2012 ss
2012 ss.docx
Animal Disease Tracebility Information Systems, Agreements, and Reports
OMB: 0579-0259
SUPPORTING STATEMENT
0579-0259
ANIMAL DISEASE TRACEABILITY DATA SYSTEMS, AGREEMENTS, AND REPORTS
Previously NATIONAL ANIMAL IDENTIFICATION SYSTEM
2013
A. Justification
1. Explain the circumstances that make the collection of information necessary. Identify any legal or administrative requirements that necessitate the collection. Attach a copy of the appropriate section of each statute and regulation mandating or authorizing the collection of information.
The Animal Health Protection Act (AHPA) of 2002 is the primary Federal law governing the protection of animal health. The law gives the Secretary of Agriculture broad authority to detect, control, or eradicate pests or diseases of livestock or poultry. The Secretary may also prohibit or restrict import or export of any animal or related material if necessary to prevent the spread of any livestock or poultry pest or disease.
The AHPA is contained in Title X, Subtitle E, Sections 10401-18 of P.L. 107-171, May 13, 2002, the Farm Security and Rural Investment Act of 2002.
On February 5, 2010, USDA announced it would develop a new, flexible framework for animal disease traceability in the United States, and undertake several other actions to further strengthen its disease prevention and response capabilities. USDA decided to offer a new approach to animal disease traceability after concluding national listening tours on the National Animal Identification System (NAIS).The changes responded directly to the feedback.
The framework provides the basic tenets of an improved animal disease traceability capability in the United States and will:
Only apply to animals moved in interstate commerce.
Be administered by the States and Tribal Nations to provide more flexibility.
Encourage the use of lower-cost technology.
Be implemented transparently through Federal regulations and the full rulemaking process.
USDA is adapting these tenets for animal disease traceability while using investments previously made in the NAIS on information systems, official animal identification devices, and other areas where States and Tribes had achieved progress through cooperative agreements. Therefore this renewal, while now supporting the new Animal Disease Traceability (ADT) framework, continues to account for several activities previously covered in 0579-0259.
USDA has had various programs that provided animal identification and traceability. Historically, disease programs achieved adequate levels of identification to meet traceability needs. As certain diseases have neared eradication, participation in those programs has decreased; thus, USDA needs other methods to maintain traceability.
USDA withdrew the NAIS in February 2010 in response to feedback received during national listening tours conducted in 2009. The revised approach, referred to as the ADT framework, set the direction for having USDA establish basic national standards while giving States and Tribes the flexibility to implement traceability solutions that work at local levels.
The traceability program remains essential in helping animal health officials protect U.S. livestock and poultry from disease spread and retaining access to domestic and foreign markets. Timely response to disease outbreaks will:
Slow disease spread and reduce associated economic impact.
Lessen disruption to producers and animal owners.
Speed lifting of quarantine and movement restrictions.
Lessen the likelihood of animal depopulation.
Increase consumer confidence.
A system that can trace an animal’s movements throughout its lifetime is fundamental to controlling any animal disease threat, foreign or domestic. The ADT framework focuses on interstate movements, with States and Tribes determining requirements for those movements. The basic data APHIS acquires through the ADT system will help achieve timely animal movement tracebacks and trace forwards when responding to an animal disease concern.
Information System
USDA continues to provide information systems that States and Tribes may use to implement their ADT plans. The USDA Animal and Plant Health Inspection Service (APHIS) will maintain information systems and provide them to States and Tribes that wish to use them. Producer premises and animal data within the ADT framework necessary to support the framework’s objectives will be maintained and controlled at the discretion of States and Tribes.
Cooperative Agreements
Development and implementation of the ADT framework continues to be a partnership involving USDA, States, Tribes, and industry. States and Tribes enter into cooperative agreements with USDA to implement their traceability activities. Traceability performance standards are being developed that will measure and document tracing capability that results from the ADT framework. Agreements will continue to be necessary as USDA develops and implements this cooperative program.
APHIS is asking OMB to approve, for 3 years, of its use of these information collection activities in connection with its efforts to carry out the ADT framework.
Indicate how, by whom, how frequently, and for what purpose the information is to be used. Except for a new collection, indicate the actual use the agency has made of the information received from the current collection.
APHIS uses the following information collection activities to conduct an Animal Traceability program which provides the necessary information needed in investigations of livestock diseases.
The ADT information system, initially developed to support the NAIS, is being maintained as an option that States and Tribes may use to support their traceability plans. The systems support the identification of premises, recording information on official animal identification devices and animal events.
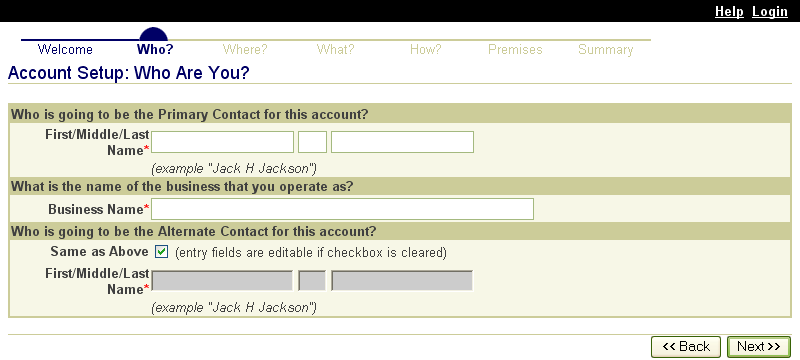
Premises Identification
The premises identification databases provide a system that can issue premises identification numbers to locations where animals are raised (such as farms and ranches) and held for other purposes (such as markets and clinics). The States and Tribes administer the identification of premises within their geographic areas. APHIS has provided the Standardized Premises Identification System (SPIS). SPIS online information system for States and Tribes to use to do this. Forty-one States, seven Tribes, and two Territories currently use the SPIS. APHIS is responsible for its development, enhancement, maintenance, and operation.
