Ss_a_0209
SS_A_0209.doc
CDC Cervical Cancer Study (CX3)
OMB: 0920-0814
CDC’S Cervical Cancer Study (Cx3)
An Intervention Pilot Study of HPV in Illinois NBCCEDP
Supporting Statement
Part a
January 30, 2009
Contact:
Technical Monitor Vicki Benard, PhD, Epidemiologist
Telephone: (770) 488-1092
Fax: (770) 488-4639
Email: vdb9@cdc.gov
National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP)
Centers for Disease Control and Prevention (CDC)
4770 Buford Highway NE, MS K55,
Atlanta, GA 30341
Table of Contents
A.1. Circumstances Making the Collection of Information Necessary
A.2. Purpose and Use of the Information
A.3. Use of Information Technology and Burden Reduction
A.4. Efforts to Identify Duplication and Use of Similar Information
A.5. Impact on Small Businesses or Other Small Entities
A.6. Consequences of Collecting the Information Less Frequently
A.7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
A.8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A.9. Explanation of Any Payment or Gift to Respondents
A.10. Assurance of Confidentiality Provided to Respondents
A.11. Justification for Sensitive Questions
A.12. Estimates of Hour Burden Including Annualized Hourly Costs
A.13. Estimate of Other Total Annual Cost Burden to Respondents or Recordkeepers
A.14. Annualized Cost to the Federal Government
A.15. Explanation for Program Changes or Adjustments
A.16. Plans for Tabulation and Publication and Project Time Schedule
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions
List of Tables
A.1-1 Summary of IFF
A.8-1 Study Consultants
A.12-1 Estimates of Hour Burden
A.12-2 Annualized Cost to Respondents
A.14-1 Annualized Cost to the Federal Government
A.16-1 Time Schedule
List of Exhibits
Exhibit 1 Data Collection by Year
List of Attachments
A Applicable Laws or Regulations
B Federal Register Notice and Public Comments
B1 Federal Register Notice
B2 Summary of Public Comments
C Clinic Data
C1 Initial Clinic Survey
C1a Cover Letter for Initial Clinic Survey
C2 Follow-up Clinic Survey
C2a Cover Letter for Follow-up Clinic Survey
D Provider Data
D1 Baseline Provider Survey
D1a Cover Letter for Baseline Provider Survey
D2 Follow-up Provider Survey
D2a Cover Letter for Follow-up Provider Survey
D3 Outline for Planned Provider Education Intervention
E Patient Data
E1 Patient Baseline Survey
E1a Patient Recruitment Script
E1b Patient Consent Form with patient survey
E1c Patient Consent Form no patient survey
E1d Patient Enrollment Form
E2 Patient Follow-up Survey
E2a Initial Postcard
E2b Cover Letter for Patient Follow-up
E2c Reminder Postcard
E2d Script for Reminder Phone call
E3 Patient Brochure
E4 Patient Bookmark
F Chart Review Table
G Laboratory Protocol
H Analysis
H1 Variables collected
H2 Analysis tables.doc
I IRB Approval
A.1. Circumstances Making the Collection of Information Necessary
Background. This is a new Information Collection Request. The Center for Chronic Disease Prevention and Health Promotion (NCCDPHP), Center for Disease Control and Prevention (CDC), requests permission from the Office of Management and Budget (OMB) to collect information associated with a pilot study to determine whether Pap test screening intervals increase with the addition of HPV testing along with Pap testing in women over 35. The outcomes of this study will be used to inform policies on reimbursement of the HPV DNA tests for CDC’s national screening program. The study will last for 5 years; this request is for the first 3 years of data collection.
The National Breast and Cervical Cancer Early Detection Program (NBCCEDP), which was created in response to the Breast and Cervical Cancer Mortality Prevention Act passed by Congress in 1990, is both the first and thus far the only national cancer screening program in the United States. The NBCCEDP offers breast and cervical cancer screening to underserved women. Currently the program operates in all states, the District of Columbia, 4 U.S. Territories and 13 American Indian and Alaska Native tribal programs. Since 1991, the NBCCEDP has served more than 3 million women, provided more than 7.2 million screening examinations, and diagnosed 30,963 breast cancers, 1,934 invasive cervical cancers, and 101,624 precursor cervical lesions, of which 43% were high-grade. More than half the women screened through the program are 40-59 years of age and almost half of the women are from racial/ethnic minority groups. Previous studies have examined the Minimum Data Elements (OMB No. 0920-0571; exp 1/31/2010) and an Economic Analysis (OMB No. 0920-0776; exp 4/30/2009) of the NBCCEDP. Given resource limitations, the screening policies for cervical cancer in the program include an annual Pap test until a woman has had three consecutive normal Pap tests within 5 years, at which time the Pap test frequency is reduced to every 3 years.
HPV DNA testing has been approved in the U.S. as a secondary screening tool for ASCUS (Atypical Squamous Cells of Undetermined Significance), and as a primary screening tool for women 30 years of age and older, but it is not currently a reimbursable expense under program guidelines. Adopting HPV testing along with Pap testing in women over 30 could help the program better utilize resources by extending the screening interval of women who are cytology normal and HPV test negative, which is estimated to be 80-90% of women. In 2005, the NBCCEDP convened an expert panel to evaluate policies on reimbursement of the HPV DNA test as an adjunct to the Pap test for primary screening. The panel recommended that the NBCCEDP not reimburse for the HPV DNA test but instead requested that pilot studies be performed to measure the feasibility, acceptability and barriers to use of the test.
The proposed pilot study will examine whether or not there is an increase in the cervical cancer screening interval to 3 years for women in the target age range with a normal Pap test and a negative HPV DNA test. Primary goals of the study are to: (1) assess whether provider and patient education will lead to extended screening intervals for women who have negative screening results; (2) identify facilitators and barriers to acceptance and appropriate use of the HPV test and longer screening intervals; (3) track costs associated with HPV testing and educational interventions; and (4) identify the HPV genotypes among this sample of low income women. Secondary goals of the study are to: (1) assess follow-up of women with positive test results and (2) determine provider knowledge and acceptability of the HPV vaccine.
This data collection is authorized under section 301 of the Public Health Service Act (42 U.S.C. 241). The authorizing legislation is included as Attachment A.
Privacy Impact Assessment. The proposed study will involve data collection from clinics, providers, and patients in 18 clinics in the state of Illinois that have been recruited to participate in the study. In Section B (Collections of Information Employing Statistical Methods) of the Supporting Statement, details are provided regarding the types of data that will be collected and the methods that will be used. All patients included in the study will be at least 35 years of age. No children under the age of 13 will be involved in the study.
Overview of the Data Collection System. The proposed pilot study will be conducted in 18 clinics in the state of Illinois. Each clinic will be assigned to one of two study arms: intervention or control. The intervention and control arms will be matched on clinic attributes such as geographical location (urban, rural, suburban), racial/ethnic characteristics of the patient population, hospital versus non-hospital status, provider specialty mix, and patient volume. Approximately, 10,000 women between the ages of 35 and 60 who are visiting one of the participating clinics for routine cervical cancer screening will be screened to determine whether or not they meet the study eligibility criteria. We estimate that it will be necessary to screen 10,000 women to identify 8,000 who will be eligible and willing to participate in the study.
All patients who agree to participate in the study will receive an HPV DNA test in addition to the Pap test. Clinics in the intervention group will receive HPV DNA tests to administer to eligible patients presenting for a routine Pap test PLUS a multi-component educational intervention involving both health care providers and patients. Clinics in the control group will receive the HPV tests but will not receive the educational intervention. Assignments for health care providers, coordinators and patients will be determined by the study arm assignment (intervention or control) or the clinic with which they are affiliated.
As shown in Exhibit 1 (Data Collection by Year), data will be collected from a number of sources, including clinic staff, providers, and patients. Extensive data collection will occur in the first year, with ongoing follow-up for the next 3 years. CDC requests OMB approval for data collection during the first three years (Phase I) of the 5-year project.
Clinic Coordinators at each of the 18 participating clinics will be surveyed monthly for the first year of the study to obtain information regarding resources associated with participating in the study. CDC’s Cervical Cancer Study (Cx3 Study) Initial Clinic Survey (Attachment C1) will be administered one month after study initiation and CDC’s Cervical Cancer Study (Cx3 Study) Follow-up Clinic Survey (Attachment C2) will be conducted monthly from 2 months to 12 months following study initiation. Mail surveys will be used. Completed surveys will be sent to the study contractor for data entry and analysis. This data collection activity will be completed in Phase I.
