permit evaluation of height loss with aging and weight status (stable or cyclical) over time.
h. Oral Health (modified and approved for 2011-2012)
Eight oral health questions asked in NHANES 2009-10 will be continued in 2011-12. In addition, 12 questions on oral health have been added in the home to collect information on dental visits, overall satisfaction with oral health status, impact of oral health on daily living and quality of life, and oral cancer. The questions on dental visits are asked for participants aged 1 year and older. Data on younger children are collected by proxy. Questions on oral health satisfaction, quality of life, and cancer will be asked for participants aged 30 years and older. Participants 20 years and older are asked an additional question to track receipt of dental preventive instructions. This additional question is designed to address a Healthy People 2020 oral health objective.
i. Aspirin Use (new and approved for 2011-2012)
Prevention of cardiovascular disease is the most critical public health issue in the U.S. Taking low-dose aspirin regularly has been shown to significantly reduce the risk of heart attack and stroke. In addition, numerous studies have suggested that aspirin may hold promise in helping to prevent cancer. Definitive national-level surveillance data on prophylactic aspirin use is needed to inform public health policy decisions. Inclusion of the aspirin subsection questionnaire on the NHANES 2011-2012 will fill this need to provide prevalence estimates of dosage-specific aspirin use for cardiovascular disease and cancer prophylaxis in the U.S. This will enable NHANES to more comprehensively provide insight into the underlying causes of observed health disparities for this cardiovascular disease and cancer prevention modality. Eleven questions have been added on the prophylactic use of aspirin. Participants are asked if a health provider has recommended this and if they are currently doing it. Those who have not had this recommendation from a health care provider are asked if they are taking low-dose aspirin on their own to prevent disease. Those who report taking prophylactic aspirin are asked how often and what dose.
j. Muscle Pain and Injury (new and approved for 2011-2012)
To make the Creatine Phosphokinase (CPK) laboratory data more useful, a short series of questions administered during the MEC interview has been added on muscle pain and injury. Recent muscle injury or strenuous exercise can elevate the CPK. The information from these items would help provide better background prevalence estimates for CPK levels in the general population, and help establish more optimal CPK reference ranges.
k. Urologic Health
Self reported information on urinary incontinence and nocturia will continue to be collected. These data are collected during the MEC CAPI interview. NHANES will provide national estimates on the prevalence of urinary incontinence and quality of life issues for those affected.
l. Telephone interview of hepatitis C positive participants:
NHANES is the only population-based study from which prevalence data are available on hepatitis C infection. Hepatitis C virus (HCV) is the most common chronic blood borne infection in the U.S. Although there is currently no vaccine to prevent HCV transmission, there are clear recommendations for infected persons to reduce risks for transmitting HCV to others. In addition, there are important recommendations for infected persons to prevent further harm to their liver and to be medically evaluated for chronic liver disease and possible treatment. A telephone survey of NHANES participants who are anti-HCV positive has been conducted since 2001 to determine the proportion of individuals who already knew of their infection status, what they know about hepatitis C, and what actions they are taking following the report of findings letter that informed them of their infection status. Telephone follow-up interviews will continue in 2011-12.
m. Pubertal Maturation Self-assessment (new and approved for 2011-2012)
The addition of the pubertal maturation self-assessment questions to the MEC Audio Computer-Assisted Self-Interview (ACASI) interview, for participants 8 to 19 years old, has important public health relevance and will improve the utility of NHANES clinical, biomarker, and questionnaire data. Information on pubertal maturation status is useful to include in NHANES since the endocrine changes manifested in secondary sexual characteristics underlie many physiological changes during puberty. Sexual development correlates more closely with physical changes such as height, weight, bone density and certain biochemical markers than chronological age, thus facilitating assessment of bone health, and body composition in pre-adolescence and adolescence. Furthermore, early sexual maturity has been found to closely correlate with self-image and sexual behaviors, which are also assessed in the MEC interview.
A validation study at Children's Hospital National Medical Center (CNMC), Washington, DC was conducted from March to August 2009. This study involved both a self-assessment of pubertal maturation and an assessment based on a physical examination done by a trained health care professional. The study showed that the audio computer-assisted self-interview (ACASI) method is a feasible method of pubertal maturation self-assessment for children as young as 8 years of age. There was good/excellent agreement between boy’s self-assessment and the examiner’s assessment of their genital stage (weighted kappa: 0.65) and pubic hair stage (weighted kappa 0.78). For girls there was excellent agreement between girl’s self-assessment and the examiner’s assessment of their breast stage (weighted kappa 0.81) and pubic hair stage (0.78). Over three-fourths of the participants thought it was easy to choose a drawing and almost 100% found the computer easy to use. Additional details about this study are found in Attachment 12a.
n. Other Interview Information
The NHANES interviews include questions that are included in other population surveys. Typically, these questions are used as covariates in data analyses rather than to compute national prevalence estimates. Some examples in NHANES are the Demographic (DMQ), Income (INQ), Health Insurance (HIQ), Housing Characteristics (HUQ), Health Care Utilization (HCQ), and Occupation (OCQ) sections.
Additional questions are included in the survey to assess such topics as reproductive health, risk behavior, and diet behavior in the U.S. population. Brief descriptions of the major NHANES supporting interview sections are provided.
Alcohol Use (modified and approved for 2011-2012): Questions on alcohol use are included for all participants 12 years and older. The questions are designed to ascertain quantity, and frequency of use for quantifying alcohol intake; to identify nondrinkers, light drinkers, and former heavy drinkers; and to determine the frequency of heavy drinking occasions among current drinkers. Data on alcohol intake during the previous day will also be obtained as part of the 24-hour dietary recall. The definition of binge drinking differs by gender. To correctly categorize women as binge drinkers the section was modified to ask about 4 or more drinks per occasion for women and 5 or more for men.
Cigarette and Tobacco Use (modified and approved for 2011-2012): Participants ages 12 and up will be asked questions about their history of cigarette and tobacco use. Additionally, questions about tobacco use in the last 5 days will be asked. The information about recent tobacco use is critical to the interpretation of the serum cotinine data. The pre - 2011 questionnaire is different for those 12-19 versus those 20 and older. We have changed the ages of these so that the 18 and 19 year olds are asked the same questions as those 20 and older.