States and Tribes may also choose to use a compliant premises identification system. These are either State systems or third-party systems evaluated and determined to be compliant with ADT data standards. These systems are also available online and operate the same way the SPIS does; however, States or third parties administer, develop, enhance, maintain, and operate these systems.
Minimal premises information is forwarded automatically by the information systems to the national repository. Premises owners and operators supply the necessary information to the States or Tribes using the communication method most convenient to them (such as the Internet, hard copy, fax, email, or telephone call). After the initial information is entered, the premises information in the database is updated if premises go out of business, come into existence, change ownership, or experience any other noteworthy changes to their operations that should be recorded.
Updates to Premises Identification Records
Updateable premises information maintained on the SPIS includes:
The PIN.
The name of the entity.
The owner or appropriate contact person.
A contact phone number.
The premises address.
The date the operation was activated.
The date the operation changed ownership or ceased operations.
The reason for this event.
The names and phone numbers of previous contact persons.
This data is automatically maintained for 20 years at the State level in the SPIS. This will give animal health officials the proper contact reference when the current contact person was not associated with the premises during the period being researched in a traceback situation.
Contact information maintained in the SPIS opens the lines of communication between producers and animal health officials, which is critical in preventing the spread of disease. State animal health officials can query their respective databases to determine what locations are in a general area during a disease outbreak or incident and can then use the contact information to inform producers and animal owners about potential disease exposure and steps they should take to protect their animals. Although it is impossible to predict how many disease traceback investigations, emergency responses, or disease outbreaks might occur in the next 3 years that would cause State animal health officials to access premises information, these occasions are relatively infrequent and localized.
Nonproducer Participant Registration
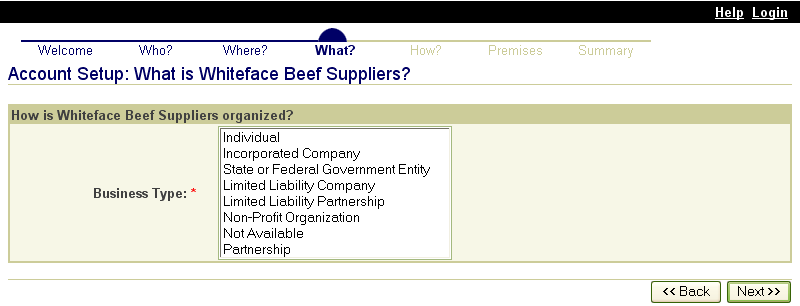
In addition to the premises identification, the ADT framework provides for the issuance of Nonproducer Participant Numbers (NPN) to entities that do not hold or manage livestock, but are otherwise involved with the food animal industry and could become a vital investigative link during an emergency traceback. These entities include State animal health officials, accredited veterinarians, AIN managers or resellers (individuals or firms responsible for distributing AIN devices to producers), official identification device manufacturers (companies that manufacture official identification devices), diagnostic laboratories, livestock buyers and dealers, and others who submit data to information systems.
Official Identification Device Applications and Approved Identification Device Manufacturer Agreements
(This includes applications for all official identification devices provided for through the ADT, primarily AIN devices, visual only eartags, radio frequency identification (RFID) eartags, and RFID injectable transponders)
Approved identification device manufacturers are companies authorized by APHIS to manufacture approved identification devices. They are responsible for the overall production and quality of the devices. Approved identification device manufacturers may only produce devices with the official identification numbers as defined in the agreement.
Approved device manufacturers must:
Complete and submit the Official Identification Device Manufacturer Application.
Abide by the terms and conditions set forth in the agreement.
Imprint the official identification number, the official shield or emblem, and other print criteria as specified in the device application or as otherwise provided by APHIS.
Maintain the uniqueness of the official identification numbers they are permitted to use.
Report the shipment of all AIN devices to the AIN Management System according to established protocols within 24 hours of shipment.
Have an operational computerized system for AIN devices that communicates with the AIN Management System and is compatible with ADT standards to maintain the necessary information, including a database of the manufacturer product codes for all devices that contain an AIN device.
Be able to support the distribution of their official identification devices.
Agree to discontinue the printing of any identification numbering system as prescribed by USDA if USDA phases out the numbering system.
Maintain a current inventory of official identification devices and make it available to USDA on request. (Note: This does not require additional recordkeeping; the manufacturer must simply agree that USDA has the right to review the current inventory of AIN devices at any time.)
Designate nonproducer participants they wish to use as AIN device managers into the AIN Management System.
APHIS provides device manufacturer applications which can be requested by contacting ADT staff directly via email or phone. Device manufacturers provide information about their devices, which APHIS uses to determine whether to approve the company’s or entity’s device as an official device for use in the ADT system.