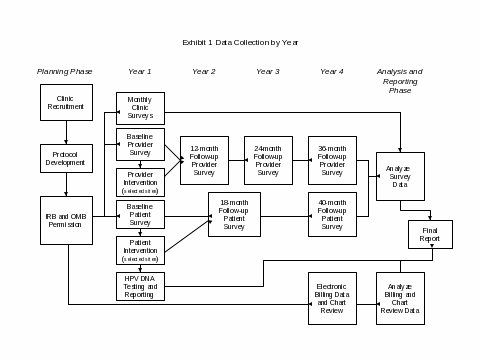
All providers (approximately 70) who routinely perform Pap tests at the participating clinics will be surveyed at four points in time to assess knowledge, attitudes, and beliefs regarding cervical cancer screening practices. CDC’s Cervical Cancer Study (Cx3 Study) Baseline Provider Survey (Attachment D1) will be administered prior to study initiation and CDC’s Cervical Cancer Study (Cx3 Study) Follow-up Provider Survey (Attachment D2) will be conducted 12 months, 24 months, and 36 months following study initiation. Mail surveys will be used. The surveys will be mailed to the participating clinics and distributed to providers. The completed surveys will be returned to the study contractor for data entry and analysis. In Phase I, we will complete the baseline and two of the three planned follow-up surveys.
A sample of 2,600 patients (1,300 in each study arm) will be asked to complete a baseline survey and two follow-up surveys to assess knowledge, attitudes, beliefs, and behavior regarding cervical cancer screening. CDC’s Cervical Cancer Study Patient Survey–Baseline Survey (Attachment E1) will be administered in the clinic prior to the patient’s HPV test by clinic staff. The completed baseline surveys will be returned to a central collection point in sealed envelopes and forwarded to the study contractor for data entry and analysis. CDC’s Cervical Cancer Study Patient Survey–Follow-up Survey (Attachment E2) will be administered by mail 18 months and 40 months following the date of study enrollment. The study contractor, Battelle, will conduct the mailing, track the responses, and enter and analyze the data. In Phase I, we will complete the baseline and one of the two planned follow-up surveys.
In Phase I of the study, each patient enrolled in CDC’s Cervical Cancer Study (Cx3 Study) will receive an HPV test in addition to her scheduled Pap test. The specimen for the Pap test will be collected, shipped, tested, and reported in accordance with the usual procedures in place in the clinic. A second specimen will be collected by the provider during the exam for shipment and testing at the CDC HPV laboratory. Clinic staff will be responsible for labeling and shipping the specimen. CDC lab staff will provide the results (positive or negative) to Battelle using the Laboratory User Network Application (LUNA) that was developed by CDC for tracking and reporting laboratory test results. Battelle will merge the test result data from LUNA with patient names and prepare a laboratory report for each patient. Battelle will send the reports to the clinic/provider. The clinic/provider will inform the patient of the results of the HPV test following their standard reporting procedures. Any follow-up suggested by the test results will also be arranged for and completed by the clinic according to their standard procedures. As part of the study, clinics will receive additional HPV tests to use for follow-up purposes. The CDC HPV lab will also conduct HPV typing on the specimens but these results will only be provided to Battelle for use in data analysis and not to the clinic/provider for use in clinical care. If patients consent to long-term storage of their specimen, CDC will store the specimens submitted to the CDC HPV lab for a period of up to 10 years.
Finally, at the time of study enrollment, patients will be asked to give consent for study personnel to access their medical and billing records. Billing records provide the information necessary to determine whether or not adjunct HPV DNA testing leads to extended screening intervals for women with negative results. During Phase II, clinic staff will be asked to provide information about clinic visits for each patient for a period of 40 months following the date of study enrollment. Clinic staff will also review the medical records of all women with a positive test outcome (i.e., abnormal Pap and/or positive HPV) to determine what type of follow-up care is received.
Items of Information to be Collected. Each proposed data collection activity is listed in Table A.1-1 below, with specific reference to the presence or absence of Information in Identifiable Form (IFF). Data collection instruments referred to are provided as attachments to this information request.
Baseline and follow-up clinic surveys. Participating clinics will be surveyed monthly (a baseline survey and 11 follow-up surveys) during the first year of the study. The initial clinic survey (Attachment C1) will request information about the clinic patient population and practice characteristics, as well as the staff time associated with participating in the Cx3 Study. The follow-up clinic surveys (Attachment C2) will primarily collect information regarding the staff time associated with participating in the Cx3 Study. No IFF information is requested in the clinic surveys.
Baseline and follow-up provider surveys. All providers (approximately 70) who routinely perform Pap tests at the participating clinics will be surveyed at four points in time to assess knowledge, attitudes, and beliefs regarding cervical cancer screening practices. A baseline provider survey (Attachment D1) will be administered at study initiation and follow-up provider surveys (Attachment D2) will be conducted 12 months, 24 months, and 36 months following study initiation. IFF information included in the surveys is limited to date of birth (month/year).
A.1-1 Summary of IFF
IIF Category |
Form Name(s) |
Special Safeguards* |
Name |
Patient Enrollment Form |
Stored separately from study data through use of an ID system |
Date of Birth (month/year) |
Patient Enrollment Form |
Stored separately from study data through use of an ID system |
Baseline provider survey Follow-up provider surveys Baseline patient survey Follow-up patient surveys |
Survey data stored outside Web STAR and separately from enrollment data. Only Battelle staff has access. |
|
Mailing Address |
Patient Enrollment Form |
Stored separately from study data through use of an ID system |
Phone Numbers |
Patient Enrollment Form |
Stored separately from study data through use of an ID system |
Medical Information and Notes |
LUNA |
Stored separately from patient enrollment data containing names and contact information |
* The provider survey data will be protected by Certificate of Confidentiality
Patient consent and enrollment forms. Approximately 8,000 women between the ages of 35 and 60 who are visiting one of 18 participating clinics for routine cervical cancer screening will be recruited for the study. Using a patient recruitment script (Attachment E1a), patients will be recruited for participation in the study by clinic staff at the clinic as they await a previously scheduled Pap test. Women who meet the eligibility criteria for inclusion in the study will be asked to sign one of two study consent forms—either the patient consent form including the patient survey (Attachment E1b) or the patient consent form not including the patient survey (Attachment E1c)—depending upon whether or not the patient was selected for the patient survey. The consent form will provide consent to receive the HPV test, to have clinic staff provide information from their medical and billing records, and (if selected) complete baseline and patient follow-up surveys. The consent form also asks patients whether or not they are willing to allow the CDC to store their HPV specimen for up to 10 years after the end of the study for possible future testing. If a patient agrees to participate in the study, she will be asked to complete a patient enrollment form (Attachment E1d). The patient enrollment form contains the following IFF categories: name, date of birth (month/year), clinic patient ID number, mailing address, and phone numbers.
Pap and HPV DNA testing. Each patient enrolled in the Cx3 Study will receive an HPV test in addition to her scheduled Pap test. The specimen for the Pap test will be collected, shipped, tested, and reported in accordance with the usual procedures in place in the clinic. A second specimen will be collected by the provider during the exam for shipment and testing at the CDC HPV laboratory. Clinic staff will be responsible for labeling and shipping the specimen using pre-assigned unique study IDs. Assigned study IDs will accompany the biological specimens and the reporting of study results. The data collection contractor, Battelle, will have the link between the ID and patient identifying data, but this link will be stored separately from the clinical data. Battelle will merge the test results with patient names and prepare a laboratory report for each patient. These reports will be sent to the clinic/provider. The clinic/provider will inform the patient of the results of the HPV test following their standard reporting procedures. Any follow-up suggested by the test results will be arranged for and completed by the clinic according to their standard procedures. HPV and Pap test results will be entered into LUNA for tracking and analysis by lab staff (see discussion of LUNA below). No patient identifying data will be included in LUNA.
Patient baseline and follow-up surveys. A sample of 2,600 patients (1,300 in each arm) will be asked to complete a baseline and two follow-up surveys to assess knowledge, attitudes, beliefs, and behavior regarding cervical cancer screening. The patient baseline survey (Attachment E1) will be administered in the clinic prior to the patient’s HPV test. The patient follow-up surveys (Attachment E2) will be administered by mail 18 months and 40 months following the date of study enrollment. The patient survey data will be stored in a database without identifying information. As described under enrollment form above, identifying information will be stored separately.
