0990-HospitalProgram_Part B-Statistical Methods
0990-HospitalProgram_Part B-Statistical Methods.doc
The Hospital Preparedness Program
OMB: 0990-0391
Office of Preparedness and Emergency Operations Division of National Healthcare Preparedness Programs
Supporting
Statement for Paperwork Reduction Act Submissions
for the Division
of Preparedness Planning on behalf of the National Healthcare
Preparedness Program Hospital
Preparedness Program Data Collection
U.S.
Department of Health and Human Services 330
C ST., SW, Rm 5615
Washington
DC 20201
(202)
245-0974
cliffon.smith@hhs.gov
Cliffon
Y. Smith, MPA Public
Health Analyst State
and Local Initiatives Team
U.S.
Department of Health and Human Services
Assistant
Secretary for Preparedness and Response Office
of Preparedness and Emergency Operations Division
of Preparedness Planning
June,
2011



Table of COntents
A. justification.....…………………………………………………………………………1
1. Circumstances Making the Collection of Information Necessary 1
2. Purpose and Use of the Information Collection 5
3. Use of Information Technology and Burden Reduction 6
4. Efforts to Identify Duplication and Use of Similar Information 7
5. Impact on Small Businesses or Other Small Entities 7
6. Consequences of Collecting the Information Less Frequently 8
7. Special Circumstances Relating to the Guidelines of 5 CFR § 1320.5(d)(2) 8
8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency 9
9. Explanation of Any Payment or Gift to Respondents 9
10. Assurance of Confidentiality Provided to Respondents 9
11. Justification of Sensitive Questions 10
12. Estimates of Annualized Burden Hours and Costs 10
13. Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers 11
14. Annualized Cost to the Federal Government 11
15. Explanation for Program Changes or Adjustments 12
16. Plans for Tabulation and Publication and Project Time Schedule 12
17. Reason(s) Display of OMB Expiration Date is Inappropriate 14
18. Exceptions to Certification for Paperwork Reduction Act Submissions 14
1. Respondent Universe and Sampling Methods 14
2. Procedures for the Collection of Information 15
3. Methods to Maximize Response Rates and Deal with Nonresponsiveness 15
4. Test of Procedures or Methods to be Undertaken 16
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data 16
C. LIST OF APPENDICES
Appendix A: Pandemic and All Hazards Preparedness Act (PAHPA)
Appendix B: End of the Year Progress Report (Sample Collection)
Appendix C: Hospital Preparedness Sample Table for Data Analysis
OMB Supporting Statement
A. JUSTIFICATION (Sections 1 – 18)
1. Circumstances Making the Collection of Information Necessary
The Program Evaluation Section (PES), part of the Department of Health and Human Services (HHS), Assistant Secretary for Preparedness and Response (ASPR), Office of Preparedness and Emergency Operations (OPEO), Division of Preparedness Planning (DPP), in conjunction with the Hospital Preparedness Program (HPP) in the Division of National Healthcare Preparedness Programs, is seeking clearance by the Office of Management of Budget (OMB) for a Generic Data Collection Form to serve as the cornerstone of its effort to assess awardee performance under the HPP Cooperative Agreement (CA) Program. Performance data are gathered from awardees as part of their Mid-Year and End-of-Year Progress Reports and other similar information collections (ICs), such as surveys to address which have the same general purpose, account for awardee spending and performance on all activities conducted in pursuit of achieving the HPP Grant goals. As healthcare system preparedness evolves, the Hospital Preparedness Program (HPP) has been driving the development of healthcare coalitions to improve national preparedness, response and community resilience. The program’s emphasis on a standardized, all-hazards approach supports the seamless coordination among healthcare facilities in the case of an emergency event. The program will collect information, not intended to measure coalition performance, but aimed to generate baseline info about existing coalitions.
This data collection effort is crucial to HPP’s decision-making process regarding the continued existence, design and funding levels of this program. Results from these data analyses enable HPP to monitor healthcare emergency preparedness and progress towards national preparedness goals. HPP supports priorities outlined by the National Preparedness Goal (the Goal) established by the Department of Homeland Security (DHS) in 2005.1 The Goal guides entities at all levels of government in the development and maintenance of capabilities to prevent, protect against, respond to and recover from major events. Additionally, the Goal will assist entities at all levels of government in the development and maintenance of the capabilities to identify, prioritize and protect critical infrastructure.