Reproductive Health and History: Information about women’s reproductive health is essential for evaluating their health status and the relationship of menopausal status to chronic disease. A personal private interview is conducted with females 12 years and older. Information is obtained on age at menarche, pregnancy history, history of breast feeding, history of hysterectomy and oophorectomy, menopausal status and symptoms of menopause, and use of exogenous hormones (oral contraceptives, hormone replacement therapy). Most of the data collected in this questionnaire section will be used as covariates for other analyses.
Sexual Behavior: The information on sexual behavior is key to reducing the risk of STDs. Such behaviors include delaying onset of sexual intercourse by adolescents, minimizing number of sexual partners and utilizing barrier contraceptives. Sexual behavior, as well as other risky behaviors such as drug use was first included in NHANES III for use in analysis of serologic markers of sexual disease and has remained a part of the survey in each year. Participants 14 -59 years are asked about age of first intercourse, number of sexual partners, use of condoms, and history of sexually-transmitted diseases. The questions on sexual behavior are included to provide for targeting risk reduction efforts; assessing the results of such efforts; and improving current understanding of the epidemiology of STDs.
Participants 60-69 are asked a selected subset of these questions including, types of sexual behavior they have engaged in, age of first intercourse, and number of sexual partners in their lifetime.This will provide further information about risk factors in the aging cohort for infection with hepatitis C.
Drug Use: Questions on drug use are included for participants 14-59 years. The questions focus on lifetime use of street drugs or recreational drugs and the intravenous use of these drugs. Additional questions on age of initiation of drug injection, duration of injection drug use, and lifetime history of drug treatment are included in this section. No measurements for the presence of drug metabolites will be conducted. The use of drugs has been demonstrated to be a risk factor for sexually transmitted diseases. Injection drug use is also a risk for blood borne pathogens such as HIV, HBV and HCV. Information on drug use is necessary along with sexual behavior questions to develop a profile of risk-taking behavior.
Participants ages 60-69 will be asked a selected subset of these questions. They are only asked if they have ever used street drugs or recreational drugs and the intravenous use of these drugs. This will provide information about risk factors for infection with hepatitis C for this age group.
Responding to Emerging Public Health Issues, New Technology and Future Survey Options
One objective of the continuous NHANES is to provide a mechanism to respond to emerging and re‑emerging public health topics. The content of the survey is modified biannually to accomplish this objective. Survey modifications may include removing or “cycling out” survey content that has been in the survey for multiple years, modifying existing survey content to include new target age groups, modified data collection methods, the use of updated technology, and the addition of new interview, laboratory, and examination components and topics. The NHANES Program utilizes a public proposal solicitation process to develop recommendations for survey content. The process and proposal guidelines are posted on the NHANES website (http://www.cdc.gov/nchs/about/major/nhanes/research_proposal_guidelines.htm). NCHS disseminates the information to survey collaborators, federal agencies, and NHANES data users.
The Division of Health and Nutrition Examination Surveys (DHANES) anticipates that new technology will be adopted during future data collection activities. NCHS staff design, plan, implement and evaluate numerous methodology projects to evaluate new technology proposed for use in NHANES. For example, new questionnaire modules and examination component protocols are often pre-tested in-house and in the field prior to full survey implementation. This process may include cognitive testing of questions as well as pilot testing of components in the actual NHANES environment. Past experience has shown that one to three years of preparatory work may be required to fully test and prepare a new NHANES examination component for the survey. New equipment must be installed, calibrated, and tested; software must be installed and tested; database variables and data processing procedures must be developed and documented; data security provisions must be developed, tested, and approved; and training manuals, staff training, and quality control procedures must be developed.
2011-12 Pilot Tests (Completed in 2010)
Several protocols are being tested to be included in the 2011-2012 NHANES, after Ethics Review Board and OMB approval. As noted above, testing will occur within the current data collection whenever possible. When necessary, testing among paid volunteers will also be included. Informed consent will be modified as appropriate for the pilot or methodological test. . If the pilot or methodological test is deemed successful it will be included in the NHANES 2011-12 subject to OMB and ERB approval. DHANES plans pilot and methodological tests only for content fully expected to be successfully implemented on the NHANES survey. A report of each test is produced after completion of the pilot or methodological project.
The methodological studies and pilot tests planned include the following (for which OMB approval has been received):
Pre examination methodological data collection
Text Messaging Pilot Study (ages 12+)
Pilot tests of 2011-12 Examination Center Components:
Cognitive Functioning (ages 60+)
Oral Health (ages 2+)
Pubertal Maturation (ages 8-19)
Muscle Strength (ages 6+)
Sagittal Abdominal Diameter (SAD) (ages 8+)
Creatinine Phosphokinase (ages 12+)
Chemosensory Variation and Impairment (ages 40+)
Post examination methodological data collection:
Physical Activity Monitor (PAM) (ages 6+)
24 Hour Urine Sodium Validation Study (ages 20-79)
2013-14 Pilot Tests
The survey expects to continue conducting pilot studies for future cycles of continuous NHANES. During 2011-2012, pilot studies will be conducted to prepare for implementation during NHANES 2013-2014. Plans for future pilot studies have not been finalized. One methodologic study for future consideration is a 24 hour urine sodium study.
Sodium consumption in the US population is well above recommended limits. It is widely believed that the high sodium intake may be partially responsible for the high prevalence of hypertension in the US population. Efforts on many fronts in the public health community are targeting a reduction in sodium in the food supply. NHANES monitors the dietary intake of the US but dietary recalls are considered inadequate measures of dietary sodium intake at this time. Urinary excretion better characterizes sodium consumption but is extremely variable. Several studies have concluded that casual urine samples correlate sufficiently with 24 hour urine collections to allow for monitoring population intake of sodium. NHANES currently collects two urine specimens under conditions more rigid than ‘casual’. One is a timed urine collection and the second is the first morning void. NHANES is considering adding a 24 hour urine collection to its current urine protocol in future years (after 2011-12) to monitor population sodium intake.
A change package would be submitted to OMB before undertaking any methodological and/or pilot studies.