AIN Device Manufacturer Updates and Recordkeeping
After obtaining an NPN, entities update information as the need arises (such as when a contact person or phone number changes). Nonproducer participants who submit information to the AIN Management System routinely maintain records associated with their animal identification activities for 5 years. This recordkeeping is standard business procedure, and is often required by Federal regulations if the entity or individual is involved in other USDA programs. This information is maintained because it could provide critical information during a disease traceback.
AIN Device Managers Registration and Agreement
AIN device managers are individuals, organizations, or companies that provide AIN devices to livestock owners or another AIN device manager or reseller. The AIN device manager must have an established relationship with an AIN device manufacturer.
To be an authorized AIN Device Manager, the individual or firm must agree to:
Abide by the terms and conditions set forth in the AIN Device Manager Agreement (Note: The agreement simply asks the individual or firm to abide by the terms or conditions listed below. All of the information about the manager or reseller will already be in the AIN Management System because only entities with an NPN will be eligible to become AIN device managers.)
Maintain an ongoing list of inventoried AIN devices received from an authorized AIN manufacturer device manufacturer or AIN manager and make that list available to USDA on request. (Note: This list does not require additional recordkeeping; the manager must simply agree that USDA has the right to review the current inventory of AIN devices at any time.)
Validate the PIN or NPN of the premises or entity that is to receive AIN devices and provide such PIN or NPN to the entity shipping the tags. The shipper reports the distribution record to the AIN Management System or to the AIN device manufacturer for orders shipped direct from the manufacturer.
Submit a record to the AIN Management System of all AINs they possess and ship or deliver to a premises, in accordance with prescribed protocols.
Educate customers on the proper use of official identification devices.
The AIN device managers will apply online using the AIN Management System, confirming the AIN device manufacturers or other device managers with whom they have an established relationship. The entity will be recognized as an AIN manager on confirmation of an established relationship with an AIN device manufacturer.
AIN Device Manager Updates
AIN device managers provide updated information regarding changes in status (contact person’s name, phone number, etc.) as the need arises. This information is maintained because it could provide critical information during a disease traceback.
Cooperative Agreements
USDA has been working with States, Tribes, and industry to advance animal disease traceability since June 2004. USDA has provided funding through cooperative agreements to participants to further develop and implement the system components. USDA and APHIS have used Government-wide fund allocation (SF-424, SF-424A, and SF-424B) and lobbying disclosure (SF-LLL) forms in processing cooperative agreement request packages.
Cooperative Agreement Application
APHIS provides Federal support for ADT implementation activities and infrastructure within participating States, Tribes, and territories through cooperative agreements. Cooperative agreement awards require quarterly reporting and Federal oversight of the successful completion of the goals and objectives outlined in the cooperative agreement workplan (a part of the application package).
ADT cooperative agreement funding is provided to advance animal disease traceability. Each participant will be required to evaluate, describe, and identify animal disease traceability risks within State or Tribal boundaries. Workplans will describe how each applicant will reduce those risks and advance animal disease traceability. Because States, Tribes, and territories have made varying progress to date, this approach will allow each applicant the flexibility needed to advance animal disease traceability appropriately for its individual situation.
State/Tribe Quarterly Reports
States and Tribes give APHIS quarterly accomplishment reports. The reports include accomplishments achieved as defined in their cooperative agreement workplans. Additionally, reporting the results of tracing capabilities based on the defined traceability performance measures documents progress on disease traceability. Statistics relative to interstate movements of livestock are also reported.
Recordkeeping
Producers and operators of feedlots, markets, buying stations, and slaughter plants routinely maintain records associated with their animal movement activities for 2 to 5 years. This recordkeeping is standard business procedure, and is often required by Federal regulations if the entity or individual is involved in other USDA programs. These records could provide critical information during a disease traceback investigation.
3. Describe whether, and to what extent, the collection of information involves the use of automated, electronic, mechanical, or other technological collection techniques or other forms of information technology, e.g., permitting electronic submission of nresponses, and the basis for the decision for adopting this means of collection. Also describe any consideration of using information technology to reduce burden.
Information on premises, nonproducer participant and animal identification devices, and animal event information are all submitted to APHIS electronically. State, Tribe, territory and industry cooperator participants in cooperative agreements submit quarterly reports electronically to the regional offices.
Premises Identification
SPIS
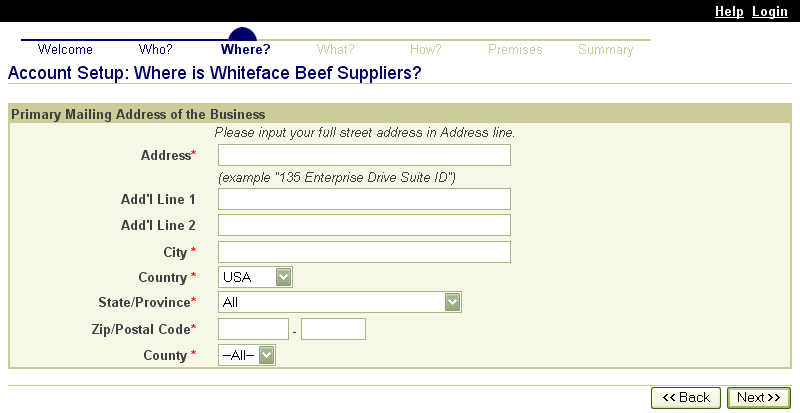
APHIS provides the SPIS to States and Tribes that elect to use the system. Each State using the SPIS has its own URL to provide access. Data for States and Tribes is partitioned so that users who log into their State or Tribal system can only access information pertaining to that State or Tribe.