This collection is authorized by section 2802(b) of the Public Health Service (PHS) Act, as amended by the Pandemic and All-Hazards Preparedness Act (PAHPA) (P.L. 109-417).2 PAHPA authorizes HHS to award cooperative agreements to enable eligible organizations to improve surge capacity and enhance community and hospital preparedness for public health emergencies (the full text of PAHPA is included in Appendix A). Medical surge, as defined by Medical Surge Capacity and Capability Handbook (MSCC) states that surge capacity is the ability to provide adequate medical evaluation and care in events that severely challenge or exceed the normal medical infrastructure of an affected community (through numbers or types of patients).3 PAHPA outlines administrative and financial annual reporting requirements for awardees, so that HHS can monitor the performance of awardees and assure proper expenditure of funds. In addition, Section 201 of PAHPA mandates the achievement of measurable evidence-based benchmarks and objective standards:
‘‘(g) ACHIEVEMENT OF MEASURABLE EVIDENCE-BASED BENCHMARKS AND OBJECTIVE STANDARDS.— ‘‘(1) IN GENERAL.—Not later than 180 days after the date of enactment of the Pandemic and All-Hazards Preparedness Act, the Secretary shall develop or where appropriate adopt, and require the application of, measurable evidence-based benchmarks and objective standards that measure levels of preparedness with respect to the activities described in this section and with respect to activities described in section 319C– 2. In developing such benchmarks and standards, the Secretary shall consult with and seek comments from State, local and tribal officials and private entities, as appropriate. Where appropriate, the Secretary shall incorporate existing objective standards. Such benchmarks and standards shall— ‘‘(A) include outcome goals representing operational achievement of the National Preparedness Goals developed under section 2802(b); and ‘‘(B) at a minimum, require entities to— ‘‘(i) measure progress toward achieving the outcome goals; and ‘‘(ii) at least annually, test, exercise, and rigorously evaluate the public health and medical emergency preparedness and response capabilities of the entity, and report to the Secretary on such measured and tested capabilities and measured and tested progress toward achieving outcome goals, based on criteria established by the Secretary.”
Performance measure data represent the extent to which awardees have met outcome goals and objectives that can be demonstrated through exercises and drills. These data allow HPP to identify strengths and gaps in healthcare emergency preparedness and supply it with a ready source to distribute “best practices” and ‘lessons learned”, a request made by awardees so that they can learn from each other. PAHPA also gives HPP authority to withhold funds to recipients who fail to meet the performance benchmarks specified in Section 201:
‘‘(5) WITHHOLDING OF AMOUNTS FROM ENTITIES THAT FAIL TO ACHIEVE BENCHMARKS OR SUBMIT INFLUENZA PLAN.—Beginning
with fiscal year 2009, and in each succeeding fiscal year, the Secretary shall— ‘‘(A) withhold from each entity that has failed substantially to meet the benchmarks and performance measures described in paragraph (1) for the immediately preceding fiscal year (beginning with fiscal year 2008), pursuant to the process developed under paragraph (4), the amount described in paragraph (6).”
As a result, HPP must collect the data elements proposed in this data collection effort to measure awardee performance against benchmarks and standards and to determine future funding amounts.
Program Description
In 2002, the U.S. Department of Health and Human Services (HHS) established the National Bioterrorism Hospital Preparedness Program (NBHPP) “to enhance the ability of hospitals and healthcare systems to prepare for and respond to bio-terror attacks on civilians and other public health emergencies, including pandemic influenza and natural disasters”. Administered initially by the Health Resources and Services Administration (HRSA), in March 2007, the NBHPP was transferred to ASPR / OPEO, as required by PAHPA. Now known simply as the Hospital Preparedness Program (HPP), it is administered by the Division of National Healthcare Systems Preparedness Programs within ASPR.
Since FY 2002, the Hospital Preparedness CA Program (hereafter referred to as HPP) has provided funding to all 50 states; the District of Columbia; the three metropolitan areas of New York City, Los Angeles County and Chicago; the Commonwealths of Puerto Rico and the Northern Mariana Islands; the territories of American Samoa, Guam and the U.S. Virgin Islands; the Federated States of Micronesia; and the Republics of Palau and the Marshall Islands. Approximately 85% of the nation’s hospitals participate in HPP. The grant helps to increase the capacities and capabilities of hospitals and other supporting healthcare entities to plan for, respond to and recover from mass casualty events. These funds are used to help States, territories and the specified municipalities meet the National Preparedness Goal and the following goals as outlined in section 319C-2 of the PAHPA:
Integration: Insure the integration of public and private medical capabilities with public health and other first responder systems
Medical capacity and capability: Increase the preparedness, response capabilities and surge capacity of hospitals, other health care facilities (including mental health facilities), trauma care and emergency medical service systems, with respect to public health emergencies
At-risk individuals: Be cognizant of and prepared for the medical needs of at-risk individuals in their community in the event of a public health emergency
Coordination: Minimize duplication of, and ensure coordination between, Federal, State, local and tribal planning, preparedness, response and recovery activities (including the State Emergency Management Assistance Compact)
Continuity of operations: Maintain vital public health and medical services to allow for optimal Federal, State, local and tribal operations in the event of a public health emergency.
Description of Data Collection Effort
Generic Form
Healthcare systems preparedness is essential during an event or disaster. In the wake of the September 11th attacks, increased attention has been given to the critical role healthcare systems readiness plays in responding to and recovering from threats both natural and man-made. Consequently, as part of its funding agreements with awardees, HPP has maintained specific reporting requirements about how well awardees have accomplished meeting the Level 1 and 2 healthcare preparedness sub-capabilities and the overarching requirements such as staff training and the conduct of exercises, targeted for development during the HPP grant period of performance.