A Community HANES has been an area of interest for a number of years. If the opportunity arises, we would like to undertake methodological and other studies under this approval. This request permits NCHS the option to plan and test a Community HANES (C-HANES) project. C-HANES is a survey mechanism to address health status issues of defined populations (e.g., race/ethnic groups and/or small geographic areas) for which the standard, cross-sectional NHANES is inappropriate or infeasible. The age groups surveyed may be broad or may be restricted to certain subgroups, depending on the community’s needs. C-HANES should provide rapid access to health data. Typically, the time elapsed from the start of a project to data dissemination will be less than two years. The C-HANES can also provide a means to bring an examination center to the sample person through the use of smaller mobile examination units than the MECs used in the main survey.
The C-HANES interview and examination components and their protocols will be similar to those of previous NHANES. However, new components and new data collection methods may be added depending on the objectives of the survey and the population surveyed. The data collection system will include some or all of the following: interviews in the home; interviews at the examination center; physiological, medical, and dental examinations; and biological specimen collection. Space, time, and resources will be, as usual, the limiting factors for what can be done in the survey and where.
CDC is including burden hours to accommodate a C-HANES project involving up to 1,000 participants (Section 12, Table 1, line 2). This project would include an interview and examination component similar to the current NHANES, but no post-examination components. Pilot tests or methodological studies to develop new NHANES components are also included in the line 2 burden. OMB would be notified of any such projects through a change package.
Also included in this category is the completion of the 2012 National Youth Fitness Survey. The Secretary of Health and Human Service has dedicated funds to Obesity Prevention and Fitness, one of four critical priorities for the Department. The Obesity Prevention and Fitness priority advances activities to improve nutrition and increase physical activity to promote healthy lifestyles and reduce obesity related conditions and costs. Funds have been used to conduct an NHANES National Youth Fitness Survey (NYFS) in 2012, simultaneously with the regular NHANES. This survey is conducted among children 3-15 years old. The study takes place in the same locations as the full NHANES, but is conducted in a separate mobile examination trailer customized specifically for the NYFS. The NYFS is being conducted with children who are not participants in the full NHANES. A full description of the NYFS may be found in the following attachments: Attachment 13a NYFS Supporting Statement A, Attachment 13b NYFS Supporting Statement B, Attachment 13c NYFS 2012 Protocol, and Attachment 13d NYFS 2012 Questionnaire.
Privacy Impact Assessment Information
A Privacy Impact Assessment was submitted on September 8, 2011. The NHANES continues to collect personal identifying information, on a confidential basis, needed to recontact respondents and to match respondents to administrative records such as the National Death Index. The ability to track respondents and match to other records greatly expands the usefulness of the data at very low cost.
Only those NCHS employees, specially designated agents, and our full research partners, who must use the personal information for a specific purpose, can use such data.
The collection of information in identifiable form requires strong measures to ensure that private information is not disclosed in a breach of confidentiality. All NCHS employees as well as all contract staff receive appropriate training and sign a “Nondisclosure Statement.” Staffs of collaborating agencies are also required to sign this statement and outside agencies are required to enter into a more formal agreement with NCHS. The transmission and storage of confidential data are protected through procedures such as encryption and carefully restricted access.
3. Use of lnformation Technology and Burden Reduction
The majority of NHANES data, approximately 95%, are collected from respondents electronically, which helps reduce burden. NHANES uses survey information technology architecture (SITA) that supports fully automated and integrated information technology, relying on innovation and modern tools, and state of the art technology and information science. SITA provides increased capabilities that allow processing of complex data with significantly less editing than in previous NHANES surveys.
SITA provides NHANES with access to all data that are collected, much of which is available in real-time. The nature of the survey requires that data be accessible at multiple sites including contractor facilities, MECs, field offices, laboratories, and NCHS headquarters. SITA supports all phases of the survey including: 1) survey planning and design, 2) data collection, 3) data receipt, control and quality assurance, 4) reporting of survey results to survey participants, 5) data review, editing and analysis, 6) generation and documentation of public use data products, 7) tracking of survey respondents, and 8) generation of status reports on all aspects of the survey.
Technologic approaches such as monitoring examination flow and the use of CAPI methods have allowed increased amounts of data to be collected during the same period compared to earlier surveys.
4. Efforts to Identify Duplication and Use of Similar Information
NHANES is a unique source of health information on the U.S. population. Each year health interview and examination data are obtained. There are no other studies that collect the detailed health, dietary, laboratory and examination data that NHANES does. Duplication of effort is avoided through contacts and discussions with numerous Federal Government agencies during the content development and planning stage of NHANES. The organizations contacted are listed in Attachment 3 of this clearance request.
5. Impact on Small Businesses or Other Small Entities
Only individuals will be asked to participate. No small businesses will be involved in this data collection.
6. Consequences of Collecting the Information Less Frequently
Prior to 1999, NHANES was conducted periodically. There was a twelve year interval between the starting dates of NHANES II and III and there was an 11 year interval between the beginning of NHANES III and the current NHANES. New data items could only be added at the start of a survey cycle. These long intervals created major gaps in data availability. In addition, they made it difficult to introduce new topic areas into NHANES because the demand for data on the most highly prevalent conditions became acute during the intervals between the periodic surveys.
Because data needs and health concerns change rapidly, policy makers need current information to plan and evaluate Healthy People objectives, prevention and treatment programs, and the impact of legislative reform. To address these needs, in 1999 NHANES began continuous data collection. This reduces the potential for gaps in objective data needed by epidemiologists, health care planners, public health officials, and health policy analysts to answer policy and research questions.
Nutrition monitoring legislation explicitly calls for continuous coverage to monitor nutrition changes as they occur (see Attachment 1). Major changes in the consumption of food can occur with the successful marketing of new products or products marketed with specific health claims. Continuous data collection will facilitate timely evaluation of these changes. Continuous data collection will permit more frequent updates of reference standards and more timely development of reference standards for new diagnostic procedures. Emerging and re-emerging health problems can be added to the content of NHANES more readily if data are being collected continuously. Continuous collection of objective data will permit more timely evaluation of Healthy People objectives that require this data. Continuous data collection allows for greater flexibility in addressing all the objectives of NHANES and coverage of more population subgroups. The objectives of NHANES support the need for the continuous collection of NHANES data.
Respondents will participate in the data collection only one time. This may include follow-up studies. Currently, two telephone interviews occur after the examination. There is a follow-up study involving a few hundred participants with positive hepatitis C tests in which participants are asked to complete a short telephone interview as described in Section B.2., Data Collection Procedures. A second dietary recall is obtained on all survey participants.