Electronic billing data and chart review. At the time of study enrollment, patients will be asked to give consent for study personnel to access their medical and billing records. Billing records will provide the information necessary to answer the primary study question, namely does HPV testing as an adjunct to Pap with provider and patient education lead to extended screening intervals for women with negative results. Billing data and chart review data (Attachment F) will obtained more than 36 months into data collection and thus are not covered under this request.
Identification of Website(s) and Website Content Directed at Children Under 13 Years of Age. The Laboratory User Network Application (LUNA), a web-based system that was developed by CDC, will be used for tracking and reporting laboratory test results. In particular, LUNA will be used to:
Store patient study identification number, whether or not the patient is a BCCP patient, date of last Pap test, last Pap test results;
Store Pap test results, as entered by clinic staff;
Track the shipment of HPV tests from the clinics to the CDC HPV lab; and
Store HPV test results, as provided by the CDC HPV lab.
LUNA will utilize the CDC Secure Data Network (SDN). Access to LUNA will be limited to CDC, clinic, and Battelle staff directly involved in carrying out the study. The web-based tracking system will not contain personal identifying information, nor will it contain survey data. All personal identifying information will be recorded on a patient enrollment form. The patient enrollment form will include: patient name; patient study identification number; clinic name; provider name; patient date of birth (month/year); and patient contract information (e.g., address, telephone number) for patients who are selected to complete the baseline and follow-up patient surveys. The information reported on the patient enrollment forms will be key-entered and stored in a separate data file from the data provided from LUNA. Hard copies of the patient enrollment forms will be stored in a locked file cabinet along with copies of the signed patient consent forms. Reports of HPV test results will be generated by merging data from the patient enrollment forms with data on HPV test results stored in LUNA. A report of the HPV test results for each patient will be sent to the clinic/provider.
There are no websites with content directed at children under 13 years of age or any other segment of the public. All users of LUNA (including clinic coordinators) must register with CDC and be issued a digital certificate that will allow them access to LUNA.
A.2. Purpose and Use of Information Collection
The results of this study will provide information regarding the extent to which providers are willing to extend the cervical cancer screening interval to 3 years for women in the target age range with a normal Pap test and a negative HPV DNA test. It will also provide information regarding whether provider and patient education will lead to extended screening intervals for women who have negative screening results. In addition, the study results will provide information regarding the level of knowledge regarding cervical cancer screening among low-income, underserved women—who represent the demographic most needy of highly sensitive screening methodologies that can increase the likelihood of detecting cervical dysplasia at less frequent screening intervals. The findings from this study will help inform policy regarding the HPV DNA test on a national level for cervical cancer screening in the NBCCEDP.
The NBCCEDP has been responsible for screening an increasing number of women over the past 15 years, serving 626,018 women in FY06, the last year for which complete data are available. Fiscal year appropriations have likewise increased over this period, with over $200 million allocated to this program in FY06. The agency is committed to the ongoing support of this national program. Without the information that will be gained from this study, the national screening program will have no information for informing policy regarding the HPV DNA test. Failure to set reimbursement policies could have a major impact on the efficient allocation of program resources to reach underserved women in the years ahead.
Privacy Impact Assessment Information. The proposed pilot study will collect data needed to inform reimbursement policies for the NBCCEDP related to cervical cancer screening. Specifically, this pilot study will enroll clinics and their physicians and patients into two study arms to compare outcomes associated with routine Pap testing coupled with HPV testing for women age 35-60 years coming in to the clinic for a regular screening Pap. Goals of the study are to: (1) assess whether provider and patient education will lead to extended screening intervals for women who have negative screening results; (2) identify facilitators and barriers to acceptance and appropriate use of the HPV test and longer screening intervals; (3) track costs associated with HPV testing and educational interventions; (4) identify the HPV genotypes among this sample of low income women; (5) assess follow-up of women with positive test results; and (6) determine provider knowledge and acceptability of the HPV vaccine.
To meet these study objectives survey data will be collected from clinics, providers, and patients. Enrollment data and Pap test and HPV test results for enrolled patients will also be obtained. IIF data collected is summarized in Table A.1-1 in Section A.1 (Circumstances Making the Collection of Information Necessary). A series of unique patient ID numbers will be provided to each clinic participating in the study. Clinics will be sent bar-coded labels to be used to identify all data collection forms, as well as the HPV test that will be shipped to the CDC HPV lab. Staff will assign a patient ID to each patient at the time of enrollment and affix this ID to the consent form, the HPV test tube, the patient chart, and the baseline survey. The enrollment form will be sent directly to the study contractor (Battelle). Follow-up surveys will be mailed by Battelle to patients using their pre-assigned ID. Responses will be returned directly to Battelle for data entry. Clinic staff will not have access to the surveys or to the database containing individual responses. Provider surveys will be distributed by clinic staff. Each provider will be provided with a postage-paid return envelope and instructed to send their completed survey to Battelle for data entry and analysis. Clinic staff will not have access to the surveys or to the database containing individual responses.
The unique ID system and the protocol for data handling are designed to protect the information that is collected from accidental disclosure. To further ensure the confidentiality of the baseline and follow-up provider survey data, the project will obtain a Certificate of Confidentiality from CDC. The IRB concluded that with this certificate in place, this project presents minimal risk to study participants. Documentation of Battelle IRB approval of the study is provided as Attachment I.
A.3. Use of Information Technology and Burden Reduction
CDC has contracted with Battelle Centers for Public Health Research and Evaluation (Battelle) to collect, manage, and analyze all data for this study. Information technology tools will be used in three ways to reduce the burden of participation. First, as described in Section A.1 (Circumstances Making the Collection of Information Necessary), we will utilize LUNA to assist with tracking and reporting the shipment of HPV specimens. More specifically, LUNA will be used to: (1) track the shipment of HPV tests from the clinics to the CDC HPV lab and (2) store Pap test results and HPV test results entered by clinic staff and CDC HPV lab staff. Second, Battelle staff will develop a survey tracking database that will be used to monitor clinic, provider, and patient surveys. This tracking database will tell Battelle study staff when to mail follow-up surveys and reminders. Third, Battelle will use electronic methods in the fourth year of data collection to collect and analyze billing records obtained from the clinics.
The clinic surveys, provider surveys, and patient surveys will use hardcopy data collection methods. This method was selected for several reasons. For the patients, a combination of in-person (baseline) and mail was selected because of the low-income population served by these clinics. We anticipate that access to computers may be problematic in this population. According to the U.S. Census Bureau, 62% of households had access to the internet in 2003, although the proportion with access was half for households with income less than $25,000 (31%) (U.S. Census Bureau, 2005). For the clinics and their providers, mail survey was indicated as a preferred mode during early discussions with the clinics about their participation in the study. The clinic survey may require input from a number of sources to complete because of its focus on clinic resources utilized in conducting the study. Hardcopy will facilitate the compilation of this information from various sources within the clinic. Mail survey for providers is preferred for ease of administration given the small number of clinics and providers involved. Because all of the providers are employed by the participating clinics, it will be efficient to distribute the surveys within their existing mail systems.
A.4. Efforts to Identify Duplication and Use of Similar Information
In our efforts to find this information through consultation with medical care providers, researchers and a review of the literature, we were not able to address this issue of HPV DNA testing in a low income, uninsured group of women to inform policy for the NBCCEDP.
In 2002, the FDA approved the HPV DNA test with cervical cytology for cervical cancer screening in women 30 years of age and older. Since the approval, several organizations such as the American College of Obstetrics and Gynecologists and the American Cancer Society support HPV testing for use in combination with cervical cytology. A cost-effectiveness analysis has shown that additional costs associated with introducing HPV testing in conjunction with cytology could be offset by an increase in the screening interval among HPV-negative, cytology normal women because of the low risk of precancer and cancer (Goldie, 2004). However, in the United States, the common practice is annual cytology, either a Pap smear or liquid-based cytology. Some questions have been raised regarding the acceptability of longer screening intervals among those at low risk of disease. Several European prospective studies are examining the acceptability of HPV testing and increasing the screening interval. However, there are no current studies in the U.S. performed in a real-life setting among women who are racially/ethnically diverse.
The use of the HPV DNA test in conjunction with cervical cytology is advocated based on the very high negative predictor value of the combined HPV DNA plus Pap test, usually 99.9 to 100 percent (Lorincz and Richart, 2003). The HPV DNA test has been proved to be reproducible, and is simple to perform (Ratnam, et al, 2000). One benefit to using HPV testing as a adjunct to cervical cytology for screening women is that it identifies not only women with concurrent cervical disease, but also those at risk of developing disease in the future (Wright, et al, 2004). Because of the large number of women with low-grade Pap tests who are HPV negative and thus at lower risk of cervical precancer and cancer, the opportunity is present to safely mange these women with a less intensive follow-up.