States and Tribes may enable their SPIS for public access so premises owners and operators with Internet access can enter their premises information online. For producers who do not have Internet access, States and Tribes also allow them to submit premises information for entering into the SPIS by mail, fax, email, or phone.
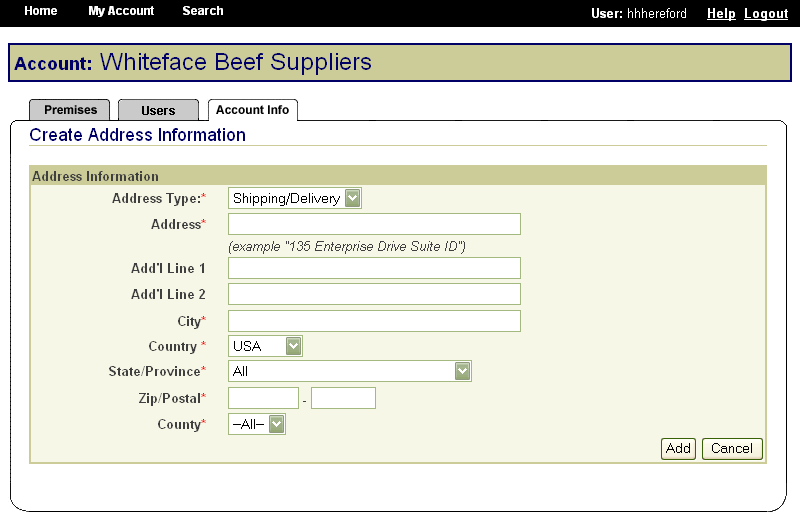
When premises information is entered in the SPIS, whether by a premises owner or operator or by a State or Tribal administrator, the information is entered directly into the State premises identification system database. At a minimum, the address, city, state and ZIP code is also forwarded to the Premises Allocator/Repository. On address validation, the Premises Allocator assigns a premises number. These electronic transactions occur automatically on submission of premises information. Having only one data entry operation eliminates problems and errors that arise from reentering data at another stage of the registration process.
These screen shots from the SPIS are typical of the whole application. The successive screens allow either a premises owner or operator or a State administrator working on the owner or operator’s behalf to submit premises information directly to the State premises identification system and to the Premises Repository/Allocator. Data is entered only once, and some data is stored in both the State and Federal databases. Thus, there is no redundancy in data input, no errors from repeated input of the same data, and no paperwork. This ensures an effective, efficient method for premises registration. |
|
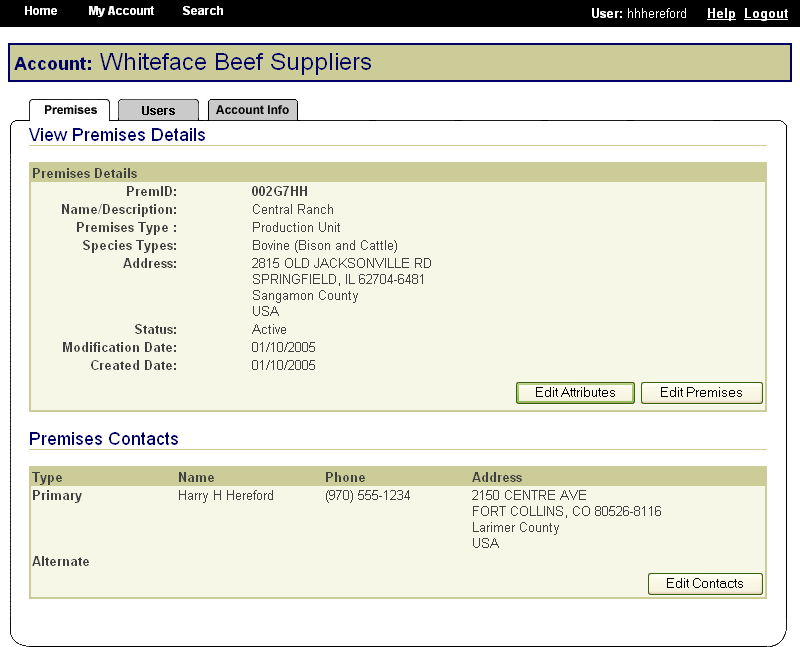
In the same way, State animal health officials who need to view premises information to carry out their duties during a traceback can instantly obtain the required information through the SPIS. State officials can access information for premises locations within their State. This screen is typical of the kind of report that an authorized user can access. |
|
Compliant Premises Identification System (CPIS)
States may also use a CPIS approved by APHIS. Such systems are similar to the premises registration system provided by APHIS.