There are no legal obstacles to reducing the burden.
7. Special Circumstances Relating to the Guidelines for 5CFR1320.5
This data collection fully complies with regulation 5CFR1320.5.
8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
a. Federal Register Notice
In compliance with 5 CFR 1320.8(d), a notice soliciting comments on the collection for NHANES was published in the Federal Register on June 6, 2012, volume 77, number 109, pp. 33463- 33464. See Attachment 2a for a copy of the notice. Public comments were received. See Attachment 2b for further details.
b. Outside Consultation
The content of NHANES is developed with input from numerous DHHS agencies (including NIH, FDA, and CDC), non-DHHS Federal agencies (including EPA, USDA, and HUD), non-government organizations, and individuals. The DHHS Data Council has been kept informed of the future NHANES plans. The DHHS Office of the Assistant Secretary for Planning and Evaluation has been briefed about the NHANES. Additionally, NCHS’s Board of Scientific Counselors has been informed on future planning.
NHANES is a collaborative undertaking. Broad input is sought from data users and interested parties to maximize the utility of the survey data. Extensive consultations occur in meetings with NHANES collaborators and interested agencies. A formal research proposal solicitation process occurs prior to content planning and development. The NHANES proposal guidelines are posted on the NHANES website NHANES website (http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/analytical_guidelines.htm).
The major efforts taken to support collaboration processes are described below. New content proposals were solicited for the 2011-2012 data collection cycle by publishing the proposal guidelines on the NHANES website. Members of the NHANES user community received letters inviting them to submit research proposals. Correspondence was sent to dozens of persons who have expressed interest in being kept informed of NHANES activities. Over 20 proposals were received in response to this solicitation. The responses ranged from a request to add one data item to requests to add entire examination components such as taste and smell measures, a revised oral health examination, and a cognitive functioning assessment.
NCHS staff made numerous presentations throughout the year at major medical and public health professional meetings as well as internal meetings organized by Federal agency research staff. The meetings provide an excellent forum for updating stakeholders on survey research activities and data products.
DHANES hosted an NHANES Consortium Meeting of our Federal collaborators and data users on April 7, 2010. The purpose of the meeting was to provide updates on NHANES topics such as nutrition monitoring activities, survey design features, recent survey content and proposed content for the 2011-12 survey, using the NCHS Research Data Center and the NHANES tutorial, and accessing surplus specimens. Guests in attendance, either in person or via web conference included representatives from the National Institutes of Health (NIH) , the United States Department of Agriculture (USDA) the Federal Drug Administration (FDA), The Office of the Assistant Secretary for Planning and Evaluation (ASPE) and the Centers for Disease Control and Prevention in Atlanta and Morgantown. Another consortium meeting covering these topics and more was held for non-federal stakeholders on May 4, 2010. In addition to the topics covered at the April 7th meeting, a representative from the CDC Foundation spoke at the 5/4 meeting to talk about how non-governmental entities can support CDC and NHANES. The May 4th meeting was conducted as a web-conference only, with participants from not-for-profit institutions and advocacy groups, universities, state and local government agencies, and private corporations all over the country.
9. Explanation of any Payment or Gifts to Respondents
To maximize response rates for the examination, NHANES participants have been remunerated, for their examination participation, since the 1970s. Remuneration began after a study was conducted to test the effect of remunerating sample persons who participated in NHANES I. The response rate for those who were told they would receive remuneration was 82%. The response rate for those who were not told they would receive remuneration was 70%. Results of the study were published as "A Study of the Effect of Remuneration Upon Response in the Health and Nutrition Examination Survey, United States," Vital and Health Statistics, Series 2-No.67. During NHANES II another study was conducted, this time on the effect of increasing remuneration. It showed that those who were told they would receive $20 after their examination had an examination rate of 79% while those who were told they would receive $10 had an examination rate of 74%.
In NHANES III (1988‑94) differential remuneration was successfully used to get participants to come to the examination session (morning, afternoon, or evening session) they were randomly assigned to. In prior NHANES, much data were lost due to failure of the participants to attend the randomly assigned session.
Continuous NHANES began in 1999 and the response rate was only 72%, therefore a remuneration study was undertaken in 2000. The basic comparison groups were the current level of remuneration plus a level approximately 50 percent higher. After 5900 observations the overall response rate was the same in both groups. Interviewers were not blinded to the remuneration and their primary objective is to get the participant to the examination center. Comments made during the debriefing suggested that interviewers spent more time convincing the lower remuneration group to be examined.
The response rates, for participants examined, for 2009 and 2010 to date are 78% and 74% respectively. The response rates to the examination from 1999-2010 are presented in the graph below.
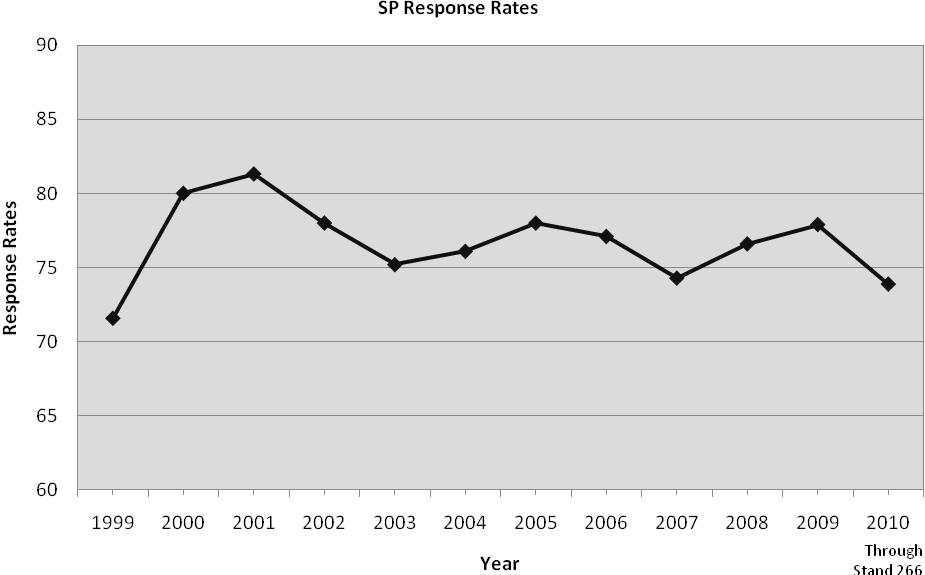 Below
are the 2011-2 remuneration rates. The only change from 2009-10 is
the addition of the $40 remuneration for the return of the physical
activity monitor (PAM). This is the same level of remuneration that
was used the PAM in 2003-06. Additionally, the remuneration for the
Flexible Consumer Behavior Survey telephone interview has been
removed because we are not doing that in 2011.