In recent studies it has been reported that conventional Pap tests are only about 50-60 percent sensitive in detecting high-grade CIN and cervical cancer, and are less sensitive for lower-grade lesions (Nanda, et al, 2000; Fahey, Irwig, and Macaskill, 1995). Because of this lower sensitivity, the Pap test needs to be repeated with great regularity to necessitate effectiveness. Because of occasional pathology misdiagnoses and consequential possible Pap test litigation, it is vital to have a screening test that can discriminate between patients with and without cervical neoplasia, and those patients that are at risk for developing disease (Lorinez and Richart, 2003). However, most women who develop cervical cancer do so because of lack of screening rather than errors in cytodiagnosis (Nanda, et al, 2000). The combination of HPV and Pap tests avoids the greatest number of invasive cervical cancer cases and deaths, measured biennually (Mandelblatt, et al, 2002).
Changing Provider and Patient Behavior. The educational intervention component of this pilot project provides opportunities to foster a change in the attitudes, beliefs, and practices of patients and providers through behavior change reinforcement and knowledge acquisition. The overall goal of the planned intervention is to increase patient and provider awareness of cervical cancer screening guidelines and intervals, thereby directly impacting the regularity of cervical cancer screening among women participating in the project. The proposed outcome objective aims to increase cervical cancer screening intervals to 3 years for women 35 and older who present a normal Pap test and a negative HPV DNA test. In essence, this objective is designed to decrease patient cervical cancer screening visits to clinic sites, a concept that is contrary to what past social marketing campaigns and patient education interventions sought to communicate: to have a Pap test annually. A majority of primary care and women’s health providers are aware of these changes in guidelines and some are fearful that, among other reasons, they may lose patients to attrition with increased screening intervals, or miss valuable opportunities to screen rarely or never screened women who rarely make office visits. It is vital that the advantages of the use of the HPV DNA test, such as higher sensitivity to identify cervical neoplasia, and the cost-effectiveness of adjunct testing be communicated to providers and patients alike, using culturally appropriate and evidence-based methods. They also need to understand that the aim is to reduce the frequency of the Pap test but not the annual well woman visit.
Public programs and studies that incorporate health communication and education strategies targeting patients and providers are vital if new testing strategies are to be adopted. Knowledge regarding HPV transmission, the relation between HPV and cervical disease, treatment and management, as well as the impact on clinical practice are necessary for both patients and providers, and have been recommended as future research initiatives (Wright, et al, 2004). This pilot project in Illinois will not only incorporate the provider as a central component to knowledge acquisition in the community, but will also study the adoption and adherence to new screening guidelines by patients and providers among a diverse population, and will observe the effects of behavior change through evidence-based educational interventions.
Justification for study:
Previous studies have indicated that multi-component interventions that target both the patient and provider have been successful in achieving objectives and goals for improved cancer screening coverage and increase in knowledge (Curbow, et al, 2004; Dignan, et al, 1994; Jenkins, et al, 1999; Taylor, et al, 2002; Dietrich, et al, 1989; Shelley, et al, 1991; Suarez, et al, 1993). A study published in 2006 implemented in rural Arkansas to influence primary care provider’s cancer screening practices in medically underserved and rural areas cited using academic detailing, which is physician education to assure accurate screening guideline knowledge; patient education to increase awareness of risk factors and regular screening; and patient generated screening questionnaires to prompt discussion between patients and providers to improve screening among the underserved. Academic detailing visits between the provider and a retired physician occurred every 6-8 weeks to reinforce expected behavior led to an increase in physician knowledge about cancer screening. For the patient education component, education centers were created in the waiting areas including brochures and a 45 minute video and patient interviews indicated increased knowledge led to increased intent to receive preventive cancer screening. Results from the education intervention for the physician found a 100% correct response rate upon post-testing from the academic detailing sessions with the physician educator. This study illustrates that the inclusion of health care providers is vital to reducing health disparities (Rutledge, et al, 2006). Because no studies have examined the extent to which patient and provider education is effective in changing attitudes and behavior regarding HPV DNA testing in a low income, uninsured group of women, this pilot study is necessary to inform policy for providing cervical cancer screening to women served by the NBCCEDP.
A.5. Impact on Small Businesses or Other Small Entities
No small businesses will be involved in this data collection.
A.6. Consequence of Collecting the Information Less Frequently
This request is for a one time study that follows providers and patients in selected clinics for 3 years after enrollment. This information is essential to inform future CDC reimbursement policies for the NBCCEDP. The duration of the study is necessary to answer the study questions related to screening intervals. Specifically, the study will answer whether or not there is an increase in the cervical cancer screening interval to 3 years for women in the target age range with a normal Pap test and a negative HPV DNA test. Clinic staff will provide information monthly related to the costs of implementing the study during the first year. Providers will complete a baseline survey and 3 follow-up surveys (one each year) to assess changes in attitudes and behaviors related to cancer screening. Patients will complete a baseline and 2 follow-up surveys (18 months and 40 months) to assess changes in their attitudes and behaviors. This level of follow up is required to answer the study questions and complete the analyses described in Section A.16 (Plans for Tabulation and Publication and Project Time Schedule). There are no legal obstacles to reduce the burden.
A.7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
This request fully complies with the regulations 5 CFR 1320.5.
A.8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A. The Federal Register Notice (Attachment B1) for the proposed data collection was posted in the Federal Register on March 7, 2008, Volume 73, page 12449-12451. On May 2, 2008 a comment was received from Dr. Lee H. Hilborne, President of the American Society for Clinical Pathology. A summary of the public comment and CDC response is included provided as Attachment B2.
B. The study protocol, including the survey instruments, sampling plans, and data collection procedures were designed in collaboration with researchers at Battelle Centers for Public Health Research and Evaluation through Contract No. 200-2002-00573, Task Order 6, entitled “Developing a Protocol for a Follow-Up Study of HPV Testing Among Women Undergoing Routine Cervical Cancer Screening.”
Four consultants provided input to CDC and Battelle in the development of the study protocol, data collection instruments and patient and provider intervention materials. The names, titles, telephone numbers, and email addresses of the consultants—along with their organizational affiliations—are provided in Table A.8 – 1.
A.8-1 Study Consultants
Year |
Consultant |
Agency/Organization |
2007
|
George
F. Sawaya, MD
Phone
415-502-4090 |
University of California, San Francisco Departments of Obstetrics, Gynecology and Reproductive Sciences; Epidemiology and Biostatistics |
2007 |
Allen J. Dietrich, MD Professor Phone: 603-653-3648 |
Dartmouth Medical School Department of Community and Family Medicine |
2007 |
Shalini Kulasingam, PhD Assistant Professor Phone: 919-286-3399 kulas002@mc.duke.edu |
Duke University School of Medicine Department of Obstetrics and Gynecology |
2007 2008 |
Philip
Castle, PhD, MPH |
National
Cancer Institute, NIH, DHHS |
A.9. Explanation of Any Payment or Gift to Respondents
Patients will receive $5 for each survey completed (baseline plus 2 follow-ups at 18 and 40 months). Providers will receive $50 for each survey completed (baseline plus 3 follow-ups at 12, 24 and 36 months). Clinics will receive a gift with a value of $10 for completing monthly surveys (monthly for 1 year). We understand that high response rates are critical to ensure valid and precise survey estimates and reduce potential for bias.
There is clear and consistent evidence that monetary remuneration significantly increases response rates to mail, telephone and face-to-face surveys, and experts on survey methods recommend their use (Dillman, 1978; Dillman 2000; Sudman, 1985). Church (1993) and Singer and colleagues (1999) have published meta analyses comparing the response rates of mail and interviewer-mediated surveys with and without monetary incentives. These studies have clearly shown that even a nominal gratuity increases response rates, and that the amount of the incentive is positively correlated with response rate (Kropf, et al., 1999; Hopkins and Gullickson, 1992; Fox et al., 1988; Harvey, 1987). Furthermore, combining other measures to increase response (e.g., sending advance letters, repeated follow-up with non-respondents) with monetary payments has been shown to produce higher response rates than payments alone or other types of incentives without payments (Collins et al., 2000; Yamarino, et al, 1991).