Into this generic data collection form (i.e., End-of-Year Progress Reporting template – see Appendix B), each awardee is required to provide responses related to demographic information (such as the number of facilities and hospital beds within a state), administration, budget and sub-capability information as well as performance measure data, including medical surge and coalitions. Administrative data includes a description of activities and progress as well as budget elements, such as proposed, obligated, and spent funds related to required emergency preparedness capabilities. In addition, the reports collect additional data elements necessary for further analysis. provides a summary of the data elements in these data collection tools.
Table 1. Summary of HPP Program Mid-Year and End-of-Year Report Elements
-
Administration and Capability Elements
Performance Measure Elements
Demographic Information
Administration / Budget
Level I sub-capabilities
Level II sub-capabilities
Additional considerations and emerging items of interest as related to the HPP Guidance
Bed tracking system reporting capacity
Redundant and two-way communication
Emergency volunteer system reporting capabilities
Fatality management
Hospital evacuation
Timely completion of reporting requirements
Medical Surge
Coalitions
Additional data elements
Future Information Collections (ICs)
Review of the literature, external evaluations conducted on the program, as well as analysis of our own HPP data, indicates that leveraging the collaboration of local community resources and partners becomes more and more important to successful preparedness and response models among healthcare entities, especially in the presence of fiscal constraints. The UPMC determined that the support and nurturing of local healthcare coalitions is one of the single most important contributions of HPP, and even greater emphasis on coalition building and implementation is increasingly more germane as HPP moves forward. As the program direction has evolved, the current collection has ended and a new collection has been developed to assess preparedness and program growth. With the new MY and EOY routine collections, Program will initiate GenICs every other month, to test the feasibility of the new collections and to determine if any further data collection might be useful. The GenICs will be supplemental collections that are within scope of the routine collections and will be triggered during the grant period; and, initiated to conduct pretesting of approved/new surveys, as well as other basic methodological research to gain insight into aspects of data quality. The MY and EOY collections will be collected for all awardees; where GenICs will be a determined subset of awardees.
Consequently, it may be necessary to occasionally probe deeper than would be expected in the HPP Mid-Year or End-of-Year Reports, in order to gain enough baseline information to quantify future progress. Please note, that currently, the routine MY and EOY reports collect the same information. The MY differs from the EOY, as we use the MY collection to gauge awardee progress and need for technical assistance. Future information collections that help identify these coalition attributes may be developed to meet this new program emphasis.
Additionally, to reduce administrative burden on awardees, there is a need to develop
reporting forms and templates that allow awardees and ASPR to more easily capture the data
and other information already provided in the grant application at other times during the
yearly grant cycle, and onsite visits by project and field officers (e.g. pre-populating some
elements of the mid-year and end- of-year reporting). Such reporting will systematically
capture relevant information in a format that allows for easy access and use within a number
of related grant business processes, including Grants management, Program and project
management, and performance metrics and evaluation. A standardized program-specific
application addendum will facilitate such data retrieval and decrease overall government
administration costs.
2. Purpose and Use of the Information Collection
The current generic data collection instrument included in Appendix B represents a concerted effort to develop a uniform system for reporting, which serves to improve the program’s ability to monitor progress in healthcare system preparedness, and report program accomplishments. HPP uses the information gained from the administration of this tool to monitor awardees’ compliance with program requirements and the development of selected healthcare preparedness capabilities.
Reflecting PAHPA’s emphasis on performance measurement and accountability, this data collection effort also helps determine healthcare emergency response capabilities. These data include indicators used to calculate performance measures, such as the number of surge beds, or the number of preparedness exercises and drills conducted during the project period, and other information needed to assess the nation’s state of healthcare preparedness. While previous data collection efforts are focused almost exclusively on capacity, describing the number of beds or personnel available or types of equipment and systems purchased, this data effort aims at measuring capability, measuring demonstrable performance during a simulated or actual disaster. This generic clearance and similar information collections to come under it, but not yet named, will continue to monitor emergency response capabilities, healthcare coalitions and related budget information. By using capability based performance metrics, HPP can provide more focused technical assistance.
The data provides HPP with the ability to review progress and generate a variety of analytic reports on financial and programmatic objectives, including comparisons of awardee-specific performance relative to an awardee’s Federal region, the nation and the 50 States. Financial analyses allow project officers to see intra-regional distribution of the extent to which spending aligns with program goals. The measure of impact of the HPP Program informs future decisions regarding funding and expectations of awardees. Consequently, the reporting increases HPP’s ability to quickly and efficiently analyze data, identify trends, provide technical assistance, make timely program decisions and provide awardees, HHS, Congress and other Federal agencies with data about the HPP Program, including information for the OMB Performance Assessment Rating Tool (PART) and GPRA, to measure progress against program performance measures.
3. Use of Information Technology and Burden Reduction
All of the data are collected and submitted electronically in order to reduce burden on the awardees. The Mid-Year and End-of-Year Reports are submitted via a user-friendly online interface that includes drop-down menu boxes, some pre-populated cells and data validation features to facilitate data entry and to increase data accuracy, quality and completeness. In addition, HPP can easily migrate the data submitted electronically into a data warehouse for archival purposes which can be used to conduct trend analysis over time. This data collection effort reduces the possibility of lost and missing data that could result from paper submission, and allows for timely and accurate data analysis that is not possible with paper submission. The data collection tools are developed to increase the utility, integrity and objectivity of the data, and will be collected and maintained consistently with OMB and HHS information quality guidelines.
