Revised Results Worksheet
Centers for Disease Control and Prevention Performance Evaluation Program for Mycobacterium Tuberculosis/Non-tuberculosis Mycobacteria Drug Susceptibility Testing Program
NewFormTB Questions.1
MPEP for M. TB and non-tuberculous Mycobacteria Drug Susceptibility Testing Results Worksheet
OMB: 0920-0600
 U.S.
DEPARTMENT OF HEALTH AND HUMAN SERVICES
U.S.
DEPARTMENT OF HEALTH AND HUMAN SERVICES

Centers for Disease Control and Prevention
Coordinating Center for Infectious Diseases, Mail Stop G-23
Atlanta, Georgia 30333
OMB Form NO. __0920-0600_
Exp. Date _05/13/2013
DRUG SUSCEPTIBILITY TESTING PROGRAM FOR
MYCOBACTERIUM TUBERCULOSIS
WARNING:
The culture panel provided in this survey consists of viable
strains of Mycobacterium
tuberculosis Complex (MTBC)
only, some of which are drug-resistant. The cultures in the panel
should be considered hazardous and capable of transmitting
infection. Testing should only be done if the recommended safety
procedures are followed as described in the Centers
for Disease Control and Prevention’s Biosafety
in Microbiological and Biomedical Laboratories, 2007, 5th Edition.
This manual can be accessed at
http://www.cdc.gov/od/ohs/biosfty/bmbl5/BMBL_5th_Edition.pdf
This manual recommends use of Biosafety Level 3 practices when
testing MTBC
cultures.
GENERAL INSTRUCTIONS
PLEASE READ ALL INSTRUCTION SHEETS COMPLETELY BEFORE PROCEEDING WITH ANY CULTURE EVALUATION.
Check the contents of your package. It should contain:
Cover letter
Results Worksheet for recording testing results with instructions.
(3) Laboratory Information Change Form for recording any changes to laboratory information.
(4) Shipping container with five (5) cultures labeled “TB Cultures.” The culture tubes are labeled with individual identification codes.
If the contents of your package are not complete, or if additional cultures are required, please call XXXXX, Project Officer at CDC at 404-498-XXXX immediately.
INSTRUCTIONS FOR ENTERING RESULTS
Results must be entered in the on-line data entry system only no later than XX/XX/XXXX. You will need your TPEP number and password. If you have forgotten or misplaced your password please contact Ms Sandra Neal at 404 498-2238.
1. After testing your samples, enter your results at the CDC Tuberculosis Drug Susceptibility Website: http://wwwn.cdc.gov/mpep/mtbds/login.aspx
2. Please verify laboratory information and make any changes on the Website or on the enclosed Laboratory Information Change Form.
3. If you can not use the on-line data entry system, please complete the Results Worksheet and contact the project officer at (888) 465-6062 or 404-498-2238.
4. For multiple choice questions beginning on page 4 of the Results Worksheet, fully blacken the circle to the left of the appropriate answer. Please do not use checks marks () or cross marks (X) within the circles.
MTBC Results Worksheet
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
Centers for Disease Control and Prevention
Coordinating Center for Infectious Diseases, Mail Stop G-23
Atlanta, Georgia 30333
OMB Form NO. __0920-0600_
Exp. Date _05/13/2013
CDC DRUG SUSCEPTIBILITY TESTING PROGRAM FOR MYCOBACTERIUM TUBERCULOSIS RESULTS FORM
Report your results Online
(password required) at: http://wwwn.cdc.gov/mpep/mtbds/login.aspx TPEP
number: ____________ (you will need this to enter your results
online) DEADLINE
for submission XXXX, 2010
Please note: Treat these cultures in the same manner that you routinely treat MTBC isolates, with the following exception:
If you test second-line drugs in your laboratory, test these isolates (regardless of results from testing first-line drugs) using those second-line drugs normally used to test patient isolates. This will provide you with an opportunity to evaluate your performance for testing second-line drugs.
WARNING:
The culture panel provided in this survey consists of viable
strains of Mycobacterium
tuberculosis Complex
(MTBC) only,
some of which are drug resistant. The cultures in the panel should
be considered hazardous and capable of transmitting infection.
Testing should only be done if the recommended safety procedures are
followed as described in the Centers
for Disease Control and Prevention’s Biosafety
in Microbiological and Biomedical Laboratories, 2007, 5th Edition.
This manual can be accessed at
http://www.cdc.gov/od/ohs/biosfty/bmbl5/BMBL_5th_Edition.pdf
This manual recommends use of Biosafety Level 3 practices
when testing M.
tb cultures.
If
you do not have the capacity to enter your results online or if you
need assistance contact: XXX-XXX-XXXX The
Project Officer can be contacted at: Sneal@cdc.gov
or 404
498-2238
Please indicate changes to your laboratory information on the enclosed Laboratory information Change Form and return to project officer.
Person(s) Completing Form:
1. Name: ___________________________________________________________
2. Title: ___________________________________________________________
MTBC Worksheet
3. Please indicate the primary classification of your laboratory. (Please blacken only one circle.)
 Hospital
Hospital
[e.g., city, county, district, community, state, regional, military, Veterans Administration, Federal government
(other than military), privately-owned, university, HMO/PPO-owned and operated, religious-associated]
 Health
Department
Health
Department
[e.g., city, county, state, regional, district, national reference laboratory]