Previous research suggests that monetary incentives may be especially effective in recruiting low-income and minority respondents. For example, analyses by Singer, Van Hoewyk, and Maher (2000) indicate that a $5 incentive paid to a random half of households in a random digit dialed telephone survey brought a higher percentage of low-education respondents into the sample. Our proposed study will include a high percentage of African American and Hispanic women. We feel that it will be particularly important to obtain a high response rate from these minority populations—and to maintain high response rates, particularly to the 18-month and 40-month follow-up surveys.
Finally, in addition to increasing survey response rates, a few studies have examined the impact of incentives on data quality (Shaw et al., 2001; Shettle and Mooney, 1999; Singer, et al, 2000). For example, experiments reported by Singer and associates (2000) indicate that promised and prepaid incentives reduce the tendency of older people and nonwhites to have more item missing data, resulting in a net reduction in item nonresponse. These studies suggest that offering an incentive may improve data quality in the sense that respondents who were provided incentives had less item-missing data and provided longer open-ended responses compared with respondents who were not provided incentives.
A gift incentive was chosen for the clinic survey rather than a monetary incentive so that the benefit could be shared with other staff who contributed to its completion. Both the patient and provider survey respondents are individuals and cash payment was selected as the preferred incentive. Research indicates that mailing incentives along with the questionnaire raises response rates more effectively than promising an incentive upon receipt of a completed questionnaire (Dillman, 2000). Therefore, for the follow-up survey, Battelle will mail the incentive as part of the initial questionnaire mailing to further improve response rates.
Several CDC studies have provided a monetary incentive to respondents. For example, a recent study entitled “Preventive Cardiac Health Care Knowledge, Beliefs, and Behaviors in Female Carriers of Duchenne/Becker Muscular Dystrophy” (OMB No. 0920-0718) provided $5 to each of 1,477 women who participated in a mail survey. These women were selected from mailing lists of the Muscular Dystrophy Association (MDA) or the Parent Project Muscular Dystrophy (PPMD) organization. Another CDC study entitled the “Arthritis Health Condition Effects Survey (ACHES)” (OMB No. 0920-0673) provided a $5 incentive to individual who responded to a random digit dial telephone survey. Finally, the CDC study entitled the “Study to Explore Early Development (SEED)” (OMB No. 0920-0741) involved incentives to families with young children, many of which included children with autism or other developmental disabilities. In this longitudinal study, incentives ranged from $25 included in the enrollment packet, to $30 included in questionnaire packets, to $80 for clinic visits.
In other recent studies targeting physician and other medical office and hospital-based respondents, incentives have been provided as compensation for their time and inconvenience and in recognition of their contributions to the studies’ goals. Examples include:
HPV Provider Survey: Knowledge, Attitudes, and Practices About Genital HPV Infection and Related Conditions (OMB No. 0920-0629)
Sponsor: Division of STD Prevention, National Center for HIV, STD, and TB Prevention
Incentive Payment: $50
Population: a national sample of 7,000 clinicians from 9 specialties
Response Rate: 81%
Survey of Endoscopic Capacity at the State Level (OMB No. 0920-0590)
Sponsor: Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion
Incentive Payment: $40
Population: the universe of physician practices, ambulatory survey centers and hospitals that perform flexible sigmoidoscopy and/or colonoscopy to screen for CRC in 15 selected states
Response Rate: 80%
A.10. Assurance of Confidentiality Provided to Respondents
For the purposes of the clinic survey, the respondent is an organizational entity, not an individual. Although a contact person completes the clinic survey on behalf of the respondent organization, the contact person is speaking from their role as an employee, and does not report personal information. The name of the contact person is not maintained in study data files.
For both the provider and patient surveys Battelle must maintain the link between both provider names and patient identifying information and their respective participant ID numbers. These links will be used for tracking survey mailings, and to link responses to all follow-up surveys. While Battelle will have the capability to link responses to individual participants, this capability will only be present until data collection is completed. At that point, the tracking files will be destroyed and there will be no way to link responses to individuals. The link between identifying information and ID numbers will be stored securely and separately.
To further protect patient confidentiality, patient identifying information contained in the enrollment form will be kept in a separate data file that will be available only to authorized project staff. Patient clinical data will be stored in LUNA on the CDC Secure Data Network SDN. Access to LUNA will be granted only to clinic, Battelle, and CDC project staff that have been issued digital certificates.
Data will be treated in a confidential manner and will not be disclosed. Neither the names of respondents nor the institutions they represent will be identified in published reports or publicly available data. Completed paper surveys (for clinic respondents, provider respondents, and patient respondents) will be stored in locked file cabinets in Battelle offices. All electronic files will be password protected and accessible only to authorized project staff. Measures to safeguard data will be emphasized in written and verbal training procedures for project personnel. To protect the confidentiality of the provider survey data, the project has requested a Certificate of Confidentiality from CDC. The IRB concluded that with this certificate in place, this project presents minimal risk to study participants. Documentation of Battelle IRB approval of the study is provided as Attachment I.
Privacy Impact Assessment Information.
A. This submission has been reviewed by staff in the CDC Information Collection Request Office, who determined that the Privacy Act applies to some components of the information collection. The applicable System of Records Notice (SORN) is 09-20-0136, Epidemiologic Studies and Surveillance of Disease Problems.
B. A Certificate of Confidentiality has been requested from the Associate Director for Science, CDC. This is authorized by section 301[d] under the Public Health Service Act. In addition, the contractor Battelle will use security controls to protect against unauthorized access, modification, destruction or disclosure of data through access control and authentication. Security controls will protect privacy and confidentiality of personal identifying information (PII) and personal health information (PHI) through technical controls, administrative controls and physical controls.
Technical Controls. Enrollment and survey data collected during performance of this study will be stored in Battelle’s SQL Server databases on Local Area Networks (LANs) behind firewalls. Each subject is assigned a unique subject ID that is the unique identifier on all analytic and survey data records, assuring that personal identifying information is not stored with the data and all analysts are blinded to the subject’s identity. The link between personal identifying information and the assigned ID is stored in a separate secured database table with controlled access. Analytical data sets may be stored on analysts’ PCs when they are working with the data. All Battelle PCs are currently Windows XP Professional, Service Pack 2 and access is controlled.
Physical Controls. All servers are located in secure controlled access areas. Physical access to Battelle offices during non-office hours requires possession of an electronic card. During office hours all visitors can only enter through a staffed reception area where they are logged in and must be escorted at all times while on the premises. Within each Battelle office are additional secure areas that have secured access at all times. All server rooms require 24-hours electronic card access. Each electronic card is programmed for a specific user and provides that user with access to all areas to which they are authorized. Battelle offices also have alarm systems monitored by professional security agencies that are activated when the offices are vacant. Authorized users have individual access codes and all access, including invalid attempts, are logged. In addition to these general security measures, sensitive material is stored in locked file cabinets when not in use. Only office administrators and staff authorized to work with these materials have keys to these file cabinets. Battelle staff are trained in these policies and periodically reminded of their importance. Battelle staff members are required to lock their computers when away from their desk using Windows XP Task Manager. Password-protected auto-locking is configured to activate after 10 minutes of inactivity.
Administrative Controls. Battelle’s IT division maintains an intranet site on Cybersecurity Policies and Procedures that is accessible by all employees. This site includes staff responsibilities for protecting data and security requirements for protection of the network, PCs, mobile devices and the data residing on them. In addition, the IT division frequently sends emails to all staff reminding them of specific security issues, such as use of the internet, remote access, email safety, etc. Battelle is in the process of developing its own IT Security Awareness training.
SQL Server databases are backed up nightly to a folder on the server’s hard drive and integrity is verified upon completion. The folder containing these full database backups is then backed up to tape as part of our network backup plan. The network backups provide nightly incremental backups and full backups on weekends for all data stored on Battelle LANs and WANs. Tapes are stored offsite at secure contracted facilities. Permissions to project databases are limited to staff members assigned to work on the project. Non-technical project staff can only access the data indirectly through applications and are authenticated by username and password when logging into the application. All PC-based files, folders, and applications are backed up nightly to a secure server in encrypted format using Connected DataProtector software. Laptops are backed up using this software when staff reconnects to the Battelle network. Files remain encrypted while stored and only the owner of the files and the IT administrator has the encryption key. Staff can elect to backup or restore files at any time in addition to the automatic backup. A Battelle technical staff member is responsible for transferring data to CDC and participating clinics in a secure manner and for receiving data from these agencies and securing it. Identifying information is always stored and transferred separately from analysis data. Records will be retained and destroyed in accordance with the applicable CDC Records Control Schedule.