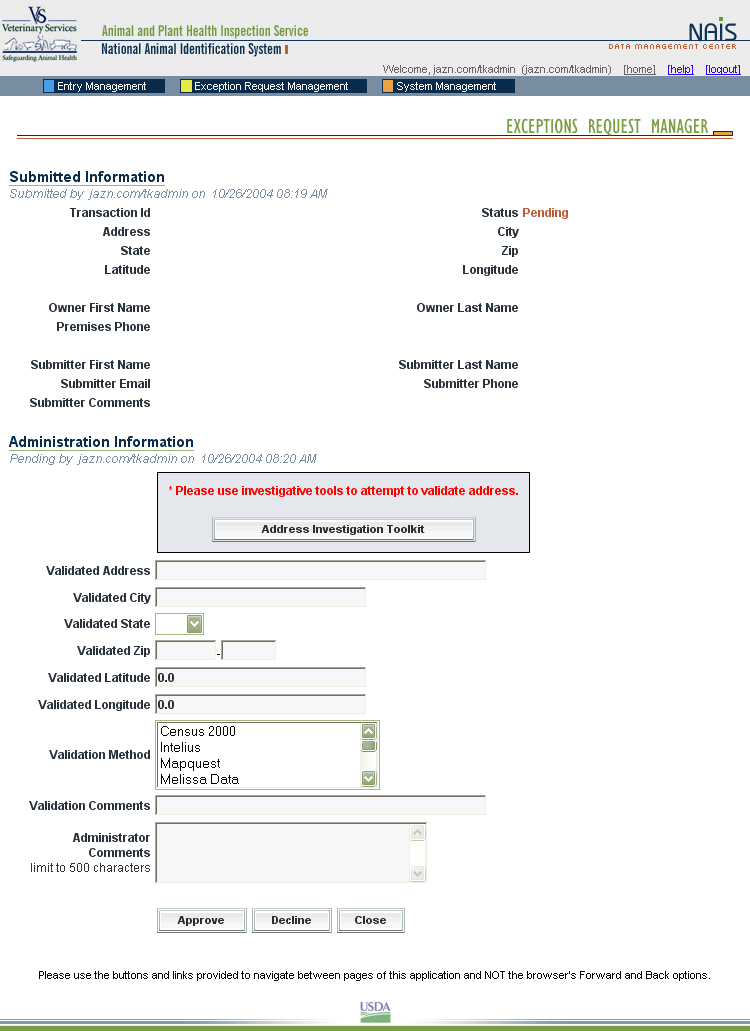
Exceptions Processing – DMC
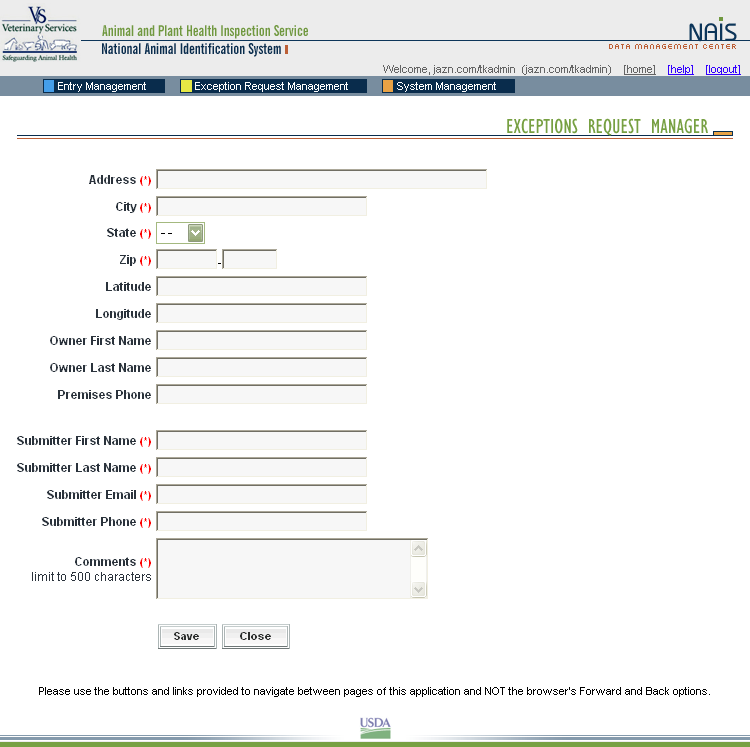
When a premises address is submitted to the Premises Allocator through the SPIS, and the submitted address does not validate, it becomes necessary to process the premises address as an exception. The user sends relevant premises information, including a property description, address information, and GPS coordinates (latitude and longitude). This information is submitted via email to the ADT Help Desk, which processes the exception request. Any additional information that may be required from the premises owner or operator to process the request is obtained either via email or over the phone.
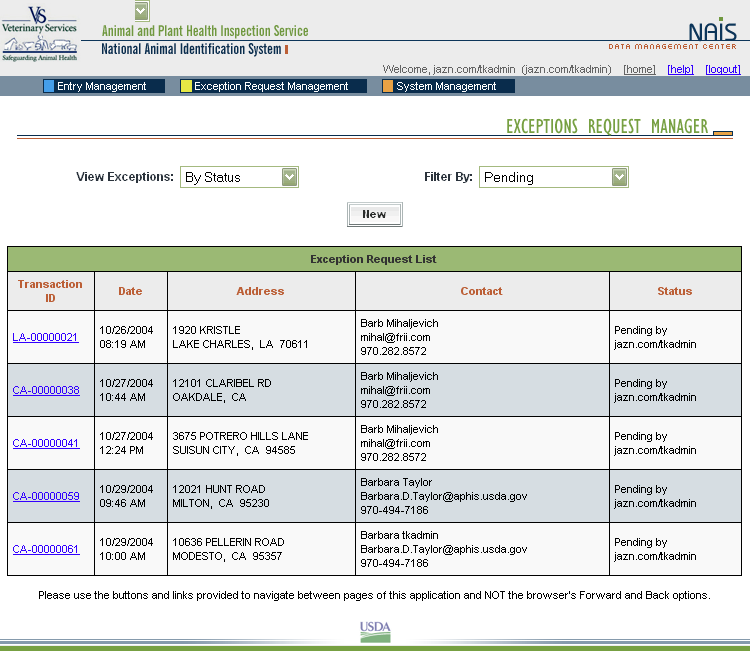
When premises are submitted for exceptions processing, an exceptions manager enters the data received from the administrator for the State where the premises are located into the Data Management Center (DMC). After researching the address, using a resource toolkit of address databases, the exceptions manager will either grant or deny the exception, depending on whether the premises’ location can be accurately identified.
