NCI response to questions
NCI Response for 0925-0046.docx
Formative Research, Pretesting, and Customer Satisfaction of NCI's Communication and Education Resources (NCI)
NCI response to questions
OMB: 0925-0046
NCI Response to OMB Pass back Questions re: 0925-0046 #14, #15, and #16
0925-0046-14
Please provide a justification for the proposed incentive amount ($75). Based on the estimated time involved, we typically recommend no more than $50/hour.
To assess the remuneration rate for this specific activity, OCE had their research contractor, AED, contact five qualitative research recruiting firms to ask whether or not they would be able to complete this specific recruit if the remuneration amount was set at $50. AED also asked what the minimum remuneration amount would be to successfully recruit the 24 female breast cancer patients/survivors required for this study. The scope of work and characteristics of participants were described.
While the cost of recruitment varied, we were strongly cautioned that a remuneration amount of $50 will likely not be sufficient to complete this recruit in a timely and cost efficient manner given the current market. Responses from these firms call into question whether a $75 dollar incentive will be sufficient to secure in-person recruits. A reimbursement of $100 per recruit for in-person tests appears to be more appropriate given the concerns expressed and range of feedback.
Below are the four responses from qualitative research recruiting firms received the week of June 1, 2011 (one firm did not respond to our query):
We suggest $100 for remote participants, $150 for in-person. The remote participants will need to spend two hours, one to prepare for the interview, one for the interview itself, even without going anywhere, and they will still need to make themselves available at a specific time. Obviously, more money should be paid to entice people to make the extra effort to come in-person, in that their commute will likely take an additional hour or more… Our recruitment rate would be $200 per person. We will distribute the announcement about the study to both our local DC area participant database and to our nationwide participant database.
At a $50 incentive, our recruitment rate would be $300 per person. It would likely take us more time to initially recruit the needed participants, because there would be fewer people willing to go to the trouble to participate for the lesser incentive. In addition, experience has shown that there are more no shows, and thus participants needing to be replaced, when we pay this small an incentive. I would also expect that participants will be less diligent in reviewing the material before the interviews if they don’t feel they are being adequately compensated.
If the incentive is the one we recommend, we would definitely bid on the work. If the incentive is $50, we would consider bidding, primarily because AED is one of our favorite clients and we would hesitate to miss out on an opportunity to support them. However, at that incentive level, we may decide to pass, especially if we are busy with other projects at the time.
Suggested Incentive: In Person Interview - $125.00 per person; Phone/Web Interview - $100.00 per person. Our rationale is based on the current rates for this area. Consumer/general population focus groups typically have an honorarium of $75 for 90-120 minutes. This is a niche audience and to get them to commit and to show we suggest the higher incentive. The total time commitment is no less than that of a focus group. Also, IDIs are often scheduled during the day and the incentive is higher to compensate for any time the participant may miss from work.
Recruiting & Confirming - $175.00 per recruit. [This firm] is currently in the progress of recruiting several groups of breast cancer patients/survivors who were diagnosed within the past 5 years.
With an incentive of $50 we would not be able to guarantee any specific number of recruits. Our cost per recruit would not change, however, there would be an administrative fee of $1000 if we were to begin recruitment but not able to complete the recruit based on the incentive.
50.00 for remote is fine but we strongly recommend 100.00 for the on site particularly given the location and distance respondents will have to travel - traffic and parking costs too.
[Recruitment cost] 150.00 per recruit
If it [the incentive] was to be 50.00 for offsite I would not feel comfortable guaranteeing a show rate. We have done this for AED before as well as other organizations and the 100.00 is what has been done in the past for on site.
[Recommended incentive] $75, this area expects it. Lower incentive doesn’t induce commitment.
Additionally for OMB No. 0925-0046-02 (approved on 8/13/2010), the respondents included cancer patients had cancer within the past 5 years. The justification memo stated that:
“In order to gauge the remuneration rates typical in today’s market, OCE has contacted 8 focus group facilities to ask what the minimum remuneration amount would be required to achieve a feasible turn-out to conduct the focus groups with the general public. We were cautioned that a remuneration amount of $60 will not be enough given the current market. Here is a sample of responses we received from focus group facilities regarding this issue:
“The minimum incentive amount that we will accept for a focus group of 90 minutes to 2 hours is $75. This is a standard amount in most major markets across the country, and show rates are greatly jeopardized if the incentive is reduced, and recruiting fees are higher because the refusal rate is higher. We need to ensure that all potential respondents agree to attend, and that the show rate is as high as possible.”
