Attachment E - EIP information
AttachmentE_0920-0852_SupplementalInformationOnly.docx
Prevalence Survey of Healthcare Associated Infections (HAIs) and Antimicrobial Use in U.S. Acute Care Hospitals
Attachment E - EIP information
OMB: 0920-0852
2011 HAI & ANTIMICROBIAL USE POINT PREVALENCE SURVEY: EIP TEAM ANTIMICROBIAL USE FORM
CDC ID: - |
Survey date: // |
Date form completed: // |
Data collector initials: ________
|
|
|
**Check here if no antimicrobials were administered on the survey date or the calendar day prior to the survey date (*be sure to consider whether dialysis qualification applies—see Primary Team/EIP Team Data Collection Form). Otherwise, fill in information, complete pages 1 AND 2 of form.
**Check here if >6 antimicrobial agents administered on the survey date or the calendar day prior to the survey date (*be sure to consider whether dialysis qualification applies—see Primary Team/EIP Team Data Collection Form), AND enter additional antimicrobial agents on another Antimicrobial Use Form.
This is Antimicrobial Use Form # ______ out of a total of ______ Antimicrobial Use Form(s) for this patient.
Therapeutic site codes: BJI = Bone or joint, BSI = Bloodstream infection, CNS = Central nervous system, CVI = Cardiovascular (other than BSI), DIS = Systemic, disseminated infection, ENT = Eyes, ears, nose, throat (includes upper respiratory infection, GTI = Gastrointestinal tract, HEB = hepatic and biliary system infections (including pancreas), IAB = intraabdominal infection other than GTI and HEB (e.g., spleen abscess), LRI = Lower respiratory infection, REP = Reproductive tract infection, SST = Skin or soft tissue infection (includes muscle infection), UTI = Urinary tract infection, UND = Undetermined, Other = specify other site.

Drug |
Route (check one): |
Rationale (check all that apply):
|
|
If Rationale is “Treatment of active infection,” then complete the following: |
||||
|
Clinician-defined therapeutic site (check all that apply): |
|
Infection onset (check all that apply): |
|||||
|
IV or IM Oral/enteral Inhaled
|
Medical prophylaxis Surgical prophylaxis
Non-infectious None documented |
|
BJI BSI CNS CVI DIS ENT |
GTI HEB IAB LRI REP |
SST UTI UND Unknown Other: _______
|
AND |
Your hospital Other healthcare facility Community Unknown |
Drug |
Route (check one): |
Rationale (check all that apply):
|
|
If Rationale is “Treatment of active infection,” then complete the following: |
||||
|
Clinician-defined therapeutic site (check all that apply): |
|
Infection onset (check all that apply): |
|||||
|
IV or IM Oral/enteral Inhaled
|
Medical prophylaxis Surgical prophylaxis
Non-infectious None documented |
|
BJI BSI CNS CVI DIS ENT |
GTI HEB IAB LRI REP |
SST UTI UND Unknown Other: _______
|
AND |
Your hospital Other healthcare facility Community Unknown |
Drug |
Route (check one): |
Rationale (check all that apply):
|
|
If Rationale is “Treatment of active infection,” then complete the following: |
||||
|
Clinician-defined therapeutic site (check all that apply): |
|
Infection onset (check all that apply): |
|||||
|
IV or IM Oral/enteral Inhaled
|
Medical prophylaxis Surgical prophylaxis
Non-infectious None documented |
|
BJI BSI CNS CVI DIS ENT |
GTI HEB IAB LRI REP |
SST UTI UND Unknown Other: _______
|
AND |
Your hospital Other healthcare facility Community Unknown |
Continued on page 2
2011 HAI & ANTIMICROBIAL USE POINT PREVALENCE SURVEY: EIP TEAM ANTIMICROBIAL USE FORM (continued)
CDC ID: -
Drug |
Route (check one): |
Rationale (check all that apply):
|
|
If Rationale is “Treatment of active infection,” then complete the following: |
||||
|
Clinician-defined therapeutic site (check all that apply): |
|
Infection onset (check all that apply): |
|||||
|