4. Efforts to Identify Duplication and Use of Similar Information
HPP is the only Federal program that provides preparedness funds to hospitals and other healthcare facilities (e.g., nursing homes, etc.), through State Departments of Health. The States collect data from HPP participating facilities on activities undertaken, and equipment purchased with HPP funds, aggregate it up to the State level, and report it to ASPR. During the development of the data collection templates, HPP researched similar programs, conducted key stakeholder interviews, and performed searches of relevant literature to identify potential duplication of the data. While other government agencies provide grants related to emergency preparedness, they focus on entities other than hospitals and other healthcare facilities.
For example, the Centers for Disease Control and Prevention (CDC) issues Public Health Preparedness (PHEP) grants that ultimately serve local Public Health Departments in order to increase public health preparedness, but does not fund healthcare preparedness, such as in hospitals, etc. Keenly aware of the potential for duplication, HPP participates actively in interagency working groups with other Federal preparedness organizations, including DHS, CMS, DOT, HRSA, VA, DoD, and CDC. Participation in these groups generally fosters interagency communication, and increases awareness of other agencies’ activities, minimizing the potential for duplication. Additionally, HPP actively collaborates with CDC and other Federal Partners in joint metrics working groups in order to maximize efficiency between HPP and PHEP, and align program definitions and measurement approaches, which results in a much more united Federal face to our data collection efforts. Future plans include development of standardized application forms that can be used by both HPP and PHEP programs.
5. Impact on Small Businesses or Other Small Entities
The data collection is aimed at jurisdictional health departments administered by 50 States, the 4 major cities, and the 8 US Territories, which do not constitute small businesses. However, the 62 awardees will need to collect information on the activities of their participating hospitals and other healthcare organizations, only some of which may be small businesses, non-profits, or other small entities. To minimize the impact on these groups, data are collected only twice during the year. HPP has allocated between 2 and 5 percent of funding for administrative costs to support efforts such as data collection.
6. Consequences of Collecting the Information Less Frequently
This is an ongoing data collection effort, and awardees are requested to participate at least semiannually. This reporting frequency allows HPP to monitor progress throughout the year and enables awardees to demonstrate their ability to plan and spend their allotted funding more efficiently and with less risk of returning unused money. By collecting data every year, HPP can analyze trends over time and plan strategically for long-term outcomes. If this collection is conducted less frequently, HPP will not be able to accurately measure and assess the impact of the Program against the stated objectives. Since healthcare preparedness financial resources are slowly diminishing over time, collecting timely data is important to prevent weaknesses in healthcare preparedness and to allow corrections in performance as necessary. Due to the constantly evolving threats (including terrorism and natural disasters), ongoing data collection is essential to reassess risks and vulnerabilities.
7. Special Circumstances Relating to the Guidelines of 5 CFR § 1320.5(d)(2)
The proposed data collection efforts fully comply with all guidelines of 5 CFR § 1320.5 (d) (2). The information collection will not be conducted in a manner:
Requiring respondents to report information to the agency more often than quarterly;
Requiring respondents to prepare a written response to a collection of information in fewer than 30 days after receipt of it;
Requiring respondents to submit more than an original and two copies of any document;
Requiring respondents to retain records, other than health, medical, government contract, grant-in-aid, or tax records, for more than three years;
In connection with a statistical survey that is not designed to produce valid and reliable results that can be generalized to the universe of the study;
Requiring the use of a statistical data classification that has not been reviewed and approved by OMB;
That includes a pledge of confidentiality that is not supported by authority established in statute or regulation, that is not supported by disclosure and data security policies that are consistent with the pledge, or which unnecessarily impedes sharing of data with other agencies for compatible confidential use; or,
Requiring respondents to submit proprietary trade secrets, or other confidential information unless the agency can demonstrate that it has instituted procedures to protect the information’s confidentiality to the extent permitted by law.
8. Comments in Response to the Federal Register Notice and Efforts to Consult
Outside the Agency
The 60day FRN was published on September 16, 2011; Vol 76, Page number 55066, no comments have been received.
During previous years of the program, HPP has received comments from awardees regarding the data reporting templates. Awardees have reported understanding the need to collect and report on data and are appreciative of the HPP’s attempts to standardize data collection and pre-populate as many data fields as possible in advance.
In addition, HPP holds monthly conference calls with awardees to provide technical assistance on the measures and address any questions or concerns they may have about these data collection efforts.
PES collaborates with awardees in various capacities, including the creation of a Metrics and Measures WorkGroup (2009) whose members include awardee stakeholders and organizations that represent health and preparedness. PES sponsored a National Healthcare Preparedness Evaluation and Improvement Conference (2009) to build partnerships, provide feedback on performance progress, and vet products and materials to engage awardees in improving products and services, while reducing burden. Additionally, PES participates in regular calls, meetings, and conferences with the Association of State and Territorial Health Officials (ASTHO), the National Association of County and City Health Officials (NACCHO), and the American Hospital Association (AHA). PES leverages these relationships to obtain views on the availability of data, frequency of collection, the clarity of instructions and record keeping, disclosure, reporting format and data elements. The current collection, 09900326 was discontinued. We are requesting approval for the current form, which will be a generic collection, so that the program can receive a new approval for the next round of program collections.