Once an address exception is entered into the DMC, the steps of address validation, research, and exceptions processing are all conducted through the Web-based DMC, which is linked to the Premises Allocator/Repository. Links have been created to other databases to permit research without reentering data, eliminating input errors.
|
|
Animal Identification
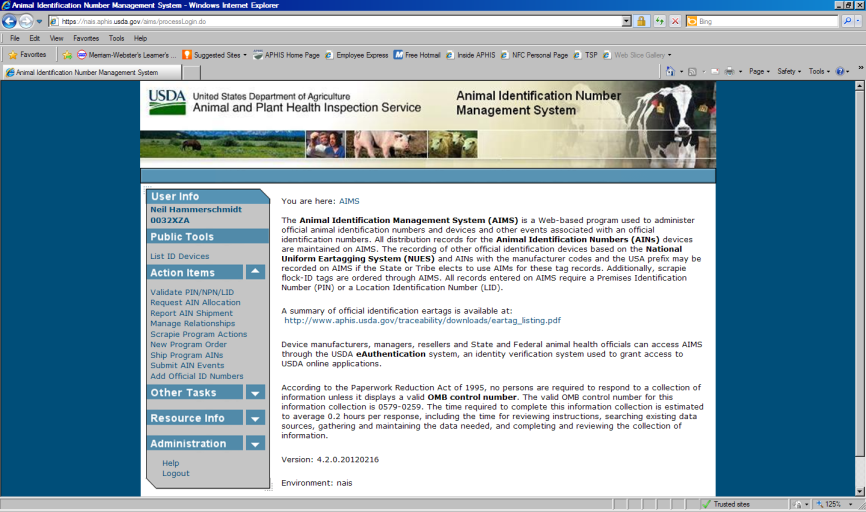
Animal Identification Management System
The Animal Identification Management System (AIMS) allocates Animal Identification Numbers (AINs) at the request of AIN device manufacturers. A record of AINS allocated to each manufacturer is automatically generated on AIMS. Whenever an AIN is shipped to another entity such as an AIN manager, an AIN reseller, or a producer’s premises, the shipment is recorded in AIMS. Those records include the PIN or NPN of the entity that distributed and received the device and the date of distribution.
|
|
Nonproducer Participants
Device manufacturers, managers, and resellers (distributors) are referred to as nonproducer participants. Each nonproducer participant obtains a Nonproducer Participant Number (NPN) through the premises registration system in the State where the headquarters is located (see screenshots under SPIS above, for example of screens that nonproducer participants would fill out online). For example, if the company’s corporate office is in Kansas, it will obtain an NPN through the Kansas premises registration system. All NPNs are unique seven-character numbers similar to PINs.
AIN Device Manufacturers and Managers
USDA has provided information technology to allow manufacturer and managers to select the most convenient application method for them. Once entities have an NPN (see above), identification device manufacturers, AIN device managers, and resellers use AIMS to indicate their interrelationships; this allows them all to be part of the AIN device distribution process. AIN device managers, once they have obtained their NPN, indicate in the system which device manufacturers they have a marketing agreement with. The system then automatically brings up the following online agreement:
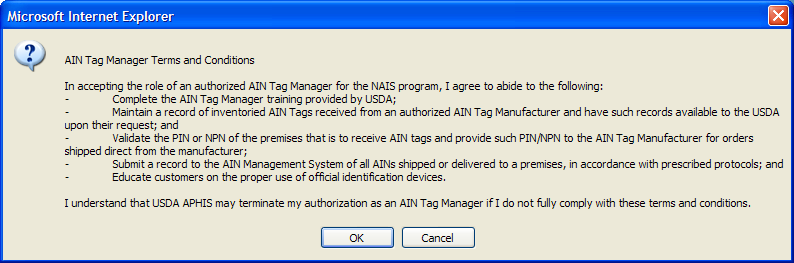
The online reseller agreement is identical except that all references to “manager” are changed to “reseller.”
Animal Trace Processing System
Working with State and industry partners, APHIS developed the Web-based portal that State and Federal animal health officials will use to request information from the animal tracking database (ATD) administrators to address animal disease events. Known as the Animal Trace Processing System, this information system provides secure access and auditing functions and is now operational at the Federal level. However, full integration of participating ATDs, and full development of the query form that State and Federal animal health officials will use, are not yet complete. As with the AIN Management System, participation in the ATPS will be completely electronic.
4. Describe efforts to identify duplication. Show specifically why any similar information already available cannot be used or modified for use for the purpose described in item 2 above.