“We recommend a $75 incentive across the board even though $85 is the typical incentive for daytime groups. These costs are the norm in our market, so it would be extremely difficult to go any lower than $75. We feel that the incentive fits are market, and the time commitment you are asking for. A higher incentive keeps respondents committed to doing the group, and gives some incentive for responding to our calls.”
“In order to have a good show rate to produce the necessary results for your research we would highly recommend offering $75.00 per person for groups after 5pm and $100.00 per person for groups before 5pm. These are the standard incentive costs for research in the DC area.”
Based on these responses, we are proposing providing a remuneration rate of approximately $75 for focus group participants from the general public, and $150 for those triad participants who are participating in the groups because of their specialization in the health care field.”
For OMB No. 0925-0046-07 (approved on 1/21/2011), the respondents included males who had heard of a PSA test but did not have cancer. Again, they were asked to participant in a focus group. The justification memo was revised based on feedback from OMB (revision dated January 11, 2011), stated that:
“Participants will receive $75 as a thank you for their participation. For intensive forms of interviews (that is, cognitive interviews, focus groups, and usability tests), participants generally receive remuneration, for several reasons: (1) Eligibility criteria for participants are usually specific, and receiving an incentive will help attract participants; (2) Intensive forms of interviews require an unusual level of mental effort and a significant amount of time; and (3) Participants are being asked to travel to a focus group facility, which involves transportation and possibly parking expenses.
After a discussion with the contractors, they are concerned that anything less than $75 will have a substantial impact on the ability to successfully recruit participants for the focus groups. They indicate that the focus groups will be conducted during the day, which mean participants may miss work. Thus a low incentive may reduce the recruitment for younger, still working men to participate and therefore bias the findings. They also indicate that reducing the incentive will mean that more people will need to be recruited in order to get enough people to show up. Because there are costs associated with recruiting each participant, even for no-shows, needing to recruit more would substantially increase the cost of the project.”
0925-0046-15
Please explain the utility of question #9:
9. How much do you agree or disagree with the following statement?
The [INSERT TRIAL NAME] trial is less interesting to me now than when it opened.
Evidence in the literature and with oncologists confirms that “low interest” in the science of a trial is a key reason why oncologists do not recommend a trial to patients. Often, a trial is initially interesting to the oncology field when it is conceptualized, but the length of time it takes to open and run a trial can render a trial obsolete or moot in the PIs’ minds (i.e., it started off interesting to them but they lost interest as time went on). In sum, there is evidence that once a trial is no longer interesting, the trial will fail to accrue its goals—solutions exist to improving this barrier if identified. This question – combined with the others in the survey—will allow us to identify the level of interest in the trial in present day and identify ways that we can address any concerns oncologists have about the trial.
0925-0046-16
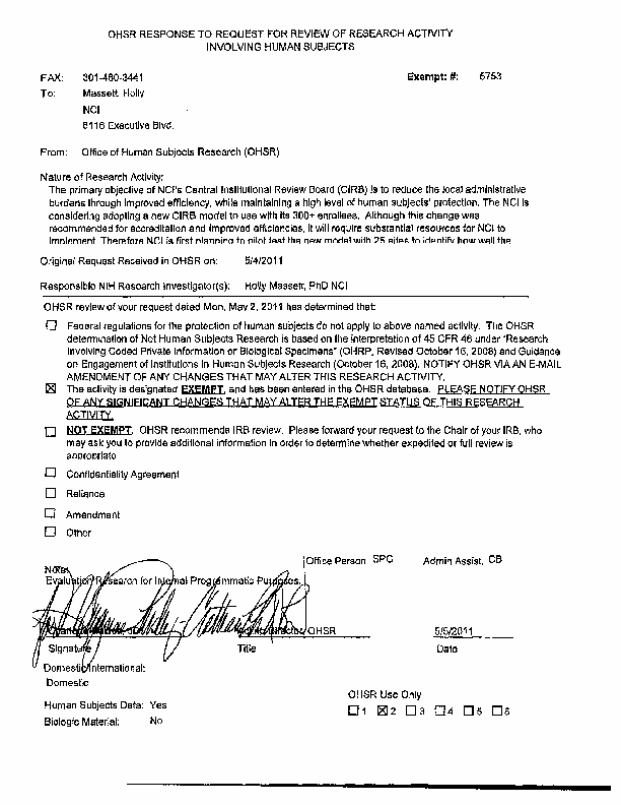
What is the status of the Office of Human Subjects Research exemption request mentioned on page 4 of the justification memo?
The proposed research for #16 has been submitted and been labeled ‘exempt’ by OHSR (see attachment below)).
Per the language at the beginning of the baseline questionnaire (The information you provide will be kept private under the Privacy Act.), please confirm that the Privacy Act applies to this collection (this was not mentioned in the justification memo).