9. Explanation of Any Payment or Gift to Respondents
HPP will not provide any payment, gifts or reimbursement to respondents for time spent completing data collection forms.
10. Assurance of Confidentiality Provided to Respondents The Privacy Act does not apply because the data to be collected do not involve individuals (i.e., citizens, patients, etc.). The data only capture aggregated program funded activities and equipment purchases made by State, Territorial, and City Health Departments, hospitals and other healthcare entities participating in HPP in the administration of the HPP program, and using Federal HPP funds for preparedness activities. All of this type of information is subject to FOIA disclosures. A statement of confidentiality is not required, since the program does not request any data that are identifiable to any individual.
To increase data privacy and security, responses are stored in a secure, password-protected online location, which can only be accessed by authorized individuals. HPP stores all data collected in a database located within secure facilities. HPP ensures that no confidential or pre-decisional information is shared with any entities outside of ASPR.
11. Justification of Sensitive Questions
This data collection effort does not involve questions related to private matters or personal sensitive information.
12. Estimates of Annualized Burden Hours and Costs
The Mid-Year, and End-of-Year Reports, and Similar Information Collections are completed by all awardees (i.e., one response per grantee organization). Each report will be administered once during the year. A small group of awardees were polled on the amount of time required to gather and enter the information. Table 2 indicates the total estimated annual burden for both the generic HPP data collection activity and for all future HPP-PHEP activities in terms of time. The generic HPP collection and the future HPP-PHEP information collections will take approximately 80.5 hours to complete. Based on 62 total respondents, the total annual burden is estimated to be 4,991 hours for HPP grantees.
Table 2. Estimated Annual Burden Hours for the Generic HPP and Future Collection Activities
-
Data Collection Activity
Number of Respondents
Number of Responses
Response Time (hours)
Total Annual Burden Hours
(for all awardees)
MY
62
1
25
1550
EOY
62
1
25
1550
Other GenICs
62
1
30.5
1891
TOTAL
4,991
To estimate cost of the burden, we know that HPP provides funding for the HPPCoordinators, who will be responsible for collecting and reporting HPP data. The total burden hours are 4,991. The rate and cost burden for the data collection activities are summarized in Table 3. The total annualized cost burden for the respondent for data collection is $131,962.04.
Table 3. Estimated Annualized Cost Burden for the Generic HPP and Future Collection Activities
-
Data Collection Activity Forms
Total Burden Hours
Annual Respondent Costs
MY
1550
$43,836.17
EOY
1550
$43,836.17
Other GenICs
1891
$44,289.70
TOTAL
4,991
$131,962.04
The Total Burden
13. Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers
Time and effort will be the only burden to respondents who participate in the evaluation. Awardees will not incur any other direct financial costs related to start-up or maintenance for these data collection initiatives. Awardees do not have to purchase any additional equipment or computer systems for this data collection effort.
14. Annualized Cost to the Federal Government.
The following table outlines the cost for each data collection activity. These costs are estimated by multiplying the number of hours to complete each task by the wage rate of the staff responsible for the task. The overall cost is approximately $86,312.50 each for developing and collecting the Mid-Year Reports and for the End-of-Year Reports. This includes developing the Mid-Year Report templates, distributing the templates and collecting, analyzing and reporting survey results. The estimated annual cost to the Federal Government for the administration of this data collection effort for three (3) years is $517,875.00. In future years, the number of awardees, the number of questions on the progress and reporting form, and the number of agencies using the form may be updated which may impact burden and cost. It is the goal of the program to change the forms as minimally as possible.
Table 4. Cost of the Proposed Data Collection Effort
-
Data Collection Activity
Cost
Mid-Year Report
Develop Mid-Year Report tool
$31,250
Distribute Mid-Year Report and collect results (44.05 Avg. Hourly Wage x 560 hrs)
$24,668.00
Analyze and report Results (44.05 Avg. Hourly Wage x 560 hrs)
$30,394.50
Subtotal
$86,312.50
End of Year Report
Develop End-of-Year Report tool
$31,250
Distribute End-of-Year Report and collect results (44.05 Avg. Hourly Wage x 560 hrs)
$24,668.00
Analyze and report results (44.05 Avg. Hourly Wage x 560 hrs)
$30,394.50
Subtotal
$86,312.50
Grand Total
$172,625.00
15. Explanation for Program Changes or Adjustments
This is a new generic data collection.
16. Plans for Tabulation and Publication and Project Time Schedule
HPP has plans for both tabulation and publication of the results and performance of the Program. Overall, the steps in the evaluation plan are as follows:
Assess data validity
Assess data for completeness
Review data state by state to assess changes from one time period to the next
Assess data from states in aggregate at the regional and national levels for changes in the data from one time period to the next
Summarize and report data for standard reports, congressional briefings and other special reports as required by HPP or other Federal agencies.