Below
are the 2011-2 remuneration rates. The only change from 2009-10 is
the addition of the $40 remuneration for the return of the physical
activity monitor (PAM). This is the same level of remuneration that
was used the PAM in 2003-06. Additionally, the remuneration for the
Flexible Consumer Behavior Survey telephone interview has been
removed because we are not doing that in 2011.
Examination payment
Subgroup |
2011-12 Payment |
16 and older assigned session |
$125 |
16 and older not assigned session |
$90 |
12-15 assigned session |
$75 |
12-15 not assigned session |
$60 |
Under 12 |
$40 |
Post-primary examination payment
Dietary Phone Follow Up $30
Urine albumin/creatinine ratio collection in the home $40
Physical Activity Monitor (new) $40
If a family has one or more children under the age of 16 and no parent/guardian has been selected into the sample, a $20 incentive is provided to accompany the child(ren) to the MEC. If participants must hire a sitter to care for children, elderly, or handicapped persons to be examined in the MEC, they are reimbursed at $5.25 an hour up to 6 hours. Participants also receive a transportation allowance for driving to the MEC, or a taxi is provided
SP transportation allowance
TRANSPORTATION ALLOWANCE 2007–2012 |
||
SP Transportation Allowance Mileages to MEC |
Cities |
Rural Areas |
<16 miles |
$30 |
$25 |
16–30 miles |
$45 |
$40 |
31–59 miles |
$55 |
$50 |
>60 miles |
$70 |
$65 |
Other efforts are made to maintain and increase response rates on a day-to-day basis (See Section B. 3. Methods to Maximize Response Rates and Deal with Nonresponse).
Potential remuneration changes may be requested during the 2011-12 NHANES. In the future, it may be necessary to test methods to encourage more participants to accept weekday MEC appointments, to avoid overly crowded exam sessions. Currently weekend appointments are frequently filled within the first two weeks at an exam site. As more participants are scheduled for exams, the already full weekend exam sessions may become overbooked with participants who are only available on weekends. Overbooking can result in incomplete data due to not having enough time to get all participants through their schedule of exams. Finding incentives to encourage participants to accept a weekday examination will ease the strain on the weekend sessions and increase the likelihood of having complete data on everyone who is MEC examined. NCHS is instituting and considering other non-monetary mechanisms to shift more appointments to weekdays. For example, modifying exam sessions hours on select days of the week to avoid participants having to travel during rush hours or changing exam schedules so that some sessions include the lunch hour to reduce the amount of leave participants might have to take off from work. If NCHS is unsuccessful in shifting more appointment to weekdays we may return to OMB with a change request for differential remuneration based on the day of the week. That is, offering higher remuneration for weekday MEC visits than for weekend appointments to make a weekday appointment more attractive to participants with schedules flexible enough to attend either a weekend or weekday MEC session.
Over the last 2 years we have continued our efforts to recruit more experienced interviewing staff, increased the training and retraining time for obtaining respondent cooperation and refusal conversion techniques, and trained field supervisory staff on more one-on-one mentoring of interviewers on non-response conversion techniques. At each stage of contact within a primary sampling unit (PSU) we have increased our attention on activities to reduce nonresponse. The PSU address listers must note every locked building, gated community, and college campus during their visit 4 months before field operations and notify the field operations team. The NCHS advance visit to PSUs has increased the emphasis on building community support during their pre-data collection visit. We have increased training of field office staff on appointment reminder calls and dealing with participants who call with questions and concerns. We’ve increased our efforts to get local media coverage and local endorsements.
In late 2009 we increased the number of interviewing staff from 27 to 30. We had had 27 interviewers since 1980. Additionally, we have begun working collaboratively with the Asian Pacific Island American Health Forum to develop recruitment strategies to attract Asian interviewing staff to maintain our high response rate after we begin oversampling Asians in 2011.
10. Assurance of Confidentiality Provided to Respondents
The Privacy Act of 1974 (5 U.S.C. 552a) “requires the safeguarding of individuals”, and Section 308(d) of the Public Health Service Act (42 U.S.C. 242m) requires the safeguarding of both individuals and establishments against invasion of privacy. Contractors who collect information identifying individuals and/or establishments must stipulate the appropriate safeguards to be taken regarding such information. The Privacy Act also provides for the confidential treatment of records of individuals, which are maintained by a Federal agency according to either individual’s name or some other identifier. This law also requires that such records in NCHS are to be protected from “uses other than those purposes for which they were collected.”
The confidentiality of individuals participating in NHANES is protected by section 308(d) of the Public Health Service Act (42 USC 242m), which states:
"No information, if an establishment or person supplying the information or described in it is identifiable, obtained in the course of activities undertaken or supported under section...306,...may be used for any purpose other than the purpose for which it was supplied unless such establishment or person has consented (as determined under regulations of the Secretary) to its use for such other purpose and (1) in the case of information obtained in the course of health statistical or epidemiological activities under section...306, such information may not be published or released in other form if the particular establishment or person supplying the information or described in it is identifiable unless such establishment or person has consented (as determined under regulations of the Secretary) to its publication or release in other form..."
In addition, legislation covering confidentiality is provided according to section 513 of the Confidential Information Protection and Statistical Efficiency Act of 2002 (CIPSEA) (PL-107-347), which states:
“Whoever, being an officer, employee, or agent of an agency acquiring information for exclusively statistical purposes, having taken and subscribed the oath of office, or having sworn to observe the limitations imposed by section 512, comes into possession of such information by reason of his or her being an officer, employee, or agent and, knowing that the disclosure of the specific information is prohibited under the provisions of this title, willfully discloses the information in any manner to a person or agency not entitled to receive it, shall be guilty of a class E felony and imprisoned for not more than 5 years, or fined not more than $250,000, or both.”
Consequently, all information collected in NHANES will be kept confidential, with an exception for suspected child abuse.