C. The respondent to the clinic survey, as described above, is an organization and not a person. The clinics have consented to be in the study and have entered into a contractual arrangement for their participation. The clinic survey is part of their obligation under the study contract. No further consent is sought at the time that the survey is distributed.
Providers within a study clinic are asked to participate in the study by their clinic. Participation includes conducting HPV tests in addition to already scheduled Pap tests and, if in the education arm of the study, participating in provider education and providing education to their patients at the time of the exam. Separate consent will be obtained for participation in the baseline and follow-up surveys. Provider surveys will be distributed by the on-site clinic coordinator to the providers within that clinic. The consent language for the survey is contained in the cover letter for the baseline provider survey (Attachment D1a), as well as in the cover letter for the follow-up provider survey (Attachment D2a). Completing the survey and returning it in the envelope provided will be taken as indication of consent. This will be true for both the baseline and follow-up surveys.
Patient enrollment and consent will be the responsibility of clinic staff. Clinic staff serving in this capacity will be trained by Battelle to perform this function. Following the patient recruitment script (Attachment E1a), the clinic staff member will approach a potentially eligible woman in the waiting room as she awaits her previously scheduled Pap test. The staff member will notify the woman that the clinic is participating in a cervical cancer screening study for the CDC and that she may be eligible for inclusion in the study. If the woman is eligible according to the 5 screening questions included in the script, the patient will be invited to enroll in the study. If she is not eligible, she will be thanked for her time and is informed that her provider will see her as soon as possible for her scheduled appointment. If she is eligible, the staff member will hand her one of two consent forms—one for patients who will be surveyed (Attachment E1b), another for patients that will not be surveyed (Attachment E1c). The consent form will be in either English or Spanish, as the patient prefers. The staff person will answer any questions that the patient may have about the consent form or the study. If the patient agrees to participate, she will be asked to sign the consent form. The staff member will also sign the consent form. A copy of the consent form will be provided to the patient, another will be kept in the medical record, and a third copy will be provided to Battelle.
After the consent form is signed, the staff member ask the patient to complete the patient enrollment form (Attachment E1d). If the woman is selected for the patient survey, contact information must be included on the enrollment form to allow Battelle to contact her for follow-up surveys. The staff member will then hand the patient the baseline survey, an envelope, and a pencil. She will be instructed to complete the survey, put it in the envelope, seal the envelope, and return the envelope to the staff member, at which time she will receive the $5 incentive. After she has completed and returned the survey (or after enrollment if she is not selected for the survey), the staff member will ask her to please be seated and that the provider will see her as soon as possible. The follow-up surveys will be conducted by mail. The consent language is repeated in the cover letter for the patient follow-up surveys (Attachment E2b).
D. The respondent to the clinic survey, as described above, is an organization and not a person. The clinics have consented to be in the study and have entered into a contractual arrangement for their participation. Completion of the initial clinic survey (Attachments C1) and the follow-up clinics surveys (Attachment C2) are part of the clinic obligation under the study contract. However, provider surveys will be completed on a voluntary basis. The providers are informed of this in writing in the cover letter for the baseline provider survey (Attachment D1a), as well as in the cover letter for the follow-up provider surveys (Attachment D2a). Likewise, patient participation in the study is voluntary. If a patient is selected for the patient survey, the patient consent form (Attachment E1b) asks for their consent for both baseline and follow-up surveys. This consent, as described, is in person and is written and signed. Specific language stating the voluntary nature of their participation is in the consent form. The follow-up surveys will be conducted by mail. The consent language is repeated in the cover letter for the patient follow-up surveys (Attachment E2b).
A.11. Justification for Sensitive Questions
Topics typically considered to be of a sensitive nature include sexual practices, alcohol or drug use, religious beliefs or affiliations, immigration status, and employment history. No information is being requested regarding these practices. The patient surveys involve the collection of information that may be considered sensitive by a portion of respondents, such as data concerning sex partners and income. The provider surveys involve the collection of information that may be considered sensitive by a portion of respondents, such as data regarding professional practices as they relate to professional guidelines. Thus, although some information may be considered sensitive by a portion of respondents, the information is required for the planned analyses and use of survey results. As described in Section A.10 (Assurance of Confidentiality Provided to Respondents), appropriate measures to safeguard respondent privacy have been instituted, including obtaining a Certificate of Confidentiality from CDC to protect the confidentiality of the provider survey data.
A.12. Estimates of Annualized Burden Hours and Costs
A total of 18 clinics will participate in the study. All clinics are expected to complete the initial and 11 follow-up surveys. It is expected that the staff person assigned to complete the survey will be a nurse and that the initial survey will take 2 hours to complete and the follow-up survey 1 hour. All 70 physicians enrolled in the study at these participating clinics are expected to complete a baseline survey and 3 follow-up surveys. The third follow-up survey will take place in year four and thus is not included in this burden table. Both physician surveys are estimated to take 30 minutes to complete. The study will enroll 8,000 women in the study. To obtain this level of participation, we estimate that 10,000 women will be screened. Only 2,600 of these women will be asked to complete a baseline and 2 follow-up surveys. An 80% retention is expected from baseline to first follow-up and from first to second follow-up. In addition, an estimated 90% of these will be Pap normal and HPV negative and thus will be eligible to be included in analyses to determine the effect on screening interval. Thus the total number of surveys anticipated in each wave is as follows: 2,600 (baseline); 2,340 (eligible for interval analysis); 1,872 (first follow-up); 1,498 (second follow-up). Only the baseline and first follow-up is included in Table A.12-1 because permission is being requested for only the first 3 years (Phase I) at this time. The estimated annualized burden hours for respondents participating in Phase I of the study are 1,006.
A.12-1 Estimates of Hour Burden
Type of Respondent |
Form Name |
No. Respondents |
No. Responses per Respondent |
Average Burden per Response (in hours) |
Total Burden Hours |
Clinic Coordinators
|
Initial Clinic Survey |
6 |
1 |
2 |
12 |
Follow-up Clinic Survey |
6 |
11 |
1 |
66 |
|
Health Care Providers |
Baseline Provider Survey |
23 |
1 |
30/60 |
12 |
Follow-up Provider Survey |
23 |
2 |
30/60 |
23 |
|
Patients |
Patient Screening Script |
3,333 |
1 |
5/60 |
278 |
Patient Enrollment Form |
2,667 |
1 |
5/60 |
222 |
|
Baseline Patient Survey |
867 |
1 |
20/60 |
289 |
|
Follow-up Patient Survey |
624 |
1 |
10/60 |
104 |
|
|
Total |
|
|
|
1,006 |
The cost to respondents for the study is shown in Table A.12 – 2. There are no costs to respondents other than their time to participate. Hourly wage rates were obtained for the state of Illinois for providers and clinic staff using the mean wage for general and family practitioners and registered nurses, respectively. These rates were obtained on February 1, 2008 at the following website: http://www.bls.gov/oes/current/oes_il.htm#b29-0000. We assumed an hourly wage rate of $10, ($2 above the 2009 Illinois state minimum wage) for the patients. Using these estimates, the total annualized cost to respondents for the first 3 years of the study is $13,403.
A.12-2 Annualized Cost to Respondents
Type of Respondent |
Total Burden Hours |
Hourly Wage Rate |
Respondent Cost |
Health Care Providers |
35 |
67.16 |
$2,351 |
Patients |
893 |
10 |
$8,930 |
Clinic Coordinators |
78 |
27.21 |
$2,122 |
Total |
|
|
$13,403 |
A.13. Estimate of Other Total Annual Cost Burden to Respondents or Recordkeepers
The data collection entails no additional costs to respondents or recordkeepers.
A.14. Annualized Cost to the Federal Government
This project has been fully funded by CDC. The project costs are shown in Table A.14-1. These total costs include (1) contract costs for Battelle for data collection and to subcontract with the clinics to conduct the study, (2) the cost of CDC staff to provide oversight to the study, and (3) the cost of CDC HPV lab to perform HPV DNA testing. The total contract cost for carrying out the project is $1,223,915 over a period of 36 months. The annualized contract cost is $407,970. The CDC oversight costs include personnel costs of Federal employees involved in oversight, estimated at $143,978.40 (25% of an FTE at GS-13, 10% Grade 05 Commissioned Corps Medical Officer, 5% of an 3 FTEs at GS-13) over the entire 36-month project period. The CDC Lab costs include personnel costs of Federal employees involved in conducting the HPV DNA testing estimated at $131,052.60 (5% of an FTE at GS-15, 5% of an FTE at GS-13, 5% of an FTE at GS-15 and 100% FTE at GS-11) over the entire 36-month project period. Thus, the total cost to the government over 36 months is $1,498,946 and the average annualized cost to the government is $514,647.60.