Tabulation
HPP will aggregate data and perform simple descriptive and summary statistical analyses on the data. Using funding data from the Mid-Year and End-of-Year Reports, and other information collections as related, the proposed and obligated funding will be compared to funding spent to ensure compliance with the awardees’ applications and program requirements. In addition, HPP will analyze spending over time, spending by capability and spending by state. The performance measure data will be monitored over time to determine if awardees are meeting benchmarks and objectives and to identify trends in preparedness planning. Our current analyses summarize each element in regards to the number of participating hospitals or awardee participation for the nation and each awardee and region.
Publication
In May 2011, ASPR released a Progress Report on healthcare systems preparedness that resulted from HPP funding. The report highlights progress made by the 62 awardees since the beginning of the HPP Program. The report is being used to build partnerships, advance preparedness activities through local and State legislatures, inform the Congress, and target HPP grant resources most effectively. The report is available online at, Public Health Emergency, PHE.GOV.
The progress reports and performance measure results will be consolidated in a comprehensive report for HPP. Additional reports for OPEO, ASPR, HHS, Congress and other Federal agencies will be provided as necessary. The data will also be made publicly available online, as required in Section 201 on Improving State and Local and Public Health Security in the PAPHA law:
“COMPILATION AND AVAILABILITY OF DATA.—The Secretary shall compile the data submitted under this section and make such data available in a timely manner on an appropriate Internet website in a format that is useful to the public and to other entities and that provides information on what activities are best contributing to the achievement of the outcome goals described in subsection (g).’’
, the Data Collection Schedule, outlines the major milestones in the data collection timeline.
Table 5. Data Collection Schedule
Activity |
Schedule |
Submit Federal Register Notice and Obtain OMB Clearance |
June, 2011 – September, 2011 |
Project and Budget Period |
July. 1, 2011 – June 30, 2012 |
Data Collection Mid Year 2011 Report End of Year 2011 Report |
Four (4)months after OMB clearance of new collection Twelve (12) months after OMB clearance of new collection |
Data Analysis Mid Year 2011 Report End of Year 2011 Report |
Five (5) months after clearance of new collection Thirteen (13) months after clearance of new collection |
Provide Evaluation Report Mid Year Report End of Year Report |
Six (6) months after clearance of new collection Fourteen (14) months after clearance of new collection |
17. Reason(s) Display of OMB Expiration Date is Inappropriate
Not Applicable. OMB expiration dates will be displayed on all data collection materials.
18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification.
B. Statistical Methods
1. Respondent Universe and Sampling Methods
The applicable population (universe) is all of the awardees for the program (i.e., 62 of 62 awardees). All awardees participate in progress reporting because data on each entity are integral for monitoring progress and measuring national preparedness. Sampling is not appropriate as there are too few recipients to employ a psychometrically sound sampling strategy. The anticipated response rate is 100 percent for standard MY and EOY collections, since participation is a requirement for receiving funding from HPP (please see B.3 for further justification of this expected response rate).
2. Procedures for the Collection of Information
For each HPP grant funding period, the HPP awardees are notified via the HPP Listserv that MY, EOY, and similar information collection forms are available and to submit online.. Awardees have approximately 3 months in advance of the due date to gather the requested information and complete and submit the report. Throughout this time, the HPP Project and Field Officers and PES are actively involved and provide technical support, as needed.
Data are reported and collected then reported into ASPR’s Program Evaluation and Planning Interface (PEPI). The data in PEPI are checked for accuracy (validity and completeness), after which the data are transformed into variables that are usable for analysis. Standard analytic techniques and simple descriptive analyses are conducted to draft reports on data status before completing a comprehensive data analysis using a variety of standard statistical programs, Statistical Analysis System (SAS), SPSS, GIS, Excel and Access.
PES employs descriptive statistics to describe the basic features of the data. Univariate analysis is used to examine: distribution (frequency), central tendency (mean, median, and mode) and dispersion (range, variance, and standard deviation). In some cases, HPP performs correlations to describe the degree of relationship between two variables. These methods, together with simple graphics analysis, serve as the basis for the analysis. This data collection effort does not require any statistical method for sample stratification, sample selection or estimation, since 100% of the awardees will be completing the standard MY and EOY data collection reports and other GenICs are voluntary.
3. Methods to Maximize Response Rates and Deal with Non-responsiveness
One of the conditions for receiving HPP funding is completing appropriate reporting requirements as outlined in HPP program guidance. This requirement increases the likelihood of achieving a response rate of 100 percent. In addition, HPP designed the electronic data collection tools for ease of use. Clear and concise instructions are included with the reports to help maximize response rates. HPP personnel also discuss reporting requirements during monthly conference calls with awardees, answering any questions and providing reminders for the due dates of the reports. They provide instructions and guidance on how to complete the report and tools and answer general questions as needed throughout the data collection effort. HPP Project Officers and PES monitor response rates and work with awardees to ensure completion of reports. HPP and PES began sharing awardee-specific data summaries and snapshot analyses with awardees to validate the accuracy of the information.