The information has been determined to be covered by the Privacy Act (see attachment below for a copy of the email indicating this).
Please explain how this GenIC fits within the scope of the umbrella generic (Formative Research, Pretesting, and Customer Satisfaction of NCI's Communication and Education Resources) as it does not appear to involve communications materials or education resources.
The intent of the OMB request for sub-study #16 is to pretest the satisfaction of a new CIRB program among oncology researchers in the field. The intent is also to formatively test new materials that CIRB might use to communicate with future enrollees. Though the justification memo did not clearly articulate how the sub-study fits under the umbrella generic package, we would like to clarify.
In reviewing the most recently approved SSA for this umbrella package, the document states that research conducted under this SSA is: 1) to conduct formative research and pretesting activities to ensure that messages have the potential to be received, understood, and accepted by those for whom they are intended; and 2) to assess customer satisfaction of NCI’s programs and products. The findings from the research proposed in #16 will be used to do both of these activities and will aid the CIRB team in: 1) assessing the degree that the enrollees are satisfied with the new program compared to the current program; and if satisfied, use the formative research and pretesting to 2) develop a communication plan to roll out the new program; 3) identify the needs and challenges of future enrollees toward the new program and thereby be able to address them effectively and increase participation; and 4) better tailor the CIRB communication materials and forms (which will be pretested during follow-up phone calls) based on the formative research findings and input from the pilot participants. In the context of its pilot program, this sub-study will be helpful in identifying and understanding the interests, behaviors, and needs of the CIRB membership population. This formative research will be integral to CIRB in developing its program and all relevant, related communication materials. The market research proposed here is designed to assess satisfaction and needs of the CIRB audiences specifically to pretest its communication materials and use the findings to develop a communication plan and messaging to ensure maximum adoption of the new program should it meet be perceived as more satisfactory over the original program.

DATE: June 6, 2011
TO: Holly A. Massett, Ph.D.
Associate Director, Office of Market Research and Evaluation
Office of Communications and Education, NCI
Nina Goodman, Project Officer
Office of Communications and Education, NCI
FROM: NIH Privacy Act Officer
SUBJECT: Applicability of the Privacy Act: Generic Sub-study, “A Pilot Study to Test a Proposed New Model for the NCI’s Central Institutional Review Board (CIRB) Participating Institution”
I have reviewed the NCI submission to OMB which involves a 9-month pilot study with twenty-five Cooperative Group sites that will implement and follow the new CIRB “independent” review model’s processes and commitments, recommended by the Association for the Accreditation of Human Research Protection Programs (AAHRPP). Twenty of the groups will be randomly selected from among those already using the current CIRB process of a “shared responsibility” model. Five of the sites not currently enrolled in CIRB were selected based on their decision to volunteer to be part of the pilot study of the new model. The Institutional Review Boards (IRB) of those five sites each identified five participants who are currently involved in their local IRB process, to participate in the survey. The findings of the survey will be critical in determining how changes to the pilot program can lead to greater adoption of the new model and help NCI decide whether or not it should invest additional resources to fully transition to the new model or continue to use the current one.
I have determined the Privacy Act will apply to this data collection, which includes the collection of personally identifiable information (PII) such as name, organization, position at organization, opinions and experiences from the Cooperative Group participants (IRB Chair, IRB Staff person, IRB Administrator/Director, Principal Investigator and Research Coordinator).
The participants nominated through their institution based on their knowledge and experience with IRB procedures and ability to react to the new model will be sent an e-mail link directing them to a survey link. Though the link will be unique to each individual and connected to the data for their institution, the participant names and identifying information will be stored separately and not be connected to survey responses. Therefore, it will not be possible to match the contact information from the consent form with the experiences/opinions provided on the questionnaires and study-specific worksheets. Survey responses will be combined across all responses and reported in the aggregate.
Page 2 – NCI/OCE CIRB Pilot Study
However, a database will reside behind the survey web-link that is designed to retrieve participant contact information for the purpose of conducting a follow-up to the baseline survey only.
The data collection is covered by NIH Privacy Act Systems of Record 09-25-0156, “Records of Participants in Programs and Respondents in Surveys Used to Evaluate Programs of the Public Health Service, HHS/PHS/NIH/OD.”
If you have any questions, please contact my office at (301) 496-2832.
Karen M. Plá
Enclosure
cc: Vivian Horovitch-Kelly, NCI PRA Liaison
6/6/2011 Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | 0925-0046-14 |
| Author | Vivian Horovitch-Kelley |
| File Modified | 0000-00-00 |
| File Created | 2021-02-02 |
© 2026 OMB.report | Privacy Policy