Privacy Impact Assessment Information
The NCHS Privacy Act Coordinator and the NCHS Confidentiality Officer have reviewed this package and have determined that the Privacy Act is applicable. This study is covered under Privacy Act System of Records Notice 09-20-0164 (“Health and Demographic Surveys Conducted in Probability Samples of the U.S. Population”).
An Advance Letter (Attachment 4) is mailed to each household in the sample segments announcing the impending arrival of an NHANES interviewer and explaining the confidential treatment of their responses. The informed consent documents for the interview, the
examination and the stored specimens each repeat the confidentiality assurance (Attachment 5).
It is the responsibility of all employees of NCHS, including NCHS contract staff, to protect and preserve all NHANES data (this includes all oral or recorded information in any form or medium) from unauthorized persons and uses. All NCHS employees as well as all contract staff have received appropriate training and made a commitment to assure confidentiality and have signed a “Nondisclosure Affidavit”. Staffs of collaborating agencies are also required to sign this statement and agencies are required to enter into a formal Designated Agent Agreement with NCHS before access to non-public data is permitted. It is understood that protection of the confidentiality of records is a vital and essential element of the operation of NCHS, and that Federal law demands that NCHS provide full protection at all times of the confidential data in its custody. Only authorized personnel are allowed access to confidential records and only when their work requires it. When confidential materials are moved between locations, records are maintained to insure that there is no loss in transit and when confidential information is not in use, it is stored in secure conditions.
NCHS policy requires physical protection of records in the field, and has delineated these requirements for the data collection contractor. The contractor also has its own policy and procedures regarding assurance of confidentiality and a pledge that all employees involved in NHANES must sign. The contractor provides all safeguards mandated by Privacy ACT and Confidentiality legislation to protect the confidentiality of the data. The contractor’s data security procedures comply fully with security requirements delineated by the Information Resources Management Office of CDC.
It is NCHS policy to make NHANES data available via public use data files to the scientific community. Confidential data will never be released to the public. For example, all personal information that could be potentially identifiable (including participant name, address, survey location number, sample person number), are removed from the public release files. The NCHS Disclosure Review Board reviews all files that will be released, to assure that directly or indirectly identifiable data are not included.
11. Justifications for Sensitive Questions
Objective data of a sensitive nature are described in this section. Content of a sensitive nature in the examination is discussed in the NHANES informed consent document (Attachment 5).
a. Social Security Number
Social Security Number (SSN) of all participants, children through adults, is requested in the household interview as a key item. The information is used to link administrative and vital records, such as the National Death Index (NDI), to the survey information. Additionally, in 2011-2012 NHANES will continue to use the SSN to link with Food Stamp Program and Women, Infants and Children Program administrative records from the USDA.
Permission to link is obtained from respondents as follows:
“The Department of Health and Human Services will conduct statistical research by combining {your/his/her} survey data with vital, health, nutrition and other related records. {Your/SP’s} social security number is used only for these purposes and the Department will not release it to anyone, including any government agency, for any other reason. Providing this information is voluntary and is collected under the authority of Section 306 of the Public Health Service Act. There will be no effect on {your/his/her} benefits if you do not provide it.”
ONLY READ IF ASKED. [Public Health Service Act is title 42, United States Code, section 242k.]
b. CMS Health Insurance Claim Number
Participants covered by Medicare will be asked to provide the CMS Health Insurance Claim Number. This will be used to link to Medicare records for further health research and also to link with other records for possible recontact of NHANES participants.
Permission to link is obtained from respondents as follows: “This number is needed to allow Medicare records of the Centers for Medicare and Medicaid Services (CMS) to be easily and accurately located and identified for statistical or research purposes. We may also need to link it with other records in order to re-contact you. Except for these purposes, the Department of Health and Human Services will not release your Health Insurance Claim Number to anyone, including any other government agency. Providing the Health Insurance Claim Number is voluntary and collected under the authority of the Public Health Service Act. Whether the number is given or not, there will be no effect on your benefits. This number will be held in strict confidence. [The Public Health Service Act is 42 USC 242k.]”
c. Residency Status
Information about country of birth and length of residency in the U.S. is requested and may be sensitive for recent immigrants. This information is important in analyzing health and nutrition data because acculturation may be related to use of the health care system, diet, and health practices. Additionally, recent immigrants may not have access to health, nutrition, and income assistance programs that affect access to health care and health and nutrition status. Interviewers will be trained to reassure participants that the information is confidential and will be used for statistical reporting only.
d. Other Content
Some of the NHANES research topics include potentially sensitive questions or examinations. In the informed consent procedure, all sample persons are advised of the voluntary nature of their participation in the survey or any of its components. Again during the physical examination, each sample person is reminded that he or she can refuse to answer questions or undergo any parts of the examination that are objectionable.
All questions and procedures have been reviewed by the NCHS Ethics Review Board (formerly called the NCHS Institutional Review Board) and the data collection contractor’s institutional review board for issues of sensitivity and safety (see Attachment 6). The potential sensitivity of questions and procedures is an evaluation criterion in determining content of the survey. The multipurpose nature of NHANES makes it necessary to exclude topics so sensitive that they may interfere with participation.
Questions and procedures thought to be of a sensitive nature are listed below. Most of these are questions commonly asked in health care settings. Within the Mobile Examination Center, answers to sensitive questions are obtained privately in contrast to the household survey.
i. Sexual behavior and sexually transmitted diseases: Several sexually transmitted diseases are part of the NHANES—herpes simplex I and II, HIV, hepatitis B and C, chlamydia and selected strains of human papilloma virus (HPV). Information is obtained through questionnaires, exams, and lab tests. It is essential to clarify risk factors and identify at-risk population subgroups associated with infection in order to plan and evaluate prevention programs. This requires self-reported information on sexual behavior combined with prevalence data on infection. The relationship between the sexually transmitted diseases and the individual risk factors is critical to understanding changes in the prevalence of the diseases.
The questions on sexual behavior were developed in consultation with other centers of CDC and are similar to those developed by the National Survey on Drug Use and Health (OMB 0930-0110), National Survey of Family Growth (NSFG)(OMB 0920-0314) and those used in NHANES III. These questions will be administered using A-CASI methods in a private room.