APHIS has exclusive responsibility for implementing the ADT system. To carry out this responsibility APHIS has established a premises registration system that ensures all animal production units and holding facilities are identified with a unique PIN so these locations are accurately listed. The ADT system must have premises information that reflects animal production units or holding facilities so the system can determine animals that came in contact with subject animals. While other systems identify tracts of land for other reasons (such as farm payment programs), the records often do not align with animal production units. Premises information from other sources, when available, was integrated into the NAIS premises system that APHIS has adapted for the ADT framework.
The PIN allows the system to determine what animals came in contact with a subject animal at a given location, and the movement records for such animals provide the traceback data needed by animal health officials. In the past, most of the information available at markets and other premises was maintained in hard copy. The online system provides immediate access to information necessary to animal health officials when initiating an investigation.
During the transition period, current official identification devices and numbering systems will be grandfathered into the ADT system to avoid re-identification of any animals. The AIN eventually will be the sole numbering system for individual animals in the administration of Federal disease programs to avoid having multiple numbers for the same animal. The AIN may also be used for industry programs the producer elects to participate in.
If the collection of information impacts small businesses or other small entities, describe any methods used to minimize burden.
The information APHIS collects for this program is the minimum needed to protect U.S. livestock and poultry from the spread of disease. APHIS estimates that 75 percent of the respondents are considered small entities.
Describe the consequence to Federal program or policy activities if the collection is not conducted or is conducted less frequently, as well as any technical or legal obstacles to reducing burden.
If the information was collected less frequently or not collected, APHIS would be unable to effectively detect disease in the U.S. livestock population, to prevent disease spread within the United States, and to eliminate certain animal diseases from the United States.
Explain any special circumstances that require the collection to be conducted in a manner inconsistent with the general information collection guidelines in 5 CFR 1320.5.
requiring respondents to report information to the agency more often than quarterly;
requiring respondents to prepare a written response to a collection of information in fewer than 30 days after receipt of it;
requiring respondents to submit more than an original and two copies of any document;
requiring respondents to retain records, other than health, medical, government contract, grant-in-aid, or tax records for more than three years;
In order to carry out an effective traceability program, APHIS is requiring respondents to maintain records for 3-5 years.
in connection with a statistical survey, that is not designed to produce valid and reliable results that can be generalized to the universe of study;
requiring the use of a statistical data classification that has not been reviewed and approved by OMB;
that includes a pledge of confidentiality that is not supported by authority established in statute or regulation, that is not supported by disclosure and data security policies that are consistent with the pledge, or which unnecessarily impedes sharing of data with other agencies for compatible confidential use; or
requiring respondents to submit proprietary trade secret, or other confidential information unless the agency can demonstrate that it has instituted procedures to protect the information's confidentiality to the extent permitted by law.
No other special circumstances exist. This information collection is conducted in a manner consistent with the guidelines established in 5 CFR 1320.5.
Describe efforts to consult with persons outside the agency to obtain their views on the availability of data, frequency of collection, the clarity of instructions and recordkeeping, disclosure, or reporting form, and on the data elements to be recorded, disclosed, or reported. If applicable, provide a copy and identify the date and page number of publication in the Federal Register of the agency's notice, soliciting comments on the information collection prior to submission to OMB.
APHIS engaged in productive consultations with the following individuals concerning the information collection activities associated with this project:
David Hecimovich
Animal ID Program Manager
Washington State Department of Agriculture
2nd Floor, Natural Resources Building
1111 Washington St. SE
Olympia, WA 98504-2560
(360) 725-5493
Cell: (360) 507-6383
DHecimovich@agr.wa.gov
Dr. Robert Fourdraine
Wisconsin Livestock Identification Consortium
135 Enterprise Drive, Suite ID
Verona, WI 53593
(608) 848-1907
rfourdraine@wiid.org
Dr. Patrick Webb
National Pork Board
1776 N.W. 114th Street
Des Moines, IA 50325
(515) 223-3441
On Monday, December 31, 2012, APHIS published in the Federal Register a renewal notice and request for comments (60-day). During that time, APHIS recieved 3 comments from concerned citizens. These comments reflect opinions about big government and the use of tax dollars. None of these comments pertain to paperwork.
Explain any decision to provide any payment or gift to respondents, other than
remuneration of contractors or grantees.
This information collection activity involves no payments or gifts to respondents.
Describe any assurance of confidentiality provided to respondents and the basis for the assurance in statute, regulation, or agency policy.
No additional assurance of confidentiality is provided with this information collection. However, the confidentiality of information is protected under 5 U.S.C. 552a.
Provide additional justification for any questions of a sensitive nature, such as sexual behavior or attitudes, religious beliefs, and other matters that are commonly considered private. This justification should include the reasons why the agency considers the questions necessary, the specific uses to be made of the information, the explanation to be given to persons from whom the information is requested, and any steps to be taken to obtain their consent.
This information collection activity will ask no questions of a personal or sensitive nature.
Provide estimates of the hour burden of the collection of information. Indicate the number of respondents, frequency of response, annual hour burden, and an explanation of how the burden was estimated.
• Indicate the number of respondents, frequency of response, annual hour burden, and an
explanation of how the burden was estimated. If this request for approval covers more than one form, provide separate hour burden estimates for each form and aggregate the hour
burdens in Item 13 of OMB Form 83-I.
See APHIS Form 71. Burden estimates were developed from discussions with State and Tribal animal health authorities; producers; nonproducer participants such as accredited veterinarians, designated laboratories, and AIN device managers; and owners and operators of feedlots, markets, buying stations, and slaughter plants.