4. Test of Procedures or Methods to be Undertaken
Several sections of the Mid-Year and End-of-Year reports have been previously utilized by HPP during past years of the program, so length of time for completion was estimated from previous awardee experience. The reports have evolved over the years based on awardee and Project Officer feedback about the ease of data entry and analysis. The performance measure section of the reports was reviewed by a small subset of awardees to determine the amount of time it would take to complete.
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data
Statistical Aspects of Design and Data Collection/Analysis Contacts
Ms. Margaret Sparr, M.S.Ed., MPA, Branch Chief
Section Chief for Evaluation, State and Local Initiatives Team
US Department of Health and Human Services (HHS)
Assistant Secretary for Preparedness and Response (ASPR)
Office of Preparedness and Emergency Operations (OPEO)
330 C ST., SW, Room 5615
Washington DC 20201
(202) 245-0771
margaret.sparr@hhs.gov
APPENDIX A
Pandemic and All-Hazards Preparedness Act (PAHPA)
(See Attached)
APPENDIX B
Hospital Preparedness Cooperative Agreement
End-of-Year Progress Report (Sample Collection)
(See Attached)
APPENDIX C
Sample Table for Data Analysis
Our current analyses summarizes each element in regards to the number of participating
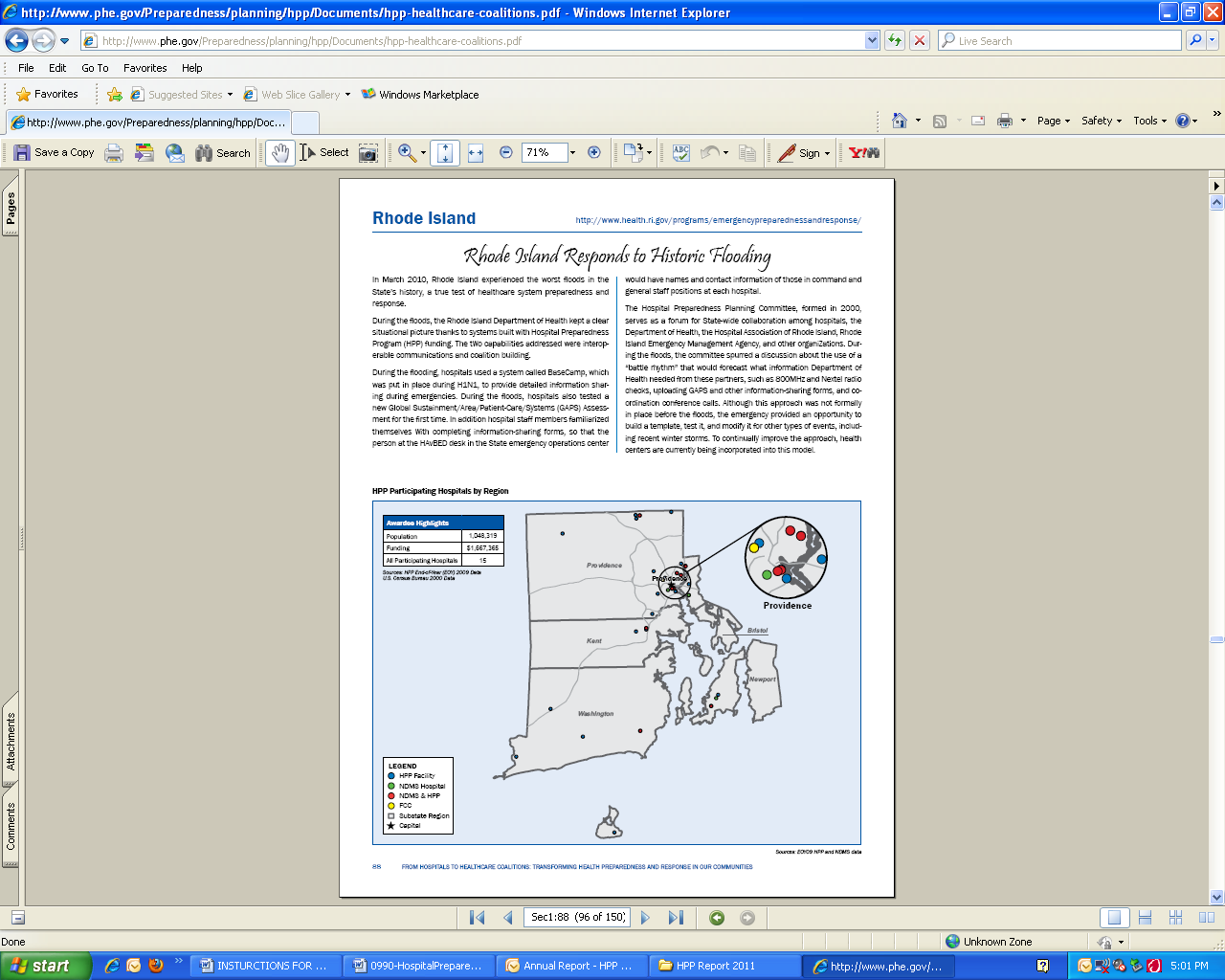
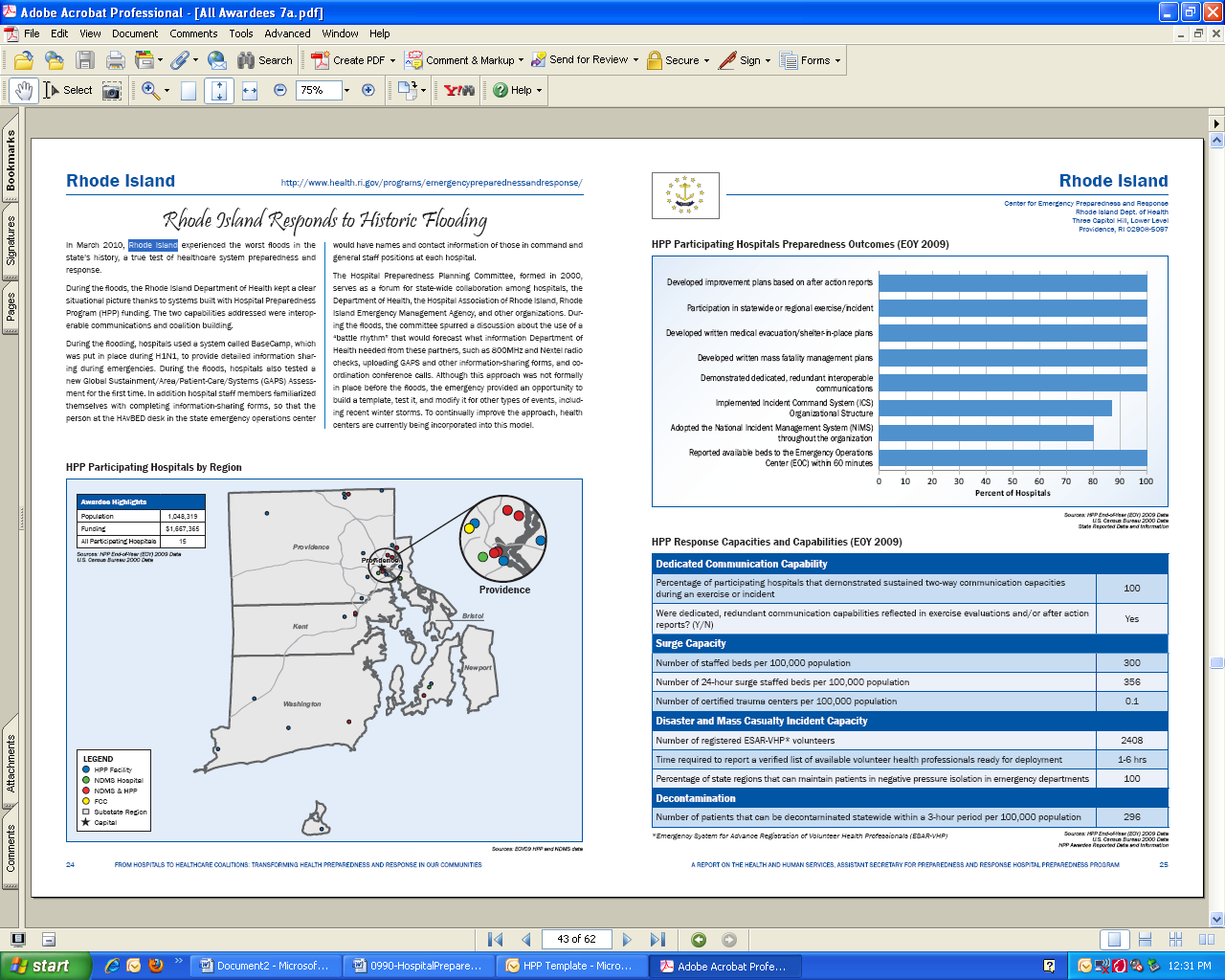
hospitals or awardee participation for the nation and each awardee and region. The following url provides a direct link to the current HPP Progress Report: http://www.phe.gov/Preparedness/planning/hpp/Documents/hpp-healthcare-coalitions.pdf
1 U.S. Department of Homeland Security. (2005, Mar. 31). Interim National Preparedness Goal. Retrieved September 25, 2007
from http://www.ojp.usdoj.gov/odp/docs/InterimNationalPreparednessGoal_03-31-05_1.pdf.
2 U.S. Congress. (2006, Jan.). Pandemic and All-Hazards Preparedness Act S.3678. Retrieved September 28, 2007 from
http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=109_cong_bills&docid=f:s3678enr.txt.pdf.
3 U.S. Department of Health and Human Services. (2009). Medical Surge Capacity and Capability: The Healthcare Coalition in Emergency Response and Recovery. Retrieved June 3, 2011 from http://www.phe.gov/Preparedness/planning/mscc/Documents/mscctier2jan2010.pdf.
| File Type | application/msword |
| File Title | Supporting Statement for Paperwork Reduction Act Submissions |
| Author | cys |
| Last Modified By | Cliffon Smith |
| File Modified | 2012-03-13 |
| File Created | 2012-03-13 |
© 2026 OMB.report | Privacy Policy