Questions on sexual activity are asked of males and females 14 years and older. It is important to ask these sensitive questions because many teenagers are sexually active and because sexual activity is a risk factor for disease transmission. NHANES is the only national health survey that assesses sexually transmitted disease exposure and prevalence in U.S. youth and adults using biologic specimens. The results of tests for sexually transmitted diseases will not be mailed to examinees for reasons of confidentiality. Examinees will be given a toll-free number they can call with the use of a self-selected password, to obtain their results.
ii. Drugs, alcohol, and tobacco: Drug, alcohol, and tobacco use are risk factors for many of the health conditions studied in NHANES. Questions are asked in the MEC of persons 14 years of age and older concerning the use of alcohol, marijuana, and cocaine; participants 12 and older will be asked about alcohol consumption and tobacco use. Similar questions were asked in NHANES III. The MEC interview is conducted in a private room. Illicit drug use, tobacco, and alcohol questions are administered to youth 12-19 years of age in A-CASI mode.
iii. Reproductive health and menstruation: Questions on reproductive health history and use of oral contraceptives, asked of females 12 years and older, may be considered sensitive by some respondents. Privacy is assured by asking the questions in the MEC interview room.
Age of first menstruation will be obtained for females 8 years and older. This question will be asked of parents of girls 8 to 11 years of age, and will be self-reported for all females 12 years and older. Information on menarche for 8-11 years of age is necessary for interpretation of biochemical and hematological assessments. As a safety screen for the dual X-ray absorptiometry (DXA), a pregnancy test will be performed on menstruating females ages 8-11 and all females 12 through 59 years. This will be explicitly addressed in the informed consent document.
iv. Mental health: Adolescents and adults of all ages will be asked a short depression screening module called the Patient Health Questionnaire or the "PHQ-9." The questions are taken from the depression module of the PRIME-MD, a self-administered questionnaire that was first used in clinical setting. The interviews will be conducted with full confidentiality in a private room in the mobile examination center by specially trained interviewers. Participants will also be told that they may terminate the interview at any time.
v. Male and female urologic health: Conditions such as urinary incontinence and gynecologic infections affect millions of Americans. The information collected in NHANES is critical to understanding the magnitude of these problems and their impact on health and quality of life. The interviews will be conducted with full confidentiality in a private room in the mobile examination center by specially trained interviewers. Participants will also be told that they may terminate the interview at any time.
vi. Pubertal Maturation: The pubertal maturation module conducted among participants ages 8-19, may be considered sensitive by some respondents. Privacy is assured by having respondents self-administer these questions while alone, in the MEC interview room.
Standard NHANES consenting procedures will be followed. In addition, designated staff at the MEC will meet with parents or proxies of children aged 8-17 years and participants aged 18 and 19 years regarding the Pubertal Assessment module. Parents and participants will be asked to read the appropriate Pubertal Maturation Assessment Informational Flyer (Attachment 12b). The MEC physicians will be trained to share age and gender appropriate drawings with parents and participants as requested and to answer general questions regarding puberty. The designated MEC staff will record that the parents or participants were given the flyers and the opportunity to read the flyers, see the drawings, and ask questions. Participants will be blocked from the MEC Interview until this has been completed.
Vaginal Swabs: Women ages 14-59 years will be requested to collect a self-obtained vaginal swab. The swab will be used to test for human papilloma virus (HPV). Survey participants will perform the swab collection in a private bathroom.
Future content: As discussed in the Responding to Emerging Public Health Issues, New Technology and Future Survey Options portion of section A.2., during the conduct of NHANES, new content may be pilot-tested or added, as new diagnostic procedures become available or as new conditions emerge. This content will be handled in similar fashion to that discussed above in the introduction to this section (A. 11d Other Content). Information will be explicitly discussed in the informed consent document if the content is considered sensitive, and appropriate privacy and confidentiality safeguards included.
12. Estimates of Annualized Burden Hours and Costs
a. Time Estimates
This submission requests OMB approval of data collection, specifically to complete the 2011-2012 NHANES, the 2012 NYFS, and for testing modules for the 2013-2014 survey.
Approximately one-quarter year of data collection is needed to complete the 2011-2012 cycle. Approximately 3,850 respondents participate in some aspect of the full survey (Attachment 9). Of these many complete the screening portion and are then screened out of the sample. Some additional respondents complete the screener and the household interview sections, but decline to be examined. The remaining approximately 1,300 participate in the screener, household interview and physical examination. The majority of these people also participate in a second dietary recall interview. A very small number participate in a hepatitis C follow-up. Averaging the burden across all respondents, at these varying levels of participation, results in an average burden of 2.4 hours. (The respondents who participate in all aspects of the survey can expect an estimated burden of 6.7 hours as documented in the signed informed consent documents [attachment 5].) The burden for this activity is 9,240 hours.
The completion of the NYFS will have approximately 1,037 respondents in this quarter for a total burden of 1,037 hours. In addition, up to 1,000 additional persons (non-NHANES respondents) might participate in tests of procedures or special studies. The average burden for these special study/pretest respondents is 3 hours for a total of 3,000 hours of burden (Attachment 11).. The burden for special studies and the completion of the NYFS is combined in Table 1, line 2, for a total of 4,037 hours.
The overall burden for the completion of all projects is 13,277 hours. When a full year of data is collected, the burden was calculated at 49,626 hours
TABLE 1 – ANNUALIZED BURDEN HOURS AND COSTS
Type of Respondent |
Number of Respondents |
Number of Responses per respondent |
Average Burden per Response (in hours) |
Total Burden Hours |
1. NHANES Respondents |
3,850 |
1 |
2.4 |
9,240 |
2. Special study/pretest participants |
2,037 |
1 |
2 |
4,037 |
Total |
|
|
|
13,277 |
b. Cost to Respondents
The hourly wage rate of $21.74 per person is based on income from wages and salary from the Bureau of Labor Statistics: http://www.bls.gov/oes/current/oes_nat.htm#00-0000. This wage rate for all persons was used since respondents do not fall into a single economic or occupational category. The total cost was $288,642 or $49.03 per respondent. (NOTE: There are no out-of-pocket costs to survey participants. Participants are remunerated for their time as well as for child care and transportation expenses.)
13. Estimate of Other Total Annual Cost Burden to Respondents or Record Keepers
None.