• Provide estimates of annualized cost to respondents for the hour burdens for collections of information, identifying and using appropriate wage rate categories.
APHIS estimates the total annual cost to these respondents to be $1,283,633. APHIS arrived at this figure by multiplying the total burden hours (47,054) by the estimated average hourly wage of the above respondents ($27.28) from the U.S. Department of Labor.
The average hourly rate is derived from the U.S Department of Labor; the Bureau of Labor Statistics' 2012 Occupational Employment Statistics available at: http://www.bls.gov/oes/current/oessrcst.htm
State animal health officials and accredited veterinarians: $43.87 (veterinarians)
Animal producers, market/buying station operators, and feedlot operators: $21.97 (first-line supervisors of farm workers)
Laboratory staff: $35.66 (animal scientists)
Device manufacturers: $23.30 (computer numerically controlled machine tool programmers)
Slaughter plant personnel: $11.63 (slaughters and meat packers)
Provide estimates of the total annual cost burden to respondents or record keepers resulting from the collection of information, (do not include the cost of any hour burden shown in items 12 and 14). The cost estimates should be split into two components: (a) a total capital and start-up cost component annualized over its expected useful life; and (b) a total operation and maintenance and purchase of services component.
No annual cost burden is associated with capital and startup costs, operation and maintenance expenditures, and purchase of services.
Provide estimates of annualized cost to the Federal government. Provide a description of the method used to estimate cost and any other expense that would not have been incurred without this collection of information.
The annualized cost to the Federal Government is estimated at $51,410 (see APHIS Form 79).
Explain the reasons for any program changes or adjustments reported in Items 13 or 14 of the OMB Form 83-1.
|
Requested |
Program Change Due to New Statute |
Program Change Due to Agency Discretion |
Change Due to Adjustment in Agency Estimate |
Change Due to Potential Violation of the PRA |
Previously Approved |
Annual Number of Responses |
106,890 |
0 |
-1,390,260 |
17,902 |
0 |
1,479,248 |
Annual Time Burden (Hr) |
47,054 |
0 |
-650,390 |
-17,646 |
0 |
715,090 |
Annual Cost Burden ($) |
0 |
0 |
0 |
0 |
0 |
0 |
The overall burden decreased 668,036 burden hours and 1,372,358 responses due to both program changes (-650,390 hours) and adjustments (-17,646 hours).
Adjustments
Even though APHIS’ burden figures stayed the same for the SF Agreements forms (SF-424, 424A, 424B, and LLL) (State) there is an adjustment of + 188 burden hours due to a change in who carries the burden. Previously, APHIS only listed the burden hours, but would subtract them from the total because another Government agency carried the burden. Currently, each agency carries their own burden for these forms until they are approved as common forms.
The following adjustments resulted in increases in burden because of consolidated animal identification methods (some other activities were removed [see below]) and general adjustments in burden:
AIN Device Manufacturer Updates and Recordkeeping (business) (+141 hours)
AIN Device Managers Registration and Agreement (business) (+1,075 hours)
AIN Device Managers Updates (business) (+2,375 hours)
There are adjustments in burden for the following burden items. The respondents decreased because of the change of the system and better automation.
Premises Identification (business) (-20,000 hours)
Updates to Premises ID Records (business) (-1,250 hours)
Nonproducer Participant Registration (business) (-175 hours)
The Cooperative Agreement Application and Quarterly Reports (State) stayed the same; however, the recordkeeping burden for Cooperative Agreement Application decreased 18 hours because the annual hours per recordkeeper decreased .25 hours due to better methods of recordkeeping (adjustment).
There are also adjustments that occurred due to a change in rounding and miscellaneous actions.
Program Changes
Official ID Device Applications is new (program change) but replaces some burden items that were removed from the last renewal (business) (+108 hours).
The change from the NAIS to the ADT has resulted in a number of burden changes including discontinuing activities. Many activities are now managed by the States and Tribes. In addition, APHIS no longer requires reporting of animal movements to premises, so APHIS no longer tracks individual and group/lot movement records. Finally, APHIS has consolidated its tracking methods for issuance of the various forms of identification. The following burden items were removed (program change):
AIN Device Manufacturer Applications, Agreements, and Training (business) (-65 hours)
Manufacturers’ Tags (business) (-13 hours)
Visual Only Eartags (business) (-13 hours)
RFID Eartags (business) ( -13 hours)
RFID Injectable Transponders (business) (-13 hours)
AIN Device Managers Training (business) (-300 hours)
Individual Group and Movement Records (business) (-650,000 hours)
Application for Evaluation of Animal Tracking Database (ATD) (States) (-68 hours)
For collections of information whose results are planned to be published, outline plans for tabulation and publication.
APHIS has no plans to publish information it collects in connection with this program.
If seeking approval to not display the expiration date for OMB approval of the
information collection, explain the reasons that display would be inappropriate.
There are no Agency forms associated with this information collection.
Explain each exception to the certification statement identified in the "Certification for Paperwork Reduction Act."
APHIS can certify compliance with all provisions of the Act.
Collections of Information Employing Statistical Methods
No statistical methods are associated with the information collection activities used in this
program.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | SUPPORTING STATEMENT 0579-0165 |
| Author | Kay Brown |
| File Modified | 0000-00-00 |
| File Created | 2021-01-31 |
© 2026 OMB.report | Privacy Policy