IV or IM Oral/enteral Inhaled
|
Medical prophylaxis Surgical prophylaxis
Non-infectious None documented |
|
BJI BSI CNS CVI DIS ENT |
GTI HEB IAB LRI REP |
SST UTI UND Unknown Other: _______
|
AND |
Your hospital Other healthcare facility Community Unknown |
Drug |
Route (check one): |
Rationale (check all that apply):
|
|
If Rationale is “Treatment of active infection,” then complete the following: |
||||
|
Clinician-defined therapeutic site (check all that apply): |
|
Infection onset (check all that apply): |
|||||
|
IV or IM Oral/enteral Inhaled
|
Medical prophylaxis Surgical prophylaxis
Non-infectious None documented |
|
BJI BSI CNS CVI DIS ENT |
GTI HEB IAB LRI REP |
SST UTI UND Unknown Other: _______
|
AND |
Your hospital Other healthcare facility Community Unknown |
Drug |
Route (check one): |
Rationale (check all that apply):
|
|
If Rationale is “Treatment of active infection,” then complete the following: |
||||
|
Clinician-defined therapeutic site (check all that apply): |
|
Infection onset (check all that apply): |
|||||
|
IV or IM Oral/enteral Inhaled
|
Medical prophylaxis Surgical prophylaxis
Non-infectious None documented |
|
BJI BSI CNS CVI DIS ENT |
GTI HEB IAB LRI REP |
SST UTI UND Unknown Other: _______
|
AND |
Your hospital Other healthcare facility Community Unknown |
Check one of the boxes below and follow the corresponding instructions:
If Rationale for ANY antimicrobial drug administered to the patient is “None documented” or “Treatment of active infection” GO TO HAI FORM.
If Rationale for EVERY antimicrobial drug administered to the patient is “Medical prophylaxis,” “Surgical prophylaxis” or “Non-infectious”
DON’T fill out HAI Form. Data collection complete.
2011 HAI & ANTIMICROBIAL USE POINT PREVALENCE SURVEY: EIP TEAM HAI FORM
CDC ID: - |
Survey date: // |
Date form completed: // |
Data collector initials: __________ |
Does the patient have an HAI (check one)? |
|
No data collection complete Yes complete the table and questions below.
|
|
Enter only one HAI on each HAI Form. This is HAI Form # _____ out of _____ total HAI Forms for this patient.
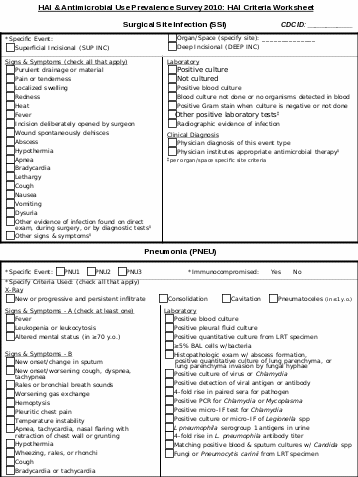
HAI |
Specific Site |
Device and Procedure Information |
Comments |
|||
UTI |
SUTI ABUTI OUTI |
Catheter-associated? No Yes |
|
|||
PNEU |
PNU1 PNU2 PNU3 |
Ventilator-associated? No Yes |
|
|||
BSI
|
LCBI
|
Central line-associated? No Yes |
|
|||
SSI |
SUP INC DEEP INC ORGAN/SPACE (for ORGAN/SPACE, specify site : ___________) |
Operative procedure category code:
|
|
|||
BJ
|
BONE JNT DISC |
|
|
|
||
CNS |
IC MEN SA |
|
|
|
||
CVS |
VASC ENDO |
CARD MED |
|
|
||
EENT |
CONJ EYE EAR |
ORAL SINU UR |
|
|
||
GI |
GE GIT HEP |
IAB TRANS NEC CDI |
|
|
||
LRI |
BRON LUNG |
|
|
|||
REPR |
EMET EPIS |
VCUF OREP |
|
|
||
SST |
SKIN ST BURN |
DECU BRST UMB |
PUST CIRC |
|
|
|
SYS |
DI |
|
|
|
||
Enter the symptom/sign onset date for this HAI: // OR Unknown OR Not collected
Enter the therapy start date for this HAI: //
OR check one: Unknown Not collected No therapy given
Was there a Secondary Bloodstream Infection associated with this HAI? No Yes Unknown
Enter up to three pathogen codes for this HAI: 1) ________ 2)________ 3) _________ OR No pathogen identified
Enter the CDC location of attribution for this HAI: _______________ Unknown Not applicable (i.e., SSI)
Continued on page 2
2011 HAI & ANTIMICROBIAL USE POINT PREVALENCE SURVEY: EIP TEAM HAI FORM (continued)
|
|
Antimicrobial Susceptibility Testing—Instructions:
Check the appropriate box(es) to indicate which of the pathogen(s) below (if any) caused this HAI. “E. coli”=Escherichia coli; “E. faecium”=Enterococcus faecium; “E. faecalis”=Enterococcus faecalis; “P. aeruginosa”=Pseudomonas aeruginosa; “S. aureus”=Staphylococcus aureus.