14. Annualized Cost to the Federal Government
This project is a multi-year, continuous survey, with survey planning, data processing and analysis, and data collection occurring simultaneously. These figures are broad estimates based on past NHANES data collection budget estimates. Staff costs were primarily based on Division of Health and Nutrition Examination Surveys personnel costs, which were obtained from the NCHS Financial Management Office. A proportion of these costs are paid by funds transferred to the CDC budget from collaborating agencies. It is estimated that about 30 percent of survey costs will be covered through this support from agencies outside of NCHS.
Table 1. Estimated survey cost per year
Category |
Annualized Cost |
Equipment, exam centers, data collection and processing, contracts, labs/readings |
$35,000,000
|
NCHS staff costs for survey planning, data analysis and overhead |
$6,000,000 |
NCHS printing, travel, supplies, etc. for NHANES staff |
$200,000 |
Total |
$41,200,000 |
15. Explanation for Program Changes or Adjustments
The currently approved burden is 49,626 hours. The requested burden is 13,277, a decrease of 36,349 hours. The decrease is due to the fact that only one-quarter year of data needs to be collected to complete the project.
16. Plans for Tabulation and Publication and Project Time Schedule
The data collected in NHANES will be released in several formats: public release Internet data sets, NCHS Vital and Health Statistics and Data Briefs, the CDC Morbidity and Mortality Weekly Report, CDC’s National Report on Human Exposure to Environmental Chemicals, Congressionally mandated annual reports such as Health U.S. and Healthy People 2010, and journal articles. Data will be presented and disseminated at professional meetings. The schedule is below in Table 16-1. The NCHS National Conference on Health Statistics provides an excellent means of notifying data users about upcoming data releases, showcasing the latest data releases, and providing tutorials on how to use NHANES data. Data will also be analyzed upon request by Federal health and nutrition policy committees, such as the U.S. Dietary Guidelines Advisory Committee and the National Academy of Sciences.
Analyses and presentations will be made by NCHS staff and other CDC staff in collaboration with consultants and staff from other Federal agencies and collaborating organizations. Schools of public health, research organizations, and individual researchers can analyze the NHANES data independently by using the public use data files. The release of public use files for the 2011-2012 NHANES is scheduled to begin by November 2013.
Published reports and presentations will address numerous topics in the areas of health status, nutritional status, and survey methodology. The prevalence of selected conditions and diseases will be established and normative distributions will be produced for many physiological and biochemical characteristics by age, sex and other demographic characteristics. Other reports will focus on the interrelationships between health conditions and risk factors assessed in NHANES or specific hypotheses that have been previously reported in studies involving population groups and subgroups.
Although the annual NHANES samples are nationally representative, analysts will need to pool data from several years to produce reliable estimates for most variables and health parameters of interest. Examples of analyses are listed below. This is not intended to be a complete listing of all planned analyses.
a. Health Status: Cross sectional and trends reports on the prevalence of conditions and risk factors such as hypertension, diabetes, urologic disease, smoking, infectious disease, physical activity, sexual behavior, abuse of alcohol and drugs and many other conditions are planned. Descriptions of dietary intake, physical activity, functional impairments, and distributions of biochemical measures including trends data are also planned.
b. Nutritional Status: NHANES measures both dietary intake and physiological and anthropometric measures of nutritional status. There will be reports on topics such as iron deficiency anemia, obesity, dietary supplements use, dietary intake, and deficiencies of selected biochemical indicators such as folate, and antioxidants. Comparisons of nutritional status and health status will also be done.
c. Special Reports: Information from the study will also be released in Health, U.S. and in reports by special groups such as the National Cholesterol Education Panel, the National Nutrition Monitoring and Related Research Program, the National High Blood Pressure Education Program, etc.
d. Methodology Reports: There will be NCHS reports describing all aspects of the design, content, conduct, and quality control of NHANES. The expected variability of estimates emanating from the study is covered in Part 2 of Section B of this supporting statement. This information will be published with each report. The NHANES implementation schedule, which includes the dates for availability of data, is shown below.
DHANES provides Analytic Guidelines to be used by NCHS staff and others using the NHANES public use data files. The NHANES Analytic Guidelines are included in Attachment 7. They are also available on the NHANES website (http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/analytical_guidelines.htm). Additionally, the Division of Health and Nutrition Examination Statistics has developed Tutorials to assist analysts in learning to use the NHANES data optimally. The tutorials are located at http://www.cdc.gov/nchs/tutorials/ . As resources permit new topics are added to the tutorial related to some of the more complex data applications. Upcoming tutorial modules include one that addresses using the Environmental Chemical data sets and another will assist users with the Physical Activity Monitor data collected from 2003-2006.
Table 16-1 PROPOSED TIME SCHEDULE: NHANES PLANNING, DATA RELEASE, AND REPORTING ACTIVITIES
|
|
2011 |
2012 |
2013 |
2014 |
Type of Activity |
Planning Activities |
Solicit/pilot proposals for 2013-2014 |
Pilot test new 2013-2014 components |
|
|
Tabular Reports* |
2009/2010 data |
|
2011/2012 data |
|
|
Micro-data Files (internet) |
|
|
2011/2012 first release in November |
|
|
Dietary Data** |
|
|
|
2011/2012 data |
|
Research Data Center Access*** |
|
|
|
2011/2012 data |
* Produce limited “early release” data tables on specific topics of public health significance (special request).
** Additional separate release of NHANES dietary recall data in accordance with DHHS/USDA survey integration plans.
***NHANES variables not released on micro-data files due to disclosure risks. See information on NCHS Research Data Center (http://www.cdc.gov/rdc/) and the NCHS Policy on Release of Micro Data (http://www.cdc.gov/nchs/data/NCHS%20Micro-Data%20Release%20Policy%204-02A.pdf).
17. Reason(s) Display of OMB Expiration Date is Inappropriate
We have several forms that are triplicate, NCR-type pages pasted into glossy, multi-page brochures, which require considerable advance time for printing. To save substantial printing costs, since 1999 OMB has granted an exception from printing the expiration date on these forms for data collection. We request that exemption be continued through the term of this clearance.
18. Exceptions to Certification for Paperwork Reduction Act Submissions.
There are no exceptions to the certification.
| File Type | application/msword |
| Author | Vicki Burt |
| Last Modified By | Conner, Catina (CDC/OD/OADS) |
| File Modified | 2012-08-28 |
| File Created | 2012-08-28 |
© 2026 OMB.report | Privacy Policy