Independent
[e.g., commercial, commercial manufacturer of reagents, HMO satellite clinic, reference laboratory (non- government affiliated)]
O ther
ther
[e.g., university-associated research, Federal government research (nonmilitary), privately-funded research]
4. In the last calendar year (January 1 - December 31, XXXX), how many Mycobacterium tuberculosis complex (MTBC) isolates (excluding quality control isolates) did your laboratory test for drug susceptibilities? (Please write the number of Mycobacterium tuberculosis isolates your laboratory tested for susceptibility in the boxes below.)
|
|
|
|
|
|
MTBC isolates:
The following questions
pertain to the receiving and testing of the culture panel. In most cases,
blacken the circle corresponding to your response in the circle
provided to the left of the answer. Some questions may require more
than one response; please blacken all that apply. In some cases,
you will be asked to fill in the boxes to the right of the answer
with an appropriate comment or number.
5. On what date was the culture panel received in your laboratory?
-
/
/
Month
Day Year
MTBC Worksheet
6. What was the condition of the cultures in the panel when they arrived?
( Please
blacken only one circle.)
Please
blacken only one circle.)
Satisfactory
Broken
Other (please explain): _______________________________
7. What method(s) was used in your laboratory to perform drug susceptibility testing on the MTBC isolates in this shipment? (Please blacken all that apply.)
Agar
Proportion (Middlebrook 7H10)
Agar Proportion (Middlebrook 7H11)
Radiometric (BACTEC)
VersaTREK Myco
MGIT System
Lowenstein Jensen (LJ) proportion method
Molecular Method (please specify): _______________________________
Other (please specify): __________________________________________
8. If your laboratory uses more than one method for testing this shipment’s isolates for first-line drugs for MTBC susceptibility, please indicate the primary method (NOT confirmatory method) that is used. (Please blacken only one circle.)
Agar
Proportion (Middlebrook 7H10)
Agar Proportion (Middlebrook 7H11)
Radiometric (BACTEC)
VersaTREK Myco
MGIT System
Lowenstein Jensen (LJ) proportion method
Molecular Method (please specify): _______________________________
Other (please specify): __________________________________________
MTBC Worksheet
9. If you use Middlebrook 7H10 or 7H11 media as either a primary or secondary method of MTBC drug susceptibility testing, your media is: (Please blacken all that apply.)
p urchased
“commercially-prepared” containing anti-tuberculosis
drugs
urchased
“commercially-prepared” containing anti-tuberculosis
drugs
prepared in-house with disks containing anti-tuberculosis drugs
prepared in-house by reconstituting and adding anti-tuberculosis drugs
Not Applicable
Note: Question 10 is a new question, to be asked one time only for informational purposes.
10 a. In your opinion, is there a need for offering performance evaluation of NTM strains?
 Yes
Yes
No
10 b. If yes - For your laboratory, would it be more advantageous to offer evaluation of:
Rapidly growing NTM
Slowly growing NTM
Continue to the next page.
MTBC Worksheet
11. For each antimicrobial that you use routinely to determine the susceptibility of M. tb, record a test method, the concentration of the antimicrobial and a result (R=Resistant, S=Susceptible, O=Other). If the isolates in the panel were tested using more than one concentration of an antimicrobial, record those results on lines that correspond to the antimicrobial you are testing (Example 1). If you need more lines than are provided for that antimicrobial, please record results in the blank lines provided at the bottom of the result page. Do not cross out an existing antimicrobial and write another drug name over it (example 2).
If you are testing an antimicrobial not listed on the result page, record the entire drug name (no abbreviations), a concentration and a result in the blank lines provided at the bottom of the result page. Please make sure that each result is recorded on a provided line and not written in the margins outside the form. Make a copy of the result page if you do not have enough room on the provided page to record all results.
Other responses related to susceptibility results such as Borderline, Contaminated, No Growth, etc. can be abbreviated and recorded to the right of the "O" selection in the result columns (examples 1 and 3).
1. Following are examples of CORRECTLY reported M. tb results.
Isoniazid |
A |
|
|
0 |
. |
1 |
|
R |
R |
R |
Isoniazid |
A |
|
|
0 |
. |
2 |
|
R S O |
R S O |
R S O |
Isoniazid |
A |
|
|
1 |
. |
0 |
|
R S O |
R S O |
R S O NG |
2. Following are examples of INCORRECTLY reported M. tb results.