A.14-1 Annualized Cost to the Federal Government
Battelle Contract Costs |
Total 36- months |
Annualized Costs |
Personnel |
444,379 |
148,126 |
Data Collection materials/services |
554,384 |
184,794 |
Subcontract |
225,152 |
75,050 |
Total Contract Costs |
1,223,915 |
407,970 |
CDC Costs |
|
|
CDC Oversight |
143,978.40 |
47,992.80 |
CDC HPV lab |
131,052.60 |
58,684.80 |
Total CDC Costs |
275,031.00 |
106,677.60 |
Cost to Federal Government |
1,498,946 |
514,647.60 |
A.15. Explanation for Program Changes or Adjustments
This is a new data collection.
A.16. Plans for Tabulation and Publication and Project Time Schedule
A. Tabulation Plan
Calculation of Sampling Weights. HPV testing will be offered to all eligible patients who are seen over a 12-month period at all clinics or until 8,000 tests are used, whichever occurs first. No sampling weights will be needed because this represents the population of eligible women.
Patient surveys will be administered to a subset of women. Starting on the same date in all clinics, patient surveys will be offered to each patient who agrees to HPV testing until a sample of 2,600 women have been accrued across all clinics. In order to give clinics and providers time to adjust to the study, the commencement of patient surveys will not begin until the second month of the intervention is underway for all clinics. Because this sampling procedure maintains the same proportions as found in the population of eligible women, no sampling weights will be needed.
Given their promise of participation, we expect 100% response rates to surveys from clinics and providers therefore no weights will be created for these groups.
Data Analysis. Data will be pooled across clinics, providers and patients. The survey data will be examined with univariate, bivariate and multivariate analyses, including analyses at both the provider- and patient-level. First, univariate analysis will be conducted on all items in the survey questionnaires. Second, bivariate analyses will be conducted to examine overall associations between key constructs. Third, multivariate analyses will be conducted in selected instances to determine the independent and mediating influence of factors. Tests of significance will be conducted with methods which adjust for clustering of participants within clinic and provider.
Scale constructs will be computed with data from the patient and provider surveys. Prior to creating these indices, we will make appropriate transformations of the questionnaire scales to ensure that low values are indicative of low support and high values are indicative of high support for HPV and Pap outcome measures. The questionnaire responses will also be standardized (subtracting the mean and dividing by the standard deviation) to ensure that they are all on the same scale. Factor analysis will be conducted to examine factor structure and Cronbach’s alpha’s will be calculated. Inter-item correlations will be conducted on all items designed to be measuring these constructs, to ensure that convergent validity exists, yet items account for separate variances.
The goals of this study are to:
Assess whether the use of provider and patient education will lead to extended screening intervals for the women who have negative screening tests;
Identify facilitators and barriers to acceptance and appropriate use of the HPV test and longer screening intervals among women who have negative screening tests;
Track costs associated with HPV testing and educational interventions;
Identify prevalence of HPV genotypes;
Secondary goals of this study are to:
Assess follow-up of women with positive test results.
Examine provider knowledge and acceptability of HPV vaccine.
Table Shells. Attachment H1 provides tables which summarize the measures that will be included in the clinic, provider, and patient surveys in order to achieve the goals of the study. In addition to the survey instruments, information will be gathered from an extraction of (1) clinic electronic medical billing records for each woman who received an HPV test to assist in the determination of the screening interval, and (2) patient medical records to examine follow-up treatment provided for women with positive test results. The discussion below provides an overview of the analyses to be performed.
Descriptive analyses
Examples of table shells that have been created to display the results of the analyses are included in Attachment H2. Table Shell 1 provides an example of the types of measures that will be used to describe the characteristics of the clinic, patient and provider samples, and to compare these characteristics across the intervention and control sites. Significance of differences will be tested with Pearson chi-square for categorical measures and ANOVA for continuous measures. Analysis plans for each study goal are outlined below.
Assess whether provider and patient education will lead to extended screening intervals for women who have negative screening results. Examination of the intervention on extended screening intervals will apply two methods: logistic regression, and event history analysis. The initial models will run standard logistic regression with the dependent variable indicating whether the next Pap was conducted 30 months or more after the baseline Pap versus all other. This analysis will then be expanded to multinomial logistic regression where multiple outcomes can be examined. Four categories for the dependent variable will be defined in the initial analyses: (1) Pap conducted at 30 months or more; (2) Pap conducted at less then 30 months; (3) no follow-up Pap conducted; and, (4) lost to follow-up. Sensitivity analyses will be conducted by comparing the impact of outcome definition on estimates and study conclusions. In addition, event history analysis will be applied to examine the impact of the intervention on a continuous measure of months to next Pap. All of these methods will use patient-level data.
Identify facilitators and barriers to acceptance and appropriate use of the HPV test and longer screening intervals. These analyses add provider and patient survey data to identify factors which influence the acceptance and appropriate use of the HPV test and longer screening intervals. Table Shell 2 provides an example of the types of measures that will be used to examine differences in key constructs which might impact the appropriate use of the HPV test and the extension of the screening interval including knowledge, beliefs and attitudes at the baseline and the initial follow-up survey. Because the baseline patient- and provider-surveys are administered prior to the intervention training and the provision of guidelines to control sites, it is expected that there will be few differences between the control and intervention sites at baseline, and greater differences at follow-up. Significance of differences will be tested with Pearson chi-square for categorical measures and ANOVA for continuous measures.
Multivariate analyses of patient-level outcomes will be completed in two steps. First, bivariate associations will be calculated. Second, independent variables which had a significant bivariate association will be entered following a forward stepwise procedure. The analyses of screening intervals will apply the best models identified in the paragraph above for the first study goal. The method applied to models examining the acceptance and appropriate use of the HPV will depend on the structure of the dependent variable under consideration. Logistic regression will be used for categorical outcomes and linear regression for continuous outcomes. For each analysis where the intervention had a significant bivariate association with the outcome, further analysis will be conducted to see the impact of adding other bivariate significant independent variables into the equation including a measure indicating intervention site. Interactions between key constructs and treatment group will be considered. For instances where the analysis will focus on changes in repeated measures with patient-level data, either a generalized estimating equations approach or a generalized linear mixed models approach will be used.
Table Shell 3 provides examples of measures that will be used to examine the independent effect of constructs on lengthening the screening interval. The column under the heading Biviarate Models will present the estimate and significance level of models which include only the single construct in the estimation model. Those constructs which are significant at the bivariate level will be considered for inclusion in the multivariate analysis. The estimates which are presented in the Multivariate Model column are only for those constructs which were found to have a significant independent effect.
In addition to the patient-level analyses outlined above, provider-level analyses will be conducted to examine changes in provider responses of constructs related to the acceptance and appropriate use of the HPV test and to extending screening intervals. Initial analyses will examine percent and mean distributions at baseline. Changes in provider responses to scales and the impact of the intervention will be examined longitudinally through the use of repeated measures ANOVA. Table Shell 4 provides an example of the presentation of repeated measures across four time points.
Track costs associated with HPV testing and educational interventions. We will begin by estimating the incremental labor cost associated with HPV testing for each clinic. The three main cost components for clinics include (1) training, (2) testing, and (3) evaluation. The third is not a cost component that will be incurred if the program is expanded to other clinics. Total labor cost of HPV training and testing activities will be computed by multiplying staff hours for a particular labor category by the average hourly rate for the category. The total labor cost will be divided by the number of women receiving the HPV test at each clinic to estimate an average cost per patient and this value will be used for estimating the potential cost of expanding the demonstration to more women and clinics. We will also calculate the indirect patient cost per health clinic visit. The total cost of a clinic visit to the patient will be the combination of time cost, travel cost, child care cost and any co-payment amount for the clinic visit. Time cost includes time spent traveling to and from the clinic and time spent at the clinic. The value of the patient’s time will be calculated based on her wage rate reported in the survey. If she is not currently working or has not worked recently, the value of her time will be based on the estimated value for housekeeping services (Haddux, Teutsch, Shaffer, and Dunet, 1996). The average indirect patient cost for a clinic visit will be used to examine potential savings to patients if screening intervals are extended among women with negative results. If there are fewer visits to the clinic because the screening interval for cervical cancer is increased using the HPV DNA test, then this may offset some of the clinic staff costs associated with training in HPV DNA testing and the testing itself.