Check the appropriate susceptibility test results for the antimicrobial agents listed: S=sensitive/susceptible. I=intermediate, R=resistant, N=not tested.
Antimicrobial agent abbreviations: AMK=amikacin, AMP=ampicillin, AMPSUL=ampicillin/sulbactam,CEFEP=cefepime, CEFOT=cefotetan, CEFTAZ=ceftazidime, CEFTRX=ceftriaxone, CIPRO=ciprofloxacin, CLINDA=clindamycin, COL/PB=colistin or polymyxin B, DAPTO=daptomycin, DOXY=doxycycline, ERYTH=erythromycin, GENT=gentamicin, IMI=imipenem, LEVO=levofloxacin, LNZ=linezolid, MERO=meropenem, OX=oxacillin, PENG=penicillin G, PIP=piperacillin, PIPTAZ=piperacillin/tazobactam, QUIDAL=quinupristin/dalfopristin, RIF=rifampin, TETRA=tetracycline, TIG=tigecycline, TMZ=trimethoprim/sulfamethoxazole, VANC=vancomycin.
Check here if NONE of the organisms below are pathogens for this HAI (data collection is now complete).
Acinetobacter baumannii other |
AMK |
AMPSUL |
CEFEP |
CEFTAZ |
CIPRO
|
COL/PB |
GENT |
IMI |
LEVO |
MERO |
PIPTAZ |
TOBRA |
TIG |
|||||||||||||
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
|
E. coli |
AMK |
AZT |
CEFEP |
CEFOT |
CEFTAZ |
CEFTRX |
CIPRO |
GENT |
IMI |
LEVO |
MERO |
TOBRA |
||||||||||||||
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
|||
Positive test for extended-spectrum beta lactamase (ESBL) production? Yes No Unknown |
Positive test for carbapenemase production? Yes No Unknown |
|
||||||||||||||||||||||||
E. faecium |
AMP |
DAPTO |
LNZ |
PENG |
QUIDAL |
VANC |
||||||
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
|
E. faecalis |
AMP |
DAPTO |
LNZ |
PENG |
VANC |
|||||
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
|
Klebsiella pneumoniae oxytoca other |
AMK |
AZT |
CEFEP |
CEFOT |
CEFTAZ |
CEFTRX |
CIPRO |
GENT |
IMI |
LEVO |
MERO |
TOBRA |
|||||||||||||
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
||
Positive test for extended-spectrum beta lactamase (ESBL) production? Yes No Unknown |
Positive test for carbapenemase production? Yes No Unknown |
|
|||||||||||||||||||||||
P. aeruginosa |
AMK |
AZT |
CEFEP |
CEFTAZ |
CIPRO |
GENT |
IMI |
LEVO |
MERO |
PIP |
PIPTAZ |
TOBRA |
||||||||||||
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
|
S. aureus |
CLIND |
DAPTO |
DOXY |
ERYTH |
GENT |
LNZ |
OX |
QUIDAL |
RIF |
TETRA |
TMZ |
VANC |
||||||||||||
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
S I |
R N |
|
Enter the vancomycin MIC (in mcg/ml):
______________ Unknown Not collected |
Check vancomycin MIC test method: E-test Vitek 2 Vitek Legacy Phoenix MicroScan dried overnight panels Unknown Not collected Other: ___________________________ |
|||||||||||||||||||||||
FORM IS COMPLETE

Phase3_AntimicrobialUseForm_v1_20101210 page 1 of 2
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | fxe9 |
| File Modified | 0000-00-00 |
| File Created | 2021-02-01 |
© 2026 OMB.report | Privacy Policy
 Treatment
of active infection
Treatment
of active infection