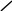
Isoniazid |
A |
1 |
2 |
- |
. |
- |
0 |
R |
R |
R |
Isoniazid |
A |
|
|
|
. |
|
|
R |
R |
R |
MTBC Worksheet
These are the results for M. tuberculosis complex testing.
**Please provide the Test Method, the Concentration, and the Test Results for each line reported. |
|||||||||||
12. (Continued) Use the blank lines provided at the end of the form for other drugs or additional concentrations. |
A=Agar Proportion B=BACTEC C=L-J Proportion D=MGIT O=Other: (Choose only one) |
Please list each concentration |
Culture Identification Codes(Fill in ONE letter for each culture) R=Resistant, S=Susceptible, O=Other Please indicate any other responses in the space providedFor example: B=Borderline, C=Contaminated, NG=No Growth, |
||||||||
Antimicrobial |
Test Method |
Conc. μg/mL |
O |
P |
Q |
R |
S |
||||
Isoniazid |
A |
|
|
|
|
|
|
|
R S O |
|
|
Isoniazid |
A |
|
|
|
|
|
|
|
R S O |
|
|
Isoniazid |
A |
|
|
|
|
|
|
|
R S O |
|
|
Isoniazid |
A |
|
|
|
|
|
|
|
R S O |
|
|
Rifampin |
A |
|
|
|
|
|
|
|
R S O |
|
|
Rifampin |
A |
|
|
|
|
|
|
|
R S O |
|
|
Rifampin |
A |
|
|
|
|
|
|
|
R S O |
|
|
Pyrazinamide |
A |
|
|
|
|
|
|
|
R S O |
|
|
Pyrazinamide |
A |
|
|
|
|
|
|
|
R S O |
|
|
Pyrazinamide |
A |
|
|
|
|
|
|
|
R S O |
|
|
Ethambutol |
A |
|
|
|
|
|
|
|
R S O |
|
|
Ethambutol |
A |
|
|
|
|
|
|
|
R S O |
|
|
Ethambutol |
A |
|
|
|
|
|
|
|
R S O |
|
|
Streptomycin |
A |
|
|
|
|
|
|
|
R S O |
|
|
Streptomycin |
A |
|
|
|
|
|
|
|
R S O |
|
|
Streptomycin |
A |
|
|
|
|
|
|
|
R S O |
|
|
Ethionamide |
A |
|
|
|
|
|
|
|
R S O |
|
|
Ethionamide |
A |
|
|
|
|
|
|
|
R S O |
|
|
Kanamycin |
A |
|
|
|
|
|
|
|
R S O |
|
|
Kanamycin |
A |
|
|
|
|
|
|
|
R S O |
|
|
Capreomycin |
A |
|
|
|
|
|
|
|
R S O |
|
|
Capreomycin |
A |
|
|
|
|
|
|
|
R S O |
|
|
Cycloserine |
A |
|
|
|
|
|
|
|
R S O |
|
|
Cycloserine |
A |
|
|
|
|
|
|
|
R S O |
|
|
p-Aminosalicylic acid |
A |
|
|
|
|
|
|
|
R S O |
|
|
p-Aminosalicylic acid |
A |
|
|
|
|
|
|
|
R S O |
|
|
Amikacin |
A |
|
|
|
|
|
|
|
R S O |
|
|
Amikacin |
A |
|
|
|
|
|
|
|
R S O |
|
|
Ofloxacin |
A |
|
|
|
|
|
|
|
R S O |
|
|
Ofloxacin |
A |
|
|
|
|
|
|
|
R S O |
|
|
Ciprofloxacin |
A |
|
|
|
|
|
|
|
R S O |
|
|
Ciprofloxacin |
A |
|
|
|
|
|
|
|
R S O |
|
|
A |
|
|
|
|
|
|
|
R S O |
|
|
|
|
A |
|
|
|
|
|
|
|
R S O |
|
|
|
A |
|
|
|
|
|
|
|
R S O |
|
|
|
A |
|
|
|
|
|
|
|
R S O |
|
|
|
A |
|
|
|
|
|
|
|
R S O |
|
|
Note: Please provide the complete drug name when filling in additional spaces.
Public reporting burden of this collection of information is estimated to average 6 minutes per response, including the time for reviewing
instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of
information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays
a currently valid OMB Control Number. Send comments regarding this burden estimate or any other aspect of this collection of information,
including suggestions for reducing this burden to CDC/ATSDR Reports Clearance Officer, 1600 Clifton Road NE, MS D-74, Atlanta, Georgia, Attn:
30333; PRA 0920-0600
| File Type | application/msword |
| File Title | CDC DRUG SUSCEPTIBILITY TESTING PROGRAM FOR MYCOBACTERIUM TUBERCULOSIS and NON-TUBERCULOUS MYCOBACTERIA |
| Author | Michael Walsh |
| Last Modified By | sxw2 |
| File Modified | 2010-10-14 |
| File Created | 2010-10-14 |
© 2026 OMB.report | Privacy Policy

 B C O
B C O

 S O
S O
 S O
S O
 S O
S O B C O
B C O

 S O
S O

 R S O
R S O