Identify the HPV genotypes among this sample of low income women. Percent distributions by HPV genotype will be computed for all women, and among the subgroup enrolled in the NBCCEDP. In addition, a summary variable will be created to identify the following result groups: (1) presence of a high risk HPV genotype (16, 18, 31, 33, 35, 39, 45, 51, 56, 58, 59 and 68); (2) presence of other low risk HPV genotypes; and, (3) no HPV detected. The statistical significance of subgroup differences will be tested with Pearson chi-square. Table Shell 5 provides an example of a cross-classification of HPV risk group by age, ethnicity and race.
Assess follow-up of women with positive test results. Separate descriptive analyses will be performed for women with results in the following three categories: (1) HPV positive and Pap abnormal; (2) HPV positive and Pap normal; and, (3) HPV negative and Pap abnormal. With data from a medical chart review, the type of follow-up procedures along with the respective visit date and result will be examined and compared to the American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines. Table Shell 6 provides an example presentation of the distribution of follow-up tests and procedures performed for each of the three groups.
Examine provider knowledge and acceptability of HPV vaccine. Analyses will examine percent and mean distributions at baseline. Changes in provider responses and the impact of the intervention will be examined longitudinally through the use of repeated measures ANOVA for continuous measures and chi-square for categorical measures. Table Shell 7 provides an example of the presentation of baseline results.
B. Publication Plan
Technical reports will be prepared to summarize project activities and the results of the data analysis. The results of the study will also be disseminated to various stakeholders through the publication of manuscripts in peer-reviewed journals and through presentations at professional meetings.
C. Project Time Schedule
A.16-1. Time Schedule
Activity |
Schedule (months after OMB clearance) |
Preparation for data collection and site training |
Months 1-2 |
Site training Provision of provider intervention to intervention sites Provision of guidelines to control sites |
Month 3 |
Clinic Surveys Mailed monthly for 12 months starting in the month following site training |
Months 4-15 |
Provider Surveys |
|
Baseline survey (prior to intervention and guidelines) |
Month 2 |
Follow-up surveys at 12, 24 and 36 months |
Months 15, 27, and 39 |
Patient Surveys |
|
Baseline survey |
Months 5-10 |
18 month follow-up survey |
Months 23-28 |
40 month follow-up survey |
Months 45-50 |
HPV Testing and Reporting of Pap and HPV Results |
|
HPV specimens collected in clinics |
Months 4-15 |
Patient enrollment forms containing personal identifiers and contact information are completed and faxed to Battelle |
Months 4-15 |
Pap test dates and results entered into the tracking database by clinics |
Months 4-15 |
HPV results entered into the tracking database by CDC lab |
Months 4-15 |
HPV results are linked to patient personal identifiers by Battelle and are sent to clinics for entry into patient medical charts |
Months 4-15 |
Tracking Pap/HPV follow-up with clinics |
|
Extraction of electronic billing records from clinics |
Month 56 |
Medical chart review to determine follow-up treatment provided for patients with positive test results |
Months 50-56 |
Data Coding and Analysis |
|
Data coding, entry and cleaning |
Months 2-58 |
Data analysis |
Months15-58 |
Publication of Results |
Months 58-60 |
A.17. Reason(s) Display of OMB Expiration Date is Inappropriate
No exemption from display of expiration date is requested.
A.18. Exceptions to Certification for Paperwork Reduction Act Submissions
No exceptions to certification are sought.
Reference List (in order cited)
Lorincz AT, Richart RM. Human papillomavirus DNA testing as an adjunct to cytology in cervical screening programs. Arch Pathol Lab Med 2003 August;127(8):959-68.
Ratnam S, Franco EL, Ferenczy A. Human papillomavirus testing for primary screening of cervical cancer precursors. Cancer Epidemiol Biomarkers Prev 2000 September;9(9):945-51.
Wright TC, Jr., Schiffman M, Solomon D et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol 2004 February;103(2):304-9.
Nanda K, McCrory DC, Myers ER et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med 2000 May 16;132(10):810-9.
Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol 1995 April 1;141(7):680-9.
Mandelblatt JS, Lawrence WF, Womack SM et al. Benefits and costs of using HPV testing to screen for cervical cancer. JAMA 2002 May 8;287(18):2372-81.
Stange KC, Fedirko T, Zyzanski SJ, Jaen CR. How do family physicians prioritize delivery of multiple preventive services? J Fam Pract 1994 March;38(3):231-7.
Curbow B, Bowie J, Garza MA et al. Community-based cancer screening programs in older populations: making progress but can we do better? Prev Med 2004 June;38(6):676-93.
Dignan M, Michielutte R, Wells HB, Bahnson J. The Forsyth County Cervical Cancer Prevention Project--I. Cervical cancer screening for black women. Health Educ Res 1994 December;9(4):411-20.
Jenkins CN, McPhee SJ, Bird JA et al. Effect of a media-led education campaign on breast and cervical cancer screening among Vietnamese-American women. Prev Med 1999 April;28(4):395-406.
Taylor VM, Hislop TG, Jackson JC et al. A randomized controlled trial of interventions to promote cervical cancer screening among Chinese women in North America. J Natl Cancer Inst 2002 May 1;94(9):670-7.
Dietrich AJ, Carney-Gersten P, Holmes DW et al. Community screening for cervical cancer in New Hampshire. J Fam Pract 1989 September;29(3):319, 321, 323.
Shelley JM, Irwig LM, Simpson JM, Macaskill P. Evaluation of a mass-media-led campaign to increase Pap smear screening. Health Educ Res 1991 September;6(3):267-77.
Suarez L, Nichols DC, Brady CA. Use of peer role models to increase Pap smear and mammogram screening in Mexican-American and black women. Am J Prev Med 1993 September;9(5):290-6.
Rutledge W, Gibson R, Siegel E et al. Arkansas special populations access network perception versus reality – cancer screening in primary care clinics. Cancer 2006 October 15;107(8 Suppl):2052-60.
U.S. Census Bureau. Computer and Internet Use in the United States: 2003. Current Population Reports P23-208. 2005.
Dillman, DA. 1978. Mail and Telephone Surveys. New York, NY: John Wiley & Sons.
Dillman, DA. 2000. Mail and Internet Surveys. New York: John Wiley & Sons, Inc.
Sudman S. Mail surveys of reluctant professionals. Evaluation Review 1985; 9(3):349-360.
Church, AH. 1993. Estimating the effect of incentives on mail survey response rates: a meta-analysis. Public Opinion Quarterly, 57:62-79.
Singer, E, Gebler, N, Raghunathan, T, Van Hoewyk, J, McGonagle, K. 1999. The effect of incentives on response rates in interviewer-mediated surveys. Journal of Official Statistics, 15: 217-230.
Kropf,ME, Scheib, J, Blair, J. 1999. The effect of alternative incentives on cooperation and refusal conversion in a telephone survey. Presented at the 54th Annual Meeting of the American Association for Public Opinion Research, St. Petersburg, Florida, May 13-16, 1999.
Hopkins KD, Gullickson AR. Response rates in survey research: a meta-analysis of the effects of monetary gratuities. J Experimental Education 1992; 61(1):52-62.
Fox, RJ, Crask, MR, Kim, J. 1988. Mail survey response rate: A meta-analysis of selected techniques for inducing response. Public Opinion Quarterly, 52:467-491.
Harvey, L. Factors affecting response rates to mailed questionnaires: A comprehensive literature review. 1987. Journal of Marketing Research, 29(3):341-353.
Collins, RL, Ellickson, PL, Hays, RD, McCaffrey, DF. 2000. Effects of incentive size and timing on response rates to a follow-up wave of a longitudinal mailed survey. Evaluation Review, 24(4):347-63.
Yammarino, FJ, Skinner, SJ, Childers, TL. 1991. Understanding mail survey response behavior: A meta-analysis. Public Opinion Quarterly, 55:613-619.
Singer, E, Van Hoewyk, J, Maher, MP. 2000. Experiments with incentives in telephone surveys. Public Opinion Quarterly, 64(2):171-88.
Shaw, MJ, Beebe, TJ, Jensen, HL, Adlis, SA. 2001. The use of monetary incentives in a community survey: impact on response rates, data quality, and cost. Health Services Research, 35(6):1339-1346.
Shettle, C, Mooney, G. 1999. Monetary incentive in government surveys. Journal of Official Statistics, 15: 231-250.
| File Type | application/msword |
| Author | Battelle |
| Last Modified By | tfs4 |
| File Modified | 2009-03-05 |
| File Created | 2009-03-05 |
© 2026 OMB.report | Privacy Policy